Abstract
Background
Postoperative complications after lung resection are common and fatal. The immediate effects of postoperative complications are related to poor prognosis; however, the long-term effects have not been assessed. Thus, this investigation aimed to clarify the long-term effects of postoperative complications among patients with resected non-small cell lung cancer (NSCLC).
Methods
This retrospective cohort study included 345 patients with resected NSCLC from a single institution. We used the Clavien-Dindo classification to classify postoperative complications. Postoperative complications were defined as complications with a Clavien-Dindo grade of ≥2. The Kaplan-Meier method was used to evaluate survival. Prognostic factors were analyzed using a Cox proportional hazard model.
Results
There were 110 patients with postoperative complications (31.9%). The 5-year overall survival (OS), recurrence-free survival (RFS), and cause-specific survival (CSS) rates were significantly lower in patients with complications than in those without complications [OS: 66.1%, 95% confidence interval (CI): 55.4–74.8% vs. 78.0%, 95% CI: 71.8–83.1%, P=0.001; RFS: 48.8%, 95% CI: 38.1–58.7% vs. 70.8%, 95% CI: 64.2–76.4%, P<0.001; CSS: 82.7%, 95% CI: 72.8–89.3% vs. 88.2%, 95% CI: 82.8–92.0%, P=0.005]. The 5-year OS was lower in the pulmonary complication group than in the other complication group (58.1%, 95% CI: 40.0–72.4% vs. 70.5%, 95% CI: 56.6–80.6%, P=0.033). Postoperative complications were indicated as a poor prognostic factor for OS (hazard ratio, 1.67; 95% CI: 1.11–2.53; P=0.002).
Conclusions
Postoperative complications were associated with unfavorable OS because of the worse prognosis of postoperative pulmonary complications.
Keywords: Non-small cell lung cancer (NSCLC), complication, surgery, prognosis, long-term effect
Introduction
Lung resection is a curative treatment for early lung cancer (1). The advantages of radical lobectomy were first reported by Cahan 40 years ago (2), and it has become the standard surgical approach for non-small cell lung cancer (NSCLC). However, postoperative complications following lung resection for NSCLC are common and unavoidable, with a reported incidence of 9.0–53.4% (3-6).
Short-term mortality is closely associated with the severity of postoperative complications (1,3). Postoperative complications are well documented to be a poor prognostic factor, with immediate negative effects (7,8). However, the long-term effect of postoperative complications on survival and recurrence is controversial. It is vital to elucidate the long-term impact of postoperative complications on patients with NSCLC in order to help with the management of these patients.
In this study, we aimed to examine the long-term effect of postoperative complications following lung resection and to demonstrate the prognostic impact of complication types in NSCLC patients with postoperative complications.
Methods
This study was obtained approved by the ethics committee of our hospital (approval number 201711-05), and it was performed in accordance with the Declaration of Helsinki. The requirement for patient consent was waived because this was a retrospective cohort study.
Patients
We retrospectively investigated 363 patients with NSCLC who underwent lung resections in our institution between April 2007 and December 2016. Among them, 11 patients who had incomplete resection, five who had a surgery-related death, which was defined as an event within the hospital stay or within 30 days postoperatively, and two who received induction chemotherapy were excluded. Patients with surgery-related death were excluded because the purpose of this investigation was to evaluate the long-term effect, not the short-term effect, of postoperative complications. Ultimately, 345 consecutive patients were included.
Baseline characteristics were compared between patients with and without postoperative complications. Tumor-node-metastasis staging was determined based on the eighth edition of the International Association for the Study of Lung Cancer Staging System (9).
Postoperative complications
Postoperative complications were classified using the Clavien-Dindo system, which categorizes postoperative complications into five major groups according to the intervention required for patient care: grade 1, requiring no pharmacological intervention other than antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy; grade 2, requiring pharmacological intervention with drugs other than those allowed for grade 1 complications; grade 3, requiring further intervention with or without general anesthesia; grade 4, life-threatening complications and those requiring intensive care unit management; and grade 5, death of the patients (10). Grade 1 complication is regarded as a minor issue; therefore, postoperative complications in this study were defined as events with Clavien-Dindo grade ≥2 during the hospital stay or within 30 days postoperatively. Postoperative pulmonary complications were defined, according to a previous report (11), as the occurrence of atelectasis, thoracic empyema, and pneumonia requiring therapeutic intervention, with the following clinical manifestations: consolidations and atelectasis on chest radiography, elevated white blood cell counts >11.2×109/L, body temperature >38 °C, purulent sputum, and oxygen saturation <90% on room air. In addition, acute respiratory distress syndrome is not classified independently because we classified postoperative respiratory complications according to the cause of respiratory dysfunction.
Surgical procedure and postoperative follow-up protocol
All operations were performed under the observation and guidance of the supervisor of thoracic surgery. The surgical approach [open thoracotomy or video-assisted thoracoscopic surgery (VATS)] was chosen with consideration of the oncological aspects and patients’ background. Mediastinal lymph node dissection was conducted routinely during lobectomy and pneumonectomy, but lymph node sampling was only performed in high-risk patients, such as those with a low performance status and severe comorbidity, and in elderly patients.
In our institution, patients with a pathological stage ≥ IA3 received adjuvant chemotherapy. In the case of stages IA3 and IB, patients received 400 mg/day of uracil plus tegafur orally for 2 years. In the case of stage II or III, patients received three or four cycles of platinum-based chemotherapy. We evaluated the default rate of adjuvant therapy which is the proportion of patients who needed to receive adjuvant therapy but actually did not receive. Blood tests for tumor markers were performed at 2-month or 3-month intervals. Tumor recurrence was assessed using computed tomography, which was performed at 6, 12, 18, and 24 months postoperatively and yearly thereafter.
Statistical analysis
Data are shown as a range, mean, or median. Clinicopathological factors were evaluated using the χ2 test or Fisher exact test for categorical variables and Student’s t-test or Mann-Whitney U-test for continuous variables. Overall survival (OS), recurrence-free survival (RFS), and cause-specific survival (CSS) were calculated using the Kaplan-Meier method and compared using the log-rank test. Patients were censored at the date of the last follow-up when they were lost to follow-up. A Cox proportional hazard model was used for univariate and multivariate analyses to identify prognostic factors for OS. Variables with a P value <0.05 in univariate analysis were selected for inclusion in the multivariate regression model; P values <0.05 were considered statistically significant. Statistical analyses were conducted with R commander (R 3.4.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
In total, 345 patients who underwent complete NSCLC resection were included in the analysis; 243 patients (70.4%) were alive and 102 died (29.6%). The median length of follow-up for all cases was 57.4 (interquartile range, 31.3–84.3) months. Postoperative complications were observed in 110 patients (110/345, 31.9%). The clinical characteristics with or without postoperative complications are shown in Table 1. The operative time, smoking index (pack-years), incidence of chronic obstructive pulmonary disease, and incidence of histologic vascular invasion were significantly higher in the patients with complications than in those without complications (P=0.001, 0.012, <0.001, and 0.018, respectively). Forced expiratory volume in 1 second/forced vital capacity (FEV1.0%) was lower in the patients with complications than in those without complications (P<0.001). The default rate of adjuvant therapy did not differ between patients without complications and with complications (35.3% vs. 41.8%, P=0.28). Table 2 shows the causes of death; there were no significant differences between the two groups.
Table 1. Patients’ characteristics with or without postoperative complication.
| Variables | Complication (N=110), n (%) | No-complication (N=235), n (%) | P value |
|---|---|---|---|
| Age, y, mean [IQR] | 71.0 [65–76] | 70.0 [65–74] | 0.22 |
| Male sex | 81 (73.6) | 155 (66.0) | 0.19 |
| Former or current smoker | 90 (81.8) | 175 (74.5) | 0.17 |
| Smoking, pack-years, median [IQR] | 50 [10–70] | 41 [0–55] | 0.012 |
| BMI, kg/m2 [IQR] | 23.0 [20.4–25.9] | 22.6 [20.3–25.1] | 0.32 |
| CCI 0/1–2/≥3 | 17/66/27 | 59/119/57 | 0.11 |
| Comorbidity | |||
| Diabetes mellitus | 30 (27.3) | 61 (26.0) | 0.80 |
| COPD | 47 (42.7) | 57 (24.3) | 0.001 |
| Performance status ≥1 | 15 (13.6) | 228 (97.0) | 0.87 |
| CEA ≥5 ng/mL | 36 (32.7) | 76 (32.3) | 1.00 |
| FVC, mL, median [IQR] | 3,065 [2,560–3,600] | 3,005 [2,490–3,590] | 0.36 |
| FEV1.0%, %, median [IQR] | 73 [66–78] | 77 [70–82] | <0.001 |
| Operation time, min, median [IQR] | 288 [243–335] | 252 [184–314] | 0.001 |
| Surgical approach, open | 63 (57.3) | 140 (59.6) | 0.65 |
| Surgical procedure | 0.15 | ||
| Wedge | 15 (13.6) | 42 (17.9) | |
| Segmentectomy | 1 (0.9) | 12 (5.1) | |
| Lobectomy | 93 (84.5) | 179 (76.2) | |
| Pneumonectomy | 1 (0.9) | 2 (0.9) | |
| Histological type Ad/Sq/othersa | 67/36/7 | 162/56/17 | 0.22 |
| Histologic vascular invasion | 58 (52.7) | 90 (38.3) | 0.018 |
| Lymphatic invasion | 80 (72.7) | 150 (63.8) | 0.14 |
| Pleural invasion | 39 (35.5) | 85 (36.2) | 0.99 |
| Pathological lymph node metastasis | 0.59 | ||
| N0/1/2 | 89/10/11 | 200/18/17 | |
| Pathological T stage | 0.15 | ||
| T1/2/3/4 | 52/34/17/7 | 121/85/21/8 | |
| Pathological stage | 0.071 | ||
| I/II/III | 66/25/19 | 170/37/28 | |
| Adjuvant chemotherapy | 33 (30.0) | 74 (31.5) | 0.80 |
| The default rate of adjuvant therapy | 46 (41.8) | 83 (35.3) | 0.28 |
a, includes large cell carcinoma, pleomorphic carcinoma, and adenosquamous cell carcinoma. BMI, body mass index; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; CEA, carcinoembryonic antigen; FVC, forced vital capacity; FEV, forced expiratory volume; IQR, interquartile range.
Table 2. Cause of deaths.
| Variables | Complication (N=110), n (%) | No-complication (N=235), n (%) | P value |
|---|---|---|---|
| Number of deaths | 41 (37.3) | 61 (26.0) | 0.043 |
| Cause of death | 0.32 | ||
| Primary lung cancer | 21 (19.1) | 29 (12.3) | 0.26 |
| Non-primary lung cancer | 20 (18.2) | 32 (13.6) | 0.20 |
| Other cancer | 4 (3.6) | 8 (3.4) | 1.00 |
| Respiratory | 8 (7.3) | 10 (4.3) | 0.30 |
| Cardiovascular | 2 (1.8) | 3 (1.3) | 0.66 |
| Other | 6 (5.5) | 11 (4.7) | 0.79 |
Postoperative complications
Postoperative complications according to the Clavien-Dindo classification are shown in Table 3. The number of patients with postoperative complications was 110 (31.9%), and the total number of postoperative complications was 130. Twenty patients had two complications. The most common complication was pneumonia (27/130, 20.8%), followed by air leak (22/130, 16.9%) and arrhythmia (13/130, 10.0%). Thirty-nine patients met the criteria of postoperative pulmonary complications (39/110, 35.5%).
Table 3. Postoperative complications classified according to Clavien-Dindo.
| Postoperative complications | Grade 2 | Grade 3 | Grade 4 | Total number | Proportion of complications (%) |
|---|---|---|---|---|---|
| Pulmonary | |||||
| Pneumonia | 17 | 6 | 4 | 27 | 20.8 |
| Interstitial pneumonia | 1 | 0 | 2 | 3 | 2.3 |
| Atelectasis | 1 | 2 | 0 | 3 | 2.3 |
| Air leak | 0 | 21 | 1 | 22 | 16.9 |
| Thoracic empyema | 0 | 3 | 0 | 3 | 2.3 |
| Asthma attack | 6 | 0 | 0 | 6 | 4.6 |
| Upper respiratory inflammation | 3 | 0 | 0 | 3 | 2.3 |
| Bronchopleural fistula | 0 | 0 | 2 | 2 | 1.5 |
| Othersa | 6 | 6 | 0 | 12 | 9.2 |
| Cardiovascular | |||||
| Arrhythmia | 12 | 1 | 0 | 13 | 10.0 |
| Cardiac failure | 2 | 0 | 0 | 2 | 1.5 |
| Cerebral | |||||
| Cerebral infarction | 1 | 0 | 0 | 1 | 0.9 |
| Haemorrhage | 0 | 1 | 0 | 1 | 0.9 |
| Psychiatry | |||||
| Delirium | 6 | 0 | 0 | 6 | 4.6 |
| Depression | 2 | 0 | 0 | 2 | 1.5 |
| Skin rash | 3 | 0 | 0 | 3 | 2.3 |
| Others related to operative procedure | |||||
| Chylothorax | 0 | 2 | 0 | 2 | 1.5 |
| Haemorrhage | 3 | 1 | 0 | 4 | 3.1 |
| Wound infection | 0 | 3 | 0 | 3 | 2.3 |
| Intercostal neuralgia | 3 | 0 | 0 | 3 | 2.3 |
| Othersb | 9 | 0 | 0 | 9 | 6.9 |
| Sum | 75 | 46 | 9 | 130 | 100 |
a, includes 4 cases of grade 3 and 1 case of grade1 pleural effusion, 1 case of grade 3 trachea cicatricial stenosis, 2 cases of grade 2 cough, 1 case of grade2 aspiration of tooth and 3 case of grade2 acute exacerbation of COPD; b, includes 1 case of grade2 exacerbation of an inclusion body myositis, 1 case of grade2 liver damage, 2 cases of grade2 urinary tract infection, 3 cases of grade2 peptic ulcer, 1 case of grade2 drug fever, and 1 case of grade2 syndrome of inappropriate secretion of antidiuretic hormone.
Survival analysis
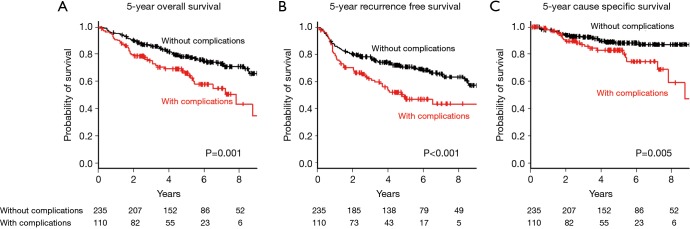
Kaplan-Maier curves for OS, RFS, and CSS in the two groups are shown in Figure 1A,B,C. The 5-year OS, RFS, and CSS were poorer in the patients with complications than in those without complications [OS: 66.1%, 95% confidence interval (CI): 55.4–74.8% vs. 78.0%, 95% CI: 71.8–83.1%, P=0.001; RFS: 48.8%, 95% CI: 38.1–58.7% vs. 70.8%, 95% CI: 64.2–76.4%, P<0.001; CSS: 82.7%, 95% CI: 72.8–89.3% vs. 88.2%, 95% CI: 82.8–92.0%, P=0.005].
Figure 1.
Kaplan-Meier survival curves of patients with and without postoperative complications: (A) 5-year overall survival; (B) 5-year recurrence-free survival; and (C) 5-year cause-specific survival.
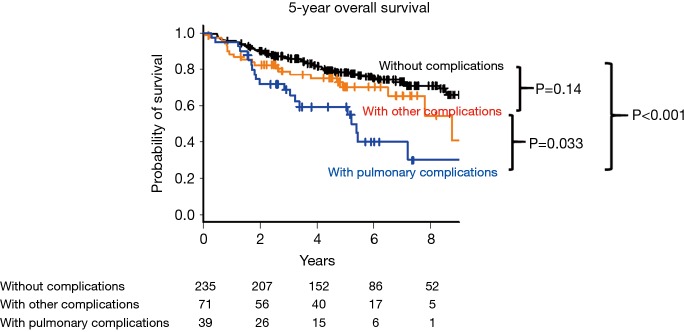
The 5-year OS was compared between the following three groups: patients without postoperative complications, patients with pulmonary postoperative complications, and patients with the other complications (Figure 2). The 5-year OS was worse in patients with postoperative pulmonary complications than in those without complications (58.1%, 95% CI: 40.0–72.4% vs. 78.0%, 95% CI: 71.7–83.0%, P<0.001). The 5-year OS in patients with pulmonary complications was worse than in those with the other complications (58.1%, 95% CI: 40.0–72.4% vs. 70.5%, 95% CI: 56.6–80.6%, P=0.033). There was no significant difference between patients with other complications and those without complications (70.5%, 95% CI: 56.6–80.6% vs. 78.0%, 95% CI: 71.7–83.0%, P=0.14).
Figure 2.
Kaplan-Meier survival curves of patients with pulmonary complications, those with other complications, and those without postoperative complications.
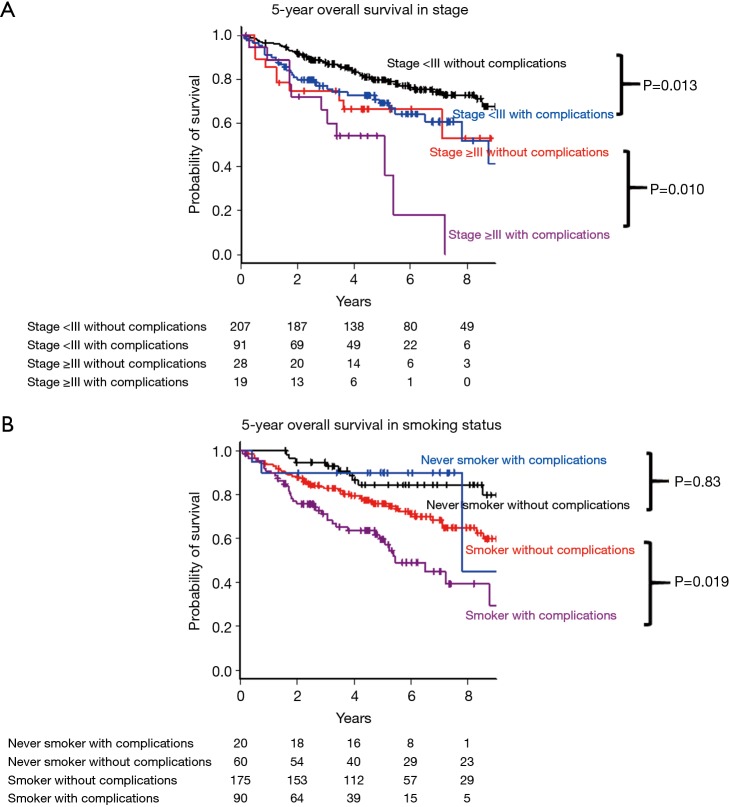
Subgroup analysis was performed with respect to pathological stage (stage <3 or ≥3) and smoking status (smoker or never smoker). In pathological stage I and II, the 5-year OS was worse in the group with complications (69.2%, 95% CI: 57.5–78.2% vs. 79.7%, 95% CI: 73.1–84.9%, P=0.013, Figure 3A), although there is no statistical difference between two groups in pathological stage ≥III (54.2%, 95% CI: 28.8–73.9% vs. 66.5%, 95% CI: 45.3–81.1%, P=0.10, Figure 3A). Concerning smoking status, the 5-year OS of the group with was lower among smoker (59.7%, 95% CI: 47.2–70.1% vs. 75.8%, 95% CI: 68.3–81.8%, P=0.019, Figure 3B), although there was no difference among never smoker (84.5%, 95% CI: 71.2–92.0% vs. 90.0%, 95% CI: 65.6–97.4%, P=0.83, Figure 3B).
Figure 3.
Subgroup analysis for the 5-year overall survival among patients with and without postoperative complications: (A) in pathological stage; (B) in smoking status.
Analysis of the prognostic factors in the Cox proportional hazard model
The results of the univariate and multivariate survival analyses of prognostic factors for OS are shown in Table 4. Covariates with P<0.05 in univariate analysis were included in the multivariate analysis. The Cox proportional hazard model for OS, which was adjusted for these covariates, showed that the presence of postoperative complications was associated with a poorer OS (hazard ratio, 1.67; 95% CI: 1.11–2.53; P=0.015).
Table 4. Cox proportional hazards model of overall survival.
| Variables | Univariate Cox model | Multivariate Cox model | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | 1.04 | 1.01–1.07 | 0.004 | 1.03 | 1.00–1.06 | 0.050 | |
| Male sex | 1.88 | 1.18–3.00 | 0.008 | 0.83 | 0.45–1.51 | 0.53 | |
| Former or current smoker | 2.80 | 1.56–5.05 | <0.001 | 2.11 | 0.97–4.59 | 0.060 | |
| PS ≥1 | 2.00 | 1.18–3.39 | 0.010 | 1.51 | 0.87–2.62 | 0.15 | |
| Pathological stage | |||||||
| II (vs. I) | 2.12 | 1.32–3.38 | <0.001 | 1.65 | 0.99–2.75 | 0.055 | |
| III (vs. I) | 2.77 | 1.66-4.61 | <0.001 | 2.26 | 1.29–3.98 | 0.005 | |
| CCI | |||||||
| 1–2 (vs. 0) | 2.32 | 1.22–4.43 | 0.011 | 1.66 | 0.83–3.34 | 0.15 | |
| ≥3 (vs. 0) | 3.47 | 1.76–6.83 | <0.001 | 2.18 | 1.03–4.60 | 0.041 | |
| COPD | 1.66 | 1.12–2.48 | 0.013 | 0.96 | 0.62–1.50 | 0.87 | |
| Histology; Sq | 1.42 | 0.92–2.17 | 0.11 | – | – | – | |
| Vascular invasion | 1.49 | 1.01–2.20 | 0.047 | 1.01 | 0.66–1.54 | 0.96 | |
| Lymphatic invasion | 1.45 | 0.94–2.22 | 0.090 | – | – | – | |
| Pleural invasion | 1.56 | 1.06–2.31 | 0.024 | 1.16 | 0.75–1.78 | 0.50 | |
| CEA ≥5 ng/mL | 2.28 | 1.55–3.37 | <0.001 | 1.75 | 1.16–2.65 | 0.007 | |
| Surgical procedure, wedge | 1.06 | 0.65–1.73 | 0.82 | 0.70 | 0.40–1.21 | 0.20 | |
| Surgical approach, open | 1.04 | 0.75–1.43 | 0.83 | – | – | – | |
| Adjuvant chemotherapy | 1.02 | 0.67–1.54 | 0.94 | – | – | – | |
| Postoperative complication | 1.92 | 1.28–2.86 | 0.002 | 1.67 | 1.11–2.53 | 0.015 | |
CCI, Charlson comorbidity index; CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease.
Discussion
We revealed three important results: first, OS, RFS, and CSS decreased in patients with postoperative complications compared to those in patients without postoperative complications. Second, postoperative pulmonary complications were related to worse OS than other complications. Third, the incidence of postoperative complications was found to be an unfavorable prognostic factor in the multivariate analysis. The novelty of our study is that it focused on the long-term effect of postoperative complications on patients with NSCLC, excluding those with surgery-related deaths.
The rate of postoperative complications following lung resection was previously reported to be 9.0–53.4% (3-6). Moreover, according to the Clavien-Dindo classification, postoperative complications occur at a rate of 39.0–53.4% (3,5). In the present study, the incidence of postoperative complications was 31.9%, which is considered acceptable compared to that reported by other institutions. The Clavien-Dindo classification has proven to be extremely reliable and is used by thoracic surgeons worldwide (12). Complications defined by this grading system were associated with prolonged hospital stay (5), mortality, and increased readmission rates (13). We excluded grade 1 postoperative complications because they require no therapeutic intervention.
We showed that postoperative complications were associated with poorer OS, RFS, and CSS. This result is in agreement with the findings of previous reports regarding the influence of postoperative complications on long-term prognosis in NSCLC patients (6,11,14,15). A large, retrospective cohort study reported that postoperative complications were associated with poor OS and CSS (6). Another prospective study showed that postoperative pulmonary complications were associated with poor OS owing to an increase in deaths due to non-primary lung cancer (11). Furthermore, two retrospective cohort studies reported that postoperative pulmonary complications were associated with increased cancer recurrence, resulting in poor RFS and DFS (14,15). In a large population study, Wang and colleagues reported that major lung complications were associated with a poor OS and DFS among patients who had undergone VATS lobectomy (15). There are two explanations for the association between postoperative complications and poor prognosis: (I) an increase in postoperative deaths due to non-primary lung cancer in patients after postoperative complications; and (II) accelerated cancer development and recurrence after postoperative complications.
Regarding the first hypothesis, Lugg et al. revealed that postoperative pulmonary complications were related to poor OS and increases in deaths due to non-primary lung cancer (11). They reported that non-primary cancer deaths were observed to be twice as two-fold higher in patients with postoperative pulmonary complications than in those without complications. In addition, they suggested that deaths due to respiratory dysfunction were three-fold higher in patients with postoperative pulmonary complications than in those without complications. Our study indicated the possibility of worse OS in patients with postoperative pulmonary complications compared to those with other complications. A previous retrospective cohort study indicated that postoperative pulmonary complications in patients with NSCLC increased the lifetime incidence of pneumonia after lung resections (16). These reports suggest that an increase in deaths due to non-primary lung cancer is associated with poor OS in NSCLC patients with postoperative complications.
The second hypothesis is that postoperative complications stimulate cancer development and recurrence. Previous reports have demonstrated that postoperative complications in patients with surgically resected NSCLC resulted in poor CSS (6) and RFS (14,15). The poor prognosis following postoperative complications can be biologically explained by the heightened inflammatory status following these complications. Systemic inflammation with elevated white blood cell, C-reactive protein, interleukin-6, and tumor necrosis factor-α levels may be linked to tumor progression and distant metastasis through immunomodulatory effects (17,18). The increased inflammatory status following postoperative complications promotes the adhesion of tumor cells to vascular endothelial cells and other organs; therefore, postoperative complications may have an important role in cancer recurrence and poor OS (14,19). Moreover, surgical stress suppresses the patient’s immune system though an elevation in the number of regulatory T cells (20). Blood transfusion and invasive surgical procedures following postoperative complications, which may suppress the immune system, are associated with cancer recurrence (21,22). Therefore, postoperative complications may increase the risk of cancer progression postoperatively, and this explains the poor OS, RFS, and CSS observed in the present study.
This investigation has some inevitable limitations. First, our study’s results, derived from a single institution, may not be readily generalizable to other populations. Second, the incidence of postoperative complications may change clinical decision making postoperatively; therefore, selection bias is unavoidable. Third, the number of patients in this study was relatively small, and the present study included heterogeneous patients with lobectomy, segmentectomy, wedge resection, and pneumonectomy; therefore, a large prospective cohort study is needed to evaluate the prognostic impact of postoperative complications on the long-term survival outcome. Forth, postoperative complications depend on the patients’ prototype; therefore, the worse OS in patients with postoperative complications may not be directly linked to postoperative complications. In this sense, there is a possibility that the incidence of postoperative complication is a confounding factor between patients’ variables and worse prognosis. Although we minimized it by multivariate analysis, the bias is unavoidable.
Conclusions
The present study indicated that postoperative complications, as defined by the Clavien-Dindo classification, resulted in poor OS, RFS, and CSS among patients with NSCLC who underwent lung resection. Pulmonary complications were particularly associated with worse OS. Special attention must be paid to the diligent follow-up of NSCLC patients with postoperative complications.
Acknowledgments
None.
Ethical Statement: The study was approved by the ethics committee of our hospital (approval number 201711-05). The requirement for patient consent was waived because this was a retrospective cohort study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer 2005;50:227-34. 10.1016/j.lungcan.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 2.Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [PubMed] [Google Scholar]
- 3.Zhang Z, Mostofian F, Ivanovic J, et al. All grades of severity of postoperative adverse events are associated with prolonged length of stay after lung cancer resection. J Thorac Cardiovasc Surg 2018;155:798-807. 10.1016/j.jtcvs.2017.09.094 [DOI] [PubMed] [Google Scholar]
- 4.Saji H, Ueno T, Nakamura H, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: JACS1303. Eur J Cardiothorac Surg 2018;53:835-41. 10.1093/ejcts/ezx415 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wu N, Zheng Q, et al. Prediction of Surgical Outcome by Modeling Based on Risk Factors of Morbidity After Pulmonary Resection for Lung Cancer in Older Adults. Ann Thorac Surg 2016;102:971-8. 10.1016/j.athoracsur.2016.03.116 [DOI] [PubMed] [Google Scholar]
- 6.Rueth NM, Parsons HM, Habermann EB, et al. The long-term impact of surgical complications after resection of stage I nonsmall cell lung cancer: a population-based survival analysis. Ann Surg 2011;254:368-74. 10.1097/SLA.0b013e31822150fe [DOI] [PubMed] [Google Scholar]
- 7.Farjah F, Backhus L, Cheng A, et al. Failure to rescue and pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2015;149:1365-71. 10.1016/j.jtcvs.2015.01.063 [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, McMurry TL, Isbell JM, et al. Readmission after lung cancer resection is associated with a 6-fold increase in 90-day postoperative mortality. J Thorac Cardiovasc Surg 2014;148:2261-7.e1. 10.1016/j.jtcvs.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer J Thorac Oncol 2016;11:39-51. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugg ST, Agostini PJ, Tikka T, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71:171-6. 10.1136/thoraxjnl-2015-207697 [DOI] [PubMed] [Google Scholar]
- 12.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 13.Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42. 10.1016/j.athoracsur.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 14.Nojiri T, Hamasaki T, Inoue M, et al. Long-term impact of postoperative complications on cancer recurrence following lung cancer surgery. Ann Surg Oncol 2017;24:1135-42. 10.1245/s10434-016-5655-8 [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Li X, Li Y, et al. The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer. J Thorac Dis 2017;9:5143-52. 10.21037/jtd.2017.10.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara S, Sugaya M, Onitsuka T, et al. Impact of Postoperative Pneumonia Developing After Discharge on Long-Term Follow-up for Resected Lung Cancer. World J Surg 2018;42:3979-87. 10.1007/s00268-018-4727-2 [DOI] [PubMed] [Google Scholar]
- 17.Alifano M, Falcoz PE, Seegers V, et al. Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:1161-7. 10.1016/j.jtcvs.2011.07.021 [DOI] [PubMed] [Google Scholar]
- 18.Shinohara S, Sugaya M, Onitsuka T, et al. Prognostic Impact of Postoperative C-reactive Protein for Non-small Cell Lung Cancer Following Lobectomy. Anticancer Res 2018;38:3193-8. [DOI] [PubMed] [Google Scholar]
- 19.Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 2015;157:362-80. 10.1016/j.surg.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol 2010;6:1863-81. 10.2217/fon.10.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumberg N, Heal J, Chuang C, et al. Further evidence supporting a cause and effect relationship between blood transfusion and earlier cancer recurrence. Ann Surg 1988;207:410-5. 10.1097/00000658-198804000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan H, Ye F, Wu L, et al. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg 2014;14:34. 10.1186/1471-2482-14-34 [DOI] [PMC free article] [PubMed] [Google Scholar]