Abstract
Background
This study’s objective was to evaluate and summarize the application of the IQQA-3D imaging interpretation and analysis system in uniportal video-assisted thoracoscopic anatomical segmentectomy.
Methods
We collected the clinical data of consecutive patients who underwent uniportal video-assisted thoracoscopic anatomical segmentectomy for single or multiple pulmonary nodules at Department No. 1 of Thoracic Surgery at Fujian Medical University, Fujian Union Hospital from July 2017 to November 2018. Patients were divided into two groups according to the use of IQQA: the IQQA group and non-IQQA group. General clinical characteristics, operation status, and postoperative recovery were compared between groups. Variations in the segmental bronchi, arteries, and veins of patients in the IQQA group were summarized.
Results
This study included 133 cases, 96 in the IQQA group and 37 in the non-IQQA group. There were no significant differences between groups in patient sex, age, preoperative smoking, pulmonary function, maximum lesion diameter, or pathological type (all P>0.05). The proportion of segmentectomies that were moderately difficult or complex was significantly higher in the IQQA group than in the non-IQQA group (28.1% vs. 16.2% and 29.2% vs. 13.5%, respectively; P=0.017). Despite having a higher percentage of more complicated operations, the IQQA group did not have longer operation times or increased postoperative complications. Fifty-five cases (57.3%) in the IQQA group had segmental structure variations, with a total of 73 variations. Among patients who underwent IQQA, 27 (65.9%) in the general segmentectomy group, 13 (48.1%) in the moderate segmentectomy group, and 15 (53.6%) in the complex segmentectomy group had anatomic variations; these differences were not significant.
Conclusions
Preoperative 3D reconstruction is necessary before segmentectomy, especially for patients undergoing moderate or complex segmentectomy. IQQA was safe and feasible for preoperative localization of lesions, surgical planning, and intraoperative navigation in uniportal video-assisted thoracoscopic anatomical segmentectomy and can facilitate complicated segmentectomy.
Keywords: Imaging, three-dimensional (3D), uniport, video-assisted thoracoscopic (VATS), segmentectomy
Introduction
With the recent generalization of physical examination, the discovery of pulmonary nodules is increasingly common. Segmental resection, which resects tumors while preserving lung function, is a reliable treatment method for peripheral nodules, especially for early lung cancer, benign lesions, and metastatic tumors (1-4). The challenges of segmentectomy include accurately determining the position of nodules, preoperative identification of anatomical variations, and preoperative planning of surgical procedures. Three-dimensional (3D) reconstruction imaging is favored by many physicians because of its advantages of stereo and direct viewing. Researchers have successfully applied various 3D reconstruction softwares to simplify segmentectomy (5-7). We have taken the lead in applying and reporting a different imaging interpretation and analysis system (IQQA-3D; developed by IQQA-Chest, EDDA Technology, Princeton Junction, NJ, USA), which produces 3D reconstructions of the pulmonary trachea and vessels with satisfactory outcomes (8). The aim of this study was to evaluate and summarize the application of IQQA in uniportal video-assisted thoracoscopic (VATS) anatomical segmentectomy.
Methods
Patients
This retrospective control study included a total of 133 patients with single or multiple pulmonary nodules who were admitted to the Department No. 1 of Thoracic Surgery at Fujian Medical University, Fujian Union Hospital from July 2017 to November 2018. This study was reviewed and approved by the institutional review board of our hospital, which waived the need for informed consent of the patients. All patients underwent uniportal VATS segmentectomy; 96 of these procedures were completed with IQQA.
Inclusion criteria
(I) A single type of pulmonary segmental surgery was performed, regardless of the number of pulmonary nodules. (II) All operations (segment, subsegment, combined segments or subsegments, segment + subsegment) were performed under uniport VATS. (III) Procedures were performed with or without mediastinal lymph node dissection or sampling.
Exclusion criteria
(I) Presence of severe preoperative emphysema. (II) Severe adhesions in the entire chest. (III) Resection by segmentectomy of nodules in different lobes at the same time. (IV) Right S7-10 segmentectomy, left S8-10 segmentectomy, or left S1-3 segmentectomy. (V) Incomplete data.
Case grouping
Patients were divided into two groups according to the use of IQQA: the IQQA group and non-IQQA group. Patients were further divided into three subgroups according to the difficulty of surgery: general subgroup, moderate subgroup, and complex subgroup. The general subgroup included RS1, RS2, RS1+S2, LS1+2, R/LS6, and LS4+S5, the moderate subgroup included RS7, R/LS8, and R/LS3; and the complex subgroup included R/LS9, R/LS10, subsegmentectomy, combined-segments resection except RS1+S2 and LS4+S5, combined-subsegments resection, and segment + subsegment resection.
Data collection
Collected data included patient age, sex, smoking history, maximum lesion diameter, pulmonary function, postoperative pathology, operation characteristics (resection range, mediastinal lymph node dissection, operation time, intraoperative blood loss), postoperative recovery (total chest drainage, postoperative hospital stay, postoperative complications), and anatomic variations verified with the IQQA system.
Surgical technique
Patient position
Lateral position, healthy side down.
Anesthesia
General anesthesia, double-cavity endotracheal intubation, single-lung ventilation.
Surgical incision selection
The 5th intercostal space on the midaxillary line for basal segmentectomy; the 4th intercostal space on the midaxillary line for pulmonary segmentectomy except the basal segments. Incision length was approximately 4 cm. An incision protection retractor was attached to the port.
Surgical procedure
IQQA group
Patients underwent revolution CT scanning (layer thickness, 0.625 mm) after admission. Image data in DICOM format were imported into the IQQA system, which located the pulmonary nodules and created a 3D reconstruction of pulmonary bronchi, arteries, and veins, simulating the target segment. Evaluation of margin sufficiency was performed according to the simulated resection range. The target segmental bronchus, arteries, segmental veins, and intersegmental veins were then confirmed (Figure 1). The treatment sequence of segmental bronchi, arteries, and veins was performed according to anatomical characteristics. The pulmonary hilum was dissected and the target segmental structures were identified. Segmental structures were approached in the order planned with IQQA. Segmental arteries and veins were generally ligated with #1 or #4 suture and were cut with an ultrasonic knife; segmental bronchi were transected with a stapler. Following treatment of segmental structures, the entire lung was fully expanded with 100% oxygen via double-lung ventilation. After changing to single-lung ventilation approximately 10 to 15 minutes later, the target segment remained expanded while the rest of the lung collapsed, forming an obvious intersegmental plane (ISP). The ISP was divided with a combination of ultrasonic knife and stapler. The procedure was as follows: (I) the ISP near the pulmonary hilum was separated with an ultrasonic knife along the intersegmental vein and the inflation-deflation line; (II) the peripheral ISP was divided with a linear cutting stapler along the inflation-deflation line. For indecipherable intersegmental veins, further identification and dissection were performed during the dissection of the ISP; veins running into the inflated segments must be intersegmental. Next, pulmonary leakage testing was performed. If a small air leak was found, it could be treated with fibrin sealant spray (Shanghai RAAS) and covered with absorbable polyglycolic acid reinforcement felt (NEOVEIL). In case of obvious air leakage, 3-0 or 4-0 Prolene suture was used for repair. The mediastinal lymph node (MLN) dissection policy was as follows: (I) for invasive adenocarcinoma, MLN dissection or sampling was performed; (II) for microinvasive adenocarcinoma, MLN were sometimes sampled; (III) MLN was not taken in other cases. A thoracic drainage tube (#22–28) was placed at the top of the chest through the incision, and a 12F drainage tube (ABLE disposable abdomen drainage catheter set) was placed through the 7th intercostal space on the posterior axillary line. Once good pulmonary expansion and lack of air leakage were confirmed, the tube was removed as soon as possible to relieve the associated pain and enable the patient to resume normal activities. The lower tube was removed when the drainage was near 100 mL per day.
Figure 1.
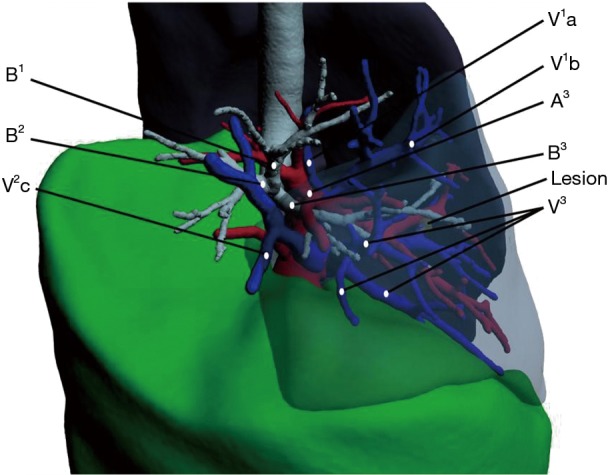
Simulated resection of right segmentectomy (S3). The targeted segmental bronchus, artery, and vein that require dissection are B3, A3, and V3. The intersegmental veins that require preservation are V1b (between S1 and S3) and V2c (between S2 and S3). B, bronchus; A, artery; V, vein.
Non-IQQA group
Patients underwent revolution CT scanning (layer thickness, 0.625 mm) after admission. The reasons that IQQA preoperative planning was not performed in this group were as follows: (I) CD-ROM format error; (II) IQQA system failure; (III) poor image quality; (VI) contraindication of contrast agent. Experienced surgeons determined the location of target nodules preoperatively on the basis of thin-layer enhanced CT. The identification of target segmental structures was performed according to surgical experience. Other procedures were the same as those in the IQQA group. Management of the ISP and thoracic drainage tubes were the same as those in the IQQA group.
Statistical analyses
SPSS 23 software (IBM Corp., Armonk, NY, USA) was used for data analysis. Continuous distributed data were expressed as mean ± standard deviation (SD); between-group differences were compared with Student’s t-test. Categorical variables were expressed as absolute frequencies and proportions (%). The χ2 or Fisher’s exact test was used to compare categorical variables. All significance tests were two-tailed, with P<0.05 considered statistically significant.
Results
General characteristics
This study included a total of 133 cases, 96 in the IQQA group and 37 in the non-IQQA group. Data on patient sex, age, maximum lesion diameter, preoperative smoking, pulmonary function, and pathological types are summarized in Table 1. There were no significant differences between the groups in these parameters (P>0.05 for all).
Table 1. General characteristics of IQQA group and non-IQQA group.
| Characteristics | IQQA group (n=96) | Non-IQQA group (n=37) | t/χ2 | P |
|---|---|---|---|---|
| Sex | 0.246 | 0.620 | ||
| Male | 37 (38.5%) | 16 (43.2%) | ||
| Female | 59 (61.5%) | 21 (56.8%) | ||
| Age (years) | 50.4±11.7 | 53.3±12.3 | 1.246 | 0.215 |
| Maximum lesion diameter (cm) | 0.85±0.37 | 0.89±0.37 | 0.599 | 0.550 |
| Smoking | 0.036 | 0.850 | ||
| Yes | 12 (12.5%) | 3 (8.1%) | ||
| No | 84 (87.5%) | 34 (91.9%) | ||
| Pulmonary function | ||||
| FEV1 (L) | 2.62±0.69 | 2.54±0.60 | 0.598 | 0.551 |
| Actual FEV1/pre-FEV1 (%) | 95.3±14.8 | 94.1±13.7 | 0.408 | 0.684 |
| Pathological types | 0.120 | 1.000 | ||
| Carcinoma in situ and benign lesions | 4 (4.2%) | 2 (5.4%) | ||
| Microinvasive adenocarcinoma | 69 (71.9%) | 26 (70.3%) | ||
| Invasive adenocarcinoma | 22 (22.9%) | 9 (24.3%) |
FEV1, forced expiratory volume in one second.
Surgical range and cases
The surgeries ranged from general to complex segmentectomy, including single segment or subsegment resection, combined segments or subsegments resection, and segment + subsegment resection. The surgical range and number of cases are shown in Table 2.
Table 2. Surgical range of IQQA group and non-IQQA group.
| Surgical range | IQQA group (n=96) | Non-IQQA group (n=37) | Total |
|---|---|---|---|
| Left lung | 45 | 18 | 63 |
| S1+2 | 9 | 7 | 16 |
| S1+2a | 1 | 0 | 1 |
| S1+2a+b | 7 | 1 | 8 |
| S1+2c | 2 | 0 | 2 |
| S3 | 1 | 0 | 1 |
| S1+2a+S3c | 1 | 0 | 1 |
| S1+2a+b+S3 | 0 | 1 | 1 |
| S4+S5 | 3 | 3 | 6 |
| S4+S5+S1+2c | 2 | 0 | 2 |
| S6 | 3 | 2 | 5 |
| S6+S* | 1 | 0 | 1 |
| S8 | 10 | 3 | 13 |
| S8a | 1 | 0 | 1 |
| S9 | 2 | 0 | 2 |
| S10 | 1 | 1 | 2 |
| S9+S10 | 1 | 0 | 1 |
| Right lung | 51 | 19 | 70 |
| S1 | 7 | 8 | 15 |
| S1+2 | 7 | 4 | 11 |
| S1a+S2a | 1 | 1 | 2 |
| S1+S3 | 1 | 0 | 1 |
| S2 | 6 | 1 | 7 |
| S3 | 9 | 3 | 12 |
| S3b | 1 | 1 | 2 |
| S2+S3a | 2 | 0 | 2 |
| S2b+S3a | 4 | 0 | 4 |
| S6 | 5 | 1 | 6 |
| S8 | 5 | 0 | 5 |
| S9 | 1 | 0 | 1 |
| S8+S9 | 2 | 0 | 2 |
Operation characteristics and postoperative recovery
There was a significant difference in surgical difficulty level between the two groups (P=0.017), with the proportion of moderate and complex segmentectomies higher in the IQQA group than in the non-IQQA group (28.1% vs. 16.2% and 29.2% vs. 13.5%, respectively). There were no significant differences between groups in other operation characteristics, including operation time. The postoperative recovery was likewise similar in the two groups (Table 3).
Table 3. Operation characteristics and postoperative recovery in IQQA and non-IQQA groups.
| Characteristics | IQQA group (n=96) | Non-IQQA group (n=37) | t/χ2 | P |
|---|---|---|---|---|
| Surgical difficulty level | 8.191 | 0.017 | ||
| General segmentectomy | 41 (42.7%) | 26 (70.3%) | ||
| Moderate segmentectomy | 27 (28.1%) | 6 (16.2%) | ||
| Complex segmentectomy | 28 (29.2%) | 5 (13.5%) | ||
| Mediastinal lymph node | 0.838 | 0.658 | ||
| Dissection | 50 (52.1%) | 16 (43.2%) | ||
| Sampling | 37 (38.5%) | 17 (45.9%) | ||
| None | 9 (9.4%) | 4 (10.8%) | ||
| Operation time (min) | 173.5±35.1 | 168.8±52.2 | 0.592 | 0.555 |
| Intraoperative blood loss (mL) | 44.3±18.2 | 42.2±14.0 | 0.637 | 0.525 |
| Total chest drainage (mL) | 523.9±365.3 | 486.2±195.5 | 0.595 | 0.553 |
| Postoperative complications | 8 (8.2%) | 1 (2.7%) | 0.598 | 0.439 |
| Pulmonary infection | 2 (2.1%) | 0 | ||
| Pulmonary leakage >7 days | 2 (2.1%) | 1 (2.7%) | ||
| Acute cerebral infarction | 1 (1.0%) | 0 | ||
| Pleural effusion | 1 (1.0%) | 0 | ||
| Postoperative hemothorax | 1 (1.0%) | 0 | ||
| Liquid pneumothorax | 1 (1.0%) | 0 | ||
| Postoperative hospital stay (days) | 4.9±3.6 | 4.0±1.3 | 1.414 | 0.160 |
Anatomic variations verified with IQQA system
A total of 73 segmental structure variations were found in 55 patients (57.3%) in the IQQA group. The mean frequency of variations was 1.3±0.6 in patients with structural variations and 0.8±0.8 in the IQQA group overall. The number of patients with variations of the segmental bronchus, segmental artery, and segmental vein was 15 (20.5%), 45 (61.6%), and 13 (17.8%), respectively (Table 4). In the IQQA group there were 27 (65.9%) patients with variations in the General subgroup, 13 (48.1%) in the Moderate subgroup, and 15 (53.6%) in the Complex subgroup; these differences were not significant (P=0.315; Table 5).
Table 4. Frequency of different anatomic variations among patients with variations (n=55), according to surgical difficulty level.
| Variable segmental structures* | General segmentectomy (n=27) | Moderate segmentectomy (n=13) | Complex segmentectomy (n=15) | Total |
|---|---|---|---|---|
| Bronchus | 6 | 6 | 3 | 15 (20.5%) |
| Artery | 20 | 11 | 14 | 45 (61.6%) |
| Vein | 10 | 0 | 3 | 13 (17.8%) |
| Total | 36 | 17 | 20 | 73 (100%) |
*For statistical convenience, anatomical differences of common anatomical structures are defined as anatomical variations. For common anatomical structures, see “Illustrated Anatomical Segmentectomy for Lung Cancer” (9).
Table 5. Anatomic variations in the IQQA group, according to surgical difficulty level (n=96).
| Patients | General segmentectomy (n=41) | Moderate segmentectomy (n=27) | Complex segmentectomy (n=28) | χ2 | P |
|---|---|---|---|---|---|
| Variation | 27 (65.9%) | 13 (48.1%) | 15 (53.6%) | 2.309 | 0.315 |
| Non-variation | 14 (34.1%) | 14 (51.9%) | 13 (46.4%) |
Among the 55 patients with variations, 38 (69.1%) had a single structural variation, 15 (27.3%) had two structural variations, and two (3.6%) had three structural variations. Segmental artery variations accounted for the majority in cases of single structural variation (76.3%). Bronchial + arterial and arterial + venous variations were predominant in cases of double structural variations. Single-structure variation accounted for the majority of variations in all segmentectomy difficulty levels, followed by double-structure variations. Variation of three structures was rare (Table 6).
Table 6. Number of structural variations according to surgical difficulty level among patients with variations (n=55).
| Patients | General segmentectomy (n=27) | Moderate segmentectomy (n=13) | Complex segmentectomy (n=15) | Total (n=55) |
|---|---|---|---|---|
| Single structural variation | 19 (70.4%) | 9 (69.2%) | 10 (66.7%) | 38 (69.1%) |
| Double structural variations | 6 (22.2%) | 4 (30.8%) | 5 (33.3%) | 15 (27.3%) |
| Three structural variations | 2 (7.4%) | 0 (0%) | 0 (0%) | 2 (3.6%) |
Discussion
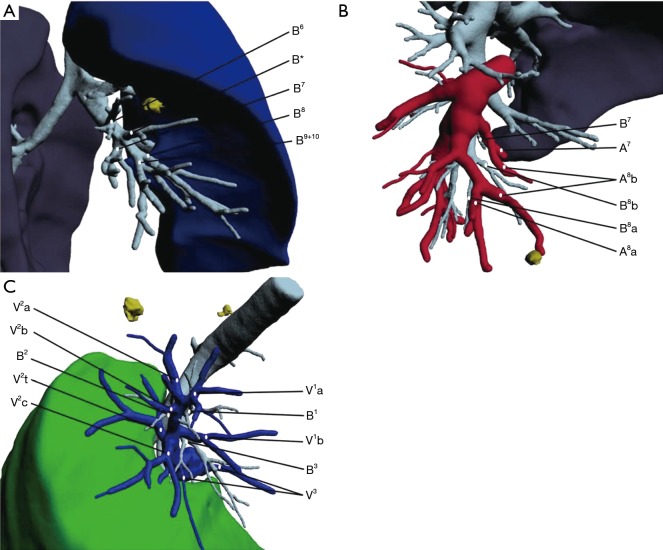
For peripheral early lung cancer, metastatic tumors, and benign lesions, segmentectomy can be an optimal treatment method which can completely remove the lesion while preserving normal lung tissue. However, the location of pulmonary nodules is very random and they can be located anywhere in the lung tissue, which poses a challenge to clinicians. Sometimes nodule location and anatomic variations may be easily determined with two-dimensional CT scan, but accurate localization is difficult in many cases. Segmental structures are complex and variable (Figure 2), which increases the difficulty of resection. Surgical procedures are not identical in different segmentectomy because of the variable anatomy. 3D reconstruction software such as the IQQA system can facilitate surgery. The system can locate nodules accurately, identify anatomical variations, help with preoperative planning, and play an important role in intraoperative navigation.
Figure 2.
Examples of segmental variation in right lung. (A) B* variation; (B) A8b from A7; (C) both V1a and V1b running into Vcenter, rather than running ventral to the hilum. B, bronchus; A, artery; V, vein.
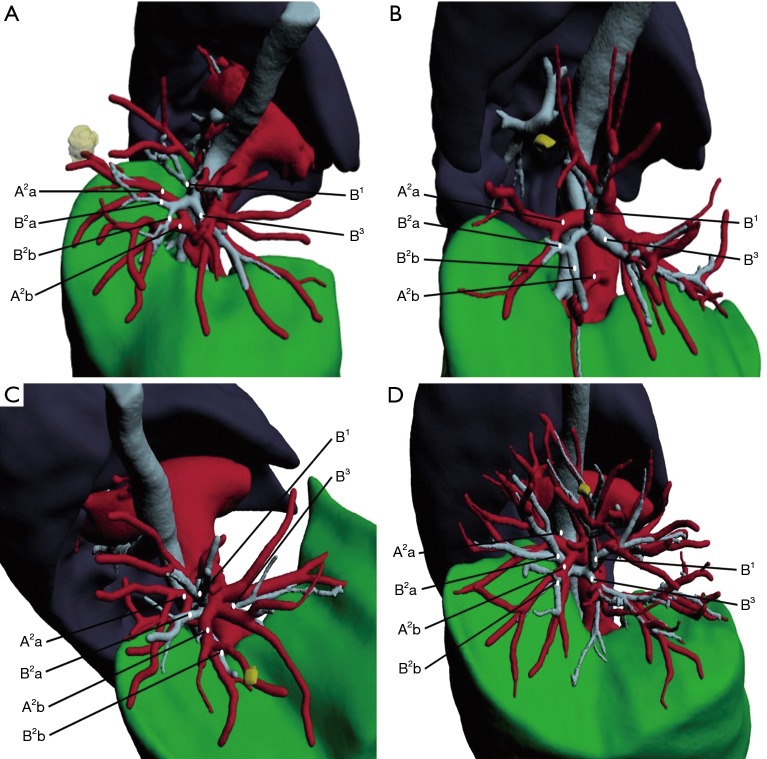
In this study the IQQA found many segmental artery, vein, and bronchus variations before surgery, identifying variations in 57.3% of patients in the IQQA group. The variable frequency of segmental artery and vein were 45 (61.6%) and 13 (17.8%), which were obviously higher than those (12% and 4% in 124 cases) reported by Hagiwara et al. (10). The reasons are as follows: most cases in his study were lobectomy (119, 96%) rather than segmentectomy (5, 4%), and the structures concerned were not subtle enough. They used 3-dimensional computed tomography software for reconstruction, showing unclear bilateral superior pulmonary vessels, because pulmonary arteries and veins are too close to each other to distinguish. We found that there were many differences about the same segmental anatomy in different cases in our research (Figure 3). Therefore, IQQA has obvious advantages in the preoperative diagnosis of anatomic variations.
Figure 3.
Different anatomy of right A2 come from different cases. (A) Both A2a and A2b from ascending artery; (B) A2a from recurrent artery, A2b from ascending artery; (C) A2a from recurrent artery, A2b from A3; (D) both A2a and A2b from recurrent artery. B, bronchus; A, artery; V, vein.
Comparing operation characteristics and postoperative recovery in the two groups, we found that the proportion of moderate and complex operations in the IQQA group was significantly higher than that in the non-IQQA group (28.1% vs. 16.2% and 29.2% vs. 13.5%, respectively). The IQQA group did not have longer operation times or increased postoperative complications, despite the higher percentage of complicated operations. Possible reasons for this finding include the following: (I) the application of IQQA provided reliable preoperative determination of nodule location, anatomical identification, and intraoperative navigation, which simplified the operation process of complicated surgery; (II) the IQQA helped doctors to understand 3D information about segmental bronchi and vessels more easily, which increased the confidence of surgeons; (III) the surgical procedure was planned in advance to reduce unnecessary exploration of surrounding structures and decrease tissue damage.
In the IQQA group, anatomic variations were present in 65.9% of patients undergoing general segmentectomy, in 48.1% of patients undergoing moderate segmentectomy, and in 53.6% of patients undergoing complex segmentectomy; these differences were not statistically significant. The prevalence of variations did not correlate with surgical difficulty. Therefore, if conditions permit, preoperative 3D imaging is helpful for surgery and is recommended for all patients undergoing segmentectomy.
Conclusions
Preoperative 3D reconstruction is necessary before segmentectomy, especially for patients undergoing moderate or complex segmentectomy. IQQA is safe and feasible for preoperative lesions localization, surgical planning, and intraoperative navigation in uniportal VATS anatomical segmentectomy and can facilitate complicated segmentectomy.
Acknowledgments
We thank Rebecca Tollefson, DVM, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Ethical Statement: The study was evaluated by the Ethics Committee of Fujian Medical University Union Hospital within which the work was undertaken and that it conforms to the provisions the Helsinki Declaration as revised in 2013.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. 10.21037/jtd.2018.02.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Pastorini A, Koryllos A, Schnell J, et al. Perioperative outcome after open and thoracoscopic segmentectomy for the treatment of malignant and benign pulmonary lesions: a propensity-matched analysis. J Thorac Dis 2018;10:3651-60. 10.21037/jtd.2018.05.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. 10.1016/j.athoracsur.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 4.Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. 10.1093/ejcts/ezu422 [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Xie B, Hu M, et al. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. 10.1093/icvts/ivw085 [DOI] [PubMed] [Google Scholar]
- 6.Yao F, Wang J, Yao J, et al. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg 2017;39:16-22. 10.1016/j.ijsu.2017.01.079 [DOI] [PubMed] [Google Scholar]
- 7.Wu WB, Xu XF, Wen W, et al. Thoracoscopic Pulmonary Sub-Subsegmentectomy Based on Three-Dimensional Images. Ann Thorac Surg 2016;102:e389-91. 10.1016/j.athoracsur.2016.04.048 [DOI] [PubMed] [Google Scholar]
- 8.Xu G, Chen C, Zheng W, et al. IQQA-3D imaging interpretation and analysis system-guided single-port video-assisted thoracic surgery for anatomical sub-segmentectomy (LS1+2a+b). J Thorac Dis 2018;10:5515-21. 10.21037/jtd.2018.08.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomori H, Okada M. Illustrated Anatomical Segmentectomy for Lung Cancer. Tokyo: Springer, 2012. [Google Scholar]
- 10.Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery†. Eur J Cardiothorac Surg 2014;46:e120-6. 10.1093/ejcts/ezu375 [DOI] [PubMed] [Google Scholar]