Abstract
Background
We aimed to investigate the association between average mean arterial pressure (a-MAP) and mortality in critically ill sepsis patients according to the presence of hypertension and previously measured blood pressure (BP).
Methods
From August 2008 to September 2014, patients with severe sepsis or septic shock presenting to the ED were categorized into four groups according to a-MAP during the initial 24 hours (group 0, a-MAP <65 mmHg; group 1, 65 mmHg ≤ a-MAP <75 mmHg; group 2, 75 mmHg ≤ a-MAP <85 mmHg; group 3, a-MAP ≥85 mmHg). A low previous BP was defined as previous a-MAP ≤85 mmHg, and a high previous BP is defined as a-MAP >85 mmHg. The primary outcome was 28-day mortality.
Results
A total of 1,395 patients were included. The 28-day mortality rates were 15.1% in patients overall, 39.7% in group 0, 18.3% in group 1, 10.1% in group 2, and 13.4% in group 3. In the regression analyses, mortality in group 2 was significantly lower compared with group 1 [odds ratio (OR), 0.33] or group 3 (OR, 0.31) in patients with hypertension. In the low previous BP group, there was greater mortality in group 3 compared to group 1 (OR, 2.42) and group 2 (OR, 3.88). In the high previous BP group, mortality was lower in group 2 compared with group 1 (OR, 0.32).
Conclusions
In critically ill sepsis patients, there were different trends in mortality according to a-MAP depending on the presence of hypertension or previous BP.
Keywords: Sepsis, septic shock, mean arterial pressure (MAP), outcomes
Introduction
Sepsis and septic shock are life-threatening problems affecting millions of people around the world due to dysregulated host responses to infection that cause organ hypo-perfusion and dysfunction (1-4). For initial resuscitation of patients with sepsis and septic shock, arterial blood pressure (BP) is a fundamental measure widely used to monitor and guide hemodynamic therapy, and early use of vasopressors with appropriate doses is an essential therapeutic component to reverse vasodilatory shock and maintain tissue perfusion (5-9).
The Surviving Sepsis campaign guidelines recommend an initial target mean arterial pressure (MAP) of 65 mmHg to maintain critical organ perfusion (5). This goal is a reasonable endpoint to maintain hemodynamic stability, but the effects of further adjustment might be variable depending on individual characteristics. In some small studies, when norepinephrine was titrated to meet a target of MAP 65 mmHg or higher, the cardiac index was increased in the higher MAP target group, but there was no difference in urinary flow, arterial lactate level, or oxygen delivery or consumption between the two groups (10-12). In a recent pilot study of a multicenter trial, the hospital mortality rate of the MAP target 60 to 65 mmHg group was lower than that of the 75–80 mmHg group in patients older than 75 years (13).
On the other hand, in a single center study, microcirculation assessed by sublingual microscopy and thenar oxygen saturation was improved when norepinephrine was titrated to MAP 85 mmHg compared to 65 mmHg in patients with previous hypertension (14). In a multicenter randomized trial comparing a low MAP target (65 to 70 mmHg) and a high MAP target (80 to 85 mmHg), the need for renal replacement therapy in the higher MAP group was significantly lower in cases of previous chronic hypertension by subgroup analysis (15). Despite this beneficial effect, there were more adverse effects such as arrhythmia in the higher target group, and there was no difference in mortality between groups.
Given these findings from previous studies, maintenance of MAP at lower or higher levels might result in different outcomes depending on previous conditions in septic shock patients, including chronic hypertension or previous BP. Personalization of an optimal MAP target is an important resuscitation strategy that requires further research, and these clinical conditions need to be considered. In this study, we aimed to investigate the relationship between average MAP during the first 24 hours of treatment and 28-day mortality in critically ill sepsis patients presenting to the emergency department (ED) according to the presence of chronic hypertension and previously measured BP.
Methods
Study design and population
This was a single-center, retrospective study of a convenience sample of patients who presented with severe sepsis or septic shock to the ED at Samsung Medical Center (a 1,960 bed, university-affiliated, tertiary referral hospital located in a metropolitan city with an annual census of 70,000), from August 2008 through September 2014.
Adult patients who met the criteria for severe sepsis and septic shock during their ED stay were registered for data collection and quality improvement activity in our institution’s registry, under an educational program called the Emergency Approach to Sepsis Treatment (EAST), which began in 2008 to highlight early recognition and timely management of severe sepsis and septic shock. Our previous studies regarding severe sepsis or septic shock have used the same registry data (16-19). The institutional review board of Samsung Medical Center approved this study (No. 2018-07-024). Informed consent was waived because this study was of a retrospective, observational, and anonymous nature. We included patients older than 18 years who presented to the ED with severe sepsis or septic shock. Severe sepsis was defined as acute organ dysfunction associated with sepsis according to the 2001 international sepsis definition (20). Septic shock was defined as acute circulatory failure (persistent arterial hypotension-systolic arterial pressure <90 mmHg, MAP <60 mmHg, or a reduction in systolic BP >40 mmHg from baseline) despite adequate fluid resuscitation (20). Patients who were younger than 18 years old, who had a terminal malignancy with poor performance, who were unresponsive to chemotherapy or radiation therapy, or who had set limitations on invasive care (e.g., patients who had previously signed a do-not-resuscitate order) were excluded from this study.
The overall treatment of patients with sepsis and septic shock was based on the Surviving Sepsis Campaign Guidelines (5). The primary hemodynamic target of resuscitation was maintenance of MAP ≥65 mmHg and lactate normalization by fluid challenge and vasopressor infusion. There was no protocol to adjust specific MAP targets in patients with hypertension or high BP values.
Data collection and outcome measurement
To identify eligible patients based on the inclusion and exclusion criteria, the research coordinator periodically screened electronic medical records using the preset criteria and also reviewed the preliminary patient list of those suspected of having severe sepsis or septic shock during an ED stay, as reported by treating ED physicians. After cases were identified, demographic and clinical data were abstracted from medical records by a single research coordinator.
Initial laboratory tests in the ED and survival data were electronically extracted from the hospital database. For patients discharged from the hospital alive prior to the 28th day of hospitalization, we used the patients’ visiting history after discharge, mortality data provided by Statistics Korea, and telephone interviews. To estimate disease severity, full Sequential Organ Failure Assessment (SOFA) scores were calculated at the time of diagnosis in the ED.
All BP values at each hour during the first 24 hours after ED arrival were extracted and treated as a missing value if there was no record. The average MAP was calculated using the sum of MAP divided by the number of hourly BP measurements during the 24 hours. Previously measured BPs were extracted from the electronic medical record at previous outpatient visits and at the time of discharge from ED or general ward admissions during the six months prior to the ED visit with a sepsis diagnosis. In the analysis using previously measured BP, only patients who had a previous BP recorded twice or more were included.
According to the average MAP (a-MAP) during the initial 24 hours, patients were categorized into four groups (group 0, a-MAP <65 mmHg; group 1, 65 mmHg ≤ a-MAP <75 mmHg; group 2, 75 mmHg ≤ a-MAP <85 mmHg; group 3, a-MAP ≥85 mmHg). Patients whose BPs were measured twice or more in the past six months were divided into two groups according to a-MAP of the extracted previous BP. The low previous BP group was defined as a previous a-MAP ≤85 mmHg, and the high previous BP group was defined as an a-MAP >85 mmHg, derived from the median MAP value of previous BP readings. The primary outcome of this study was 28-day mortality.
Statistical analysis
Standard descriptive statistics were analyzed for all variables. The results are presented as mean ± standard deviation (SD) for continuous variables and as the number of patients with percentage for categorical data. Continuous and categorical variables were analyzed by Student’s t-test, ANOVA test, and Chi-square test, as appropriate. For multiple comparisons, P values were adjusted by the Bonferroni correction. To evaluate associations between 28-day mortality and MAP groups according to the presence of previous hypertension or between previous BP groups, multivariable logistic regression analyses were performed using variables of age, gender, initial SOFA score, initial lactate level, infection source, comorbidities (selected by a cut-off of P value <0.05) and the use of vasopressors. The results are described as the odds ratio (OR) and 95% confidence interval (CI). Statistical significance was defined by a P value <0.05. STATA 14.2 software (STATA Corporation, College Station, TX, USA) was used for statistical analysis.
Results
Baseline characteristics
During the study period, a total of 1,728 patients with severe sepsis or septic shock were registered in the sepsis registry. Of these, 333 patients were excluded due to terminal malignancy (n=160), signed DNR order, or refusal of invasive procedures (n=173). A total of 1,395 patients were included in the final analysis. The baseline characteristics of the 28-day survival and non-survival groups are summarized in Table 1. The overall 28-day mortality rate was 15.1%. Overall, the mean age was 63.0±14.2 years, and the proportion of males was 56.4%. The most frequent infection source was pneumonia, followed by intra-abdominal and urinary tract infection. In patients overall, the a-MAP during the initial 24 hours was 77.2±8.7 mmHg, and the average number of extracted BP values each hour from the hospital database was 16.4±6.2. In the survival group, the 24-hour a-MAP was higher than in the non-survival group (77.7±8.5 versus 74.3±9.4 mmHg).
Table 1. Baseline characteristics of overall, survival, and non-survival groupsa.
| Characteristic | Overall (n=1,395) | Survival (n=1,184) | Non-survival (n=211) | P |
|---|---|---|---|---|
| Age | 63.0±14.2 | 62.9±14.4 | 64.1±13.7 | 0.271 |
| Male sex, No. (%) | 787 (56.4) | 667 (56.3) | 120 (56.9) | 0.885 |
| Initial SOFA score | 6.6±3.4 | 6.3±3.2 | 9.1±3.7 | <0.001 |
| Preexisting conditions, No. (%) | ||||
| Hypertension | 466 (33.4) | 402 (34.0) | 64 (30.3) | 0.304 |
| Diabetes | 321 (23.0) | 271 (22.9) | 50 (23.7) | 0.797 |
| Chronic heart disease | 152 (10.9) | 121 (10.2) | 31 (14.7) | 0.055 |
| Chronic lung disease | 80 (5.7) | 66 (5.6) | 14 (6.6) | 0.542 |
| Liver cirrhosis | 90 (6.4) | 66 (5.6) | 24 (11.4) | 0.002 |
| Chronic renal disease | 73 (5.2) | 63 (5.3) | 10 (4.7) | 0.727 |
| Metastatic malignancy | 325 (23.3) | 253 (21.4) | 72 (34.1) | <0.001 |
| Infection source, No. (%) | ||||
| Lung | 514 (36.8) | 409 (34.5) | 105 (49.8) | <0.001 |
| Abdomen | 476 (34.1) | 420 (35.5) | 56 (26.5) | 0.012 |
| Urinary tract | 172 (12.3) | 166 (14.0) | 6 (2.8) | <0.001 |
| Others | 233 (16.7) | 189 (16.0) | 44 (20.9) | 0.079 |
| Hemodynamic variables | ||||
| Initial MAP (mmHg) | 77.7±21.1 | 78.5±21.1 | 72.7±20.4 | <0.001 |
| Average of MAP over 24 hours (mmHg) | 77.2±8.7 | 77.7±8.5 | 74.3±9.4 | <0.001 |
| Heart rate (bpm) | 113.2±24.8 | 112.7±24.9 | 116.3±24.4 | 0.052 |
| Serum lactate level (mmol/L) | 4.7±2.9 | 4.4±2.5 | 6.5±4.2 | <0.001 |
| Fluid therapy within 6 hr (Ml) | 2,551±1,480 | 2,518±1,460 | 2,740±1,579 | 0.045 |
| Vasoactive drug infusions, No. (%) | ||||
| Norepinephrine | 853 (61.1) | 693 (58.5) | 160 (75.8) | <0.001 |
| Others | 238 (17.1) | 151 (12.8) | 87 (41.2) | <0.001 |
| Mechanical ventilation, No. (%) | 119 (8.5) | 70 (5.9) | 49 (23.2) | <0.001 |
| Culture growth, No. (%) | 609 (43.6) | 510 (43.1) | 99 (46.9) | 0.299 |
| RRT, No. (%) | 136 (9.7) | 74 (6.3) | 62 (29.4) | <0.001 |
a, values are mean ± SD. SOFA, Sequential Organ Failure Assessment; MAP, mean arterial pressure; RRT, renal replacement therapy.
There were 68 patients in MAP group 0, 519 in group 1, 593 in group 2, and 215 in group 3. The baseline characteristics between the MAP groups are summarized in Table 2. Overall 28-day mortality rates in the MAP groups were 39.7% in group 0, 18.3% in group 1, 10.1% in group 2, and 13.4% in group 3 (P<0.001).
Table 2. Baseline characteristics of MAP groupsa.
| Characteristic | MAP | P | |||
|---|---|---|---|---|---|
| Group 0 (n=68) | Group 1 (n=519) | Group 2 (n=593) | Group 3 (n=215) | ||
| 28-day mortality rate (%) | 27 (39.7) | 95 (18.3) | 60 (10.1) | 29 (13.4) | <0.001 |
| Average of 24 hr MAP* | 61.0±3.7 | 71.0±2.6 | 79.1±2.7 | 92.0±7.0 | |
| Age | 59.8±15.4 | 62.8±15.5 | 63.4±13.0 | 63.6±13.6 | 0.231 |
| Male sex, No. (%) | 37 (54.4) | 296 (57.0) | 327 (55.1) | 127 (59.1) | 0.754 |
| Initial SOFA score | 8.6±3.7 | 7.1±3.3 | 6.6±3.2 | 5.0±3.2 | <0.001 |
| Preexisting conditions, No. (%) | |||||
| Hypertension | 14 (20.6) | 162 (31.2) | 202 (34.1) | 88 (40.9) | 0.008 |
| Diabetes | 14 (20.6) | 114 (22.0) | 141 (23.8) | 52 (24.2) | 0.823 |
| Chronic heart disease | 13 (19.1) | 56 (10.8) | 62 (10.5) | 21 (9.8) | 0.162 |
| Chronic lung disease | 4 (5.9) | 29 (5.6) | 34 (5.7) | 13 (6.0) | 0.996 |
| Liver cirrhosis | 9 (13.2) | 41 (7.9) | 30 (5.1) | 10 (4.7) | 0.018 |
| Chronic renal disease | 4 (5.9) | 34 (6.6) | 21 (3.5) | 14 (6.5) | 0.111 |
| Metastatic malignancy | 14 (20.6) | 105 (20.2) | 156 (26.3) | 50 (23.3) | 0.111 |
a, group category: group 0, a-MAP <65 mmHg; group 1, 65 mmHg ≤ a-MAP <75 mmHg; group 2, 75 mmHg ≤ a-MAP <85 mmHg; group 3, a-MAP ≥85 mmHg. MAP, mean arterial pressure; SOFA, Sequential Organ Failure Assessment.
28-day mortality in MAP groups according to history of chronic hypertension
Among patients without previous chronic hypertension, the 28-day mortality in group 0 was 44.4%, which was significantly higher than the other groups (group 1, 17.3%; group 2, 11.5%; group 3, 12.6%). In contrast, among patients with previous chronic hypertension, group 0 (21.4%) and group 1 (20.3%) had similar mortality rates, and group 2 (7.4%) experienced significantly lower mortality rates compared with groups 1 and 3 (Figure 1).
Figure 1.
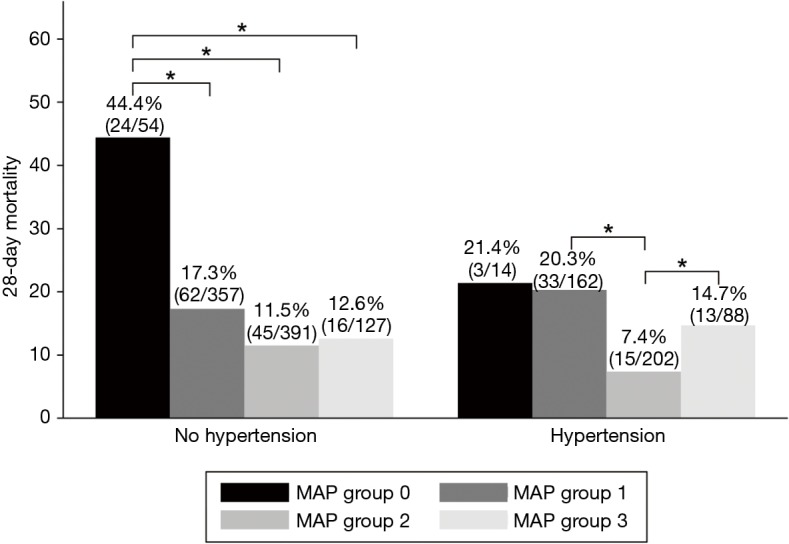
The 28-day mortality according to MAP group and the presence of chronic hypertension (*P<0.05). MAP, mean arterial pressure.
In the multivariable logistic regression analysis, in patients without chronic hypertension, 28-day mortality of group 0 was significantly increased compared to group 1 (OR, 2.80, 95% CI: 1.35–5.80, P=0.006). In contrast, in patients with chronic hypertension, there was no significant difference of 28-day mortality of Group 0 and Group 1 (OR, 1.16, 95% CI: 0.26–5.10, P=0.843) (Tables 3,S1). In patients with chronic hypertension, 28-day mortality of group 2 was significantly reduced compared to group 1 (OR, 0.33, 95% CI: 0.16–0.70, P=0.004), whereas in patients without chronic hypertension, there was no significant difference of 28-day mortality of group 1 and group 2 (OR, 0.66, 95% CI: 0.41–1.05, P=0.085). With reference to group 2 in the same regression model, Group 3 was significantly associated with higher 28-day mortality (OR, 3.16, 95% CI: 1.27–7.86, P=0.013).
Table 3. Odds ratio for 28-day mortality according to MAP group and the presence of chronic hypertension.
| Subgroup | Unadjusted OR | 95% CI | P | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|---|
| No hypertension | ||||||
| MAP group 0a | 3.80 | (2.08–6.95) | <0.001 | 2.80 | (1.35–5.80) | 0.006 |
| MAP group 1 | Refb | Base | ||||
| MAP group 2 | 0.61 | (0.40–0.93) | 0.023 | 0.66 | (0.41–1.05) | 0.085 |
| MAP group 3 | 0.68 | (0.37–1.23) | 0.211 | 1.05 | (0.53–2.09) | 0.876 |
| Chronic hypertension | ||||||
| MAP group 0 | 1.06 | (0.28–4.04) | 0.925 | 1.16 | (0.26–5.10) | 0.843 |
| MAP group 1 | Ref | Base | ||||
| MAP group 2 | 0.31 | (0.16–0.60) | <0.001 | 0.33 | (0.16–0.70) | 0.004 |
| MAP group 3 | 0.67 | (0.33–1.36) | 0.277 | 1.07 | (0.43–2.63) | 0.880 |
a, group category: group 0, a-MAP <65 mmHg; group 1, 65 mmHg ≤ a-MAP <75 mmHg; group 2, 75 mmHg ≤ a-MAP <85 mmHg; group 3, a-MAP ≥85 mmHg; b, reference. MAP, mean arterial pressure; OR, odds ratio; CI, confidence interval.
28-day mortality in MAP groups according to history of previously measured BP
For the analysis using the previously measured BPs, a total of 1,126 patients were used after excluding patients with less than two BP measurements (n=269). The mean previously measured BP overall was 85.2±5.2 mmHg. The low previous BP group (below MAP 85 mmHg) had 588 cases with an average value of 78.3±3 mmHg, and the high previous BP group (above 85 mmHg) had 538 cases with an average value of 92.8±2.8 mmHg.
Overall 28-day mortality rates in the MAP groups were 40.3% in group 0, 19.7% in group 1, 10.7% in group 2, and 16.4% in group 3 (Figure 2). In the multivariable logistic regression analysis, in patients with high previous BP, 28-day mortality of group 2 was reduced compared to group 1 (OR, 0.32, 95% CI: 0.16–0.64, P=0.001), whereas in patients with low previous BP, there was no significant difference of 28-day mortality of group 1 and group 2 (OR, 0.62, 95% CI: 0.35–1.08, P=0.093). In patients with low previous BP, there was a significant increase in 28-day mortality in group 3 compared to group 1 (OR, 2.42, 95% CI: 1.01–5.76, P=0.046) and group 2 (OR, 3.88, 95% CI: 1.55–9.71, P=0.004). In patients with high previous BP, 28-day mortality of group 0 was significantly higher compared to group 1 (OR, 3.71, 95% CI: 1.04–13.19, P=0.042) and all other groups (Tables 4,S1).
Figure 2.
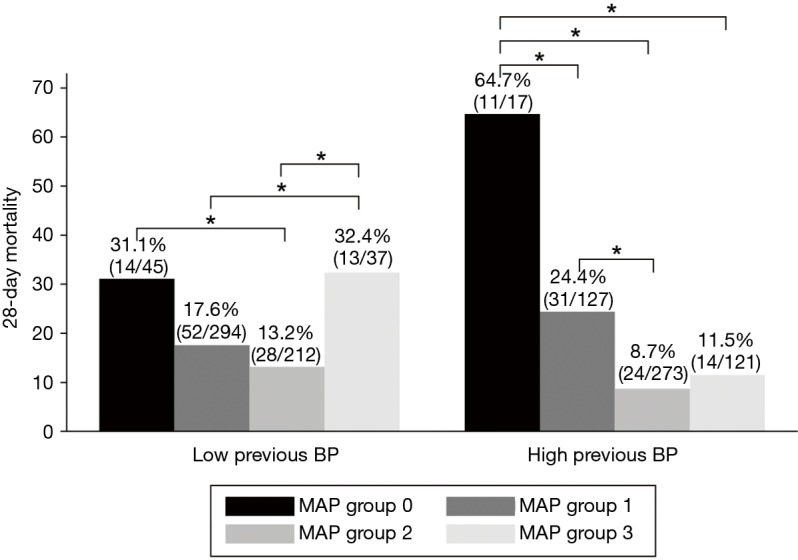
The 28-day mortality according to MAP group and previously measured blood pressure (*P<0.05). MAP, mean arterial pressure.
Table 4. Odds ratio for 28-day mortality according to MAP group and previously measured blood pressure.
| Subgroup | Unadjusted OR | 95% CI | P | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Low previous BPb | ||||||
| MAP group 0a | 2.10 | (1.04–4.22) | 0.037 | 1.56 | (0.67–3.58) | 0.293 |
| MAP group 1 | Ref | Base | ||||
| MAP group 2 | 0.70 | (0.43–1.16) | 0.174 | 0.62 | (0.35–1.08) | 0.093 |
| MAP group 3 | 2.23 | (1.05–4.73) | 0.036 | 2.42 | (1.01–5.76) | 0.046 |
| High previous BPc | ||||||
| MAP group 0 | 5.67 | (1.93–16.61) | 0.002 | 3.71 | (1.04–13.19) | 0.042 |
| MAP group 1 | Ref | Base | ||||
| MAP group 2 | 0.29 | (0.16–0.53) | <0.001 | 0.32 | (0.16–0.64) | 0.001 |
| MAP group 3 | 0.40 | (0.20–0.80) | 0.010 | 0.71 | (0.30–1.68) | 0.439 |
a, group category: group 0, a-MAP <65 mmHg; group 1, 65 mmHg ≤ a-MAP <75 mmHg; group 2, 75 mmHg ≤ a-MAP <85 mmHg; group 3, a-MAP ≥85 mmHg; b, the average MAP of previously measured blood pressure was less than 85 mmHg; c, the average MAP of previously measured blood pressure was 85 mmHg or more. MAP, mean arterial pressure; OR, odds ratio; CI, confidence interval.
Discussion
MAP is the main hemodynamic variable indicating the driving pressure for organ perfusion. MAP higher than 65 mmHg is a reasonable goal during initial resuscitation, but it may be necessary to individualize the MAP target according to changes in basal physiology depending on age and comorbidity. We observed some different trends in mortality according to a-MAP as measured during the first 24 hours of treatment depending on the presence of chronic hypertension or previous BP in critically ill sepsis patients presenting to the ED.
In this study, the prognosis was more favorable when the MAP was maintained at a relatively high level (75 mmHg ≤ a-MAP <85 mmHg) in patients with a history of chronic hypertension or with previously measured high BP. The patient's physiology, which was altered by arterial hypertension, may not be normalized even if the BP is subsequently controlled (21). Alternately, since the BP of patients diagnosed with chronic hypertension has not been well controlled, it is likely that similar results were obtained in both cases. Our results support findings from the SEPSISPAM study that a high target MAP (80 to 85 mmHg) may reduce the incidence of doubling of serum creatinine level and need for renal replacement therapy in patients with chronic hypertension (15), as well as a study from Xu et al. (12) showing that increasing MAP from 65 mmHg to normal level was associated with improved microcirculation in hypertensive septic shock patients.
On the other hand, higher MAP targets might be harmful (13,22). We also found that patients who maintained an a-MAP above 85 mmHg had higher mortality among those with chronic hypertension or relatively lower previous BP. Possible causes might be excessive vasopressor use, excessive adrenergic response of sepsis patients, and other compounding factors such as underlying diseases. Considering the results from previous studies and the present study, we suggest that increasing MAP 5–10 mmHg from 65 mmHg could be beneficial in selected patients, but excessive augmentation to more than 85 mmHg might cause adverse effects.
Previous studies have shown that there is a physiological change, such as right shift of brain autoregulation, in patients exposed to persistent arterial hypertension in chronic hypertension (8,23,24). Several studies have observed that maintaining MAP at a target higher than 65 may help preserve renal function (8,10), but there is no evidence of a higher survival rate with higher MAP (15). It is unclear whether these physiological changes in patients do not affect the mortality rate or because the physiological changes in the patient are normalized as the BP is controlled by the medication for hypertension (21,25). Accordingly, there was a need to evaluate the actual BP of the patient separately from any history of hypertension. We planned this analysis to see if it could be used as an index to individualize the MAP target if previous BP measurements were available in patients being managed for severe sepsis or septic shock.
The results of this study alone are not sufficient to suggest that the MAP target of septic shock should be individualized according to previous history of hypertension and previously measured BP. However, it seems relatively clear that individual MAP targets may need to be set, as comorbidity and physiology are different for each patient. Various patient factors may affect the prognosis and may interact with other factors. Further well-designed studies are needed to individualize MAP targets.
There are some limitations to this study that should be considered. First, this was a single-center study conducted in the ED of a tertiary care academic center. Therefore, it was subject to selection bias and may not be generalizable to other settings. Second, this study was retrospectively conducted with reference to the electronic medical record. The a-MAP values were not actual goals of resuscitation but rather observed values. It is difficult to say that BP was well maintained around the target MAP of each group because average values of MAPs measured at intervals of one hour were used for grouping. Therefore, the causal relationship between MAP and outcome may be limited. Third, only the first 24 hours of MAP were used for analysis and the effects of subsequent MAP were not considered. Fourth, the capacities of the vasopressor and possible adverse effects were not considered. Fifth, in this study, BP before the hypotensive event was included in the analysis. However, we conclude that the bias was not significant because the results were similar in the additional analysis of patients who were first enrolled by septic shock. Sixth, we need a further comparative analysis of more detailed subgroups to find clinical differences and causal factors associated with these results.
Conclusions
Mortality trends according to a-MAP during the first 24 hours of treatment varied depending on the presence of chronic hypertension or previous BP in critically ill sepsis patients. Compared with a 24-hour a-MAP ≥65 mmHg and <75 mmHg, patients with an a-MAP ≥75 mmHg and <85 mmHg showed better survival rates, particularly in patients with chronic hypertension or relatively higher previous BP. However, a 24-hour a-MAP ≥85 mmHg was associated with higher mortality in patients with chronic hypertension or relatively lower previous BP. These findings suggest that target MAP needs to be individualized in selected patients.
Table S1. Multivariable regression models for 28-day mortality according to MAP groupa.
| Characteristic & subgroup | No chronic hypertension group | Chronic hypertension group | Low previous BP group | High previous BP group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||||
| Age ≥75 years | 1.06 (0.57–1.96) | 0.836 | 1.44 (0.76–2.70) | 0.257 | 0.79 (0.40–1.55) | 0.506 | 2.31 (1.14–4.69) | 0.020 | |||
| Gender (male) | 0.57 (0.37–0.87) | 0.009 | 0.89 (0.47–1.71) | 0.746 | 0.55 (0.33–0.91) | 0.022 | 0.91 (0.50–1.66) | 0.774 | |||
| Initial SOFA score | 1.29 (1.19–1.40) | <0.001 | 1.16 (1.04–1.29) | 0.006 | 1.25 (1.14–1.37) | <0.001 | 1.31 (1.18–1.47) | <0.001 | |||
| Initial lactate level | 1.17 (1.10–1.25) | <0.001 | 1.16 (1.06–1.26) | <0.001 | 1.19 (1.10–1.29) | <0.001 | 1.16 (1.07–1.27) | <0.001 | |||
| Preexisting conditions | |||||||||||
| Liver cirrhosis | 1.13 (0.56–2.30) | 0.722 | 1.17 (0.32–4.17) | 0.807 | 1.46 (0.61–3.51) | 0.393 | 0.64 (0.21–1.94) | 0.440 | |||
| Metastatic cancer | 2.65 (1.70–4.13) | <0.001 | 2.25 (1.10–4.58) | 0.025 | 1.99 (1.19–3.30) | 0.008 | 2.95 (1.58–5.51) | 0.001 | |||
| Infection sourceb | |||||||||||
| Lung | 1.94 (1.17–3.15) | 0.007 | 3.05 (1.41–6.41) | 0.005 | 2.58 (1.42–4.71) | 0.002 | 1.41 (0.74–2.68) | 0.292 | |||
| Abdomen | Refb | Ref | Ref | Ref | |||||||
| UTI | 0.16 (0.03–0.69) | 0.014 | 0.51 (0.13–1.93) | 0.324 | 0.27 (0.06–1.27) | 0.103 | 0.22 (0.05–0.91) | 0.037 | |||
| Others | 1.42 (0.79–2.53) | 0.235 | 2.35 (0.92–6.00) | 0.072 | 1.90 (0.82–3.88) | 0.078 | 0.92 (0.39–2.14) | 0.848 | |||
| Vasoactive drug | 0.66 (0.38–1.17) | 0.160 | 2.04 (0.80–5.18) | 0.130 | 0.78 (0.40–1.52) | 0.475 | 0.77 (0.35–1.72) | 0.538 | |||
| MAP group | |||||||||||
| Group 0 | 2.80 (1.35–5.80) | 0.006 | 1.16 (0.26–5.10) | 0.843 | 1.56 (0.67–3.58) | 0.293 | 3.71 (1.04–13.19) | 0.042 | |||
| Group 1 | Ref | Ref | Ref | Ref | |||||||
| Group 2 | 0.66 (0.41–1.05) | 0.085 | 0.33 (0.16–0.70) | 0.004 | 0.62 (0.35–1.08) | 0.093 | 0.32 (0.16–0.64) | 0.001 | |||
| Group 3 | 1.05 (0.53–2.09) | 0.876 | 1.07 (0.43–2.63) | 0.880 | 2.42 (1.01–5.76) | 0.046 | 0.71 (0.30–1.68) | 0.439 | |||
a, group category: group 0, a-MAP <65 mmHg; group 1, 65 mmHg ≤ a-MAP <75 mmHg; group 2, 75 mmHg ≤ a-MAP <85 mmHg; group 3, a-MAP ≥85 mmHg; b, reference value in analysis. SOFA, Sequential Organ Failure Assessment; UTI, urinary tract infection; MAP, mean arterial pressure; OR, odds ratio; CI, confidence interval.
Acknowledgments
None.
Ethical Statement: The study was approved by institutional review board of Samsung Medical Center approved this study (No. 2018-07-024). Informed consent was waived because this study was of a retrospective, observational, and anonymous nature.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840-51. 10.1056/NEJMra1208623 [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Pereira AJ, Gleeson J, et al. Early management of sepsis. Clin Exp Emerg Med 2014;1:3-7. 10.15441/ceem.14.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin TG, Hwang SY, Kang GH, et al. Korean Shock Society septic shock registry: a preliminary report. Clin Exp Emerg Med 2017;4:146-53. 10.15441/ceem.17.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486-552. 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. 10.1056/NEJMoa010307 [DOI] [PubMed] [Google Scholar]
- 7.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. 10.1056/NEJMoa1500896 [DOI] [PubMed] [Google Scholar]
- 8.Dunser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med 2009;35:1225-33. 10.1007/s00134-009-1427-2 [DOI] [PubMed] [Google Scholar]
- 9.Badin J, Boulain T, Ehrmann S, et al. Relation between mean arterial pressure and renal function in the early phase of shock: a prospective, explorative cohort study. Crit Care 2011;15:R135. 10.1186/cc10253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourgoin A, Leone M, Delmas A, et al. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med 2005;33:780-6. 10.1097/01.CCM.0000157788.20591.23 [DOI] [PubMed] [Google Scholar]
- 11.LeDoux D, Astiz ME, Carpati CM, et al. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000;28:2729-32. 10.1097/00003246-200008000-00007 [DOI] [PubMed] [Google Scholar]
- 12.Xu JY, Ma SQ, Pan C, et al. A high mean arterial pressure target is associated with improved microcirculation in septic shock patients with previous hypertension: a prospective open label study. Crit Care 2015;19:130. 10.1186/s13054-015-0866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamontagne F, Meade MO, Hebert PC, et al. Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med 2016;42:542-50. 10.1007/s00134-016-4237-3 [DOI] [PubMed] [Google Scholar]
- 14.Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care 2011;15:R222. 10.1186/cc10462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014;370:1583-93. 10.1056/NEJMoa1312173 [DOI] [PubMed] [Google Scholar]
- 16.Hwang SY, Jo IJ, Lee SU, et al. Low Accuracy of Positive qSOFA Criteria for Predicting 28-Day Mortality in Critically Ill Septic Patients During the Early Period After Emergency Department Presentation. Ann Emerg Med 2018;71:1-9.e2. 10.1016/j.annemergmed.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 17.Joo YM, Chae MK, Hwang SY, et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency department. Clin Exp Emerg Med 2014;1:35-40. 10.15441/ceem.14.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J, Hwang SY, Jo IJ, et al. Impact of Metformin Use on Lactate Kinetics in Patients with Severe Sepsis and Septic Shock. Shock 2017;47:582-7. 10.1097/SHK.0000000000000782 [DOI] [PubMed] [Google Scholar]
- 19.Shin TG, Jo IJ, Hwang SY, et al. Comprehensive Interpretation of Central Venous Oxygen Saturation and Blood Lactate Levels During Resuscitation of Patients With Severe Sepsis and Septic Shock in the Emergency Department. Shock 2016;45:4-9. 10.1097/SHK.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8. 10.1007/s00134-003-1662-x [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Witkowski S, Fu Q, et al. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension 2007;49:1149-55. 10.1161/HYPERTENSIONAHA.106.084939 [DOI] [PubMed] [Google Scholar]
- 22.Lamontagne F, Day AG, Meade MO, et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 2018;44:12-21. 10.1007/s00134-017-5016-5 [DOI] [PubMed] [Google Scholar]
- 23.Strandgaard S, Olesen J, Skinhoj E, et al. Autoregulation of brain circulation in severe arterial hypertension. Br Med J 1973;1:507-10. 10.1136/bmj.1.5852.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Immink RV, van den Born BJ, van Montfrans GA, et al. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation 2004;110:2241-5. 10.1161/01.CIR.0000144472.08647.40 [DOI] [PubMed] [Google Scholar]
- 25.Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension 2005;45:216-21. 10.1161/01.HYP.0000153094.09615.11 [DOI] [PubMed] [Google Scholar]


