Abstract
Background
The current regimens for advanced non-small cell lung cancer (NSCLC) patients are deficient due to failings in standard treatments. This retrospective study aimed to assess the efficacy and safety of low-dose apatinib in combination with S-1 therapy in a NSCLC setting.
Methods
In this retrospective study, advanced NSCLC patients who failed standard treatment in Changzhou Cancer Hospital of Soochow University were screened for eligibility. Progression-free survival (PFS) was set as the primary endpoint. Overall response rate (ORR), disease control rate (DCR), overall survival (OS), and the safety profile were considered to be the secondary endpoints.
Results
A total of 31 eligible patients were included. The median PFS (mPFS) was 102 days (95% CI: 57–147 days). ORR was achieved in 7 patients (22.6%; 95% CI: 11.1–38.2%) and DCR was maintained in 23 patients (74.2%; 95% CI: 58.2–86.5%). The median OS (mOS) was 422 days (95% CI: 148–696 days). Patients with a history of smoking tended to have a shorter OS without significant differences (HR =4.105, 95% CI: 0.874–19.288, P=0.074). Treatment-related grade III toxicity was observed in 5 patients (16%) and common grade I or II adverse events (AEs) were fatigue (42%), hypertension (32%), and hand-foot-skin reaction (23%).
Conclusions
Combination of low-dose apatinib and S-1 could be an effective and tolerable choice for advanced NSCLC patients who are unable to benefit from standard treatment; however, further exploration in larger clinical trials is needed.
Keywords: Non-small cell lung cancer (NSCLC), apatinib, S-1, combination therapy
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common malignancies worldwide. According to the 2015 China Cancer Surveys (1), NSCLC was the leading cause of cancer-related death with a poor outcome of a 16.1% five-year survival rate (2). NSCLC is the main pathological type of lung cancer, accounting for about 85% of all patients (3). Platinum-based chemotherapy and the continued emergence of targeted therapy and immunotherapy drugs have brought more treatment options for advanced NSCLC (4). However, these drugs are only approved for first or second-line treatment, and the guidelines of NSCLC still lack a recommended third-line or beyond drug (3).
In the tumor microenvironment, neo-angiogenesis promotes cancer cells growth and metastasis, and this process depends on the activation of vascular endothelial growth factor (VEGF) signaling pathway (5). Bevacizumab (6) and ramucirumab (7), two kinds of monoclonal antibodies which inhibit VEGF signaling pathway, showed clinical benefit in advanced NSCLC as the first or second-line treatment. Anlotinib, another anti-angiogenic drug, also showed that it could significantly improve the progression-free survival (PFS) and overall survival (OS) rate compared with the placebo group in the third-line treatment of advanced NSCLC (8), suggesting anti-angiogenic drugs have tremendous clinical value for advanced NSCLC failing from multi-lines of therapies.
Apatinib, one novel tyrosine kinase inhibitor targeting VEGFR-2, inhibits the proliferation, migration, and neovascularization of endothelial cells. The usage of apatinib showed clinical benefits for advanced gastric cancer and breast cancer patients experiencing multiple lines of therapies (9,10). Efficacy of apatinib for advanced NSCLC patients who failed from second-line treatment was investigated in one phase II study, and the result showed that the apatinib group obtained a longer mPFS compared with the placebo group (11). In recent years, increasing clinical trials have reported the potential benefits of apatinib in advanced NSCLC patients after multi-line therapies (12-14). However, patients who received apatinib monotherapy with a higher dose faced more drug toxicity.
S-1, one compound preparation consisting of tegafur, gimeracil, and oteracil potassium, also demonstrated a notable treatment effect for lung cancer, including in the third-line of therapy (15-19). In addressing the clinical benefits of apatinib and S-1 single-drug therapy in lung cancer, we designed this retrospective study to further assess the efficacy and safety of low-dose apatinib combined with S-1 in advanced NSCLC patients failing from standard treatment. As far as we know, this was the first clinical trial to study the clinical value and safety of low-dose apatinib combined with S-1 treatment in this NSCLC setting.
Methods
Study design and participants
This retrospective study enrolled advanced NSCLC patients meeting the inclusion and exclusion criteria in Changzhou Cancer Hospital of Soochow University from August 2016 to March 2018.
Enrollment criteria included the following conditions: aged between 18 and 75 years; pathologically confirmed NSCLC; patients with EGFR-sensitive mutations treated by first or third generation EGFR-TKI until resistance or intolerance occurred; received low-dose apatinib combined with S-1 capsules after failure of standard treatment (apatinib 250 mg once daily; S-1 capsules 40–60 mg twice daily, days 1–14, one cycle repeated every 3 weeks, dosage depends on the patients’ surface area); patients with extracranial measurable lesions; an Eastern Cooperative Oncology Group (ECOG) performance status score 0–2; asymptomatic CNS metastases; and informed consent. Patients were excluded for the following conditions: clinical symptoms of brain metastases or meningeal metastasis.
Efficacy and safety assessments
PFS was set as the primary endpoint, and overall response rate (ORR), disease control rate (DCR), OS, and safety were the secondary endpoints. PFS was measured from the time of treatment initiation to clinical or radiographic progression or death. OS was defined as the length of time from the random assignment to death or last contact. Tumor response was assessed according to the response evaluation criteria in solid tumors 1.1 (RECIST1.1). Safety assessments consisted of recording protocol-defined adverse events (AEs) and serious AEs (SAEs), which were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
The presentation of categorical variables and continuous variables were expressed by statistical description: a percentage or mean ± standard deviation, respectively. The Cox proportional hazards model assessed univariate and multivariate analysis. The Kaplan-Meier method was used to estimate PFS and OS. For all analyses, P value <0.05 was considered statistically significant, and a confidence interval of 95% was used (95% CI). The SPSS22.0 software (SPSS, Inc., Chicago, IL, USA) was carried out for statistical analysis.
Results
Patient demographics
From August 2016 to March 2018, 31 advanced NSCLC patients were eligible as per inclusion and exclusion criteria. The baseline characteristics of the study enrolled patients are summarized in Table 1. The mean age of the study population was 60±8 years. Nineteen percent (6/31) and 81% (25/31) of patients were female and male, respectively. The percentage of pathological diagnosis of lung squamous cell and adenocarcinoma was 32% (10/31) and 68% (21/31), respectively. At the baseline, 23% (7/31) patients carried EGFR-sensitive mutations, and brain metastases accounted for 19% (6/31). Nineteen percent of (6/31) the patients experienced a second-line treatment. For patients with driver gene mutations (EGFR/ALK), the period of receiving the first-line treatment to the time administered by apatinib combined with S-1 therapy was 502±66 days. For patients without driver gene mutations (EGFR/ALK), the period in lung adenocarcinoma patients was 337±141 days, and in lung squamous carcinoma patients the period was 258±135 days.
Table 1. Patients demographics and clinical characteristic.
| Characteristic | All patients (n=31) |
|---|---|
| Gender | |
| Male | 25 |
| Female | 6 |
| Age, mean ± SD, years | 60±8 |
| Pathological diagnosis | |
| Adenocarcinoma | 21 |
| Squamous cell carcinoma | 10 |
| Smoking | |
| Yes | 20 |
| No | 11 |
| ECOG PS | |
| 0–1 | 9 |
| 2 | 22 |
| EGFR mutation | |
| Yes | 7 |
| No | 24 |
| Treatment line | |
| Second line | 6 |
| Third line or above | 25 |
| CNS metastatic | |
| Yes | 6 |
| No | 25 |
CNS, central nervous system.
Efficacy
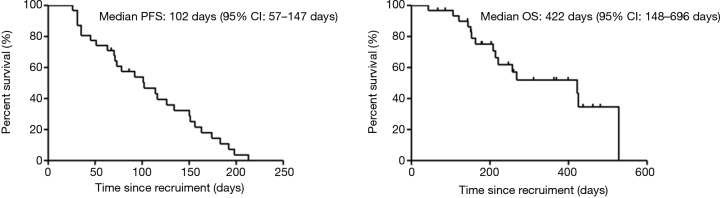
All 31 patients were included in the analysis set. The mPFS was 102 days (95% CI: 57–147 days, Figure 1), and the mOS was 422 days (95% CI: 148–696 days, Figure 1). Tumor response in study patients is summarized in Table 2. ORR was achieved in 7 (22.6%; 95% CI: 11.1–38.2%) patients, and DCR was maintained in 23 patients (74.2%; 95% CI: 58.2–86.5%). In patients with lung adenocarcinoma, mPFS in the EGFR mutation group and wild type group was 73 and 78 days, respectively (P=0.708), and mOS in the EGFR mutation group and wild type group was 425 and 422 days, respectively (P=0.776). The clinical benefit of study combination therapy was observed irrespective of sex, age, ECOG PS, EGFR mutation status, treatment line, and the presence of brain metastases. Patients with a history of smoking tended to have a shorter mOS without significant differences (HR =4.105, 95% CI: 0.874–19.288, P=0.074, Table 3).
Figure 1.
Kaplan-Meier curves of PFS and OS for low-dose apatinib combined with S-1 treatment in patients with advanced non-small cell lung cancer. PFS, progression-free survival; OS, overall survival.
Table 2. Responses assessed per RECIST version 1.1.
| Tumor response | Low-dose apatinib combined with S-1 (N=31) |
|---|---|
| Objective response, n (%; 95% CI) | 22.6% (95% CI: 11.1–38.2%) |
| Disease control rate, n (%; 95% CI) | 74.2% (95% CI: 58.2–86.5%) |
| Best overall response, n (%) | |
| Complete response | 0 |
| Partial response | 7 (22.6) |
| Stable disease | 16 (51.6) |
| Progressive disease | 8 (25.8) |
RECIST, response evaluation criteria in solid tumors.
Table 3. Prognostic factors for PFS and OS analyzed by univariate and multivariate Cox regression model.
| Variable | PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox regression model | Univariate Cox regression model | Multivariate Cox regression mode | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |||
| Sex (male vs. female) | 1.194 | 0.446–3.197 | 0.724 | 1.894 | 0.418–8.585 | 0.408 | |||||
| Age (≥65 vs. <65) | 0.724 | 0.329–1.591 | 0.421 | 2.961 | 0.943–9.300 | 0.063 | 2.109 | 0.649–6.858 | 0.215 | ||
| Smoking (yes vs. No) | 1.663 | 0.714–3.876 | 0.238 | 4.567 | 1.008–20.696 | 0.049 | 4.105 | 0.874–19.288 | 0.074 | ||
| ECOG (2 vs. 0–1) | 1.379 | 0.544–3.496 | 0.498 | 0.95 | 0.296–3.047 | 0.931 | |||||
| Pathological type (lung squamous cell vs. adenocarcinoma) | 1.842 | 0.800–4.241 | 0.151 | 0.487 | 0.167–1.415 | 0.186 | 0.515 | 0.168–1.575 | 0.245 | ||
| EGFR mutation status (mutation vs. wildtype) | 1.028 | 0.410–2.573 | 0.953 | 0.938 | 0.257–3.418 | 0.922 | |||||
| CNS metastatic (yes vs. no) | 1.626 | 0.639–4.138 | 0.307 | 0.946 | 0.260–3.447 | 0.933 | |||||
| Treatment line (third-line vs. second-line) | 1.072 | 0.404–2.842 | 0.889 | 0.419 | 0.129–1.367 | 0.419 | |||||
PFS, progression-free survival; OS, overall survival; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group.
Safety
At the data cutoff, AEs of any grade occurred in 94% of patients (29/31). AEs is summarized in Table 4. Most of the patients were tolerant, and the common grade I and grade II toxicity were fatigue (42%), hypertension (32%), and hand-foot-skin reaction (23%), consistent with reported observations (9,11). Grade III toxicity included hypertension, myelosuppression, fatigue and hand-foot-skin reaction, which occurred in 16% of patients (5/31), and disease symptoms were controlled after corresponding treatments. No cases of treatment-related death occurred until the end of the study period.
Table 4. Adverse events in the safety population.
| Event | Low-dose apatinib combined with S-1 (N=31) | |||
|---|---|---|---|---|
| Grade I–II | Grade III | Grade IV | Grade V | |
| Hypertension | 10 (32%) | 1 (3%) | 0 | 0 |
| Myelosuppression | 5 (16%) | 3 (10%) | 0 | 0 |
| Mucositis | 1 (3%) | 0 | 0 | 0 |
| Fatigue | 13 (42%) | 1 (3%) | 0 | 0 |
| Hand-foot-skin reaction | 7 (23%) | 2 (6%) | 0 | 0 |
| Hemoptysis | 3(10%) | 0 | 0 | 0 |
| Hoarseness | 2 (6%) | 0 | 0 | 0 |
| Appetite decreases | 2 (6%) | 0 | 0 | 0 |
| Proteinuria | 1(3%) | 0 | 0 | 0 |
| Oral ulcer | 1 (3%) | 0 | 0 | 0 |
| Epistaxis | 1 (3%) | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 |
Discussion
The growth of tumor cells depended on oxygen and nutrients supplied by the tumor angiogenesis (20), and VEGF signaling pathway played an important role in neovascularization (21-23). To our knowledge, VEGFR-2, one member of the VEGFR family (mainly including VEGFR-1, VEGFR-2, VEGFR-3), was considered to be the most relevant factor associated with tumor angiogenesis (24). Apatinib could destroy the interaction between VEGF-A and VEGFR-2, and inhibit the VEGF signaling pathway (25,26). Our study indicated that low-dose apatinib combined with S-1 provided effective clinical outcomes and reliable safety in advanced NSCLC patients after standard treatment failure.
One meta-analysis demonstrated that anti-angiogenic tyrosinase inhibitors plus chemotherapy could significantly improve ORR and mPFS when compared with the chemotherapy alone group for advanced NSCLC (27). In our study, the ORR in the overall assessable patients was 22.6%, while the ORR in one previous phase II trial of apatinib monotherapy in patients with advanced non-squamous NSCLC was only 12.2% (11), indicating that low-dose apatinib combined with S-1 therapy might achieve higher response rate. Recently, one study explored the clinical efficiency of apatinib (the dosage from 250 to 750 mg per day) in advanced non-squamous NSCLC after multi-lines treatments, and the mOS was 7.4 months (95% CI: 1.3–13.5) (28). Compared with this study, the mOS in our trial was 422 days (95% CI: 148–696), which showed a longer survival time. Apatinib could reverse ABCB1 and ABCG2-mediated multidrug resistance (MDR) by inhibiting their transport function, resulting in an elevated concentration of antitumor drugs in tumor cells (29). This finding may provide one possible explanation for the better anti-tumor effect of combination therapy.
Several studies explored the predictive factors useful for selecting a sub-population that was more suitable for apatinib therapy. Early anti-angiogenesis-related AEs, protein expression level of phosphorylated VEGFR2 (p-VEGFR2), and hypertension were significantly related to patients’ outcome and considered as potential predictive factors of apatinib therapy (30,31). Our study showed that patients with a history of smoking tended to have shorter mOS without significant differences (HR =4.105, 95% CI: 0.874–19.288, P=0.074). Due to the small sample size of the study, larger studies are needed to identify whether it is a potential marker.
The adverse event profiles were manageable for apatinib combined with S-1 therapy, and the incidence of grade III or VI was lower than a high dose of apatinib monotherapy (28). All patients were tolerant without any serious adverse reactions, and fatigue, hypertension, and hand-foot-skin reaction were the common grade I or II AEs.
There were some limitations in our study. As a retrospective study, the interpretations of the results are limited. The small sample size could have contributed to unavoidable selection bias, or measurement bias might have weakened the relative reliability and validity of our conclusions.
In conclusion, low-dose apatinib combined with S-1 therapy obtained effective clinical benefits and was tolerable in advanced NSCLC patients who failed from standard treatment.
Acknowledgments
The study sincerely thanks all patients who participated in the study.
Funding: This study was supported by the Natural Science Foundation of Jiangsu (Grant No. BE2016656 and BRA2018171), the Changzhou Sci & Tech Program, China (Grant No. CJ20179029 and CE20175037), the Health Talents Project for Jiangsu, China (Grant No. ZDRCC2016020), and the High-level Health Talents of Changzhou City (Grant No. 2016CZLJ009).
Ethical Statement: The Ethics Committee of Changzhou Cancer Hospital of Soochow University approved this study (approval number: 2017SY-005-01). All procedures were performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921-30. 10.1002/ijc.29227 [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-small cell lung cancer. National Comprehensive Cancer, Network 2017 v7; 2018. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 5.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013;13:871-82. 10.1038/nrc3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 7.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 8.Baihui Han, Kai Li, Qiming Wang, et al. Third-line treatment: A randomized, double-blind, placebo-controlled phase III ALTER-0303 study—Efficacy and safety of anlotinib treatment in patients with refractory advanced NSCLC. J Clin Oncol 2017;35:abstr 9053.
- 9.Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. 10.1200/JCO.2013.48.8585 [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961-9. 10.1002/ijc.28829 [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol 2012;30:abstr 7548.
- 12.Xu J, Liu X, Yang S, et al. Clinical response to apatinib monotherapy in advanced non-small cell lung cancer. Asia Pac J Clin Oncol 2018;14:264-9. 10.1111/ajco.12834 [DOI] [PubMed] [Google Scholar]
- 13.Ding L, Li QJ, You KY, et al. The Use of Apatinib in Treating Non small-Cell Lung Cancer Case Report and Review of Literature. Medicine (Baltimore) 2016;95:e3598. 10.1097/MD.0000000000003598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Zhang S, Gao G, et al. Successful treatment using apatinib with or without docetaxel in heavily pretreated advanced non-squamous non-small cell lung cancer: a case report and literature review. Cancer Biol Ther 2018;19:141-4. 10.1080/15384047.2017.1414757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka H, Okamoto I, Morita S, et al. Efficacy and safety analysis according to histology for S-1 in combination with carboplatin as first-line chemotherapy in patients with advanced non-small-cell lung cancer: updated results of the West Japan Oncology Group LETS study. Ann Oncol 2013;24:1326-31. 10.1093/annonc/mds629 [DOI] [PubMed] [Google Scholar]
- 16.Kubota K, Sakai H, Katakami N, et al. A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol 2015;26:1401-8 10.1093/annonc/mdv190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawahara M, Furuse K, Segawa Y, et al. Phase II study of S- 1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 2001;85:939-43. 10.1054/bjoc.2001.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiyama O, Taniguchi H, Kondoh Y, et al. Phase II study of S-1 monotherapy as a first-line treatment for elderly patients with advanced non-small cell lung cancer: the Central Japan Lung Study Group trial 0404. Anticancer Drugs 2011;22:811-6. 10.1097/CAD.0b013e3283440231 [DOI] [PubMed] [Google Scholar]
- 19.Ono A, Naito T, Murakami H, et al. Evaluation of S-1 as third- or further- line chemotherapy in advanced non-small-cell lung cancer. Int J Clin Oncol 2010;15:161-5. 10.1007/s10147-010-0034-0 [DOI] [PubMed] [Google Scholar]
- 20.Jayson GC, Kerbel R, Ellis LM, et al. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388:518-29. 10.1016/S0140-6736(15)01088-0 [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2002;2:795-803. 10.1038/nrc909 [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Jeong YJ, Cho HJ, et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol Rep 2017;37:777-84. 10.3892/or.2016.5296 [DOI] [PubMed] [Google Scholar]
- 23.Lv J, Sun B, Mai Z, J, et al. STAT3 potentiates the ability of airway smooth muscle cells to promote angiogenesis by regulating VEGF signalling. Exp Physiol 2017;102:598-606. 10.1113/EP086136 [DOI] [PubMed] [Google Scholar]
- 24.Koch S, Tugues S, Li X, et al. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011;437:169-83. 10.1042/BJ20110301 [DOI] [PubMed] [Google Scholar]
- 25.Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014;2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis 2017;8:e3015. 10.1038/cddis.2017.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li BT, Barnes TA, Chan DL, et al. The addition of anti-angiogenic tyrosinekinase inhibitors to chemotherapy for patients with advanced non-small-cell lung cancers: A meta-analysis of randomized trials. Lung Cancer 2016;102:21-7. 10.1016/j.lungcan.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Liang L, Nie L, et al. Efficacy, safety and predictive indicators of apatinib after multilines treatment in advanced nonsquamous nonsmall cell lung cancer: Apatinib treatment in nonsquamous NSCLC. Asia Pac J Clin Oncol 2018;14:446-52. 10.1111/ajco.12870 [DOI] [PubMed] [Google Scholar]
- 29.Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) Reverses Multidrug Resistance by Inhibiting the Efflux Function of Multiple ATP-Binding Cassette Transporters. Cancer Res 2010;70:7981-91. 10.1158/0008-5472.CAN-10-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Qin S, Wang Z, et al. Correction to: Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol 2018;11:5. 10.1186/s13045-017-0545-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141-51. 10.1007/s10549-013-2793-6 [DOI] [PubMed] [Google Scholar]