Abstract
Background
The aim of this study is to evaluate the outcomes of bioprosthetic versus mechanical valves in patients on dialysis.
Methods
All patients who underwent aortic (AVR) or mitral valve replacement (MVR) at a single institution from 2011–2017 were reviewed. Primary stratification was bioprosthetic versus mechanical valves. The primary outcome was all-cause mortality. Secondary outcomes included hospital readmission, valve reoperation rates and bleeding events. Kaplan-Meier curves were generated and Cox proportional hazards regression models were used for risk-adjustment.
Results
During the study period, 3,969 patients underwent AVR or MVR, of which 97 (2.4%) were on dialysis. In dialysis patients, unadjusted 30-day mortality was comparable between bioprosthetic (12.7%) versus mechanical (5.9%) valves (P=0.31). However, the bioprosthetic group had higher rates of 1-year (40.3% versus 15.2%; P=0.03) and 5-year mortality (67.9% versus 60.7%; P=0.02). Most patients were readmitted within 5 years with no differences between the groups (bioprosthetic 80.3% versus mechanical 100%; P=0.57). There were no valve reoperations in either group at 5 years. The 5-year readmission rate was higher in the mechanical cohort (10.5% versus 53.8%; P=0.05). Risk-adjusted analysis confirmed these findings, where mechanical valves were independently associated with reduced mortality at 1-year and 5-years.
Conclusions
Despite the limited life expectancy of patients on dialysis, mechanical valves have an intermediate term mortality benefit compared to bioprosthetic valves. This comes at the expense of a higher rate of readmission for bleeding. Although valve choice should consider multiple factors, these data suggest that mechanical valve usage in dialysis patients is reasonable.
Keywords: Dialysis, valve replacement, aortic valve, mitral valve, survival
Introduction
End-stage renal disease (ESRD) continues to be a major healthcare burden. According to the annual report from the United States Renal Data System, approximately 30 million American adults have chronic kidney disease. In 2015, 124,111 new cases of ESRD were reported, with 690,899 cumulative cases (1). The management of valvular heart disease in ESRD can be challenging. Foremost, in-hospital and long-term morbidity and mortality is extremely high for patients undergoing heart surgery who have ESRD (2,3). Furthermore, there continues to be debate on the choice of prosthetic valve. Mechanical valves offer durability but require anticoagulation, while bioprosthetic valves do not require anticoagulation but have more limited durability. Implantation of mechanical valves has been questioned due to the decreased life expectancy and platelet dysfunction in ESRD patients (4,5). However, historically, there are concerns over early calcification and failure of bioprosthetic valves in these patients as well (6,7). To date, there are few studies that have compared valve types in patients with ESRD with varying results (3,8-12). This study evaluated the impact of mechanical versus bioprosthetic valves on early and mid-term outcomes in patients with ESRD.
Methods
Study population
Adult patients aged 18 years or older undergoing either aortic valve replacement (AVR) or mitral valve replacement (MVR) at a single institution between 2011 and 2017 were included. The Society of Thoracic Surgeons Database was also used to obtain patient characteristics, risk algorithms, and mortality data. Urgent or emergent cases and endocarditis involving concomitant (other) valve surgery or coronary bypass grafting were included. This study was approved by the institutional review board (IRB approval # PRO16020002).
Baseline characteristics
Primary stratification of the study population was mechanical versus bioprosthetic valve replacement. Baseline preoperative and intraoperative characteristics were then compared between these cohorts. Preoperative variables included demographics such as age and sex, and comorbidities which included diabetes mellitus, hypertension, chronic lung disease, intravenous drug abuse, infective endocarditis, peripheral arterial disease, cerebrovascular disease, coronary artery disease, heart failure and preoperative use of an intra-aortic balloon pump. Operative variables that were compared included type of valve (AVR or MVR), approach (sternotomy versus right mini-thoracotomy), concomitant operations, cardiopulmonary bypass time, and aortic cross-clamp time.
Outcomes
The primary outcome was all-cause mortality, measured at 30 days, 1-year, and 5-years after valve surgery. Secondary outcomes included valve reoperation, hospital readmission and readmission for bleeding. Other secondary outcomes included postoperative complications such as blood product transfusion, sepsis, stroke, prolonged intubation, pneumonia, limb ischemia, pacemaker placement, gastrointestinal bleed and atrial fibrillation.
Kaplan-Meier curves were generated to evaluate unadjusted 30-day, 1 and 5-year mortality for mechanical versus bioprosthetic replacements. Kaplan-Meier curves were also generated for the secondary outcomes of valve reoperation, hospital readmission and readmission for bleeding. Cox proportional hazards regression models were developed for risk adjustment. All candidate variables were evaluated in univariate Cox regression analysis, and those variables that were associated with the outcome in univariate analysis were then incorporated in the multivariable Cox proportional hazards regression models.
All continuous data in this study are presented as mean ± standard deviation and all categorical data as number (percentage). Data analyses were performed with SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous variables were compared using the unpaired student’s t-test and categorical variables were compared using chi-square. In all analyses, a two-tailed P value of less than 0.05 was considered significant.
Results
Comparison of baseline variables
During the study period, 3,969 patients underwent AVR (n=3,118) or MVR (n=851), of which 97 (2.4%) were on dialysis. In total, there were 72 AVRs and 42 MVRs in the dialysis cohort. All patients who had concomitant AVR and MVR had the same valve type (bioprosthetic or mechanical) implanted in both positions. Demographics and comorbidities were similar except patients who received mechanical valves were significantly younger compared to patients who received bioprosthetic valves (Table 1). There was also a higher percentage of patients on immunosuppressive medications and lower percentage patients with peripheral vascular disease who received a mechanical valve. Patients who had recent or history of intravenous drug use were more likely to receive a mechanical valve (Table 1). Intraoperative data, including operative approach, concomitant procedures, cardiopulmonary bypass and aortic cross-clamp times were similar between valve choices (Table 2).
Table 1. Baseline characteristics.
| Characteristics | Total | Bio prosthesis | Mechanical | P value |
|---|---|---|---|---|
| # Patients | 97 | 63 | 34 | |
| Mitral | 42 (43.3%) | 26 (41.3%) | 16 (47.1%) | |
| Aortic | 72 (74.2%) | 50 (79.4%) | 22 (64.7%) | |
| Patient characteristics | ||||
| Age | 60.8±12.8 | 63.9±12.9 | 55.1±10.3 | 0.001 |
| Sex | ||||
| Male | 65 (67.0%) | 44 (69.8%) | 21 (61.8%) | 0.420 |
| Female | 32 (33.0%) | 19 (30.2%) | 13 (38.2%) | |
| Race | ||||
| White | 81 (83.5%) | 58 (92.1%) | 23 (67.6%) | 0.002 |
| Black | 16 (16.5%) | 5 (7.9%) | 11 (32.4%) | |
| BMI | 30.4±8.15 | 31.3±8.99 | 28.7±6.11 | 0.145 |
| BSA | 2.02±0.30 | 2.04±0.32 | 1.99±0.26 | 0.421 |
| History and risk factors | ||||
| Diabetes | 53 (54.6%) | 36 (57.1%) | 17 (50.0%) | 0.570 |
| Hypertension | 84 (86.6%) | 52 (82.5%) | 32 (94.1%) | 0.110 |
| Chronic lung disease | 40 (41.2%) | 25 (39.7%) | 15 (44.1) | 0.894 |
| IV drug abuse | ||||
| Yes | 7 (7.2%) | 2 (3.2%) | 5 (14.7%) | 0.001 |
| Recent | 7 (7.2%) | 2 (3.2%) | 5 (14.7%) | |
| Remote | 9 (9.3%) | 3 (4.8%) | 6 (17.6%) | |
| Infective endocarditis | ||||
| None | 59 (60.8%) | 38 (60.3%) | 21 (61.8%) | 0.569 |
| Treated | 8 (8.2%) | 4 (6.3%) | 4 (11.8%) | |
| Active | 30 (30.9%) | 21 (33.3%) | 9 (26.5%) | |
| Immunosuppressive medication therapy | 21 (21.6%) | 7 (11.1%) | 14 (41.2%) | 0.001 |
| History of mediastinum radiation therapy | 2 (2.1%) | 1 (1.6%) | 1 (2.9%) | 0.654 |
| History of peripheral arterial disease | 26 (26.8%) | 23 (36.5%) | 3 (8.8%) | 0.003 |
| History of CVD | 25 (25.8%) | 14 (22.2%) | 11 (32.4%) | 0.276 |
| History of heart failure | 42 (43.3%) | 26 (41.3%) | 16 (47.1%) | 0.583 |
| Any arrhythmia | 50 (51.5%) | 35 (55.6%) | 15 (44.1%) | 0.282 |
| Cardiogenic shock | ||||
| No | 94 (96.9%) | 60 (95.2%) | 34 (100%) | 0.644 |
| Yes | 1 (1.0%) | 1 (1.6%) | 0 (0.0%) | |
| Yes: at time of procedure | 1 (1.0%) | 1 (1.6%) | 0 (0.0%) | |
| Yes: not at procedure, but within 24 hrs | 1 (1.0%) | 1 (1.6%) | 0 (0.0%) | |
| Creatinine | 5.12±2.91 | 4.74±2.70 | 5.82±3.19 | 0.083 |
| Total albumin | 3.18±0.68 | 3.07±0.76 | 3.37±0.47 | 0.045 |
| Total bilirubin | 0.80±0.45 | 0.80±0.46 | 0.79±0.43 | 0.888 |
| Preoperative hemodynamics | ||||
| Ejection fraction (%) | 54.5±12.2 | 53.7±12.1 | 55.9±12.6 | 0.402 |
| Aortic stenosis | 61 (62.9%) | 42 (66.7%) | 19 (55.9%) | 0.294 |
| Mitral stenosis | 24 (24.7%) | 14 (22.2%) | 10 (29.4%) | 0.434 |
| STS risk algorithms | ||||
| Mortality | 14.6±12.2 | 16.5±14.1 | 11.3±6.64 | 0.128 |
Table 2. Operative variables.
| Variables | Total | Bio prosthesis | Mechanical | P value |
|---|---|---|---|---|
| # Patients | 97 | 63 | 34 | |
| Operation approach | ||||
| Full sternotomy | 89 (91.8%) | 58 (92.1%) | 31 (91.2%) | 0.852 |
| Partial sternotomy | 5 (5.2%) | 3 (4.8%) | 2 (5.9%) | |
| Right thoracotomy | 1 (1.0%) | 1 (1.6%) | 0 (0.0%) | |
| Right mini-thoracotomy | 2 (2.1%) | 1 (1.6%) | 1 (2.9%) | |
| Incidence | ||||
| First CV surgery | 84 (86.6%) | 57 (90.5%) | 27 (79.4%) | 0.103 |
| First re-Op CV surgery | 11 (11.3%) | 6 (9.5%) | 5 (14.7%) | |
| Second re-Op CV surgery | 2 (2.1%) | 0 (0.0%) | 2 (5.9%) | |
| Status | ||||
| Elective | 22 (22.7%) | 17 (27.0%) | 5 (14.7%) | 0.097 |
| Urgent | 71 (73.2%) | 42 (66.7%) | 29 (85.3%) | |
| Emergent | 4 (4.1%) | 4 (6.3%) | 0 (0.0%) | |
| Perfusion time (min) | 160±65.3 | 158±70.7 | 163±55.0 | 0.723 |
| Cross clamp time (min) | 123±52.5 | 124±57.4 | 122±42.8 | 0.828 |
| Pre-op IABP | 1 (1.0%) | 1 (1.6%) | 0 (0.0%) | 0.233 |
| Concomitant procedures | ||||
| CABG | 38 (39.2%) | 23 (36.5%) | 15 (44.1%) | 0.464 |
| Tricuspid | 19 (19.6%) | 11 (17.5%) | 8 (23.5%) | 0.472 |
Operative outcomes
Post-operative complications for bioprosthetic versus mechanical valves were similar (Table 3). Unadjusted 30-day mortality was also comparable for bioprosthetic and mechanical valves (12.7% and 5.9%, respectively, P=0.31) (Figure 1). In addition, 30-day hospital readmission (bioprosthetic 35.2% vs. mechanical 36.4%, P=0.99) and readmission for bleeding (bioprosthetic 3.7% vs. mechanical 3.2%, P=0.89) were not significantly different between the groups (Figures 1 and 2).
Table 3. Postoperative outcomes.
| Variables | Total | Bio prosthesis | Mechanical | P value |
|---|---|---|---|---|
| # Patients | 97 | 63 | 34 | |
| Blood product transfused | 75 (77.3%) | 49 (77.8%) | 26 (76.5%) | 0.883 |
| Sepsis | 6 (6.2%) | 3 (4.8%) | 3 (8.8%) | 0.428 |
| Stroke | ||||
| No | 96 (99.0%) | 62 (98.4%) | 34 (100%) | 0.460 |
| Yes, embolic or ischemic | 1 (1.0%) | 1 (1.6%) | 0 (0.0%) | |
| Pulmonary ventilation >24 hrs | 26 (26.8%) | 18 (28.6%) | 8 (23.5%) | 0.593 |
| Pneumonia | 7 (7.2%) | 4 (6.3%) | 3 (8.8%) | 0.653 |
| Limb ischemia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.99 |
| Pacemaker | 6 (6.2%) | 5 (7.9%) | 1 (2.9%) | 0.426 |
| GI event | 9 (9.3%) | 6 (9.5%) | 3 (8.8%) | 0.910 |
| New onset atrial fibrillation | 46 (47.4%) | 25 (39.7%) | 21 (61.8%) | 0.038 |
Figure 1.
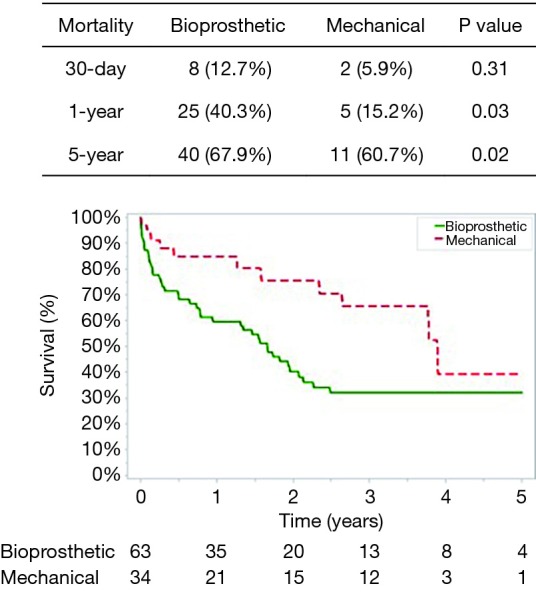
Freedom from mortality.
Figure 2.
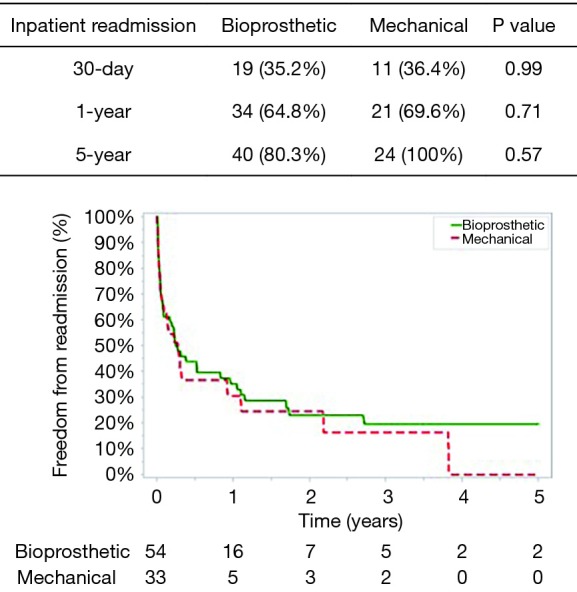
Freedom from inpatient readmission.
Long-term outcomes
The mean follow-up was similar between patients who received a bioprosthetic (1.77±1.80 years) versus mechanical valve (2.01±1.63 years) (P=0.51). No patients had valve reoperations at 5 years in either group. The 1-year and 5-year unadjusted mortality rates were higher in the bioprosthetic group (1-year: bioprosthetic 40.3% versus mechanical 15.2%, P=0.03 and 5-year: bioprosthetic 67.9% versus mechanical 60.7%, P=0.021) (Figure 1). Although 1- and 5-year hospital readmission rates were comparable, the mechanical cohort did have a higher rate of 5-year readmission for bleeding (P=0.05) (Figures 2 and 3).
Figure 3.
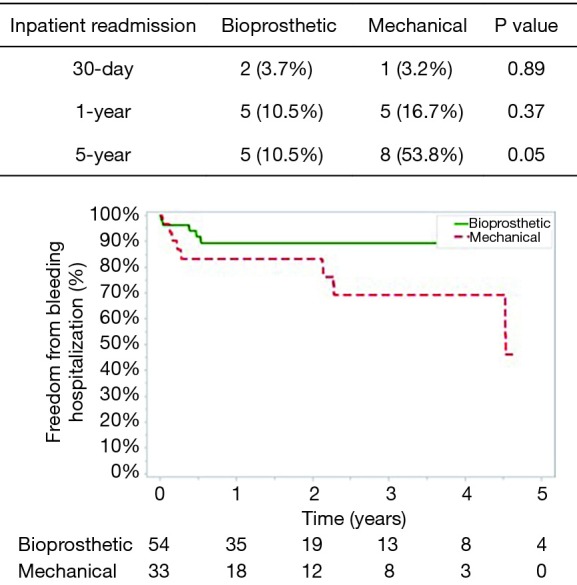
Freedom from re-hospitalization for bleeding.
After risk adjustment, the use of a mechanical prosthesis had a significant survival benefit at both 1-year (HR, 0.27, 95% CI: 0.10–0.74, P=0.010) and 5-year (HR, 0.43, 95% CI: 0.21–0.86, P=0.017) (Tables 4 and 5). Chronic lung disease as well as double valve replacement or MVR (as compared to AVR) were also shown to be strong predictors of 1- and/or 5-year mortality (Tables 4 and 5). Other variables that may have been expected to have an independent impact on mortality, such as age, gender, endocarditis, urgency of operation, heart failure and other concomitant procedures such as CABG or tricuspid valve procedures, did not have a significant impact on 1- or 5-year mortality (Tables 4 and 5).
Table 4. Association between variables and 1-year mortality [30 total deaths (25 bio-prosthesis, 5 mechanical)].
| Variables | Univariate models | Multivariate model* | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Mechanical vs. bio-prosthesis | 0.34 | 0.13–0.90 | 0.029 | 0.27 | 0.10–0.74 | 0.010 | |
| Age | 1.02 | 0.99–1.05 | 0.242 | ||||
| Female vs. male | 1.50 | 0.72–3.11 | 0.279 | ||||
| Chronic lung disease (vs. none) | 2.82 | 1.19–6.70 | 0.019 | 4.94 | 1.91–12.79 | 0.001 | |
| Infective endocarditis (vs. none) | |||||||
| Treated | 1.33 | 0.39–4.55 | 0.645 | ||||
| Active | 1.28 | 0.58–2.79 | 0.540 | ||||
| History of peripheral arterial disease | 1.62 | 0.77–3.41 | 0.201 | ||||
| Heart failure within 2 weeks | 0.97 | 0.44–2.12 | 0.938 | ||||
| Concomitant CABG | 0.71 | 0.33–1.52 | 0.378 | ||||
| Concomitant tricuspid | 1.39 | 0.60–3.24 | 0.448 | ||||
| Prior CT surgery (incidence) | 1.42 | 0.54–3.72 | 0.478 | ||||
| Status (vs. elective) | |||||||
| Urgent | 1.25 | 0.51–3.09 | 0.625 | ||||
| Emergent | 2.27 | 0.46–11.27 | 0.316 | ||||
| Procedure type (vs. AVR) | |||||||
| MVR | 2.55 | 1.11–5.90 | 0.028 | 4.91 | 1.88–12.84 | 0.001 | |
| AVR and MVR | 2.71 | 1.09–6.74 | 0.032 | 3.16 | 1.18–8.49 | 0.023 | |
*, included any variables from the univariate models with P<0.200 in the multivariate model. AVR, aortic valve replacement; MVR, mitral valve replacement.
Table 5. Association between variables and 5-year mortality [51 total deaths (40 bio-prosthesis, 11 mechanical)].
| Variables | Univariate models | Multivariate model* | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Mechanical vs. bio-prosthesis | 0.46 | 0.23–0.89 | 0.021 | 0.43 | 0.21–0.86 | 0.017 | |
| Age | 1.01 | 0.99–1.03 | 0.318 | ||||
| Female vs. male | 1.14 | 0.64–2.04 | 0.657 | ||||
| Chronic lung disease (vs. none) | 2.63 | 1.35–5.12 | 0.005 | 3.85 | 1.85–8.03 | <0.001 | |
| Infective endocarditis (vs. none) | |||||||
| Treated | 0.90 | 0.32–2.54 | 0.841 | ||||
| Active | 1.16 | 0.63–2.15 | 0.635 | ||||
| History of peripheral arterial disease | 1.37 | 0.76–2.48 | 0.294 | ||||
| Heart failure within 2 weeks | 0.62 | 0.35–1.11 | 0.107 | 0.60 | 0.32–1.12 | 0.107 | |
| Concomitant CABG | 0.77 | 0.43–1.37 | 0.374 | ||||
| Concomitant tricuspid | 1.48 | 0.75–2.89 | 0.257 | ||||
| Prior CT surgery (incidence) | 1.41 | 0.63–3.16 | 0.402 | ||||
| Status (vs. elective) | |||||||
| Urgent | 1.45 | 0.72–2.91 | 0.301 | ||||
| Emergent | 2.10 | 0.58–7.64 | 0.260 | ||||
| Procedure type (vs. AVR) | |||||||
| MVR | 1.48 | 0.74–2.96 | 0.262 | 2.58 | 1.17–5.71 | 0.019 | |
| AVR and MVR | 1.71 | 0.86–3.37 | 0.123 | 2.02 | 0.96–4.25 | 0.064 | |
*, included any variables from the univariate models with P<0.200 in the multivariate model. AVR, aortic valve replacement; MVR, mitral valve replacement.
Discussion
There is a growing population of patients with ESRD. Though survival has improved from the 1970’s to 2000’s, once a person is diagnosed with ESRD requiring dialysis, there is still a significant loss of life years. It is estimated that a 50 year old with ESRD has an average life-years lost of almost 25 years (13). Cardiovascular diseases account for approximately 50% of mortalities in patients with ESRD (1).
Cardiac surgery in patients with preoperative ESRD has been shown to be associated with increased morbidity and mortality (14-17). The overall long-term prognosis and quality of life of these patients are quite poor ((15). There continues to be a debate on the most appropriate valve choice for a patient on ESRD. First, historically, there were concerns of early calcification of bioprosthetic valves and subsequent failure in small series from the 1970’s (6,7). In addition to this, Ribeiro and colleagues showed that there is increased prevalence of cardiac valve calcification in patients on hemodialysis (18). Such studies suggest that the physiologic dysregulation of calcium in patients on hemodialysis makes them more prone to the development of calcium. These concerns manifested itself in the 1998 AHA/ACC guidelines for valve choice in dialysis patients which suggested that mechanical valves should be implanted (19).
The benefits of mechanical valves in patients with ESRD have also been questioned. These valves require anticoagulation to prevent thrombosis. The risk of major bleeding is significantly increased when patients on dialysis are placed on aspirin and warfarin (20). Furthermore, adjusted survival of these patients has been improving over the years, but is still quite dismal. The 5-year adjusted survival for a patient on hemodialysis was 34.5% in 2000 and 40.2% in 2008 (1). Due to the dismal survival rates, it is argued that patients on hemodialysis likely would not benefit from the durability of a mechanical valve. This was a conclusion of Williams and colleagues (8). In their study, they discovered minimal differences in survival of ESRD patients who receive a mechanical versus a bioprosthetic valve, even in patients who were younger than 65 years, suggesting that there is actually minimal impact of valve choice. Similar findings were discovered in the largest series utilizing the United States Renal Data System database done by Herzog and colleagues. Over a two-decade period, they identified 5,858 ESRD patients who had undergone heart valve surgery. Their study demonstrated no significant survival differences related to the choice of valve implanted (2). Their study was instrumental in changing guidelines resulting in the more liberal use of mechanical valves. Unlike guidelines from 1998, the most recent guidelines do not explicitly give suggestions on valve type for ESRD (21).
Our data showed that indeed there was a survival benefit at 1 and 5 years for patients receiving a mechanical versus a bioprosthetic valve. In addition, 1 and 5-year mortality was negatively impacted when the patient required an MVR or an AVR/MVR, or had chronic lung disease. There were no significant differences in hospital readmissions although readmissions for bleeding were higher at 5 years in the mechanical group. The mechanical cohort did have a younger patient population, and this may be because of bias on part of the surgeons to recommend a mechanical prosthesis in younger patients because of life expectancy. However, there was no difference in STS PROM between the two groups. Patients with mechanical valves were less likely to have peripheral arterial disease and this may be a surrogate for age and status at the time of surgery.
These data reaffirms the reduced survival of ESRD patients who require cardiac valve operations. However, the overall 5-year survival in this study was 35.6%, which is similar to patients on dialysis who do not require cardiac valve surgery. This supports valve surgery in patients with ESRD with acceptable longer-term mortality, particularly when the patient survives to discharge from their index hospitalization. The data also suggests that it is not unreasonable to implant a mechanical valve in patients who have ESRD requiring dialysis. However, the increased risk of bleeding while on anticoagulation must be recognized. Our study did show increased readmission rates due to bleeding in patients with mechanical valves at 5-years. The reasons for the survival benefit are unclear and quite possibly may be a result of selection bias by giving healthier ESRD patients a mechanical valve, although similarities in our demographics and preoperative comorbidities do not suggest this. Historical studies have suggested that early calcification could be a potential cause of early prosthetic failure (6,7,22). Although our study did not have any valve reoperations, it is unknown whether these patients deteriorated clinically from valve failure and were deemed to be nonoperative candidates due to incrementally higher surgical risk. Similarly, although the absolute hospital readmission rates were similar between the cohorts, the scope and severity of the clinical issues leading to readmission were not measured.
Study limitations
The principal limitation of our study is the relatively small sample size, which is a reflection of the rarity of operating on patients on dialysis who need valve surgery, particularly in the era of less invasive transcatheter therapies (23,24) and in an effort to minimize hospital mortality in this critical population (25). This may affect the ability to detect significant differences between the groups and subject the analyses to type II error. This study was also retrospective and not randomized, allowing for selection bias. The decision on who to operate on and choice of valve were individualized and made by the primary surgeon. Therefore, potentially, an ESRD patient who is expected to live longer as assessed by the surgeon, may have a higher propensity to receive a mechanical valve.
Conclusions
This study demonstrates that mechanical valves provide an intermediate term mortality benefit as compared to bioprosthetic valves in patients undergoing AVR and/or MVR. This benefit in survival must be balanced with the burden of anticoagulation and the increased risk of readmission for bleeding. Although valve choice should be individualized, these data suggest that mechanical valve usage is reasonable in patients on dialysis.
Acknowledgments
We would like to acknowledge Michael Sharbaugh, MPH, for his statistical guidance for this project.
Ethical Statement: The study was approved by the institutional review board (IRB approval # PRO16020002).
Footnotes
Conflicts of Interest: TG Gleason—Medical Advisory Board, Abbott; A Kilic—Medical Advisory Board, Medtronic, Inc.The other authors have no conflicts of interest to declare.
References
- 1.United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2017. [Google Scholar]
- 2.Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation 2002;105:1336-41. 10.1161/hc1102.100075 [DOI] [PubMed] [Google Scholar]
- 3.Thourani VH, Sarin EL, Keeling WB, et al. Long-term survival for patients with preoperative renal failure undergoing bioprosthetic or mechanical valve replacement. Ann Thorac Surg 2011;91:1127-34. 10.1016/j.athoracsur.2010.12.056 [DOI] [PubMed] [Google Scholar]
- 4.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial 2006;19:317-22. 10.1111/j.1525-139X.2006.00179.x [DOI] [PubMed] [Google Scholar]
- 5.Neild GH. Life expectancy with chronic kidney disease: an educational review. Pediatric Nephrology (Berlin, Germany) 2017;32:243-8. 10.1007/s00467-016-3383-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishbein MC, Gissen SA, Collins JJ, Jr, et al. Pathologic findings after cardiac valve replacement with glutaraldehyde-fixed porcine valves. Am J Cardiol 1977;40:331-7. 10.1016/0002-9149(77)90154-0 [DOI] [PubMed] [Google Scholar]
- 7.Lamberti JJ, Wainer BH, Fisher KA, et al. Calcific stenosis of the porcine heterograft. Ann Thorac Surg 1979;28:28-32. 10.1016/S0003-4975(10)63387-5 [DOI] [PubMed] [Google Scholar]
- 8.Williams ML, Bavaria JE, Acker MA, et al. Valve Selection in End-Stage Renal Disease: Should It Always Be Biological? Ann Thorac Surg 2016;102:1531-5. 10.1016/j.athoracsur.2016.04.092 [DOI] [PubMed] [Google Scholar]
- 9.D'Alessandro DA, Skripochnik E, Neragi-Miandoab S. The significance of prosthesis type on survival following valve replacement in dialysis patients. J Heart Valve Dis 2013;22:743-50. [PubMed] [Google Scholar]
- 10.Lucke JC, Samy RN, Atkins BZ, et al. Results of valve replacement with mechanical and biological prostheses in chronic renal dialysis patients. Ann Thorac Surg 1997;64:129-32. 10.1016/S0003-4975(97)00342-1 [DOI] [PubMed] [Google Scholar]
- 11.Boning A, Boedeker RH, Rosendahl UP, et al. Long-term results of mechanical and biological heart valves in dialysis and non-dialysis patients. Thorac Cardiovasc Surg 2011;59:454-9. 10.1055/s-0030-1271028 [DOI] [PubMed] [Google Scholar]
- 12.Chan V, Jamieson WR, Fleisher AG, et al. Valve replacement surgery in end-stage renal failure: mechanical prostheses versus bioprostheses. Ann Thorac Surg 2006;81:857-62. 10.1016/j.athoracsur.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 13.van Walraven C, Manuel DG, Knoll G. Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 2014;63:491-9. 10.1053/j.ajkd.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 14.Bechtel JF, Detter C, Fischlein T, et al. Cardiac surgery in patients on dialysis: decreased 30-day mortality, unchanged overall survival. Ann Thorac Surg 2008;85:147-53. 10.1016/j.athoracsur.2007.08.048 [DOI] [PubMed] [Google Scholar]
- 15.Jayasekera H, Pinto N, Mundy J, et al. Cardiac surgery in the presence of dialysis: effect on mid-term outcomes and quality of life. Heart Lung Circ 2011;20:105-10. 10.1016/j.hlc.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 16.Rahmanian PB, Adams DH, Castillo JG, et al. Early and late outcome of cardiac surgery in dialysis-dependent patients: single-center experience with 245 consecutive patients. J Thorac Cardiovasc Surg 2008;135:915-22. 10.1016/j.jtcvs.2007.09.027 [DOI] [PubMed] [Google Scholar]
- 17.Zimmet AD, Almeida A, Goldstein J, et al. The outcome of cardiac surgery in dialysis-dependent patients. Heart Lung Circ 2005;14:187-90. 10.1016/j.hlc.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro S, Ramos A, Brandao A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transplant 1998;13:2037-40. 10.1093/ndt/13.8.2037 [DOI] [PubMed] [Google Scholar]
- 19.Bonow RO, Carabello B, de Leon AC, et al. ACC/AHA Guidelines for the Management of Patients With Valvular Heart Disease. Executive Summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease). J Heart Valve Dis 1998;7:672-707. [PubMed] [Google Scholar]
- 20.Holden RM, Harman GJ, Wang M, et al. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol 2008;3:105-10. 10.2215/CJN.01810407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. 10.1016/j.jacc.2014.02.537 [DOI] [PubMed] [Google Scholar]
- 22.Kilic A, Bianco V, Gleason TG, et al. Hospital readmission rates are similar between patients with mechanical versus bioprosthetic aortic valves. J Card Surg 2018;33:497-505. 10.1111/jocs.13781 [DOI] [PubMed] [Google Scholar]
- 23.Kim KM, Shannon F, Paone G, et al. Evolving trends in aortic valve replacement: A Statewide experience. J Card Surg 2018;33:424-30. 10.1111/jocs.13740 [DOI] [PubMed] [Google Scholar]
- 24.Sultan I, Siki M, Wallen T, et al. Management of coronary obstruction following transcatheter aortic valve replacement. J Card Surg 2017;32:777-81. 10.1111/jocs.13252 [DOI] [PubMed] [Google Scholar]
- 25.Litwinowicz R, Bartus K, Drwilla R, et al. In hospital mortality in cardiac surgery patients after readmissions to the intensive care unit: a single-center experience with 10,992 patients. J Cardiothorac Vasc Anesth 2015;29:570-5. 10.1053/j.jvca.2015.01.029 [DOI] [PubMed] [Google Scholar]


