Abstract
Background
This quantitative meta-analysis was conducted to provide an indirect comparison of the diagnostic value of computed tomography (CT) with positron emission tomography (PET)/CT for differentiating benign and malignant solitary pulmonary nodules (SPNs).
Methods
PubMed, Embase, and the Cochrane Library were searched to identify eligible studies throughout November 2018, which differentiated benign and malignant SPNs using CT or PET/CT. The summary sensitivity, specificity, positive and negative likelihood ratio (PLR and NLR), diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC) were calculated using bivariate generalized linear mixed model and random-effects model. The diagnostic value of CT with PET/CT was indirectly evaluated using the ratio for diagnostic parameters.
Results
The sensitivity, specificity, PLR, NLR, DOR, and AUC for CT were 0.94 [95% confidence interval (CI): 0.87–0.97], 0.73 (95% CI: 0.64–0.80), 3.45 (95% CI: 2.60–4.58), 0.09 (95% CI: 0.04–0.17), 32.01 (95% CI: 15.10–67.86), and 0.89 (95% CI: 0.86–0.91), respectively. The pooled sensitivity, specificity, PLR, NLR, DOR, and AUC for PET/CT were 0.89 (95% CI: 0.85–0.92), 0.78 (95% CI: 0.66–0.86), 3.97 (95% CI: 2.57–6.13), 0.15 (95% CI: 0.10–0.20), 24.04 (95% CI: 12.71–45.48), and 0.91 (95% CI: 0.89–0.94), respectively. No significant differences were observed between CT and PET/CT for sensitivity, specificity, PLR, NLR, DOR, and AUC.
Conclusions
This study used both CT and PET/CT with a moderate-to-high diagnostic value for differentiating benign and malignant SPNs and showed no significant differences in diagnostic parameters between CT and PET/CT.
Keywords: 18F-FDG-PET/CT, benign solitary pulmonary nodules (benign SPNs), malignant solitary pulmonary nodules (malignant SPNs), diagnosis
Introduction
Lung cancer has been the leading cause of cancer incidence and mortality worldwide for several decades, accounting for nearly 13% of the total cancer cases (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases, and 15% of cases are small cell lung cancer (SCLC) (2). Although the treatment strategies, including surgery, chemotherapy, radiotherapy, and targeted therapy, have developed rapidly, the prognosis of lung cancer remains poor; the 5-year survival rate of NSCLC and SCLC is less than 15% and 1–3%, respectively (3,4). Therefore, choosing an appropriate diagnostic tool is essential to early detect and hence decrease the mortality rate of lung cancer.
Solitary pulmonary nodule (SPN) is an intraparenchymal lung lesion less than 3 cm, which is fully surrounded by lung tissue and does not correlate with lymph nodes, atelectasis, adenopathy, and pneumonia (5-7). The incidence of malignancies for SPNs ranged from 0.5% to 3.5%. It depended on patient characteristics and radiological features of nodules (8). These characteristics included age of patients, smoking status, history of cancer, nodule diameter, nodule volume, spiculated margins, and upper lobe location (9, 10). Currently, computed tomography (CT) is widely used for detecting and differentiating pulmonary nodules based on the difference in intensity against the background (11-13). However, the traditional CT for measuring tumor size may produce a large number of false positives and lead to unnecessary treatments (14-17). Moreover, the guidelines of the American College of Chest Physicians recommend that 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) is a more sensitive and specific imaging technique for differentiating benign and malignant SPNs, while the costs and availability are limited (6). A previous study showed that the combination of CT and PET with an excellent performance in differentiating benign and malignant SPNs due to the combined sensitivity for CT and specificity for PET could provide improved diagnostic value (18). However, whether the diagnostic value of PET/CT was superior than CT remains controversial CT was conducted under breath hold and maximum inspiration, while the PET/CT was conducted under continuous shallow breathing of the patient. This difference causes differing diagnostic value between PET/CT and CT for detecting benign and malignant SPNs, especially for smaller SPNs (up to 8 mm).
Several systematic reviews and meta-analyses investigated the diagnostic value of CT or PET/CT for classifying benign or malignant SPNs. However, these studies just provided the pooled diagnostic parameters; the comparisons of the two diagnostic methods were not illustrated (19-21). Therefore, this comprehensive quantitative meta-analysis was conducted to indirect compare the diagnostic value of CT with PET/CT for differentiating benign and malignant SPNs. Moreover, whether the diagnostic value differed according to country, study design, and sample size of included studies was also examined.
Methods
Data sources, search strategy, and selection criteria
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009 (22). Studies published in English and investigating the diagnostic value of CT or PET/CT for classifying benign and malignant SPNs were eligible for inclusion in this study. The electronic databases, including PubMed, Embase, and the Cochrane Library, were systematically searched for studies from their inception up to November 2018. The Medical Subject Headings and free words of the following terms were used: (“solitary pulmonary nodules” OR “SPNs” OR “pulmonary coin lesion” OR “lung nodules”) AND (“computed tomography” OR “CT” OR “PET/CT”). The reference lists from relevant review and studies were also reviewed to identify any potential eligible study.
The literature search and study selection process were conducted by two independent authors. Inconsistencies were settled through discussion between these two authors, and an additional author made the final decision. The inclusion criteria of this meta-analysis were as follows: (I) study design: prospective or retrospective; (II) patients: patients with benign and malignant SPNs; (III) diagnostic tool: CT or PET/CT; (IV) gold reference: histology or biopsy; and (V) outcomes: true and false positive, true and false negative, or data transformed into the aforementioned information.
Data collection and quality assessment
Two authors independently abstracted data and performed quality assessment. Any disagreement was resolved by these two authors referring to the original study. The collected information included first author’s surname, publication year, country, study design, sample size, size of nodules, percentage male, mean age or age range, diagnostic tool, gold standard, true and false positive, and true and false negative. The quality of included studies was assessed using Quality Assessment of Diagnostic Accuracy Studies (QUADAS) based on 14 items, and yes, no, or unclear were answered for each item (23).
Statistical analysis
The summary sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and the area under the receiver operating characteristic curve (AUC) with corresponding 95% confidence intervals (CIs) were calculated based on true positive, false positive, false negative, and true negative in each individual study. The methods for calculating sensitivity, specificity, PLR, NLR, and DOR used bivariate generalized linear mixed and random-effects modes (24). The AUC for CT and PET/CT for differentiating benign and malignant SPNs was calculated using hierarchical regression (25). The Q statistic was employed to calculate heterogeneity among included studies, and a P value less than 0.10 was regarded as significant heterogeneity (26,27). Subgroup analyses for DOR were conducted based on country, study design, and sample size. Moreover, the ratio of diagnostic parameters between CT and PET/CT or subgroups was also calculated to indirect compare the diagnostic value of CT with PET/CT (28). The publication biases for CT and PET/CT were also evaluated using funnel plots and Deeks’ asymmetry tests (29). The P value for all pooled analyses was two-sided, and a P value less than 0.05 was regarded as statistically significant. Stata software (version 10.0; Stata Corporation, TX, USA) was employed to conduct all statistical analyses.
Results
Literature search
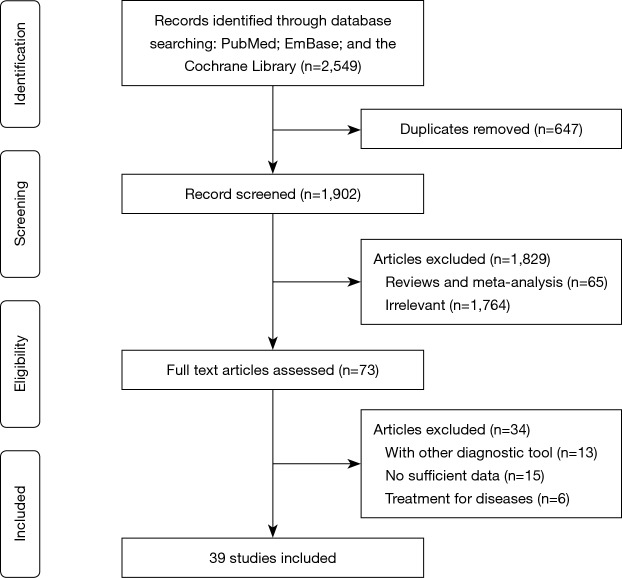
The initial electronic searches produced 2,549 records, and 647 articles were excluded due to duplicate topics. The titles and abstracts were reviewed in the remaining 1,902 studies, and 1,829 studies were excluded due to irrelevant topics or design as review or meta-analyses. The full-text was assessed for the remaining 73 studies, and finally, 39 studies were selected (30-50) for this meta-analysis (51-68). The reasons for excluding 34 studies were as follows: patients diagnosed with other diagnostic tools (n=13), lack of sufficient data (n=15), and studies evaluating treatment effectiveness (n=6). The details of study selection process are presented in Figure 1. Moreover, manual searches of references in the aforementioned studies did not yield any new eligible study.
Figure 1.
Flow diagram of study selection process.
Study characteristics
The baseline characteristics of included studies and patients are shown in Table 1. Overall, 16 studies evaluated the diagnostic value of CT, and the remaining 23 studies investigated the diagnostic value of PET/CT for classifying benign and malignant SPNs. Thirteen studies investigated the diagnostic value of contrast enhanced CT, and the remaining 3 studies evaluated the diagnostic value of CT. These studies involved a total of 3,614 patients with SPNs. Eleven studies had a prospective design, and the remaining 28 studies had a retrospective design. Seventeen studies were conducted in Western countries and the remaining 22 in Eastern countries. The quality assessment of included studies is listed in Table 2; nearly all the studies had moderate or high quality.
Table 1. Baseline characteristics of studies included in the systematic review and meta-analysis.
| Study | Publication year | Country | Study design | Sample size | Size of nodules (mm) | Percentage male (%) | Age (year) | Diagnostic tool | Gold standard | True positive | False positive | False negative | True negative |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swensen (30) | 1992 | USA | Retro | 30 | 6–30 | 53.8 | 41–79 | CECT | Histology or cytology | 21 | 1 | 1 | 6 |
| Swensen (31) | 1995 | USA | Retro | 163 | 6–40 | 57.7 | 22–78 | CECT | Histology | 110 | 12 | 0 | 41 |
| Yamashita (32) | 1995 | Japan | Retro | 32 | 6–-30 | 65.6 | 27–78 | CECT | Biopsy | 16 | 9 | 2 | 5 |
| Swensen (33) | 1996 | USA | Retro | 107 | 7–-30 | 53.3 | 29–85 | CECT | Histology | 51 | 15 | 1 | 40 |
| Potente (34) | 1997 | Italy | Retro | 25 | 6–40 | 62.5 | 39–82 | CECT | Histology | 17 | 2 | 0 | 6 |
| Swensen (35) | 2000 | USA | Pro | 356 | 5–40 | 49.2 | 21–89 | CECT | Histology | 168 | 78 | 3 | 107 |
| Yi (36) | 2004 | Korea | Retro | 131 | 8–30 | 62.6 | 24–82 | CECT | Histopathology | 69 | 28 | 1 | 33 |
| Kim (37) | 2004 | Korea | Pro | 40 | NA | NA | NA | CECT | Histology | 17 | 10 | 2 | 21 |
| Jeong (38) | 2005 | Korea | Retro | 107 | <30 | 57.9 | 22–81 | CECT | Histology | 46 | 6 | 3 | 52 |
| Iwano (39) | 2008 | Japan | Retro | 107 | 5–30 | 54.7 | 60.9 | CT | Histology | 40 | 12 | 12 | 43 |
| da Silva (40) | 2008 | Brazil | Pro | 39 | NA | NA | NA | CT | Histology | 7 | 0 | 3 | 29 |
| Bai (41) | 2009 | China | Retro | 68 | 8–30 | 55.9 | 28–79 | CECT | Immunohistochemistry | 34 | 16 | 2 | 16 |
| Fan (42) | 2012 | China | Retro | 82 | <40 | 42.7 | 40–84 | CECT | Biopsy | 57 | 4 | 7 | 14 |
| Shu (43) | 2013 | China | Retro | 144 | 8–30 | 52.8 | 28–79 | CECT | Immunohistochemistry | 60 | 16 | 12 | 56 |
| Ye (44) | 2014 | China | Pro | 87 | 5–30 | 67.8 | 21–84 | CECT | Histology | 27 | 12 | 25 | 23 |
| Chen (45) | 2018 | China | Retro | 72 | NA | 56.9 | 14–85 | CT | Histology | 39 | 9 | 3 | 24 |
| Herder (46) | 2004 | The Netherlands | Retro | 36 | <30 | 43.0 | 61.0 | PET/CT | Histopathology | 13 | 5 | 1 | 17 |
| Yi (47) | 2006 | Korea | Pro | 119 | <30 | 52.1 | 31–81 | PET/CT | Histopathology | 76 | 5 | 3 | 35 |
| Orlacchio (48) | 2007 | Italy | Retro | 56 | 6–30 | 64.3 | 63.0 | PET/CT | Histology | 20 | 0 | 6 | 100 |
| Tsushima (49) | 2008 | Japan | Retro | 53 | 8–30 | 54.9 | 49–85 | PET/CT | Histochemical | 28 | 1 | 0 | 24 |
| Degirmenci (50) | 2008 | Turkey | Retro | 46 | <30 | 50.0 | 34–83 | PET/CT | Histopathology | 16 | 4 | 10 | 19 |
| Jeong (51) | 2008 | Korea | Retro | 100 | 9–30 | 56.0 | 58.0 | PET/CT | Histology | 35 | 14 | 5 | 46 |
| Kagna (52) | 2009 | Israel | Retro | 93 | 3–30 | 75.3 | 46–90 | PET/CT | Histology | 27 | 10 | 8 | 48 |
| Schillaci (53) | 2009 | Italy | Pro | 30 | 9.5–30 | 56.7 | 36–80 | PET/CT | Histology | 14 | 1 | 4 | 11 |
| Sathekge (54) | 2010 | Belgium | Pro | 30 | 1.4–30 | 73.3 | 37–84 | PET/CT | Histology | 12 | 12 | 2 | 4 |
| Divisi (55) | 2010 | Italy | Retro | 124 | 5–15 | 73.4 | 40–72 | PET/CT | Histology | 89 | 7 | 4 | 24 |
| Nguyen (56) | 2011 | USA | Retro | 42 | 10–30 | 71.4 | 48–82 | PET/CT | Histology | 21 | 1 | 5 | 15 |
| Li (57) | 2011 | China | Pro | 96 | 4–30 | 64.6 | 57.5 | PET/CT | Histology | 58 | 9 | 2 | 27 |
| Sebro (58) | 2013 | USA | Retro | 72 | <30 | 97.2 | 69.9 | PET/CT | Histology | 58 | 6 | 3 | 5 |
| Sim (59) | 2013 | UK | Retro | 186 | 8–30 | 41.4 | NA | PET/CT | Histology | 137 | 14 | 21 | 14 |
| Zhang (60) | 2014 | China | Pro | 113 | NA | 59.3 | 26–81 | PET/CT | Histology | 68 | 6 | 9 | 30 |
| Demir (61) | 2014 | Turkey | Retro | 48 | 9–30 | 47.9 | 56.2 | PET/CT | Histology | 17 | 7 | 1 | 23 |
| Li (62) | 2014 | China | Retro | 298 | <30 | 55.0 | 59.1 | PET/CT | Histology | 199 | 31 | 49 | 19 |
| van Gómez López (63) | 2015 | Spain | Retro | 55 | <30 | 81.8 | 62.0 | PET/CT | Histology | 32 | 7 | 8 | 8 |
| Dabrowska (64) | 2015 | Poland | Pro | 71 | 8–30 | 36.6 | 45–88 | PET/CT | Histology | 37 | 1 | 12 | 21 |
| Ohno (65) | 2015 | Japan | Pro | 198 | 9–29 | 56.1 | 75.4 | PET/CT | Histology | 119 | 59 | 14 | 26 |
| Wang (66) | 2016 | China | Retro | 62 | <30 | NA | NA | PET/CT | Histology | 15 | 15 | 2 | 30 |
| Sahin (67) | 2016 | Turkey | Retro | 41 | <30 | 68.3 | 41–78 | PET/CT | Histology | 20 | 6 | 2 | 13 |
| Lee (68) | 2018 | Korea | Retro | 55 | 8–30 | 98.2 | 67.8 | PET/CT | Histology | 40 | 2 | 1 | 12 |
CECT, contrast enhanced computed tomography; CT, computed tomography; NA, not available; PET, positron emission tomography; Pro, prospective; Retro, retrospective.
Table 2. Quality assessment of the included studies using the QUADAS tool.
| Study | Question about study design characteristic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representative patient spectrum | Reporting of selection criteria | Reference standard | Absence of disease progression bias | Absence of partial verification bias | Absence of differential verification bias | Absence of incorporation bias | Description of index text execution | Description of reference standard execution | Reference standard blinded | Index test blinded | Absence of clinical review bias | Reporting of uninterpretable/intermediate results | Withdrawal | |
| Swensen | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Swensen | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Yamashita | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Swensen | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Potente | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | No | Yes |
| Swensen | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Yi | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | No | Yes |
| Kim | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Jeong | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Iwano | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| da Silva | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Bai | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | No | Yes | Yes |
| Fan | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Shu | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Ye | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Chen | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes |
| Herder | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Yi | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | No | Yes |
| Orlacchio | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Tsushima | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Degirmenci | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Jeong | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Kagna | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Schillaci | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes |
| Sathekge | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Divisi | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Nguyen | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Li | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes |
| Sebro | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Sim | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Zhang | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Demir | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Li | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| van Gómez López | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Dabrowska | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Ohno | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Wang | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Sahin | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes |
| Lee | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | No | Yes |
Sensitivity and specificity
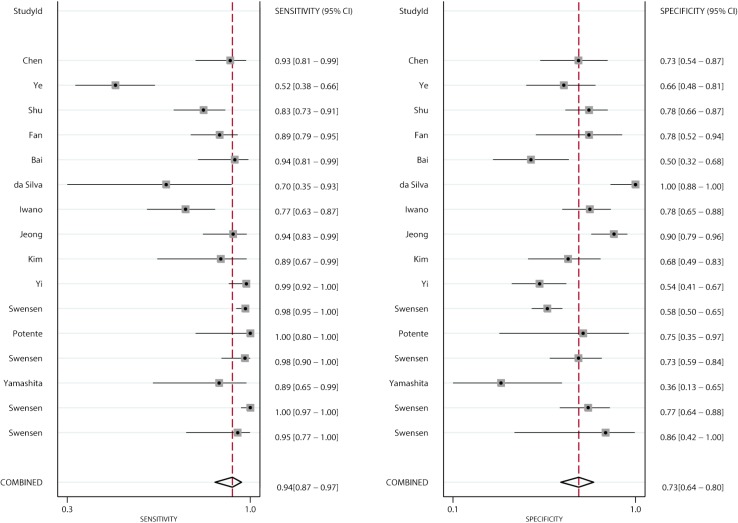
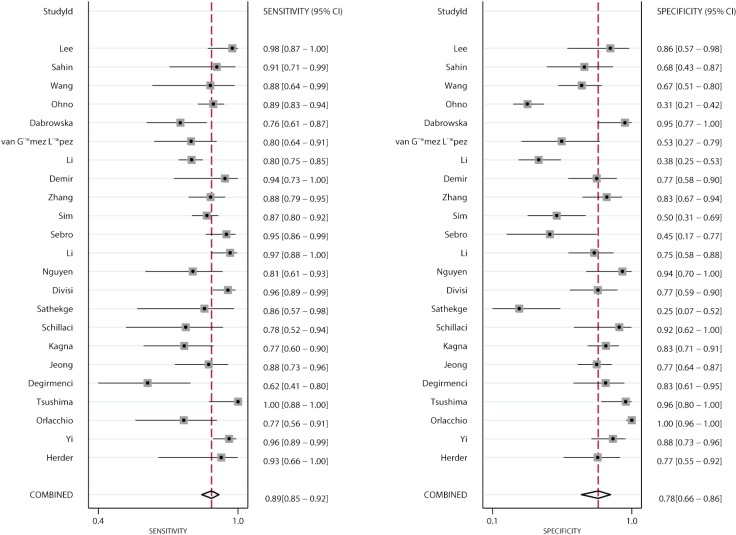
Figures 2 and 3 show the pooled sensitivity and specificity of CT and PET/CT for differentiating benign and malignant SPNs, respectively. The pooled sensitivity and specificity for CT were 0.94 (95% CI: 0.87–0.97), and 0.73 (95% CI: 0.64–0.80), respectively. Moreover, the pooled sensitivity and specificity for PET/CT were 0.89 (95% CI: 0.85–0.92), and 0.78 (95% CI: 0.66–0.86), respectively. The diagnostic value of sensitivity (ratio: 1.06: 95% CI: 0.99–1.13; P=0.111) and specificity (ratio: 0.94; 95% CI: 0.79–1.11; P=0.453) between CT and PET/CT was not statistically significant.
Figure 2.
Pooled sensitivity and specificity of CT. CT, computed tomography.
Figure 3.
Pooled sensitivity and specificity of PET/CT. CT, computed tomography; PET, positron emission tomography.
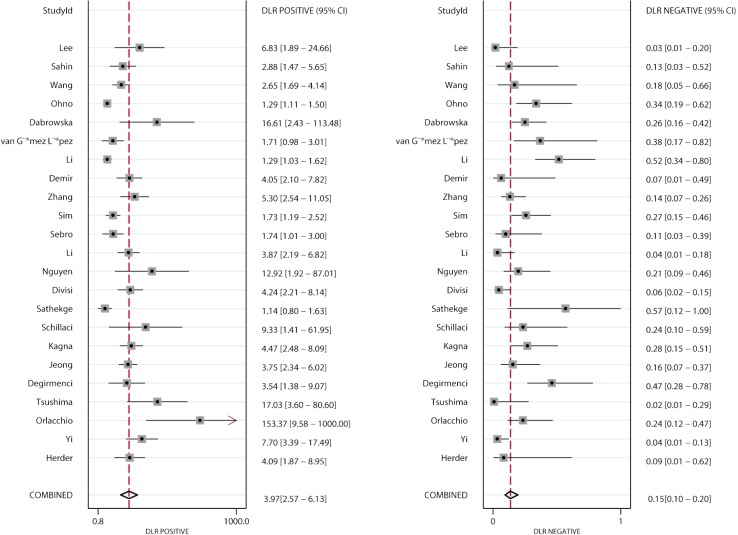
PLR and NLR
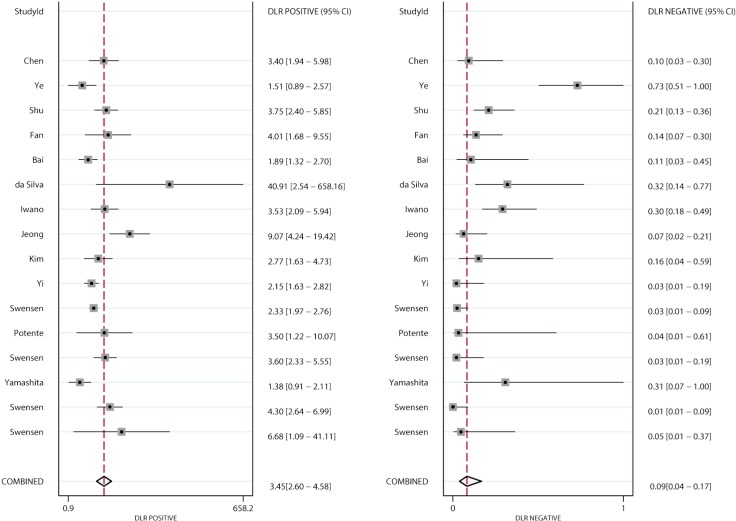
Figures 4 and 5 present the summary PLR and NLR of CT and PET/CT for differentiating benign and malignant SPNs, respectively. The summary PLR and NLR for CT were 3.45 (95% CI: 2.60–4.58), and 0.09 (95% CI: 0.04–0.17), respectively. Furthermore, the summary PLR and NLR for PET/CT were 3.97 (95% CI: 2.57–6.13), 0.15 (95% CI: 0.10–0.20), respectively. No significant differences were observed between CT and PET/CT for PLR (ratio: 0.87; 95% CI: 0.52–1.46; P=0.596), and NLR (ratio: 0.60; 95% CI: 0.27–1.34; P=0.212).
Figure 4.
Pooled PLR and NLR of CT. PLR, positive likelihood ratio; NLR, negative likelihood ratio; CT, computed tomography.
Figure 5.
Pooled PLR and NLR of PET/CT. PLR, positive likelihood ratio; NLR, negative likelihood ratio; CT, computed tomography; PET, positron emission tomography.
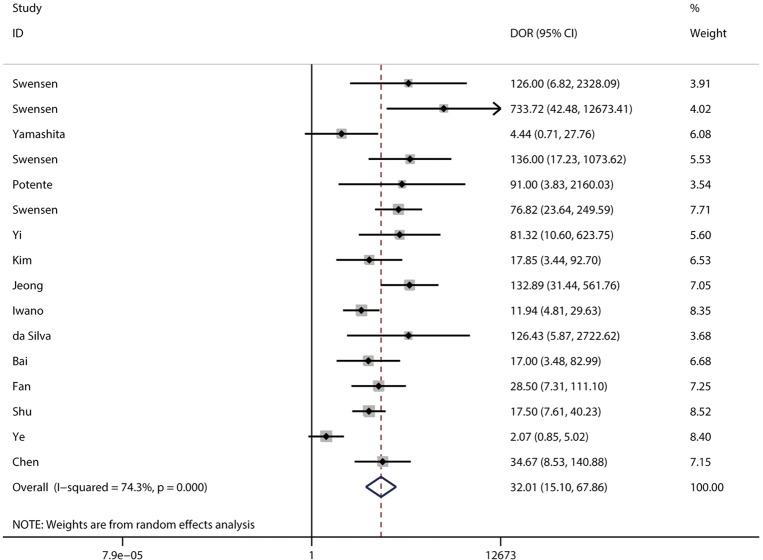
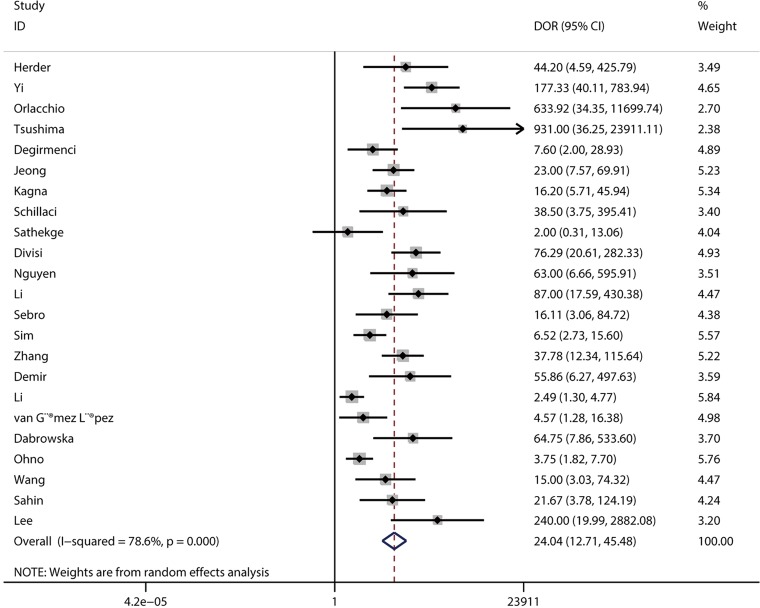
DOR and AUC
The pooled DOR for CT was 32.01 (95% CI: 15.10–67.86; Figure 6) with significant heterogeneity observed among included studies (P<0.001), while the summary DOR for PET/CT was 24.04 (95% CI: 12.71–45.48; Figure 7) with significant heterogeneity. The DOR between CT and PET/CT was not statistically significant (ratio: 1.33; 95% CI: 0.50–3.57; P=0.569). Moreover, the AUC for CT and PET/CT was 0.89 (95% CI: 0.86–0.91; Figure 8) and 0.91 (95% CI: 0.89–0.94; Figure 9), respectively. No significant difference was found between CT and PET/CT for AUC (ratio: 0.98; 95% CI: 0.94–1.02; P=0.268).
Figure 6.
Pooled DOR of CT. DOR, diagnostic odds ratio; CT, computed tomography.
Figure 7.
Pooled DOR of PET/CT. DOR, diagnostic odds ratio; CT, computed tomography; PET, positron emission tomography.
Figure 8.
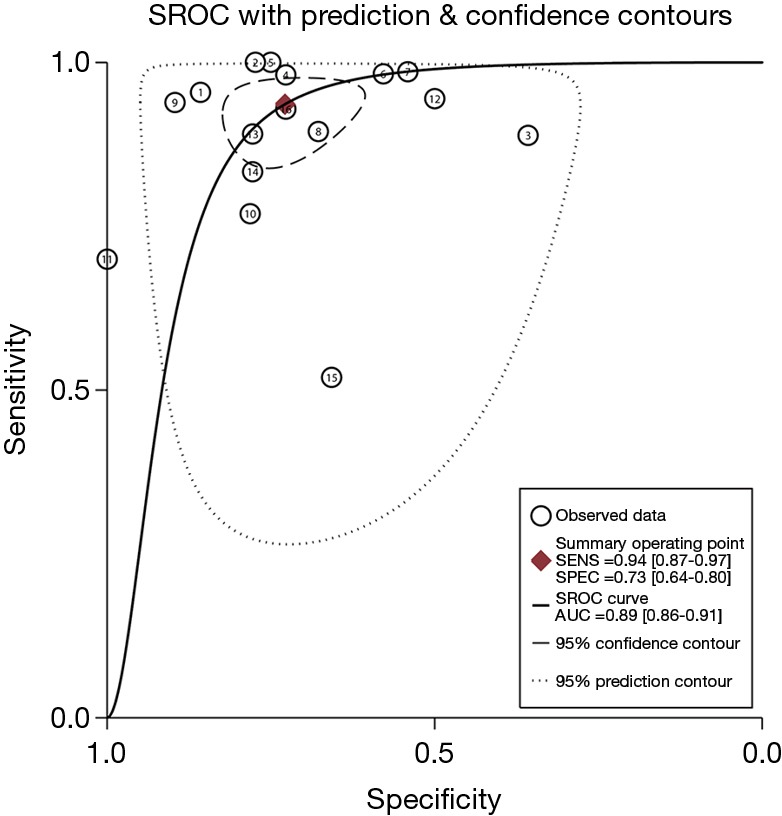
The summary ROC curve and AUC for CT. ROC, receiver operating characteristic; AUC, area under the ROC curve; CT, computed tomography.
Figure 9.
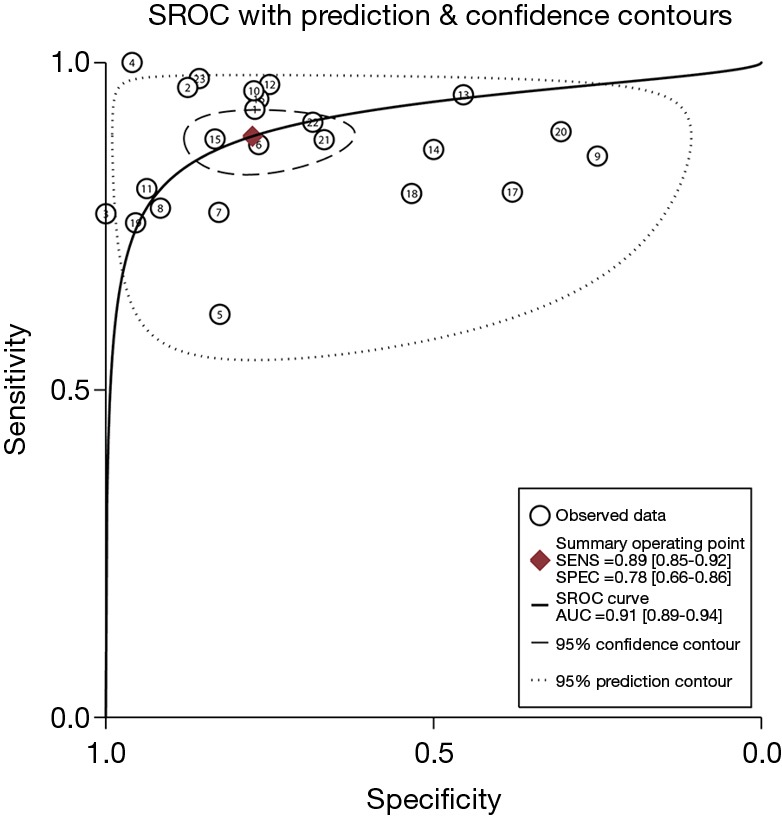
Summary ROC curve and AUC for PET/CT. ROC, receiver operating characteristic; AUC, area under the ROC curve; CT, computed tomography; PET, positron emission tomography.
Subgroup analysis
The results of subgroup analyses for DOR are presented in Table 3. CT had high DOR than PET/CT for pooled studies conducted in Western countries (ratio: 5.37; 95% CI: 1.65–17.54). Furthermore, CT had lower DOR for studies conducted in Eastern countries than in Western countries (ratio: 0.16; 95% CI: 0.05–0.50). No other significant differences between CT and PET/CT or subgroups were observed.
Table 3. Subgroup analyses for the diagnostic odds ratio of CT and PET/CT for differentiating benign and malignant solitary pulmonary nodules.
| Variable | Subgroups | Diagnostic tool | Number of studies | DOR and 95% CI | P value for heterogeneity | Ratio between CT and PET/CT | Ratio between subgroups for CT | Ratio between subgroups for PET/CT |
|---|---|---|---|---|---|---|---|---|
| Country | Eastern | CT | 10 | 17.68 (7.96–39.28) | <0.001 | 0.65 (0.19–2.27) | 0.16 (0.05–0.50) | 0.70 (0.19–2.54) |
| PET/CT | 12 | 27.22 (10.38–71.36) | <0.001 | |||||
| Western | CT | 6 | 112.92 (48.51–262.84) | 0.831 | 5.37 (1.65–17.54) | |||
| PET/CT | 11 | 21.02 (9.18–48.12) | 0.001 | |||||
| Study design | Prospective | CT | 4 | 20.95 (2.27–193.50) | <0.001 | 0.80 (0.06–10.96) | 0.59 (0.06–6.03) | 1.14 (0.24–5.44) |
| PET/CT | 7 | 26.12 (6.59–103.50) | <0.001 | |||||
| Retrospective | CT | 12 | 35.58 (17.89–70.77) | 0.012 | 1.55 (0.56–4.27) | |||
| PET/CT | 16 | 22.93 (10.92–48.15) | <0.001 | |||||
| Sample size | ≥100 | CT | 7 | 56.88 (21.18–152.74) | 0.002 | 3.00 (0.70–12.82) | 3.06 (0.73–12.86) | 1.00 (0.46–2.19) |
| PET/CT | 8 | 18.93 (6.54–54.79) | <0.001 | |||||
| <100 | CT | 9 | 18.58 (6.56–-52.67) | 0.001 | 0.68 (0.19–2.45) | |||
| PET/CT | 15 | 27.14 (12.99–56.70) | 0.002 |
CT, computed tomography; DOR, diagnostic odds ratio; PET, positron emission tomography.
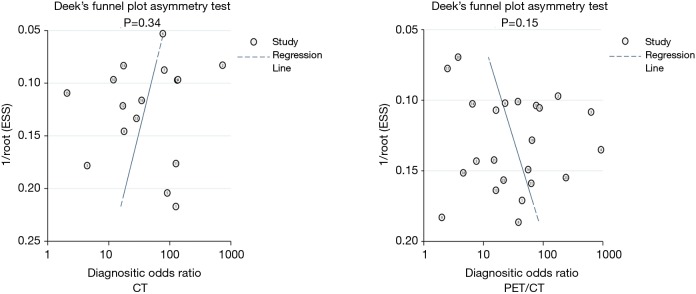
Publication bias
Publication bias was assessed for CT and PET/CT and is presented in Figure 10. No significant publication bias was found for CT (P=0.34) and PET/CT (P=0.15).
Figure 10.
Publication biases for CT and PET/CT. CT, computed tomography; PET, positron emission tomography.
Discussion
Numerous studies reported the diagnostic value of CT or PET/CT for differentiating benign and malignant SPNs. The present quantitative meta-analysis based on 39 studies was conducted to determine the diagnostic value of CT and PET/CT for classifying benign and malignant SPNs and provide the indirect comparison results for the better diagnostic tool. The findings of this meta-analysis indicated that both CT and PET/CT had a moderate-to-high diagnostic value for differentiating benign and malignant SPNs, with no significant differences between these two diagnostic tools. Moreover, CT should be recommended in Western countries due to high DOR compared with PET/CT. Finally, the DOR of CT was lower in Eastern countries than in Western countries.
Several meta-analyses have already investigated the diagnostic value of CT and PET/CT in classifying benign and malignant SPNs (19-21). Li et al. conducted a meta-analysis of 20 studies using 18F-FDG-PET and reported the sensitivity of 0.89 (95% CI: 0.87–0.91), specificity of 0.70 (95% CI: 0.66–0.73), PLR of 3.33 (95% CI: 2.35–4.71), NLR of 0.18 (95% CI: 0.13–0.25), and DOR of 22.43 (95% CI: 12.55–40.07) (19). Ruilong et al. conducted a meta-analysis of 12 studies and suggested that the pooled sensitivity, specificity, PLR, and NLR of 18F-FDG-PET were 0.82 (95% CI: 0.76–0.87), 0.81 (95% CI: 0.66–0.90), 4.30 (95% CI: 2.30–7.90), and 0.22 (95% CI: 0.16–0.30), respectively (20). Moreover, Zhang et al. indicated that the pooled sensitivity, specificity, PLR, NLR, DOR of CT were 0.89 (95% CI: 0.88–0.91), 0.70 (95% CI: 0.68–0.73), 2.88 (95% CI: 2.46–3.37), 0.16 (95% CI: 0.12–0.21), and 23.83 (95% CI: 16.18–35.11), respectively (21). The aforementioned results indicated that both CT and PET/CT had high sensitivity and moderate specificity for evaluating SPNs, while the comparison results of these two diagnostic tools were not evaluated. Therefore, this quantitative meta-analysis was conducted to obtain the comprehensive diagnostic value of CT and PET/CT for classifying benign and malignant SPNs.
Although most included studies indicated high sensitivity (>0.80) and moderate specificity (>0.70) of CT, several studies reported inconsistent results. Ye et al. indicated patients with 12.4 HU or lower washout as a cutoff value; the sensitivity and specificity for malignancy were 52.5% and 65.0%, respectively (44). Bai et al. found that the sensitivity of CT was higher while the specificity of CT was lower than expected (41). da Silva et al. indicated that CT had a sensitivity of 70.0% and a specificity of 100.0% (40). Iwano et al. suggested CT differentiating malignant from benign SPNs with a sensitivity of 76.9% and a specificity of 80% (39). The other four studies reported CT with moderate or high sensitivity, while the specificity was lower than expected (32,35-37). The potential reasons for these results could be several studies without contrast injection, which were associated with a high incidence of false positive and negative. Moreover, the experience of radiologists could affect the accuracy of CT for evaluating SPNs. Similarly, five included studies indicated PET/CT with low or moderate sensitivity (48,50,52,53,64), and eight studies reported PET/CT with low specificity for classifying benign and malignant SPNs (54,58,59,62,63,65-67). The reason for this could be that 18F-FDG was not a tumor-specific tracer, and false-positive results might be obtained in patients with inflammatory lesions (69,70).
No significant differences were found in sensitivity, specificity, PLR, NLR, DOR, and AUC between CT and PET/CT. However, the results of subgroup analyses indicated that the DOR of CT was higher than that of PET/CT in Western countries. Moreover, the DOR of CT might differ between Eastern and Western countries. However, these results might vary because of the imbalance in the number of included studies in corresponding subsets. Moreover, patient characteristics across included studies could affect the diagnostic accuracy of CT and PET/CT. Therefore, the results of subgroup analyses just provided relative results. Hence, further studies are needed to verify the diagnostic value of CT and PET/CT in classifying benign and malignant SPNs.
This study had several limitations. (I) The included studies had prospective and retrospective designs, thereby introducing potential uncontrolled selection and recall biases. (II) The size of nodules was variable across included studies, affecting the diagnostic accuracy of CT and PET/CT. (III) The skills of radiologists differed among included studies, resulting in a potential observer bias. (IV) The indirect comparison results of CT with PET/CT were based on different populations, and the results might vary due to uncontrolled heterogeneity among participants. (V) The analysis based on published studies and publication bias was inevitable.
The results of this meta-analysis indicated that both CT and PET/CT had a moderate-to-high diagnostic value for evaluating SPNs. Moreover, no significant differences in all diagnostic parameters were found between CT and PET/CT. Moreover, we noted CT was associated with high diagnostic value than PET/CT in Western countries, whereas the DOR of PET/CT in Eastern countries was non-significant high than CT. Considering the high cost and limited availability of PET/CT, CT should be recommended for differentiating benign and malignant SPNs. Future prospective studies should be conducted to directly compare the diagnostic value of CT and PET/CT in detecting benign and malignant SPNs.
Acknowledgments
Funding: This work was supported by the Zhejiang Provincial Science and Technology Department Project (grant number LGF18H180016).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91-112. 10.3322/caac.20102 [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. 10.1056/NEJMra0802714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan S, Guan Y, Zhao G, et al. Association between plasma fibrinogen and survival in patients with small-cell lung carcinoma. Thorac Cancer 2018;9:146-51. 10.1111/1759-7714.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. 10.1056/NEJMcp012290 [DOI] [PubMed] [Google Scholar]
- 6.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 7.Sim YT, Poon FW. Imaging of solitary pulmonary nodule-a clinical review. Quant Imaging Med Surg 2013;3:316-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midthun DE. Screening for lung cancer. Clin Chest Med 2011;32:659-68. 10.1016/j.ccm.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 9.Gould MK, Fletcher J, Iannettoni M. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest 2007;132:108S-30S. 10.1378/chest.07-1353 [DOI] [PubMed] [Google Scholar]
- 10.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 12.Fass L. Imaging and cancer: a review. Mol Oncol 2008;2:115-52. 10.1016/j.molonc.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 14.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. 10.1001/jama.2012.5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776-84. 10.7326/0003-4819-157-11-201212040-00005 [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Oxnard GR, Moskowitz CS, et al. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res 2010;16:4647-53. 10.1158/1078-0432.CCR-10-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birchard KR, Hoang JK, Herndon JE, Jr, et al. Early changes in tumor size in patients treated for advanced stage nonsmall cell lung cancer do not correlate with survival. Cancer 2009;115:581-6. 10.1002/cncr.24060 [DOI] [PubMed] [Google Scholar]
- 18.Chang CY, Tzao C, Lee SC, et al. Incremental value of integrated FDG-PET/CT in evaluating indeterminate solitary pulmonary nodule for malignancy. Mol Imaging Biol 2010;12:204-9. 10.1007/s11307-009-0241-0 [DOI] [PubMed] [Google Scholar]
- 19.Li ZZ, Huang YL, Song HJ, et al. The value of 18F-FDG-PET/CT in the diagnosis of solitary pulmonary nodules: A meta-analysis. Medicine (Baltimore) 2018;97:e0130. 10.1097/MD.0000000000010130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruilong Z, Daohai X, Li G, et al. Diagnostic value of 18F-FDG-PET/CT for the evaluation of solitary pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun 2017;38:67-75. 10.1097/MNM.0000000000000605 [DOI] [PubMed] [Google Scholar]
- 21.Zhang CY, Yu HL, Li X, et al. Diagnostic value of computed tomography scanning in differentiating malignant from benign solitary pulmonary nodules: a meta-analysis. Tumour Biol 2014;35:8551-8. 10.1007/s13277-014-2113-8 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25.Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 2002;21:1237-56. 10.1002/sim.1099 [DOI] [PubMed] [Google Scholar]
- 26.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK: The Cochrane Collaboration, 2008. [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodward M. Epidemiology: study design and data analysis: Chapman & Hall/CRC, 2000:252-73. [Google Scholar]
- 29.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 30.Swensen SJ, Morin RL, Schueler BA, et al. Solitary pulmonary nodule: CT evaluation of enhancement with iodinated contrast material--a preliminary report. Radiology 1992;182:343-7. 10.1148/radiology.182.2.1732947 [DOI] [PubMed] [Google Scholar]
- 31.Swensen SJ, Brown LR, Colby TV, et al. Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology 1995;194:393-8. 10.1148/radiology.194.2.7824716 [DOI] [PubMed] [Google Scholar]
- 32.Yamashita K, Matsunobe S, Tsuda T, et al. Solitary pulmonary nodule: preliminary study of evaluation with incremental dynamic CT. Radiology 1995;194:399-405. 10.1148/radiology.194.2.7824717 [DOI] [PubMed] [Google Scholar]
- 33.Swensen SJ, Brown LR, Colby TV, et al. Lung nodule enhancement at CT: prospective findings. Radiology 1996;201:447-55. 10.1148/radiology.201.2.8888239 [DOI] [PubMed] [Google Scholar]
- 34.Potente G, Iacari V, Caimi M. The challenge of solitary pulmonary nodules: HRCT evaluation. Comput Med Imaging Graph 1997;21:39-46. 10.1016/S0895-6111(96)00071-7 [DOI] [PubMed] [Google Scholar]
- 35.Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73-80. 10.1148/radiology.214.1.r00ja1473 [DOI] [PubMed] [Google Scholar]
- 36.Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004;233:191-9. 10.1148/radiol.2331031535 [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Kim HJ, Lee KH, et al. Solitary pulmonary nodules: a comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. J Comput Assist Tomogr 2004;28:766-75. 10.1097/00004728-200411000-00007 [DOI] [PubMed] [Google Scholar]
- 38.Jeong YJ, Lee KS, Jeong SY, et al. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology 2005;237:675-83. 10.1148/radiol.2372041549 [DOI] [PubMed] [Google Scholar]
- 39.Iwano S, Nakamura T, Kamioka Y, et al. Computer-aided differentiation of malignant from benign solitary pulmonary nodules imaged by high-resolution CT. Comput Med Imaging Graph 2008;32:416-22. 10.1016/j.compmedimag.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 40.da Silva EC, Silva AC, de Paiva AC, et al. Diagnosis of solitary lung nodules using the local form of Ripley's K function applied to three-dimensional CT data. Comput Methods Programs Biomed 2008;90:230-9. 10.1016/j.cmpb.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 41.Bai RJ, Cheng XG, Qu H, et al. Solitary pulmonary nodules: comparison of multi-slice computed tomography perfusion study with vascular endothelial growth factor and microvessel density. Chin Med J (Engl) 2009;122:541-7. [PubMed] [Google Scholar]
- 42.Fan L, Liu SY, Li QC, et al. Multidetector CT features of pulmonary focal ground-glass opacity: differences between benign and malignant. Br J Radiol 2012;85:897-904. 10.1259/bjr/33150223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shu SJ, Liu BL, Jiang HJ. Optimization of the scanning technique and diagnosis of pulmonary nodules with first-pass 64-detector-row perfusion VCT. Clin Imaging 2013;37:256-64. 10.1016/j.clinimag.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 44.Ye XD, Ye JD, Yuan Z, et al. Dynamic CT of solitary pulmonary nodules: comparison of contrast medium distribution characteristic of malignant and benign lesions. Clin Transl Oncol 2014;16:49-56. 10.1007/s12094-013-1039-8 [DOI] [PubMed] [Google Scholar]
- 45.Chen CH, Chang CK, Tu CY, et al. Radiomic features analysis in computed tomography images of lung nodule classification. PLoS One 2018;13:e0192002. 10.1371/journal.pone.0192002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herder GJ, Golding RP, Hoekstra OS, et al. The performance of (18)F-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules. Eur J Nucl Med Mol Imaging 2004;31:1231-6. 10.1007/s00259-004-1552-7 [DOI] [PubMed] [Google Scholar]
- 47.Yi CA, Lee KS, Kim BT, et al. Tissue characterization of solitary pulmonary nodule: comparative study between helical dynamic CT and integrated PET/CT. J Nucl Med 2006;47:443-50. [PubMed] [Google Scholar]
- 48.Orlacchio A, Schillaci O, Antonelli L, et al. Solitary pulmonary nodules: morphological and metabolic characterisation by FDG-PET-MDCT. Radiol Med 2007;112:157-73. 10.1007/s11547-007-0132-x [DOI] [PubMed] [Google Scholar]
- 49.Tsushima Y, Tateishi U, Uno H, et al. Diagnostic performance of PET/CT in differentiation of malignant and benign non-solid solitary pulmonary nodules. Ann Nucl Med 2008;22:571-7. 10.1007/s12149-008-0160-1 [DOI] [PubMed] [Google Scholar]
- 50.Degirmenci B, Wilson D, Laymon CM, et al. Standardized uptake value-based evaluations of solitary pulmonary nodules using F-18 fluorodeoxyglucose-PET/computed tomography. Nucl Med Commun 2008;29:614-22. 10.1097/MNM.0b013e3282f9b5a0 [DOI] [PubMed] [Google Scholar]
- 51.Jeong SY, Lee KS, Shin KM, et al. Efficacy of PET/CT in the characterization of solid or partly solid solitary pulmonary nodules. Lung Cancer 2008;61:186-94. 10.1016/j.lungcan.2007.12.021 [DOI] [PubMed] [Google Scholar]
- 52.Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer. Eur J Nucl Med Mol Imaging 2009;36:997-1004. 10.1007/s00259-009-1061-9 [DOI] [PubMed] [Google Scholar]
- 53.Schillaci O, Travascio L, Bolacchi F, et al. Accuracy of early and delayed FDG PET-CT and of contrast-enhanced CT in the evaluation of lung nodules: a preliminary study on 30 patients. Radiol Med 2009;114:890-906. 10.1007/s11547-009-0400-z [DOI] [PubMed] [Google Scholar]
- 54.Sathekge MM, Maes A, Pottel H, et al. Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J 2010;100:598-601. 10.7196/SAMJ.4082 [DOI] [PubMed] [Google Scholar]
- 55.Divisi D, Di Tommaso S, Di Leonardo G, et al. 18-fluorine fluorodeoxyglucose positron emission tomography with computerized tomography versus computerized tomography alone for the management of solitary lung nodules with diameters inferior to 1.5 cm. Thorac Cardiovasc Surg 2010;58:422-6. 10.1055/s-0030-1249945 [DOI] [PubMed] [Google Scholar]
- 56.Nguyen NC, Kaushik A, Wolverson MK, et al. Is there a common SUV threshold in oncological FDG PET/CT, at least for some common indications? A retrospective study. Acta Oncol 2011;50:670-7. 10.3109/0284186X.2010.550933 [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Su M, Li F, et al. The value of 18F-FDG-PET/CT in the differential diagnosis of solitary pulmonary nodules in areas with a high incidence of tuberculosis. Ann Nucl Med 2011;25:804-11. 10.1007/s12149-011-0530-y [DOI] [PubMed] [Google Scholar]
- 58.Sebro R, Aparici CM, Hernandez-Pampaloni M. FDG PET/CT evaluation of pathologically proven pulmonary lesions in an area of high endemic granulomatous disease. Ann Nucl Med 2013;27:400-5. 10.1007/s12149-013-0695-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sim YT, Goh YG, Dempsey MF, et al. PET-CT evaluation of solitary pulmonary nodules: correlation with maximum standardized uptake value and pathology. Lung 2013;191:625-32. 10.1007/s00408-013-9500-6 [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Cui LB, Tang X, et al. DW MRI at 3.0 T versus FDG PET/CT for detection of malignant pulmonary tumors. Int J Cancer 2014;134:606-11. 10.1002/ijc.28394 [DOI] [PubMed] [Google Scholar]
- 61.Demir Y, Polack BD, Karaman C, et al. The diagnostic role of dual-phase (18)F-FDG PET/CT in the characterization of solitary pulmonary nodules. Nucl Med Commun 2014;35:260-7. 10.1097/MNM.0000000000000049 [DOI] [PubMed] [Google Scholar]
- 62.Li S, Zhao B, Wang X, et al. Overestimated value of (18)F-FDG PET/CT to diagnose pulmonary nodules: Analysis of 298 patients. Clin Radiol 2014;69:e352-7. 10.1016/j.crad.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 63.van Gómez López O, García Vicente AM, Honguero Martínez AF, et al. (18)F-FDG-PET/CT in the assessment of pulmonary solitary nodules: comparison of different analysis methods and risk variables in the prediction of malignancy. Transl Lung Cancer Res 2015;4:228-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dabrowska M, Krenke R, Korczynski P, et al. Diagnostic accuracy of contrast-enhanced computed tomography and positron emission tomography with 18-FDG in identifying malignant solitary pulmonary nodules. Medicine (Baltimore) 2015;94:e666. 10.1097/MD.0000000000000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohno Y, Nishio M, Koyama H, et al. Solitary pulmonary nodules: Comparison of dynamic first-pass contrast-enhanced perfusion area-detector CT, dynamic first-pass contrast-enhanced MR imaging, and FDG PET/CT. Radiology 2015;274:563-75. 10.1148/radiol.14132289 [DOI] [PubMed] [Google Scholar]
- 66.Wang FL, Tan YY, Gu XM, et al. Comparison of Positron Emission Tomography Using 2-[18F]-fluoro-2-deoxy-D-glucose and 3-deoxy-3-[18F]-fluorothymidine in Lung Cancer Imaging. Chin Med J (Engl) 2016;129:2926-35. 10.4103/0366-6999.195468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Şahin E, Kara A, Elboga U. Contribution of nonattenuation-corrected images on FDG-PET/CT in the assessment of solitary pulmonary nodules. Radiol Med 2016;121:944-9. 10.1007/s11547-016-0681-y [DOI] [PubMed] [Google Scholar]
- 68.Lee SH, Sung C, Lee HS, et al. Is (18)F-FDG PET/CT useful for the differential diagnosis of solitary pulmonary nodules in patients with idiopathic pulmonary fibrosis? Ann Nucl Med 2018;32:492-8. 10.1007/s12149-018-1273-9 [DOI] [PubMed] [Google Scholar]
- 69.Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology 2005;236:1011-9. 10.1148/radiol.2363041310 [DOI] [PubMed] [Google Scholar]
- 70.Yang W, Fu Z, Yu J, et al. Value of PET/CT versus enhanced CT for locoregional lymph nodes in non-small cell lung cancer. Lung Cancer 2008;61:35-43. 10.1016/j.lungcan.2007.11.007 [DOI] [PubMed] [Google Scholar]