Abstract
Background
Although oxygen supplementation during bronchoscopy in patients with pre-existing hypoxemia is provided, adequacy of oxygenation may not be achieved, resulting in the occurrence of respiratory failure that requires endotracheal tube intubation. The purpose of this study was to compare high-flow nasal cannula (HFNC) with non-invasive ventilation (NIV) in patients with pre-existing hypoxemia undergoing flexible bronchoscopy (FB) on the ability to maintain oxygen saturation during bronchoscopy.
Methods
A prospective randomized study was conducted in patients who had hypoxemia [defined as partial pressure of arterial oxygen (PaO2) less than 70 mmHg at room air] and required FB for the diagnosis of abnormal pulmonary lesions. Patients were randomized to receive either HFNC or NIV during FB. The primary outcome was the lowest oxygen saturation level during FB.
Results
Fifty-one patients underwent randomization to HFNC (n=26) or NIV (n=25). Baseline characteristics in terms of age, Simplified Acute Physiologic Score II values, and cardiorespiratory parameters were similar in both groups. After receiving HFNC or NIV, oxygen saturation as measured by pulse oximeter (SpO2) increased to greater than 90% in all cases. During FB, although the lowest SpO2 was similar in both groups, the lowest SpO2 <90% tended to occur more often in the HFNC group (34.6% vs. 12.0%; P=0.057). In patients with baseline PaO2 <60 mmHg on ambient air, a decrease in PaO2 from preprocedure to the end of FB was less in the NIV group (–13.7 vs. –57.0 mmHg; P=0.019). After FB, the occurrence of SpO2 <90% was 15.4% and 4.0% in the HFNC group and NIV group, respectively (P=0.17).
Conclusions
In overall, NIV and HFNC provided the similar effectiveness in prevention of hypoxemia in hypoxemic patients undergoing FB. However, in subgroup analysis, NIV provided greater adequacy and stability of oxygenation than HFNC in patients with baseline PaO2 <60 mmHg on ambient air.
Keywords: Non-invasive ventilator (NIV), high-flow nasal cannula (HFNC), bronchoscopy, hypoxemia
Introduction
Flexible bronchoscopy (FB) is a useful tool to investigate abnormal pulmonary lesions. However, patients with these conditions usually have hypoxemia. Thus, oxygen supplementation is required during FB in patients with pre-existing hypoxemia. Even in patients without pre-existing hypoxemia, FB itself can lead to desaturation, and a previous report showed that oxygen supplementation was required in 24% of patients (1). Although conventional oxygen supplementation during bronchoscopy in these patients is provided, adequacy of oxygenation may not be achieved, resulting in termination of the procedure and the occurrence of respiratory failure requiring endotracheal tube intubation (1-3). As severe hypoxemia represents a contraindication for FB, some institutes usually intubate patients prior to the procedure, which can cause distress and torment to the patients.
Non-invasive ventilation (NIV) has been reported as preventing desaturation and post-procedure requirements for mechanical ventilation in nonventilated patients with hypoxemia undergoing FB (4-7). Compared with conventional oxygen delivery methods, NIV was reported to be superior in terms of maintaining adequate oxygen (4). Recently, the British Thoracic Society suggested the use of NIV in hypoxemic patients undergoing FB in an environment in which intubation and ventilatory support are readily accessible (8).
High-flow nasal cannula (HFNC) is a new oxygen supplement that provides higher gas flow rates than conventional low-flow oxygen systems. It can be used as an alternative to NIV in patients with hypoxemic respiratory failure (9-11). Recently, HFNC was proved to improve oxygenation in patients undergoing FB (12,13).
To our knowledge, there is only one study comparing HFNC with NIV in a limited number of hypoxemic patients undergoing FB. This study demonstrated the superiority of NIV over HFNC in maintaining adequate oxygenation before, during, and after FB (14). However, the study did not achieve the primary outcome of the lowest oxygen saturation during FB, as the inspired fraction of oxygen (FiO2) provided to the patients was high as 1.0. In addition, there was only a small number of cases but nearly achieved the primary outcome. Furthermore, FiO2 after FB was adjustable to maintain an arterial oxygen saturation of more than 90%, resulting in difficulty to compare the efficacy of the devices. Therefore, we conducted this prospective randomized study to compare the efficacy of these two oxygen delivery methods with fixed lower FiO2 throughout the study protocol in more numbers of hypoxemic patients undergoing FB.
Methods
A prospective randomized study was conducted at Ramathibodi Hospital, Mahidol University, Thailand, from September 2015 to December 2017 with patients who had hypoxemia and required FB for the diagnosis of abnormal pulmonary lesions. All participants or their legal representatives provided their written informed consent prior to enrolment. The study protocol was approved by the Ethics Committee on Human Experimentation of Ramathibodi Hospital, Faculty of Medicine, Mahidol University (ID 08-58-23), and the trial was registered at Thai Clinical Trials Registry (http://www.clinicaltrials.in.th; registration number TCTR20150828001; registration date 28 August 2015).
Population
Eligible patients were 15 years of age or older who had hypoxemia, defined as the partial pressure of arterial oxygen (PaO2) less than 70 mmHg at room air, and who required FB for diagnosis of abnormal pulmonary lesions. All participants provided their written informed consent. Patients were excluded from the study if they were not cooperative or complied with FB, had indication for intubation (unstable vital signs, severe hypoxemia that did not improve after NIV or HFNC, or unable to tolerate the NIV or HFNC), had a decreased level of consciousness and/or unconscious, were considered as high risk for aspiration, had a distorted maxillofacial structure that was not suitable for NIV or HFNC, or rejected participation in the study or withdrew from the study. After randomization, patients who were either unable to tolerate the NIV or HFNC or who had oxygen saturation as measured by pulse oximeter (SpO2) of 90% or less were also excluded.
Randomization
After enrolment, patients were randomized in a 1:1 ratio to receive either NIV or HFNC by the minimization method with stratification factors including gender, PaO2 at ambient air (≥60 vs. <60 mmHg), and distribution on chest radiographs (focal vs. diffuse).
Study intervention
All patients in the NIV group were ventilated with a dedicated NIV machine (Philips Respironics V60; Philips Respironics, Murrysville, PA, USA). The full-face mask adjunct with elastic banding was applied as the interface. A swivel connector (T-adapter) was inserted between the ventilator tubing and the face mask to allow the insertion of the bronchoscope. Ventilator parameters were set as bilevel positive airway pressure mode, with expiratory positive airway pressure (EPAP) of 5 cmH2O and inspiratory positive airway pressure (IPAP) at the level that achieved the tidal volume of 8 mL/kg or at least 10 cmH2O. The FiO2 was kept at 0.6 throughout and 30 minutes after the procedure. The peak inspiratory flow rate was also recorded.
HFNC was delivered continuously through a nasal cannula with AIRVO2 (Fisher and Paykel, Auckland, New Zealand). The inspiratory flow rate was 40 L/min, and the FiO2 was kept at 0.6 throughout and 30 minutes after the procedure, similar to the NIV group.
After initiation of NIV or HFNC, patients who were either unable to tolerate their mode of oxygen supplement or who had SpO2 of 90% or less were excluded from the study.
Bronchoscopic procedure
All procedures were performed at the general wards or intermediate care unit. Before, during, and after FB, the electrocardiogram, non-invasive blood pressure, and pulse oximeter were continuously monitored. Bronchoscopic procedures were performed using sedation with 50 µg of fentanyl and local anaesthesia with lidocaine (10% lidocaine spray for the pharynx and 1% lidocaine solution for the larynx, vocal cords, and bronchi by spray-as-you-go technique).
A flexible bronchoscope (BF-P180, Olympus, Tokyo, Japan; external diameter, 4.9 mm; channel diameter, 2.0 mm) was inserted through the oral route. The entire bronchial tree was endoscopically examined down to the level of the sub-subsegmental bronchi. The decision to perform a bronchoalveolar lavage (BAL) or other sampling technique was not part of the study and was left to the decision of the bronchoscopists. BAL was performed at the target bronchus by repeated instillation with 20 mL of normal saline and by aspiration until a total of 100–150 mL of normal saline was instilled or a returned fluid of 50 mL was collected. Subsequently, transbronchial biopsy (TBB) was obtained blindly without fluoroscopic guidance. BAL fluid was processed for cytological and microbiological analyses. After FB was accomplished, the patients maintained their mode of oxygen supplement for 30 minutes. After that, the appropriate modes were considered according to their primary physicians.
Data collection
Baseline characteristics, Simplified Acute Physiology Scores II (SAPS II) (15), as well as clinical pre-bronchoscopic diagnosis were collected. Post-bronchoscopic diagnosis was defined based on cytological and microbiological results or follow-up clinical and radiologic courses.
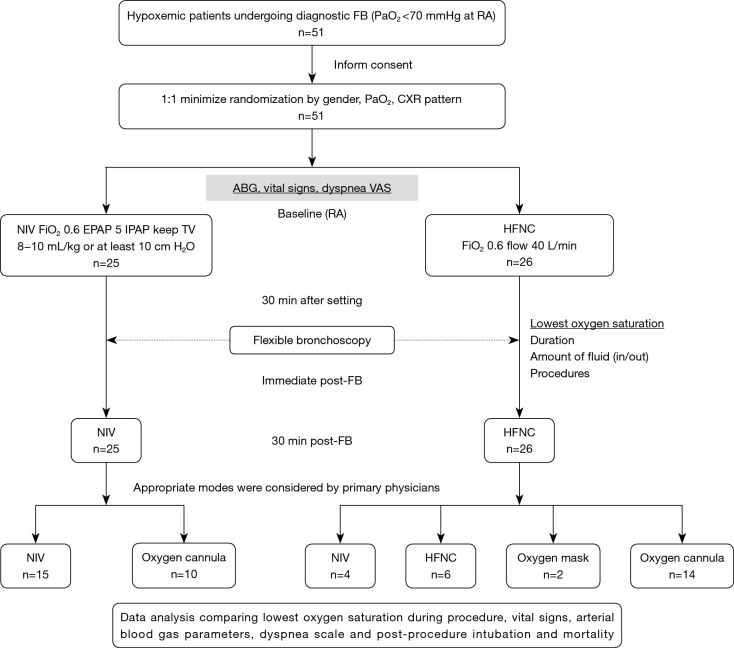
Vital signs and arterial blood gas analysis were examined at baseline (T0), 30 minutes after initiation of NIV or HFNC (T1), immediately after FB (T2), and 30 minutes after FB (T3). Visual analogue scale of dyspnoea was also assessed at T0, T1, T2, and T3 by using a 10-cm long horizontal line with anchor statements on the left (no dyspnoea) and on the right (extreme dyspnoea). The patient was asked to mark the point on the line that best corresponds to their symptom severity. The distance in centimeters (0 to 10) from the left to the point was measured and recorded as VAS. Any complications, such as haemoptysis, cardiac arrhythmias, cardiac arrest, oversedation, lowest SpO2 (defined as sustained fall in oxygen saturation for at least 20 seconds during FB), pneumothorax, and the need for endotracheal intubation (ETI) after FB, were recorded. In addition, the duration of the FB procedure, from the time the bronchoscope was introduced into the oral cavity to the vocal cord and from the vocal cord until removal from the mouth, as well as the amount of lidocaine, instilled fluid, and retrieved fluid were also collected. The details on the study workflow are shown in Figure 1.
Figure 1.
Design of the study and the flow of patients. FB, flexible bronchoscopy; PaO2, partial pressure of arterial oxygen; CXR, chest X-ray; ABG, arterial blood gas; VAS, visual analogue scale; RA, room air; NIV, non-invasive ventilation; HFNC, high-flow nasal cannula; FiO2, inspired fraction of oxygen; EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; TV, tidal volume.
Outcomes
The primary outcome was the lowest SpO2 during FB. Secondary outcomes were changes in haemodynamic parameters, arterial blood gas parameters, dyspnoea scale, and the occurrence of adverse effects.
Based on the results of the previous study (14), the lowest SpO2 was 95%±5%. To detect a 3% difference in the lowest SpO2 during FB with a power of 80% and a level of significance of 5%, 45 patients were required in each group. However, due to the difference between our study protocol and the previous study, the exact population required to achieve the statistical significance might be lesser, we planned to analyse the results when we achieved the patients more than the previous study (40 patients).
Statistical analysis
All values were expressed as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. Between-group comparisons for continuous variables were performed using Student’s two-tailed t-test or nonparametric Mann-Whitney U-test in the case of non-normal distribution. Chi-square test or Fisher’s exact test, in case of low expected frequencies, was used for comparisons of categorical variables. ETI at 7 days and mortality at 28 days after FB were assessed by the Kaplan-Meier method, and differences between the NIV and HFNC were assessed by the log-rank test. All statistical tests were two-sided, and P<0.05 was considered statistically significant. All data were analysed using the SPSS statistical software package, version 16.0 for Windows (SPSS, Chicago, IL).
Results
Fifty-one patients (mean age 58.6±15.9 years; 28 men, 23 women) were included in the study and underwent randomization to NIV (n=25) and HFNC (n=26). There were 19 and 18 patients who had baseline PaO2 <60 mmHg in the NIV and HFNC groups, respectively. At study entry, the baseline characteristics, SAPS II, pre-bronchoscopic diagnosis, final diagnosis, and haemodynamic and blood gas parameters were comparable between both groups (Table 1). PaO2 was slightly lower in the NIV group although it did not reach statistical significance (51.3 vs. 56.6 mmHg, P=0.07).
Table 1. Baseline characteristics and bronchoscopic procedure.
| Parameters | NIV (n=25) | HFNC (n=26) | P value |
|---|---|---|---|
| Male gender | 11 (39.3%) | 17 (60.7%) | 0.12 |
| Age, years | 57.2 (16.7) | 60.0 (15.3) | 0.68 |
| SAPS II score | 27.4 (9.5) | 28.1 (8.8) | 0.89 |
| Immunosuppression | 18 (47.4%) | 20 (52.6%) | 0.69 |
| CXR pattern | |||
| Focal | 3 (12.0%) | 6 (23.1%) | 0.30 |
| Diffuse | 22 (88.0%) | 20 (76.9%) | – |
| Main underlying diseases | |||
| HIV/AIDS immunocompromised host | 0 (0.0%) | 3 (11.5%) | 0.08 |
| Non-HIV immunocompromised host | 17 (68.0%) | 14 (53.8%) | 0.30 |
| Interstitial lung disease | 3 (12.0%) | 4 (15.4%) | 0.73 |
| COPD/asthma | 3 (12.0%) | 1 (3.8%) | 0.28 |
| Lung cancer | 2 (8.0%) | 5 (19.2%) | 0.24 |
| Old pulmonary tuberculosis | 1 (4.0%) | 2 (7.7%) | 0.58 |
| Chronic heart diseases | 1 (4.0%) | 2 (7.7%) | 0.58 |
| Pre-bronchoscopic diagnosis | |||
| Suspected opportunistic infection | 12 (48.0%) | 13 (50.0%) | 0.13 |
| Suspected hospital-acquired pneumonia | 6 (24.0%) | 5 (19.2%) | – |
| Suspected active interstitial lung disease | 6 (24.0%) | 2 (7.7%) | – |
| Suspected malignancy | 1 (4.0%) | 6 (23.1%) | – |
| Final diagnosis | |||
| Bacterial pneumonia | 6 (24.0%) | 2 (7.7%) | 0.06 |
| Viral pneumonia | 4 (16.0%) | 3 (11.5%) | – |
| PCP | 2 (8.0%) | 7 (26.9%) | – |
| Other fungal pneumonia | 1 (4.0%) | 3 (11.5%) | – |
| Pulmonary tuberculosis | 6 (24.0%) | 2 (7.7%) | – |
| Active interstitial lung disease | 5 (20.0%) | 3 (11.5%) | – |
| Malignancy | 1 (4.0%) | 6 (23.1%) | – |
| Physiologic parameters at baseline | |||
| Mean blood pressure (mmHg) | 89.2 (13.2) | 83.5 (14.9) | 0.15 |
| Heart rate (beats/min) | 88.5 (17.2) | 89.8 (17.2) | 0.78 |
| Respiratory rate (breaths/min) | 28.1 (14.4) | 23.6 (6.2) | 0.16 |
| pH | 7.46 (0.04) | 7.45 (0.04) | 0.74 |
| PaCO2 (mmHg) | 33.0 (6.8) | 32.8 (4.9) | 0.89 |
| PaO2 (mmHg) | 51.3 (9.4) | 56.6 (10.6) | 0.07 |
| SaO2 (%) | 85.7 (8.0) | 89.1 (5.0) | 0.07 |
| Dyspnoea at baseline by VAS | 5.2 (2.6) | 4.3 (3.1) | 0.30 |
| Bronchoscopic procedure | |||
| Time from oral cavity to the vocal cord (min:s) | 1:12 (0:44) | 1:15 (0:49) | 0.81 |
| Time from the vocal cord until removal (min:s) | 9:56 (4:47) | 11:46 (7:42) | 0.31 |
| Instilled fluid (mL) | 137.8 (52.6) | 148.6 (51.4) | 0.46 |
| Retrieved fluid (mL) | 45.1 (20.8) | 44.9 (21.8) | 0.97 |
| Lidocaine used (mL) | 13.8 (3.5) | 13.8 (3.6) | 0.99 |
| Sampling techniques | |||
| Bronchoalveolar lavage (BAL) | 13 (52.0%) | 10 (38.5%) | 0.63 |
| Transbronchial biopsy (TBB) | 2 (8.0%) | 3 (11.5%) | – |
| BAL and TBB | 10 (40.0%) | 12 (46.2%) | – |
| Inspection | 0 (0.0%) | 1 (3.8%) | – |
| Oxygen supplement 30 minutes after the procedure | |||
| NIV | 15 (60.0%) | 4 (15.4%) | 0.002 |
| HFNC | 0 (0.0%) | 6 (23.1%) | – |
| Oxygen mask | 0 (0.0%) | 2 (7.7%) | – |
| Oxygen cannula | 10 (40.0%) | 14 (53.8%) | – |
Data are presented as mean (SD) or number (%). COPD, chronic obstructive pulmonary diseases; CXR, chest X-ray; HFNC, high-flow nasal cannula; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; min, minutes; mL, millimetres; mmHg, millimetres of mercury; NIV, noninvasive ventilator; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PCP, Pneumocystis jiroveci pneumonia; pH, potential of hydrogen ion; SaO2, arterial oxygen saturation as measured by blood gas analysis; SAPS, Simplified Acute Physiology Score; sec, seconds; VAS, visual analogue scale.
Tolerance of the procedure
After initiation of NIV or HFNC, all patients were able to tolerate their mode of oxygen supplement. SpO2 could be raised to ≥90% in all cases. PaO2 and SaO2 were improved from baseline (T1–T0) in both methods but were not different among both (Table 2). In the NIV group, the mean IPAP used to achieve the target tidal volume was 12.3±2.1 cmH2O. The mean peak inspiratory flow rate was 38.6±7.9 L/min, which was similar to the inspiratory flow rate of the HFNC group (40 L/min).
Table 2. Mean differences in physiologic parameters between baseline, pre–flexible bronchoscopy, and post-flexible bronchoscopy.
| Parameter | T1–T0 | T2–T1 | T3–T2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HFNC | NIV | P value | HFNC | NIV | P value | HFNC | NIV | P value | |||
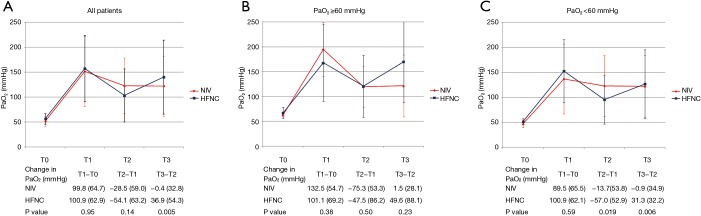
| PaO2 | 100.9 (62.9) | 99.8 (64.7) | 0.95 | −54.1 (63.2) | −28.5 (59.0) | 0.14 | 36.9 (54.3) | −0.4 (32.8) | 0.005* | ||
| T0 PaO2 ≥60 mmHg | 101.1 (69.2) | 132.5 (54.7) | 0.38 | −47.5 (86.2) | −75.3 (53.3) | 0.50 | 49.6 (88.1) | 1.5 (28.1) | 0.23 | ||
| T0 PaO2 <60 mmHg | 100.9 (62.1) | 89.5 (65.5) | 0.59 | −57.0 (52.9) | −13.7 (53.8) | 0.019* | 31.3 (32.2) | −0.9 (34.9) | 0.006* | ||
| SaO2 | 9.2 (5.2) | 11.3 (9.3) | 0.33 | −3.7 (4.4) | 0.04 (6.5) | 0.018* | −6.23 (6.0) | −2.4 (5.5) | 0.037* | ||
| T0 PaO2 ≥60 mmHg | 5.4 (2.2) | 6.0 (0.9) | 0.49 | −3.1 (5.3) | −1.2 (1.5) | 0.40 | 3.1 (4.8) | −1.0 (1.3) | 0.07 | ||
| T0 PaO2 <60 mmHg | 10.9 (5.2) | 13.0 (10.2) | 0.45 | −4.1 (4.0) | 0.4 (7.4) | 0.030* | 2.2 (4.7) | 0.05 (4.8) | 0.18 | ||
| PaCO2 | 1.0 (5.0) | 2.5 (6.0) | 0.35 | 3.5 (5.9) | 3.6 (4.5) | 0.95 | −0.8 (5.4) | −2.0 (4.2) | 0.39 | ||
| T0 PaO2 ≥60 mmHg | 4.2 (5.2) | 1.9 (7.6) | 0.49 | 1.9 (7.7) | 4.5 (2.3) | 0.44 | 0.4 (8.0) | −2.2 (1.5) | 0.23 | ||
| T0 PaO2 <60 mmHg | −0.4 (4.3) | 2.7 (5.5) | 0.07 | 4.2 (4.8) | 3.2 (5.0) | 0.58 | −1.3 (3.9) | −1.9 (4.7) | 0.67 | ||
| pH | −0.004 (0.037) | 0.001 (0.039) | 0.66 | −0.052 (0.052) | −0.041 (0.043) | 0.41 | 0.019 (0.039) | 0.017 (0.036) | 0.91 | ||
| T0 PaO2 ≥60 mmHg | −0.009 (0.040) | −0.003 (0.037) | 0.80 | −0.046 (0.039) | −0.048 (0.045) | 0.95 | 0.011 (0.059) | 0.021 (0.016) | 0.68 | ||
| T0 PaO2 <60 mmHg | −0.002 (0.036) | 0.001 (0.040) | 0.76 | −0.055 (0.043) | −0.039 (0.042) | 0.26 | 0.022 (0.027) | 0.015 (0.041) | 0.61 | ||
| MAP | 5.9 (13.6) | 5.6 (9.0) | 0.91 | 6.5 (15.6) | 0.8 (12.7) | 0.17 | −7.5 (15.6) | −2.3 (13.1) | 0.21 | ||
| T0 PaO2 ≥60 mmHg | 1.6 (11.4) | 9.1 (12.2) | 0.25 | 12.1 (16.6) | 0.3 (9.3) | 0.14 | −8.5 (12.6) | −8.8 (8.1) | 0.96 | ||
| T0 PaO2 <60 mmHg | 7.8 (14.3) | 4.4 (7.8) | 0.37 | 3.9 (15.0) | 1.0 (13.8) | 0.53 | −7.0 (17.0) | −0.2 (13.8) | 0.19 | ||
| HR | 2.9 (11.4) | 3.0 (15.9) | 0.97 | 15.9 (14.5) | 3.0 (7.5) | <0.001* | −11.9 (12.7) | −3.4 (7.8) | 0.005* | ||
| T0 PaO2 ≥60 mmHg | −1.5 (14.3) | −1.3 (8.4) | 0.98 | 27.6 (9.2) | −2.5 (7.2) | <0.001* | −14.8 (9.7) | −4.2 (11.2) | 0.08 | ||
| T0 PaO2 <60 mmHg | 4.8 (9.6) | 4.4 (17.6) | 0.92 | 10.8 (13.6) | 4.7 (6.9) | 0.10 | −10.7 (13.8) | −3.1 (6.8) | 0.040* | ||
| RR | −2.5 (3.8) | −4.0 (13.5) | 0.57 | 6.2 (6.1) | 1.9 (4.5) | 0.006* | −2.5 (5.8) | −1.9 (4.4) | 0.71 | ||
| T0 PaO2 ≥60 mmHg | −2.4 (2.7) | −1.8 (4.2) | 0.78 | 9.4 (7.4) | 1.7 (6.2) | 0.06 | −2.9 (6.7) | 4.5 (6.3) | 0.65 | ||
| T0 PaO2 <60 mmHg | −2.5 (4.3) | −4.7 (15.4) | 0.56 | 4.8 (5.2) | 1.9 (4.1) | 0.07 | −2.3 (5.6) | −1.1 (3.5) | 0.45 | ||
| Dyspnoea | −0.1 (0.7) | −0.9 (2.1) | 0.07 | 2.2 (2.5) | 1.2 (2.2) | 0.16 | −2.2 (1.9) | −0.5 (0.9) | <0.001* | ||
| T0 PaO2 ≥60 mmHg | −0.1 (0.6) | −1.3 (1.9) | 0.19 | 3.7 (3.4) | 1.5 (2.0) | 0.18 | −2.6 (2.1) | −0.3 (0.5) | 0.022* | ||
| T0 PaO2 <60 mmHg | 0.0 (0.8) | −0.7 (2.2) | 0.18 | 1.4 (1.7) | 1.1 (2.3) | 0.62 | −2.0 (1.8) | −0.5 (1.0) | 0.006* | ||
Data are shown as mean (SD). T0, baseline; T1, after HFNC or NIV initiation; T2, immediately after flexible bronchoscopy; T3, 30 minutes after flexible bronchoscopy; HFNC, high-flow nasal cannula; HR, heart rate; MAP, mean arterial pressure; NIV, non-invasive ventilation; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; pH, potential of hydrogen ion; RR, respiratory rate; SaO2, arterial oxygen saturation as measured by blood gas analysis.
The time required to pass the bronchoscope through the vocal cords was not different between the NIV and HFNC groups (Table 1). The duration of the procedure, sampling techniques, and instilled fluid were also not different. All patients tolerated the procedure well until completion.
The lowest SpO2 during FB was 94.6%±4.2% vs. 92.2%±6.6% in the NIV and HFNC groups, respectively (P=0.12). However, the lowest SpO2 <90% tended to occur more often in the HFNC group (34.6% vs. 12.0%; P=0.057). Table 2 demonstrates the mean differences in physiologic parameters between baseline, pre-FB, and post-FB. NIV provided a less decrease in oxygenation by the procedure (T2–T1) than HFNC in those who had T0 PaO2 <60 mmHg (Figure 2). In addition, this was reflected in the view of oxygenation stability immediately and 30 minutes after FB (T3–T2), which was more pronounced in the NIV group than in the HFNC group. Immediately and 30 minutes after FB, the occurrence of SpO2 <90% was 15.4% and 7.7% in the HFNC group and 4.0% and 4.0% in the NIV group, respectively (P=0.17 and P=0.57 for immediately and 30 minutes after the FB, respectively). In terms of vital signs, patients in the NIV group were able to maintain a more stable heart rate and respiratory rate than those in the HFNC group. The tolerability indirectly assessed by the dyspnoea visual analogue scale was not significantly different during T1–T0 and T2–T1, but in T3–T2, patients who were on HFNC had a decrease in dyspnoea scale score as compared with those who were on NIV.
Figure 2.
PaO2 at baseline (T0), after HFNC or NIV initiation (T1), at the end of flexible bronchoscopy (T2), and 30 minutes after flexible bronchoscopy (T3) in all patients (A), and subgroup analysis in patients with baseline PaO2 ≥60 mmHg (B) and PaO2 <60 mmHg (C). HFNC, high-flow nasal cannula; NIV, noninvasive ventilation; PaO2, arterial partial pressure of oxygen.
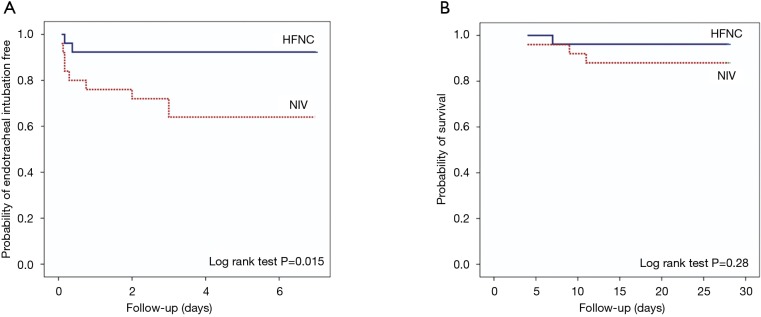
Pneumothorax occurred in one case in each group who had undergone TBB. Other complications, such as haemoptysis, cardiac arrhythmias, cardiac arrest, and oversedation, were not detected. No case required ETI immediately after FB. The first case of ETI developed at 2 and 4 hours after FB in the NIV group and the HFNC group, respectively. Within 8 hours after FB, five cases in the NIV group and one case in the HFNC group required ETI from deterioration in respiratory status without response to NIV (P=0.07). Details of these cases are summarized in Table 3. Mortality at 7 and 28 days was not different between both groups (8% and 12% in the NIV group and 3.8% and 3.8% in the HFNC group, P=0.53 and P=0.28, respectively). Kaplan-Meier analysis of ETI at 7 days and 28-day mortality are shown in Figure 3.
Table 3. Characteristics of patients who required endotracheal intubation within 8 hours after flexible bronchoscopy.
| Parameters | Patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Group | NIV | NIV | NIV | NIV | NIV | HFNC |
| Age, sex | 68 y, female | 31 y, female | 61 y, male | 68 y, female | 53 y, male | 53 y, male |
| SAPS II score | 32 | 12 | 39 | 38 | 25 | 39 |
| Immunosuppression | No | Yes | Yes | No | Yes | No |
| Final diagnosis | Bacterial pneumonia | Active interstitial lung disease | Pulmonary tuberculosis | Lung cancer | Viral pneumonia (CMV) | Pulmonary tuberculosis |
| PaO2 at baseline (mmHg) | 34 | 68 | 48 | 41 | 45 | 43 |
| PaO2 after NIV/HFNC (mmHg) | 67 | 254 | 62 | 85 | 88 | 75 |
| PaO2 after FB (mmHg) | 46 | 88 | 114 | 92 | 83 | 102 |
| PaO2 30 min after FB (mmHg) | 69 | 64 | 76 | 92 | 79 | 92 |
| Lowest SpO2 during FB (%) | 86 | 96 | 97 | 92 | 87 | 83 |
| Oxygen supplement after FB | NIV | NIV | NIV | NIV | NIV | NIV |
| Sampling techniques | BAL | Transbronchial biopsy | Transbronchial biopsy | BAL and transbronchial biopsy | BAL | BAL and transbronchial biopsy |
| Pneumothorax | No | No | No | Yes | No | Yes |
| Endotracheal tube insertion (h) | 4 | 4 | 2 | 7 | 3 | 4 |
| Hospital mortality | No | No | No | Day 11 | No | Day 7 |
BAL, bronchoalveolar lavage; CMV, cytomegalovirus; HFNC, high-flow nasal cannula; hrs, hours; FB, flexible bronchoscopy; mmHg, millimetres of mercury; NIV, non-invasive ventilator; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; SpO2, oxygen saturation by pulse oximeter; SAPS, Simplified Acute Physiology Score; sec, seconds; y, years.
Figure 3.
Kaplan-Meier analysis of endotracheal intubation free at 7 days (A) and 28-day mortality (B). HFNC, high-flow nasal cannula; NIV, non-invasive ventilation.
Discussion
To date, there have been many studies comparing the efficacy of HFNC to NIV in various conditions, such as acute hypoxemic respiratory failure (9,16), preoxygenation prior to intubation (17), postoperative cardiothoracic surgery (10), post-extubation (18), and stable hypercapnic chronic obstructive pulmonary disease (19). At present, only the study by Simon et al. has compared HFNC with NIV in hypoxemic patients undergoing FB (14).
After initiation of NIV or HFNC, similar to previous studies on hypoxemic patients (4-7,9-14,16), both methods were able to improve oxygenation from baseline. NIV has some advantages over HFNC. NIV provides EPAP, which prevents alveolar collapse (16), raises mean airway pressure (19), and decreases the work of breathing (9,10,19). IPAP (EPAP + inspiratory pressure) provides tidal volume once triggering at low effort has occurred. In addition, NIV delivers adequate high inspiratory flow at constant FiO2. Although HFNC does not intend to provide EPAP, its high flow can generate positive airway pressures in the nasopharynx, which translates to the alveoli (20). The pressure generated inside shows that an increase in flow of 10 L/min produced a 0.8-cmH2O increase in expiratory pressure during mouth-closed breathing (21). HFNC can also provide a constant high flow at a fixed FiO2. However, when inspiratory flow rates of the patient exceed the constant high-flow supplies, the additional flow from the surrounding air will be recruited, resulting in lower inspired FiO2 than the delivered gas.
At T1–T0, Simon et al. demonstrated better improvement in oxygenation with NIV than HFNC (14), and this improvement was similar in our study. This could be caused by the difference in the ventilator setting protocol. In Simon et al.’s study, NIV was set to a positive end expiratory pressure (PEEP) of 3–10 cmH2O and a pressure support of 15–20 cmH2O. Thus, with this setting, NIV took all the advantages over HFNC. In contrast, in our study, NIV was set to a PEEP of 5 cmH2O, which might be equal to the expiratory pressure created by HFNC. The mean pressure support used to achieve the target tidal volume of 8–10 mL/kg in our patients was 7.3±2.1 cmH2O, which made respiratory effort easy. Finally, the mean peak inspiratory flow rate was 38.6±7.9 L/min, which was similar to the inspiratory flow rate of HFNC (40 L/min). Therefore, it was not surprising to find similar oxygenation improvement between both groups.
HFNC has some unfavourable effects during FB. First, EPAP was shown to be lost when the mouth opened (21). Due to the closed circuit of the NIV face mask, even when patients open their mouth, EPAP would be maintained. Moreover, HFNC provides a fixed constant flow. As the respiratory rate and inspiratory effort increase during the procedure, the flow may not be sufficient, resulting in lower inspired FiO2. In contrast, with the NIV mode, the flow can be adequately provided to reach and maintain the target inspiratory pressure. However, this advantage of the NIV was shown in maintenance of oxygenation during FB only in a subgroup of patients with severe hypoxemia in our study and in Simon et al.’s study (14).
At T3–T2, oxygenation did not change in the NIV group, reflecting the oxygenation stability by NIV. In the HFNC group, oxygenation significantly increased, possibly because of the EPAP gained after the mouth closed and the decrease in the respiratory effort.
FB via NIV face mask was not more difficult than HFNC, as identified by the similarity in time required to reach the vocal cords and the total operation time. The tip was keeping the direction from the face mask connector to the oral cavity as the straight path.
ETI occurred more often in the NIV group. In previous studies, ETI within 8 hours after the FB procedure was considered as a procedure related to ETI (5,14,22). However, Trouillet et al. found PaO2 that dropped from the procedure would return to baseline within 2 hours (23). In our study, although there was no statistical significance, baseline PaO2 was lower in the NIV group, reflecting in more severe pulmonary diseases in this group. At 30 minutes after FB, PaO2 was also raised to greater than 60 mmHg in all cases who had ETI, reflecting in the efficacy of both HFNC and NIV in oxygen maintenance after FB. In addition, even in the HFNC group, NIV was prescribed by the primary physicians in four cases after 30 minutes of the procedure finish time with the intention of oxygenation improvement and decreased work of breathing. If these four cases of HFNC group had not received NIV, they would have been intubated. Finally, the first case of ETI developed after 2 hours after FB. Therefore, these adverse events would be explained by the progression of the natural course of the underlying pulmonary diseases and not as a consequence of the bronchoscopic procedure under NIV.
The limitation of our study was a relatively small number of patients. We did not achieve the primary outcome of the lowest SpO2 during FB. With our results, in order to detect a 3% difference in the lowest SpO2 during FB with a power of 80% and a level of significance of 5%, we required a sample size of 86 for each group. Therefore, we decided not to extend the study. However, we found that the effect of NIV was enhanced in the patients who had baseline PaO2 <60 mmHg in the secondary outcome. Thus, further study regarding oxygen supplementation during FB should focus on these patients.
In conclusion, NIV and HFNC are well tolerated and effective for oxygen supplementation during the FB procedure in patients with hypoxemia. NIV provided more adequacy and stability of oxygenation and cardiopulmonary parameters than HFNC only in a subgroup of patients with a baseline PaO2 <60 mmHg on ambient air. However, further studies focussing on severe hypoxemic patients are required before drawing conclusion.
Acknowledgments
None.
Ethical Statement: This prospective randomized study was approved by the Ethics Committee on Human Experimentation of Ramathibodi Hospital, Faculty of Medicine, Mahidol University (ID 08-58-23), and the trial was registered at Thai Clinical Trials Registry (http://www.clinicaltrials.in.th; registration number TCTR20150828001; registration date 28 August 2015). All participants or their legal representatives provided their written informed consent prior to enrolment.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jones AM, O'Driscoll R. Do all patients require supplemental oxygen during flexible bronchoscopy? Chest 2001;119:1906-9. 10.1378/chest.119.6.1906 [DOI] [PubMed] [Google Scholar]
- 2.Milman N, Faurschou P, Grode G, et al. Pulse oximetry during fibreoptic bronchoscopy in local anaesthesia: frequency of hypoxaemia and effect of oxygen supplementation. Respiration 1994;61:342-7. 10.1159/000196366 [DOI] [PubMed] [Google Scholar]
- 3.Attaran D, Towhidi M, Amini M, et al. The relationship between peak expiratory flow rate before bronchoscopy and arterial oxygen desaturation during bronchoscopy. Acta Medica Iranica 2008;46:95-8. [Google Scholar]
- 4.Antonelli M, Conti G, Rocco M, et al. Noninvasive positive-pressure ventilation vs. conventional oxygen supplementation in hypoxemic patients undergoing diagnostic bronchoscopy. Chest 2002;121:1149-54. 10.1378/chest.121.4.1149 [DOI] [PubMed] [Google Scholar]
- 5.Baumann HJ, Klose H, Simon M, et al. Fiber optic bronchoscopy in patients with acute hypoxemic respiratory failure requiring noninvasive ventilation--a feasibility study. Critical Care 2011;15:R179. 10.1186/cc10328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clouzeau B, Bui HN, Guilhon E, et al. Fiberoptic bronchoscopy under noninvasive ventilation and propofol target-controlled infusion in hypoxemic patients. Intensive Care Med 2011;37:1969-75. 10.1007/s00134-011-2375-1 [DOI] [PubMed] [Google Scholar]
- 7.Korkmaz Ekren P, Basarik AB, Gurgun A, et al. Can fiberoptic bronchoscopy be applied to critically ill patients treated with noninvasive ventilation for acute respiratory distress syndrome? Prospective observational study. BMC Pulm Med 2016;16:89. 10.1186/s12890-016-0236-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society Bronchoscopy Guideline Group British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68 Suppl 1:i1-i44. 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 9.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185–96. 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 10.Stéphan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: A randomized clinical trial. JAMA 2015;313:2331-9. 10.1001/jama.2015.5213 [DOI] [PubMed] [Google Scholar]
- 11.Drake MG. High-flow nasal cannula oxygen in adults: An evidence-based assessment. Ann Am Thorac Soc 2018;15:145-55. 10.1513/AnnalsATS.201707-548FR [DOI] [PubMed] [Google Scholar]
- 12.Lucangelo U, Vassallo FG, Marras E, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract 2012;2012:506382. 10.1155/2012/506382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EJ, Jung CY, Kim KC. Effectiveness and safety of high-flow nasal cannula oxygen delivery during bronchoalveolar lavage in acute respiratory failure patients. Tuberc Respir Dis (Seoul) 2018;81:319-29. 10.4046/trd.2017.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon M, Braune S, Frings D, et al. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy--a prospective randomised trial. Critical Care 2014;18:712. 10.1186/s13054-014-0712-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 16.Jaber S, Monnin M, Girard M, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomized controlled OPTINIV trial. Intensive Care Med 2016;42:1877-87. 10.1007/s00134-016-4588-9 [DOI] [PubMed] [Google Scholar]
- 17.Besnier E, Guernon K, Bubenheim M, et al. Pre-oxygenation with high-flow nasal cannula oxygen therapy and non-invasive ventilation for intubation in the intensive care unit. Intensive Care Med 2016;42:1291-2. 10.1007/s00134-016-4369-5 [DOI] [PubMed] [Google Scholar]
- 18.Hernández G, Vaquero C, Colinas L, et al. Effect of post-extubation high-flow nasal cannula vs non-invasive ventilation on re-intubation and post-extubation respiratory failure in high-risk patients. A randomized clinical trial. JAMA 2016;316:1565-74. 10.1001/jama.2016.14194 [DOI] [PubMed] [Google Scholar]
- 19.Bräunlich J, Seyfarth HJ, Wirtz H. Nasal high-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med 2015;10:27. 10.1186/s40248-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helviz Y, Einav S. A systematic review of the high-flow nasal cannula for adult patients. Crit Care 2018;22:71. 10.1186/s13054-018-1990-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007;20:126-31. 10.1016/j.aucc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 22.Maitre B, Jaber S, Maggiore SM, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients: a randomized double-blind study using a new device. Am J Respir Crit Care Med 2000;162:1063-7. 10.1164/ajrccm.162.3.9910117 [DOI] [PubMed] [Google Scholar]
- 23.Trouillet JL, Guiguet M, Gibert C, et al. Fiberoptic bronchoscopy in ventilated patients. Evaluation of cardiopulmonary risk under midazolam sedation. Chest 1990;97:927-33. 10.1378/chest.97.4.927 [DOI] [PubMed] [Google Scholar]