Abstract
Background
Direct-acting antivirals are successful in curing hepatitis C virus infection in more than 95% of patients treated for 12 weeks, but they are expensive. Shortened treatment durations, which may have lower cure rates, have been proposed to reduce costs.
Objectives
To evaluate the lifetime cost-effectiveness of different shortened treatment durations for genotype 1 noncirrhotic treatment-naive patients.
Methods
Assuming a UK National Health Service perspective, we used a probabilistic decision tree and Markov model to compare 3 unstratified shortened treatment durations (8, 6, and 4 weeks) against a standard 12-week treatment duration. Patients failing shortened first-line treatment were re-treated with a 12-week treatment regimen. Parameter inputs were taken from published studies.
Results
The 8-week treatment duration had an expected incremental net monetary benefit of £7737 (95% confidence interval £3242-£11 819) versus the standard 12-week treatment, per 1000 patients. The 6-week treatment had a positive incremental net monetary benefit, although some uncertainty was observed. The probability that the 8- and 6-week treatments were the most cost-effective was 56% and 25%, respectively, whereas that for the 4-week treatment was 17%. Results were generally robust to sensitivity analyses, including a threshold analysis that showed that the 8-week treatment was the most cost-effective at all drug prices lower than £40 000 per 12-week course.
Conclusions
Shortening treatments licensed for 12 weeks to 8 weeks is cost-effective in genotype 1 noncirrhotic treatment-naive patients. There was considerable uncertainty in the estimates for 6- and 4-week treatments, with some indication that the 6-week treatment may be cost-effective.
Keywords: cost-effectiveness, direct-acting antivirals, hepatitis C virus, shortened treatment duration
Highlights
-
•
The cost effectiveness of direct-acting antiviral treatment for chronic hepatitis C virus has been well documented, although the cost of treatment is considerable. Shortened treatment durations have been proposed to reduce costs, albeit at the expense of potentially curing fewer patients.
-
•
Shortening treatment duration from 12 to 8 weeks using direct-acting antiviral therapy is cost-effective for treatment of mild chronic hepatitis C virus in genotype 1 noncirrhotic treatment-naive patients, provided a re-treatment strategy is adopted for patients who fail first-line treatment.
-
•
There was considerable uncertainty in the cost-effectiveness estimates for the 6- and 4-week shortened treatments, with some indication that the 6-week treatment may be cost-effective, but the 4-week treatment may not. More robust evidence on the efficacy of the 6- and 4-week shortened treatment durations is needed.
Introduction
The cost-effectiveness of direct-acting antiviral (DAA) treatment for chronic hepatitis C virus (HCV) has been well documented,1, 2, 3, 4 and a wide array of DAA therapies have been approved for use internationally.5 The therapies, which are generally administered orally over 12 weeks, are successful in more than 95% of patients with chronic HCV genotype 1 (GT1).5 The advent of an effective cure has brought the potential to address HCV globally. The World Health Organization recently outlined its commitment toward eliminating HCV by 2030.6 Nevertheless, the cost of a standard 12-week treatment course is high, and variations in price exist internationally and across DAA regimens. In the United Kingdom, the price set by manufacturers initially ranged from £30 000 to £60 000 per patient,7, 8, 9 whereas in the United States, a 12-week course of treatment can cost more than $90 000 per patient.10 Although significantly lower prices have been agreed between manufacturers and healthcare payers, these prices have not been made publicly available. Shortened treatment duration is a mechanism that could be used to reduce drug costs, albeit at the expense of potentially curing fewer patients.
Recent evidence suggests that shortened treatment durations are associated with lower cure rates in GT1 noncirrhotic treatment-naive patients. Kowdley et al11 reported that the cure rate fell from 96% in patients treated for 12 weeks using a triple-DAA regimen (ombitasvir-paritaprevir-ritonavir with dasabuvir [3D]) to 88% in patients treated for 8 weeks with the same regimen. Sulkowski et al12 considered shorter treatment durations using a combination of 4 DAAs (daclatasvir, asunaprevir, beclabuvir, and sofosbuvir [DCV-Trio + SOF]) and found that 57% and 29% of patients treated over 6 and 4 weeks, respectively, cleared the virus. Other studies considered the effectiveness of existing DAA therapies over shortened treatment durations, but with the addition of an investigational nonnucleoside or protease inhibitor. For example, Kohli et al13 found that 40% of patients were cured when treated for 4 weeks using ledipasvir and sofosbuvir (LDV/SOF) plus a nonnucleoside inhibitor (GS9669).
Although cure rates are lower over shortened treatment durations, patients can usually be re-treated with an alternative, or similar, DAA regimen if first-line treatment fails. One concern with first-line treatment failure, however, is that patients can develop resistance to DAA therapies and this can affect the likelihood of future viral eradication.14 Nevertheless, much evidence suggests that noncirrhotic patients with DAA resistance, or resistance-associated polymorphisms, can clear the virus with further treatment15, 16, 17, 18, 19 even before the advent of combinations with broader antiviral activity. Wilson et al19 found that 90% of patients with DAA resistance were cured with a 12-week re-treatment using LDV/SOF, whereas 91% of patients overall were cured after shortened first-line treatment failure. Bourliere et al20 found that 97% of patients who previously failed first-line treatment cleared the virus over 12 weeks using sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX).
Given the burden of high treatment costs, and the potential to cure as many patients using shortened treatment durations (with a re-treatment strategy adopted for all patients who fail first-line treatment), the cost-effectiveness of short-course therapy needs to be considered. In this article, we compared the lifetime cost-effectiveness of different unstratified shortened treatment durations. We modeled outcomes for GT1 noncirrhotic treatment-naive patients with HCV in the United Kingdom, for whom shortened treatment has been reported in the literature, and for whom shortened treatment may be considered in the future.
Methods
We used a decision tree and Markov model to investigate the cost-effectiveness of shortened DAA treatment from the National Health Service perspective in the United Kingdom. We applied monthly cycles during the first year in the decision tree to simulate treatment outcomes and annual cycles in the Markov model to simulate the natural history of HCV. We adopted a lifetime time horizon (60 years, from an initial age of 40 years) and discounted costs and utilities at 3.5% per annum, as per the National Institute for Health and Care Excellence (NICE) guidelines.21
Target Population
The model simulated outcomes for GT1 noncirrhotic treatment-naive patients infected with HCV, combining data from subtypes 1a and 1b. We modeled outcomes for a representative population with noncirrhotic HCV in the United Kingdom, on the basis of Hartwell et al.22 At baseline, 51.1% and 48.9% of patients had mild (F0-F1) and moderate (F2-F3) liver fibrosis, respectively. At model entry, patients were aged 40 years and 70% were men (Table 1).
Table 1.
Summary of treatment, epidemiological, cost, and quality-of-life inputs for probabilistic sensitivity analyses.
| Variable | Base case | Distribution | α | β | Source |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Initial distribution of liver fibrosis | |||||
| Mild (F0-F1) | 51.1% | – | – | – | Hartwell et al22 |
| Moderate (F2-F3) | 48.9% | – | – | – | Hartwell et al22 |
| Age (y) | 40 | – | – | – | Hartwell et al22 |
| Sex, male | 70% | – | – | – | Hartwell et al22 |
| Efficacy (SVR12) | |||||
| First-line treatment | |||||
| 12 wk | 0.96 | Beta | 76 | 3 | Kowdley et al11 |
| 8 wk | 0.87 | Beta | 69 | 10 | Kowdley et al11 |
| 6 wk | 0.64 | Beta | 6 | 3 | Sulkowski et al12,∗ |
| 4 wk | 0.38 | Beta | 1 | 2 | Sulkowski et al12,∗ |
| Re-treatment | |||||
| 12 wk | 0.973 | Beta | 142 | 4 | Bourliere et al20 |
| Annual transition probabilities | |||||
| Fibrosis progression | |||||
| Mild-to-moderate | 0.025 | Beta | 38 | 1 484 | Grieve et al,23 Wright et al24 |
| Moderate-to-CC | 0.037 | Beta | 27 | 699 | Grieve et al,23 Wright et al24 |
| Nonfibrosis progression | |||||
| CC-to-DCC | 0.039 | Beta | 15 | 359 | Fattovich et al25 |
| CC-to-HCC | 0.014 | Beta | 2 | 135 | Cardoso et al26 |
| DCC-to-HCC | 0.014 | Beta | 2 | 135 | Cardoso et al26 |
| HCC-to-liver transplant | 0.020 | Beta | 98 | 4 801 | Hartwell et al22 |
| DCC-to-liver transplant | 0.020 | Beta | 98 | 4 801 | Grieve et al23 |
| Liver-related mortality | |||||
| DCC-to-liver death | 0.130 | Beta | 147 | 983 | Fattovich et al25 |
| HCC-to-liver death (first year) | 0.430 | Beta | 117 | 155 | Fattovich et al25 |
| HCC-to-liver death (subsequent year) | 0.430 | Beta | 117 | 155 | Fattovich et al25 |
| Liver transplant-to-liver death (first year) | 0.150 | Beta | 85 | 481 | Grieve et al23 |
| Liver transplant-to-liver death (subsequent year) | 0.057 | Beta | 85 | 1 407 | Bennett et al27 |
| Reinfection | 0.010 | Beta | 4 | 391 | Johnson et al3 |
| Costs | |||||
| Treatment-related costs | |||||
| 3D (monthly) | £12 140.56 | Fixed | – | – | National Institute for Health and Care Excellence8 |
| SOF/VEL/VOX (monthly) | £14 942.33 | Fixed | – | – | National Institute for Health and Care Excellence28 |
| Monitoring costs (monthly) | £162.34 | Fixed | – | – | National Institute for Health and Care Excellence8 |
| Health state costs | |||||
| SVR mild (F0-F1) | £60.36 | Gamma | 34 | 2 | Backx et al29 |
| SVR moderate (F2-F3) | £60.36 | Gamma | 34 | 2 | Backx et al29 |
| Mild (F0-F1) | £166.50 | Gamma | 13 | 13 | Hartwell et al22 |
| Moderate (F2-F3) | £612.50 | Gamma | 35 | 17 | Backx et al29 |
| CC (F4) | £951.13 | Gamma | 17 | 54 | Backx et al29 |
| DCC | £12 833.96 | Gamma | 15 | 849 | Hartwell et al22 |
| HCC (first year) | £11 436.41 | Gamma | 13 | 894 | Hartwell et al22 |
| HCC (subsequent year) | £11 436.41 | Gamma | 13 | 894 | Hartwell et al22 |
| Liver transplant (first year) | £51 769.79 | Gamma | 15 | 3 473 | Hartwell et al22 |
| Liver transplant (subsequent year) | £1 949.08 | Gamma | 14 | 136 | Hartwell et al22 |
| Adverse event costs | |||||
| Anemia | £501.58 | Gamma | 10 | 48 | Johnson et al3 |
| Rash | £166.50 | Gamma | 16 | 10 | Johnson et al3 |
| Depression | £414.17 | Gamma | 16 | 26 | Johnson et al3 |
| Neutropenia | £980.26 | Gamma | 10 | 98 | Johnson et al3 |
| Thrombocytopenia | £875.16 | Gamma | 14 | 62 | Johnson et al3 |
| Utilities | |||||
| Treatment-related utilities (penalties) | |||||
| Mild (F0-F1)—3D (monthly) | −0.001 | Fixed | – | – | Johnson et al3 |
| Moderate (F2-F3)—3D (monthly) | −0.001 | Fixed | – | – | Johnson et al3 |
| Health state utilities | |||||
| SVR mild (F0-F1) | 0.820 | Fixed | – | – | Wright et al24 |
| SVR moderate (F2-F3) | 0.710 | Fixed | – | – | Wright et al24 |
| Mild (F0-F1) | 0.770 | Beta | 141 | 42 | Wright et al24 |
| Moderate (F2-F3) | 0.660 | Log-normal | – | – | Wright et al24 |
| CC (F4) | 0.550 | Log-normal | – | – | Wright et al24 |
| DCC | 0.450 | Beta | 55 | 67 | Wright et al24 |
| HCC (first year) | 0.450 | Beta | 55 | 67 | Wright et al24 |
| HCC (subsequent year) | 0.450 | Beta | 55 | 67 | Wright et al24 |
| Liver transplant (first year) | 0.450 | Beta | 55 | 67 | Hartwell et al22 |
| Liver transplant (subsequent year) | 0.670 | Beta | 32 | 16 | Wright et al24 |
CC indicates compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; SOF/VEL/VOX, sofosbuvir/velpatasvir/voxilaprevir; SVR12, sustained virological response at 12 wk; 3D, ombitasvir, paritaprevir, ritonavir with dasabuvir.
Synthesized from Kowdley et al11 and Sulkowski et al12 (for further details, see Appendix 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011).
Treatment Comparators and Regimens
We compared 3 unstratified shortened treatment durations (8, 6, and 4 weeks) against the standard 12-week treatment duration. We considered treatment regimens currently used in the United Kingdom in our analysis. In the base-case analysis, we used a triple-DAA regimen (3D) for first-line treatment because of the availability of data on the effectiveness of shortened treatment.11 3D contains 2 fixed-dose tablets with 12.5 mg ombitasvir, 75 mg paritaprevir, and 50 mg ritonavir, which are taken daily along with 1 dose of 250 mg dasabuvir.8
We assumed that patients who failed first-line treatment were re-treated for 12 weeks, as per recent UK guidelines.30, 31 We used SOF/VEL/VOX as the salvage regimen. SOF/VEL/VOX is a nonstructural protein 5A (NS5A) inhibitor-containing regimen that is administered once daily using a fixed-dose tablet; each tablet contains 400 mg sofosbuvir, 100 mg velpatasvir (NS5A inhibitor), and 100 mg voxilaprevir (protease inhibitor).28 SOF/VEL/VOX is the currently recommended treatment regimen for patients who previously failed first-line treatment in the United Kingdom.30, 31
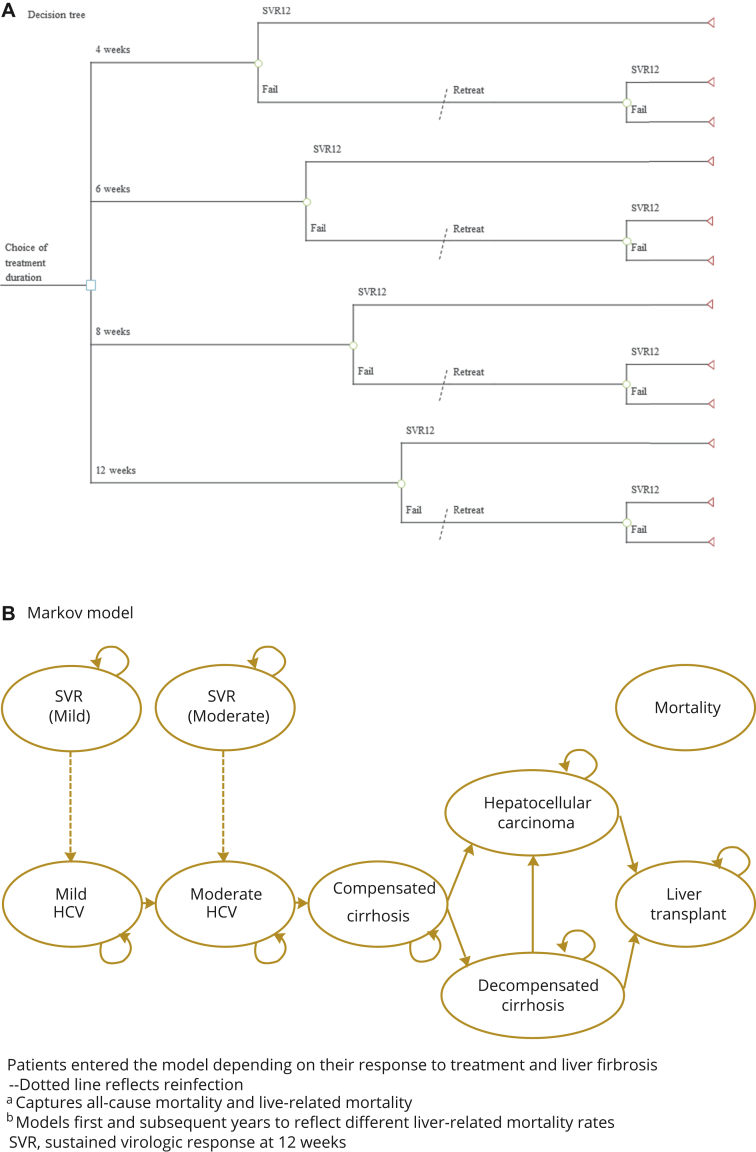
Model Structure
The decision tree was designed to capture treatment outcomes in the first year using monthly cycles (Figure 1A). Patients were assessed for sustained virological response at 12 weeks posttreatment (SVR12, effective cure), which was defined as having HCV ribonucleic acid less than 25 IU per milliliter. Patients who failed first-line treatment were re-treated at 24 weeks. All patients entered the Markov model on the basis of their response to treatment.
Figure 1.
Economic model structure: (A) Decision tree simulating treatment outcomes; (B) Markov model simulating natural disease history.
HCV indicates hepatitis C virus; SVR, sustained virological response.
The Markov model was adapted from previously validated models that characterize the natural disease history of HCV.3, 22, 32, 33 Patients entered the model on the basis of their initial distribution of liver fibrosis (mild or moderate), and whether treatment had been successful (Figure 1B). In HCV-cleared patients, we modeled potential reinfection. Patients without SVR12 could progress from mild (F0-F1) to moderate (F2-F3) to severe liver fibrosis, or compensated cirrhosis (F4). Once in this health state, patients could develop more advanced liver disease, including hepatocellular carcinoma and decompensated cirrhosis. Patients with decompensated cirrhosis were also at risk of hepatocellular carcinoma, with both groups of patients at risk of requiring a liver transplant. We modeled liver-related deaths for each of these advanced health states with 2 health states captured in the hepatocellular carcinoma and liver transplant health states to reflect the initial and subsequent risk of liver-related mortality. At any stage in the Markov model, patients could die of non–liver-related deaths.
Model Assumptions
We assumed there was no progression to more severe health states during treatment, such as compensated cirrhosis (F4), or once treatment had been successful. Only if patients became reinfected could disease progression occur. There are no clinical guidelines on the appropriate length of time patients should wait before a salvage treatment is administered. In our model, we assumed that the wait time did not affect the success of re-treatment.
Parameter Inputs
We informed the model using a synthesis of evidence, as presented in Table 1 and described herein.
Treatment-related inputs
We synthesized evidence from 2 sources to inform the efficacy of first-line 3D treatment. Kowdley et al11 reported SVR12 for 3D after 12 and 8 weeks of treatment, on the basis of a large phase 2b clinical trial with 571 patients, and found that 96% and 88% of patients, respectively, cleared the virus. Evidence on shorter treatment durations for 3D was not available; nevertheless, SVR12 for an alternative DAA regimen using DCV-Trio + SOF was reported by Sulkowski et al12 for a similar population after 6 and 4 weeks of treatment. The small phase 2 clinical trial with 28 patients reported that 57% and 29% of patients achieved SVR12 after 6 and 4 weeks of treatment, respectively. For our analysis, we assumed that the odds ratio of SVR12 after 6 and 4 weeks of treatment using DCV-Trio + SOF could be applied to 3D. We calculated the odds ratio of available data (ie, 8 vs 12 weeks and 4 vs 6 weeks), averaged these to estimate 8 versus 6 weeks, and applied these to our baseline 3D estimates to obtain predicted estimates for 6 and 4 weeks for 3D therapy. We used a Bayesian Markov chain Monte-Carlo simulation framework to pool the evidence to propagate and reflect the uncertainties in these estimates for use in probabilistic sensitivity analysis (for further details, see Appendix 1 in Supplemental Materials found at 10.1016/j.jval.2018.12.011). The estimated SVR12 was 96%, 87%, 64%, and 38% after 12, 8, 6, and 4 weeks of treatment, respectively, with uncertainty around these estimates presented in Table 1.
Bourliere et al20 provided evidence on the efficacy of re-treatment using SOF/VEL/VOX from 2 phase 3 clinical trials (POLARIS-1 and POLARIS-4). Overall, 97.3% of patients achieved SVR12; we used this parameter to inform the expected SVR12 in patients who failed any of the treatment strategies. We assessed uncertainty in this parameter using data obtained from Bourliere et al20 and beta distributions.
Treatment-related adverse events associated with DAA treatment were modeled to reflect the potential impact of these clinical events over different treatment durations. We obtained the probability of adverse events occurring from a clinical trial of 3D treatment, as reported by Johnson et al.3 To estimate the probability of these events occurring over different treatment durations, we converted the probabilities to rates and calculated the time-dependent probability for each strategy (see Appendix 2 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011). We assumed that the probability of adverse events occurring was the same for re-treatment as for first-line treatment. The adverse events included anemia, rash, depression, grade 3 or 4 neutropenia, and grade 3 or 4 thrombocytopenia.
Epidemiological inputs
The Markov model simulated the natural disease history of HCV using annual transition probabilities, which are presented in Table 1. We derived estimates from published studies on the probability of reinfection (1% per annum),3 fibrosis23, 24 and nonfibrosis25, 26 progression, liver-related mortality,23, 25, 27 and all-cause mortality, stratified by age and sex.34
Costs
The model considered both treatment-related and health state costs from the perspective of the National Health Service in the United Kingdom (Table 1). All unit costs were expressed in sterling (£) and valued at 2016/2017 prices.
The costs for the drug regimens were taken from the respective NICE technology appraisals for 3D8 and SOF/VEL/VOX (Table 1). These costs were applied on a pay-per-tablet basis, rather than a pay-per-treatment success basis. We also considered monitoring costs, and derived these from a previous technology appraisal for a similar DAA.7 We assumed that monitoring costs were the same for first-line treatment and re-treatment because these costs were not expected to vary by DAA treatment. Treatment-related costs (drug and monitoring costs) were assumed to be fixed in the model because these prices were not expected to vary in the United Kingdom. The costs associated with adverse events were taken from the study by Johnson et al.3 We assessed uncertainty in these estimates using gamma distributions.
Health state costs were derived from a previous UK evaluation of HCV by Hartwell et al22 and a cost analysis of resource use incurred by both HCV-infected and HCV-cleared patients, undertaken by Backx et al.29 Gamma distributions were assumed for all cost inputs. We updated costs to 2016/2017 prices using the Hospital and Community Health Services index35 (Table 1).
Utility weights
We derived treatment-related and health state utility estimates from published studies (Table 1). Johnson et al3 provided treatment-related utilities for 3D treatment. The quality-of-life estimates were obtained from the 5-level EuroQol 5-dimensional questionnaire, which was administered to patients participating in clinical trials for 3D treatment. For our analysis of shortened treatment, we converted the 12-week treatment estimates to reflect the monthly deterioration in quality of life because of adverse events associated with treatment. We assumed the same treatment-related utilities for SOF/VEL/VOX because these have not yet been published.
Health state utilities for HCV infection were derived from Wright et al24 and reflected the expected annual health-related quality of life associated with each HCV health state. As in the study by Wright et al,24 and other analyses,3, 22 we assumed that the health utility associated with treatment success (SVR12) was greater than the baseline utility level by a score of 0.05 for both mild and moderate liver fibrosis. We assumed that the health utility in successfully treated patients was fixed in the model. We investigated uncertainty in infected patients using beta distributions (Table 1).
Cost-Effectiveness Analyses
Base-case analysis
The base-case analysis compared the lifetime cost-effectiveness of different shortened treatment durations against the standard 12-week treatment duration for GT1 noncirrhotic treatment-naive patients in the United Kingdom. We calculated expected costs and quality-adjusted life-years (QALYs) per 1000 patients using a probabilistic analysis, with parameters sampled from predefined distributions over 10 000 simulations in Microsoft Excel software.36 The expected costs and QALYs were computed as an average over the 10 000 simulations. We calculated the expected incremental net monetary benefit (INMB) of each shortened treatment strategy relative to 12 weeks of treatment, assuming a willingness-to-pay (WTP) threshold of £20 000:
We also calculated the expected cost per cure:
where pCured is the proportion of patients cured over the treatment duration. We reported the probability that any strategy was the most cost-effective treatment strategy at different WTP thresholds using cost-effectiveness acceptability curves.
Sensitivity analyses
We considered alternative DAA regimens currently used in the United Kingdom as first-line treatment to assess the impact of different drug prices and utility scores on cost-effectiveness. These included LDV/SOF, daclatasvir plus sofosbuvir (DCV/SOF), and elbasvir/grazoprevir (ELB/GZR). (The cost and utility estimates used in these analyses are detailed in Appendix 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011.)
We assessed the impact on cost-effectiveness of lower drug prices. The base-case analysis used prices reported in the NICE technology appraisals; nevertheless, reduced drug prices were agreed by the drug manufacturers and NICE, which have not been made publicly available. In a sensitivity analysis, we reduced drug prices by 80% (to £7284.34 and £8965.40 per 12-week course for first-line treatment and re-treatment, respectively). We considered alternative discount rates in sensitivity analyses to assess the impact of lower (1.5%) and higher (5%) discount rates on cost-effectiveness findings. This was useful to assess the generalizability of our findings to other healthcare settings where different discount rates are applied. Here, we assumed the same 80% reduction in drug prices.
Scenario analyses
Because of uncertainty in the cost of treatment in the United Kingdom and elsewhere, we used a threshold analysis to investigate cost-effectiveness under different drug prices. Here, we ranged the cost from £0 to £40 000 per 12-week course for both first-line treatment and re-treatment simultaneously.
We also used threshold analyses to investigate the impact of different first-line cure rates on cost-effectiveness for each shortened treatment strategy separately, assuming the same 80% reduction in drug prices. First, we considered higher SVR rates for the 8-week treatment, holding all else constant, because the base-case rate of 87% was somewhat conservative; higher success rates over 8 weeks have been reported for other regimens,37 as well as newer DAAs.38 In this scenario, we ranged SVR12 between 86% and 96%. After the 6-week treatment, we varied the first-line cure rate between 40% and 85% (ie, between the SVR12 after 4 [38%] and 8 [87%] weeks of treatment), holding all else constant. Finally, we investigated the cost-effectiveness of the 4-week treatment at higher cure rate thresholds, constrained at 65% (ie, constrained at the SVR12 after 6 weeks of treatment).
Because of some uncertainty in the cure rate after re-treatment, we conducted a threshold analysis on this parameter also. Here, we varied the cure rate between 0% and 100% and assumed the same thresholds for each shortened treatment strategy. We assumed the same 80% reduction in drug prices in this scenario also.
Results
Base-Case Findings
The 8-week treatment generated lower expected lifetime costs and fewer QALY gains compared with the 12-week treatment. The strategy had the lowest expected lifetime cost per cure at £32 607 (95% confidence interval [CI] £29 288-£36 699). At a WTP threshold of £20 000, despite the smaller QALY gains, the strategy had the highest INMB per 1000 patients at £7737 (95% CI £3242-£11 819) because of the considerable cost savings associated with the shortened treatment strategy (Table 2). At 56%, the strategy had the highest probability of being the most cost-effective option. The 6-week treatment produced a positive expected INMB, although some uncertainty was observed because of imprecise estimates on the effectiveness of shortened treatment (INMB £1860 [95% CI −£14 517 to £15 153]). The strategy had a lower probability of being the most cost-effective at 25%. Similar uncertainty was observed in the 4-week treatment strategy, which produced a negative expected INMB (−£4735 [95% CI −£24 197 to £20 141]) because of higher overall lifetime costs and lower QALY gains. The strategy had 17% probability of being the most cost-effective.
Table 2.
Cost-effectiveness findings.
| Analysis | Costs (£) (95% CI) | QALYs (95% CI) | Cost per cure (£) (95% CI) | INMB (£) (95% CI)∗,† | P(CE)† |
|---|---|---|---|---|---|
| Base-case analysis | |||||
| 12 wk | 40 911 (38 742 to 44 007) | 15.51 (15.00 to 16.16) | 41 051 (38 788 to 44 313) | – | .029 |
| 8 wk | 32 821 (29 513 to 36 971) | 15.49 (14.98 to 16.14) | 33 194 (29 701 to 37 669) | 7 737 (3 242 to 11 819) | .558 |
| 6 wk | 37 668 (25 511 to 52 476) | 15.44 (14.92 to 16.11) | 39 048 (25 746 to 56 050) | 1 860 (−14 517 to 15 153) | .245 |
| 4 wk | 43 126 (20 506 to 59 551) | 15.38 (14.83 to 16.07) | 46 021 (20 762 to 67 835) | −4 735 (−24 197 to 20 141) | .168 |
| Sensitivity analysis (80% reduction in drug prices) | |||||
| 12 wk | 11 455 (9 951 to 13 657) | 15.51 (14.99 to 16.16) | 11 495 (9 972 to 13 721) | – | .220 |
| 8 wk | 9 738 (8 083 to 12 016) | 15.49 (14.97 to 16.14) | 9 848 (8 136 to 12 217) | 1 370 (−344 to 2 685) | .470 |
| 6 wk | 10 892 (7 617 to 14 835) | 15.44 (14.90 to 16.11) | 11290 (7 709 to 15 949) | −815 (−6 868 to 3 170) | .203 |
| 4 wk | 12 203 (6 634 to 17 020) | 15.38 (14.82 to 16.07) | 13 008 (6 706 to 19 512) | −3 197 (−12 090 to 4 291) | .107 |
CI indicates confidence interval; INMB, incremental net monetary benefit; P(CE), probability most cost-effective; QALY, quality-adjusted life-year.
Versus 12 wk.
At £20 000 willingness to pay.
Sensitivity Analyses Findings
Changing the drug regimen for first-line treatment did not affect the base-case findings, except in the case of the 4-week treatment strategy that produced a positive INMB using DCV/SOF as first-line treatment. The main driver for this change was the increased cost of first-line treatment, which increased the overall cost of the 12-week treatment. In all cases, the 8- and 6-week treatment strategies produced a positive expected INMB (see Appendix 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011). Greater uncertainty was observed when DCV/SOF was used as first-line treatment; nevertheless, the shortened treatment strategies had comparably higher probabilities of being the most cost-effective treatment strategies compared with the 12-week treatment (see Appendix 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011).
Reducing drug costs by 80% introduced some uncertainty in the results. Because of the reduced cost savings, the 8-week treatment strategy returned a considerably smaller INMB with some uncertainty observed (£1370 [95% CI −£344 to £2685]). Nevertheless, the strategy still had the highest probability (47%) of being the most cost-effective because of lower expected lifetime costs (Table 2; cost-effectiveness acceptability curves are presented in Appendix 4 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011).
The results were robust to changes in the discount rate (see Appendix 5 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2018.12.011). At the lower rate of 1.5%, the same uncertainty was observed, and the 8-week treatment strategy still had the highest probability of being the most cost-effective, at 46%. At the higher rate of 5.0%, the probability that the 8-week treatment was the most cost-effective increased to 50% and no uncertainty in the expected INMB was observed.
Scenario Analyses Findings
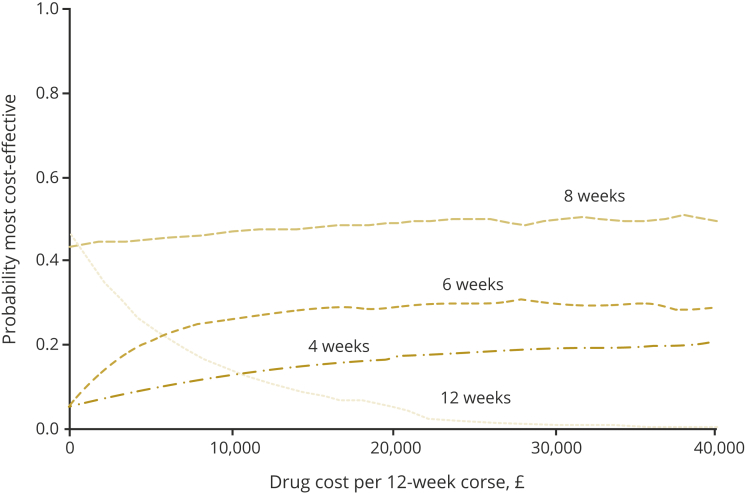
Figure 2 presents the findings from the drug-cost threshold analysis. The results are plotted using information on the probability of cost-effectiveness at £20 000 WTP. The horizontal axis presents the different drug costs. At 0 drug costs, the 12-week treatment was the most cost-effective option because of the obvious QALY advantage over the shortened treatment strategies. Nevertheless, the 8-week treatment had the highest probability of being the most cost-effective at all drug costs above 0 because of the available cost savings. At all prices higher than £6000 per 12-week course, the 6-week treatment had a consistently higher probability (>20%) than the 12-week treatment of being the most cost-effective. The 4-week treatment had a consistently low probability (<20%) of being the most cost-effective strategy at all drug prices.
Figure 2.
Probability of cost-effectiveness at £20 000 willingness to pay and different drug costs.
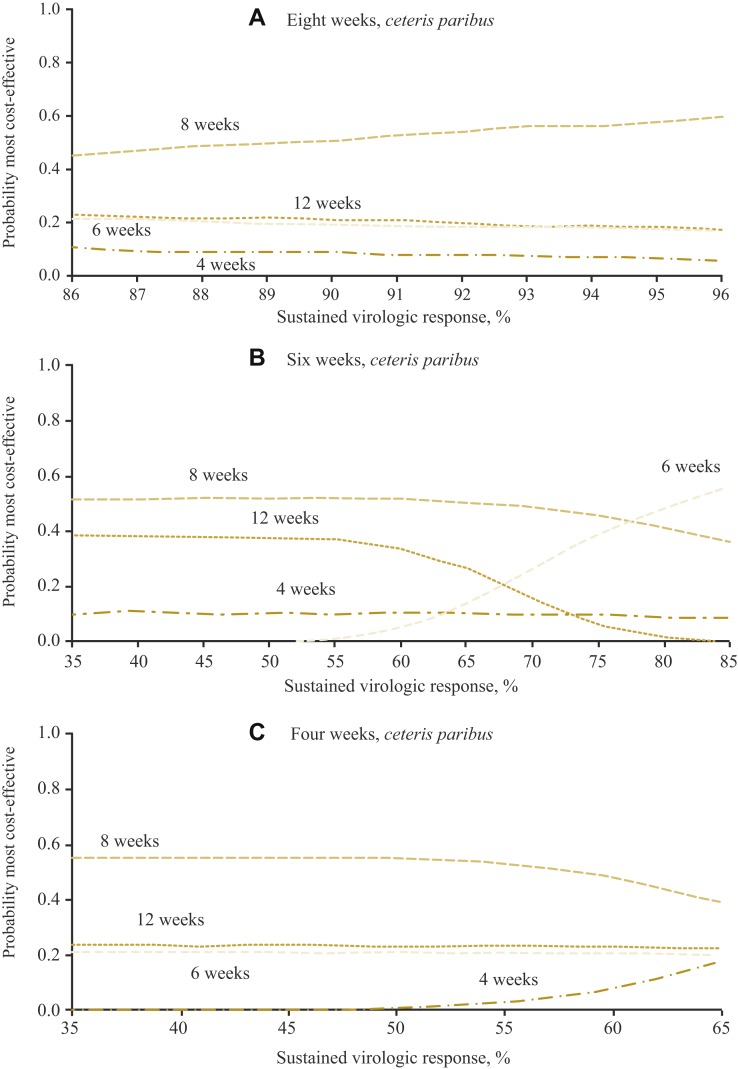
Figure 3 presents the findings from the first-line cure rate threshold analysis after 8, 6, and 4 weeks of treatment, respectively. The results are similarly plotted using information on the probability of cost-effectiveness. The probability that the 8-week treatment duration is cost-effective increases with each percentage increase in SVR12 (Figure 3A). At 96% SVR12, ceteris paribus, the probability that the strategy is the most cost-effective is 60%. If SVR12 after the 6-week treatment was 77% or higher, ceteris paribus, the strategy had the highest probability of being the most cost-effective strategy (Figure 3B). The 4-week treatment had the lowest probability of being the most cost-effective, even when SVR12 was as high as 65% (Figure 3C).
Figure 3.
Probability of cost-effectiveness at £20 000 willingness to pay and different first-line cure rate thresholds: (A) 8-wk treatment; (B) 6-wk treatment; (C) 4-wk treatment.
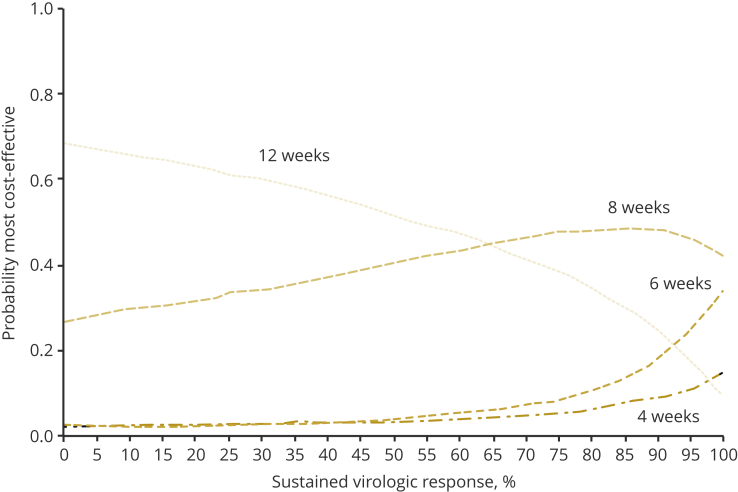
The results from the re-treatment cure rate threshold analysis are presented in Figure 4. At re-treatment cure rates higher than 65%, the 8-week treatment had the highest probability of being the most cost-effective strategy. The 6- and 4-week treatments had a higher probability of being cost-effective compared with the 12-week treatment if SVR12 after re-treatment was 92.5% and 97.5% or higher, respectively.
Figure 4.
Probability of cost-effectiveness at £20 000 willingness to pay and different re-treatment cure rate thresholds.
Discussion
Strengths and Limitations
To our knowledge, this is the first study to consider the cost-effectiveness of short-course therapy for chronic HCV. We compared 3 different shortened treatment durations using data reported in the literature for a noncirrhotic treatment-naive population. We developed a decision tree to capture treatment outcomes and adapted a previously validated Markov model to reflect the disease history of HCV. We assessed a number of DAA regimens currently used in the United Kingdom and conducted various sensitivity and scenario analyses. Our results were generally robust to these analyses.
There are limitations to this work. The evidence on the effectiveness of shortened treatment duration is limited, and often limited to DAAs not currently approved. With the exception of Kowdley et al11 who reported the effectiveness of 3D treatment, which is currently recommended for use internationally, most studies considered the effectiveness of a combination of DAA regimens,12 or explored the effectiveness of existing DAA regimens but with the addition of an investigational nonnucleoside or protease inhibitor, for example.13, 39, 40 As a consequence, these analyses have had little impact on policy. For instance, in the United Kingdom, the recommended standard treatment duration for all DAA regimens in GT1 is 12 to 16 weeks, with the exception of LDV/SOF, which is recommended for use over 8 weeks in patients with low baseline viral load, and glecaprevir/pibrentasvir, which is a new regimen that is licensed for use over 8 weeks.41 Although the evidence is limited, we used the data from Kowdley et al11 as our baseline source for efficacy data on 12 and 8 weeks of treatment and predicted the expected cure rate for this regimen over 6- and 4-week durations using data from Sulkowski et al.12
The evidence for the effectiveness of re-treatment is also limited, although most studies report considerably high success rates in GT1 noncirrhotic patients.15, 17, 18, 19, 20, 42 For our analysis, we used recent evidence from Bourliere et al20 who investigated the effectiveness of re-treatment using the now currently recommended re-treatment regimen (SOF/VEL/VOX) over 12 weeks for a similar noncirrhotic population that failed first-line therapy. In scenario analysis, we assessed lower re-treatment cure rates and found that the shortened treatment strategies were more cost-effective than the 12-week treatment, provided SVR12 after re-treatment was 65% or higher after the 8-week treatment, and 92.5% and 97.5% after the 6- and 4-week treatment, respectively.
Although the cost of DAA treatment is high, we do not know the actual price healthcare payers pay for these drugs. In our base-case analysis, we assumed the prices reported in the technology appraisals,7, 8, 9, 43 which are an exaggeration of the actual prices paid. In a threshold analysis, we varied these prices and found that the base-case findings remained generally robust at prices lower than £40 000 per 12-week course.
Finally, we took a UK perspective in this article. Although some variations in monitoring costs might exist internationally, there is little to differentiate in terms of treatment costs, patient outcomes, and disease progression. Discount rates differ across some settings; for instance, in Australia, Estonia, Latvia, Lithuania, and Ireland, the applied discount rate is 5%, whereas in Canada it is 1.5%.44 We applied these rates in sensitivity analyses and found that the results remained generally unchanged, suggesting that our findings may be generalizable to other healthcare settings that assume the same, or either lower or higher, discount rates. The findings are also likely generalizable across DAA regimens, which are generally homogeneous; DAAs have similar cure rates, comparably high treatment costs, and uniform impact on patients’ quality of life. We did not explore strategies that might stratify patients as suitable for shortened therapy at 4 and 6 weeks, although such approaches are likely to improve the cost-effectiveness of treatment.
Implications for Practice
Our findings that the 8-week (or even 6-week) shortened treatment duration is likely to be cost-effective are important in the context of clinical practice. Treating patients over shortened durations in resource-constrained settings, for example, allows scarce resources, such as staff, to be better allocated and distributed although such approaches need to be balanced against the complexity of delivering care. The decision to treat patients, particularly those with high loss to follow-up, such as chaotic drug users, is also less problematic. Treating these patients over shortened treatment durations is effective and cost-effective if a salvage treatment can be administered. This may be useful in settings in which there is a limited time available for treatment (eg, prisons); nevertheless, the decision to treat patients serving short sentences remains problematic because of loss to follow-up. Future research should identify the potential cost-effectiveness of shortened treatment durations in this context.
Although short-course therapy is not currently recommended, the cost-effectiveness of this approach is clear. Shortening treatment to 8 or 6 weeks using existing DAA therapies, such as 3D, LDV/SOF, DCV/SOF, and ELB/GZR, appears cost-effective, although some uncertainty in the 6-week treatment strategy exists. We highlight the need for further evidence on the efficacy of 6 and 4 weeks of treatment using licensed regimens, along with evidence on the success of re-treatment. Future research should also identify patients for whom shortened treatment is likely to be effective, with treatment duration optimized on the basis of baseline viral load or resistance to DAA therapies, for example, which has been shown to limit patients’ chance of viral eradication,45, 46 particularly in those with prolonged exposure to treatment previously.15
Conclusions
We compared the lifetime cost-effectiveness of 3 unstratified shortened treatment durations (8, 6, and 4 weeks) against the standard 12-week treatment, with a re-treatment strategy adopted for all patients who failed first-line treatment, for GT1 noncirrhotic treatment-naive patients in the United Kingdom. The 8-week treatment generates marginally fewer expected lifetime QALYs than the 12-week treatment duration, but is the most cost-effective option because of considerably lower expected lifetime costs arising from lower first-line treatment costs. There is considerable uncertainty surrounding the cost-effectiveness of 6 and 4 weeks of treatment because of limited evidence on efficacy, although there is some indication that the 6-week treatment may be cost-effective, whereas the 4-week treatment is likely not cost-effective.
Provided drug costs are above 0, the 8-week treatment has the highest probability of being the most cost-effective because of the available cost savings versus the standard 12-week treatment. The 6-week treatment had a higher probability than the 12-week treatment of being the most cost-effective at all drug prices higher than £6000 per 12-week course; nevertheless, the probability was generally low at approximately 30%. The 8-week treatment is highly cost-effective at higher first-line cure rates; at 96% SVR12, the strategy has 60% probability of being the most cost-effective. The 6-week treatment would be the most cost-effective option if the first-line cure rate was 77% or higher, whereas the 4-week treatment always had a low probability of being the most cost-effective, even when the first-line cure rate was as high as 65%. Shortening treatment duration is cost-effective if the re-treatment cure rate is 65% or higher after the 8-week treatment, or 92.5% and 97.5% after the 6- and 4-week treatment, respectively.
Source of Financial Support
This work was supported by the Medical Research Council STOP-HCV-1 project (grant no.: MR/K01532X/1). N. J. Welton was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at the University of Bristol, in partnership with Public Health England. G. Cooke is supported in part by the Biomedical Research Centre of Imperial College National Health Service Trust and NIHR Research Professorship. The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the NIHR, the Department of Health, or Public Health England.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.jval.2018.12.011.
Contributor Information
Christopher G. Fawsitt, Email: c.fawsitt@bristol.ac.uk.
STOP-HCV Consortium:
Eleanor Barnes, Jonathan Ball, Diana Brainard, Gary Burgess, Graham Cooke, John Dillon, Graham Foster, Charles Gore, Neil Guha, Rachel Halford, Kevin Whitby, Chris Holmes, Anita Howe, Emma Hudson, Sharon Hutchinson, William Irving, Salim Khakoo, Paul Klenerman, Natasha Martin, Benedetta Massetto, Tamyo Mbisa, John McHutchison, Jane McKeating, John McLauchlan, Alec Miners, Andrea Murray, Peter Shaw, Peter Simmonds, Chris Spencer, Emma Thomson, Peter Vickerman, and Nicole Zitzmann
Supplemental Materials
References
- 1.Chahal H.S., Marseille E.A., Tice J.A. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med. 2016;176(1):65–73. doi: 10.1001/jamainternmed.2015.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhatwal J., Kanwal F., Roberts M.S., Dunn M.A. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson S.J., Parise H., Virabhak S., Filipovic I., Samp J.C., Misurski D. Economic evaluation of ombitasvir/paritaprevir/ritonavir and dasabuvir for the treatment of chronic genotype 1 hepatitis C virus infection. J Med Econ. 2016;19(10):983–994. doi: 10.1080/13696998.2016.1189920. [DOI] [PubMed] [Google Scholar]
- 4.Najafzadeh M., Andersson K., Shrank W.H. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–419. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva, Switzerland: 2016. Guidelines for the Screening, Care and Treatment of Persons With Chronic Hepatitis C Infection. [Google Scholar]
- 6.World Health Organization . World Health Organization; Geneva, Switzerland: 2016. Global Health Sector Strategy on Viral Hepatitis 2016-2021. [Google Scholar]
- 7.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2015. Technology Appraisal Guidance [TA363]: Ledipasvir–Sofosbuvir for Treating Chronic Hepatitis C. [Google Scholar]
- 8.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2015. Technology Appraisal Guidance [TA365]: Ombitasvir–Paritaprevir–Ritonavir With or Without Dasabuvir for Treating Chronic Hepatitis C. [Google Scholar]
- 9.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2015. Technology Appraisal Guidance [TA364]: Daclatasvir for Treating Chronic Hepatitis C. [Google Scholar]
- 10.Corman S., Elbasha E.H., Michalopoulos S.N., Nwankwo C. Cost-utility of elbasvir/grazoprevir in patients with chronic hepatitis C genotype 1 infection. Value Health. 2017;20(8):1110–1120. doi: 10.1016/j.jval.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Kowdley K.V., Lawitz E., Poordad F. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370(3):222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 12.Sulkowski M.S., Flamm S., Kayali Z. Short-duration treatment for chronic hepatitis C virus with daclatasvir, asunaprevir, beclabuvir and sofosbuvir (FOURward study) Liver Int. 2017;37(6):836–842. doi: 10.1111/liv.13335. [DOI] [PubMed] [Google Scholar]
- 13.Kohli A., Kattakuzhy S., Sidharthan S. Four-week direct-acting antiviral regimens in noncirrhotic patients with hepatitis C virus genotype 1 infection: an open-label, nonrandomized trial. Ann Intern Med. 2015;163(12):899–907. doi: 10.7326/M15-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawlotsky J.M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016;151(1):70–86. doi: 10.1053/j.gastro.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Lawitz E, Flamm S, Yang JC, et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir-based regimens with ledipasvir/sofosbuvir for 24 weeks. Paper presented at: Meeting of the European Association for the Study of the Liver; April 22-26, 2015; Vienna, Austria.
- 16.Lawitz E, Poordad F, Gutierrez J, et al. C-swift retreatment final results: highly successful retreatment of GT1-infected patients with 12 weeks of elbasvir/grazoprevir plus sofosbuvir and ribavirin after failure of short-duration all-oral therapy. Paper presented at: European Association for the Study of the Liver; April 13-17, 2016; Barcelona, Spain.
- 17.Lawitz E., Poordad F., Wells J. Sofosbuvir-velpatasvir-voxilaprevir with or without ribavirin in direct-acting antiviral-experienced patients with genotype 1 hepatitis C virus. Hepatology. 2017;65(6):1803–1809. doi: 10.1002/hep.29130. [DOI] [PubMed] [Google Scholar]
- 18.Poordad F, Bennett M, Sepe TE, et al. Ombitasvir/paritaprevir/r, dasabuvir, and sofosbuvir treatment of patients with HCV genotype 1-infection who failed a prior course of DAA therapy: the quartz-i study … 95-100% SVR. Paper presented at: European Association for the Study of the Liver; April 13-17, 2016; Barcelona, Spain.
- 19.Wilson E.M., Kattakuzhy S., Sidharthan S. Successful retreatment of chronic HCV genotype-1 infection with ledipasvir and sofosbuvir after initial short course therapy with direct-acting antiviral regimens. Clin Infect Dis. 2016;62(3):280–288. doi: 10.1093/cid/civ874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourliere M., Gordon S.C., Flamm S.L. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. 2017;376(22):2134–2146. doi: 10.1056/NEJMoa1613512. [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2013. Guide to the Methods of Technology Appraisal 2013. [PubMed] [Google Scholar]
- 22.Hartwell D., Jones J., Baxter L., Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15(17) doi: 10.3310/hta15170. i-xii, 1-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieve R., Roberts J., Wright M. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55(9):1332–1338. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright M., Grieve R., Roberts J., Main J., Thomas H.C. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10(21):1–113. doi: 10.3310/hta10210. iii. [DOI] [PubMed] [Google Scholar]
- 25.Fattovich G., Giustina G., Degos F. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso A.C., Moucari R., Figueiredo-Mendes C. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52(5):652–657. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Bennett W.G., Inoue Y., Beck J.R., Wong J.B., Pauker S.G., Davis G.L. Estimates of the cost-effectiveness of a single course of interferon-alpha2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127(10):855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2018. Technology Appraisal Guidance [TA507]: Sofosbuvir–Velpatasvir–Voxilaprevir for Treating Chronic Hepatitis C. [Google Scholar]
- 29.Backx M., Lewszuk A., White J.R. The cost of treatment failure: resource use and costs incurred by hepatitis C virus genotype 1-infected patients who do or do not achieve sustained virological response to therapy. J Viral Hepat. 2014;21(3):208–215. doi: 10.1111/jvh.12132. [DOI] [PubMed] [Google Scholar]
- 30.National Health Service . National Health Service; Scotland: 2018. National Clinical Guidelines for the Treatment of HCV in Adults. [Google Scholar]
- 31.National Health Service . National Health Service; London: 2018. Specialised Commissioning Drugs Briefing: Spring 2018. [Google Scholar]
- 32.Grishchenko M., Grieve R.D., Sweeting M.J. Cost-effectiveness of pegylated interferon and ribavirin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care. 2009;25(2):171–180. doi: 10.1017/S0266462309090229. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd J., Jones J., Hartwell D., Davidson P., Price A., Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11(11):1–205. doi: 10.3310/hta11110. iii. [DOI] [PubMed] [Google Scholar]
- 34.National life tables, 2013-2015. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables Accessed June 10, 2017.
- 35.Curtis L., Burns A. Personal Social Services Research Unit, University of Kent; Canterbury, Kent: 2017. Unit Costs of Health and Social Care 2017. [Google Scholar]
- 36.Microsoft . Microsoft Corp; Redmond, WA: 2016. Microsoft Excel (2016) [Google Scholar]
- 37.Sarrazin C. The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol. 2016;64(2):486–504. doi: 10.1016/j.jhep.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Zeuzem S., Foster G.R., Wang S. Glecaprevir–pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 2018;378(4):354–369. doi: 10.1056/NEJMoa1702417. [DOI] [PubMed] [Google Scholar]
- 39.Kattakuzhy S., Wilson E., Sidharthan S. Moderate sustained virologic response rates with 6-week combination directly acting anti-hepatitis C virus therapy in patients with advanced liver disease. Clin Infect Dis. 2016;62(4):440–447. doi: 10.1093/cid/civ897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohli A., Osinusi A., Sims Z. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet. 2015;385(9973):1107–1113. doi: 10.1016/S0140-6736(14)61228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2018. Technology Appraisal Guidance [TA499]: Glecaprevir–Pibrentasvir for Treating Chronic Hepatitis C. [Google Scholar]
- 42.Lawitz E., Mangia A., Wyles D. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 43.National Institute for Health and Care Excellence . National Institute for Health and Care Excellence; London: 2016. Technology Appraisal Guidance [TA413]: Elbasvir–Grazoprevir for Treating Chronic Hepatitis C. [Google Scholar]
- 44.Attema A.E., Brouwer W.B.F., Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745–758. doi: 10.1007/s40273-018-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cloherty G., Talal A., Coller K. Role of serologic and molecular diagnostic assays in identification and management of hepatitis C virus infection. J Clin Microbiol. 2016;54(2):265–273. doi: 10.1128/JCM.02407-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itakura J., Kurosaki M., Higuchi M. Resistance-associated NS5A variants of hepatitis C virus are susceptible to interferon-based therapy. PLoS One. 2015;10(9):e0138060. doi: 10.1371/journal.pone.0138060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.