Abstract
Background
To evaluate the influence of tumor depth on preoperative computed tomography (CT) image, and resection margin length on local recurrence after pulmonary metastasectomy of colorectal cancer.
Methods
Patients undergoing thoracoscopic pulmonary wedge resection for single pulmonary metastasis of colorectal cancer origin from 2007 to 2017 were analyzed. Factors such as resection margin, tumor size and depth were analyzed. The local recurrences of two subgroups based on the pulmonary resection margin (Group 1: resection margin 1–10 mm or shorter than the tumor size, Group 2: resection margin >10 mm or at least greater than the tumor size) were analyzed.
Results
Sixty-five patients were included in this study. The local recurrence rate was 12/65 (18.5%). Median follow up period was 33 months. Median tumor size and depth on preoperative CT were 1.1 and 1.6 cm. Median length of resection margin was 0.5 cm (group 1: 0.4 cm, group 2: 1.0 cm, P<0.001). No difference was noted in 3-year local recurrence-free survival (80.8% vs. 76.7%, P=0.756) between the two subgroups. No significant correlation was noted between the length of resection margin and the tumor size and depth. However, tumor depth was an independent factor related to higher local recurrence on multivariate analysis.
Conclusions
Extent of resection margin in pulmonary metastasectomy does not seem to affect significantly on the local recurrence if complete resection is accomplished. However, preoperative tumor depth on CT image and postoperative distant metastasis seem to affect on local recurrence after pulmonary metastasectomy.
Keywords: Pulmonary metastasis, colorectal cancer, video-assisted thoracoscopic surgery (VATS), recurrence
Introduction
Pulmonary metastasectomy is considered as a potentially curative therapy and is widely being practiced worldwide as a treatment of colorectal origin lung metastasis (1-3). Unlike in primary lung cancers, wedge resection is the most common procedure used for excision (4). However, a major problem with wedge resection is the risk of local recurrence, especially at the recurrence margin. And, the local recurrence at the surgical margins which may suggest a failure in previous operation regarding the complete tumor resection, still remains a problem to be solved.
Several prognostic factors related to survival and tumor recurrence have been proposed (5-12). Among them, the completeness of tumor resection is considered as one of the main prognostic factors affecting the survival and tumor recurrence. Obviously, anatomical pulmonary resections like segmentectomy or even lobectomy showed better results with post-metastasectomy local recurrence when compared to wedge resection (4,13,14). However, colorectal cancer lung metastasis shows a high recurrence rate after resection and about 28% of these patients had recurrent pulmonary metastasectomies (15). Therefore, balance between adequate resection for preventing recurrence and limited resection to preserve pulmonary function for a possible additional pulmonary resection is required.
The selection of surgical technique for pulmonary metastasectomy is mainly decided based on the surgeon’s experience and the location of tumor on the preoperative computed tomography (CT) image. For a complete resection of metastatic tumor, Rusch et al. proposed a safety margin of 5 to 10 mm (16) and Higashiyama et al. more than 10, or even 20 mm, if possible (13). Also, Welter et al. suggested a safety distance of more than 7 mm in order to completely remove the satellite tumor cells and aerogenous spread of floating cancer cell clusters surrounding the metastatic nodule (15,17). Shiono et al. suggested wedge resection for peripheral lung nodules, and segmentectomy for more central lesions (4). Obviously, small nodules located at peripheral location near the visceral pleura usually does not become a problem. However, it is unclear whether the wedge resection might be sufficient to achieve complete tumor removal if the tumor size is larger or located at a borderline area between peripheral and central locations.
Therefore, our goal in this study was to clarify the safeness and feasibility of simple wedge resection in completely resecting the tumors with certain sizes and depth. To do so, we analyzed the prognostic significance of resection margin on the local recurrence by evaluating the impact of resection margin width, which we defined as the shortest distance between the tumor and the resection line, on local recurrence after the curative resection of colorectal origin single pulmonary metastasis. Also, we tried to evaluate the effects of tumor depth and size on the resection margin which subsequently may affect the local recurrence.
Methods
Inclusion and exclusion criteria
Patients undergoing pulmonary wedge resection for pulmonary metastasis of colorectal cancer origin from 2007 to 2017 were analyzed. Inclusion criteria for pulmonary metastasectomy was complete resection or control of primary colorectal cancer, no uncontrollable extrapulmonary metastasis. Additionally, only those patients with single metastatic nodules without mediastinal lymph node metastasis were included. Patients with insufficient medical or radiological data, or those who had metastasectomy for recurrent pulmonary metastasis were excluded from this study.
Data collection
Data including the patients’ baseline characteristics, treatment modalities, and survival results were obtained from the medical records. Preoperative image data and pathological data regarding the tumor size, depth, and resection margin were obtained from the preoperative chest CT scans and pathological reports. Preoperative CT scans were performed within one month before the operation, and all the data from the preoperative chest CT scans were based on the radiologist’s official report.
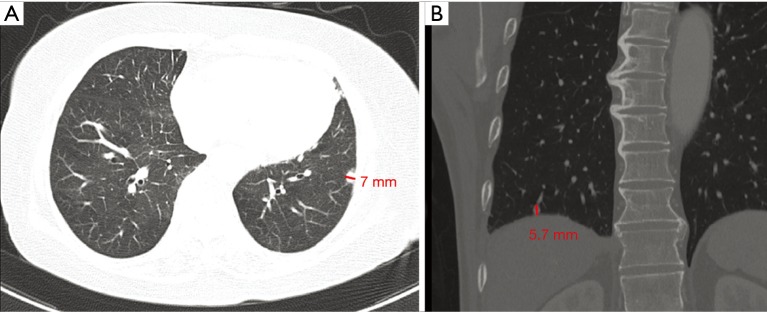
Tumor size was defined as the diameter of the tumor’s longest axis. And, tumor depth was defined as the longest perpendicular distance between the tumor’s deepest margin and the visceral pleura on either the axial or coronal view of the chest CT image (Figure 1). To have a uniform measurement of the tumor size and depth, they were all measured only from the chest CT and not positron emission tomography (PET) CT.
Figure 1.
Measurement of the tumor depth. The tumor depth (red line) was defined as the longest perpendicular distance between the tumor’s deepest margin and the visceral pleura on the CT image, including both the (A) axial and (B) coronal view. CT, computed tomography.
The achievement of complete resection was decided by the pathologist, and it was defined as the non-existence of tumor cell at the resected margin including the stapled line. Resection margin width was defined as the shortest distance between the tumor and the resected margin including the stapled line.
Postoperative follow up was performed using the chest CT scan taken every 6 months at the outpatient clinic by the either the colorectal surgeon or the oncologist.
Local recurrence was either biopsy proven or diagnosed based on the radiologist’s official report of postoperative CT image. Local recurrence was defined as the development of new tumor or contrast enhancement within or adjacent to the previous stapled zone suggesting tumor recurrence. Regional recurrence which was defined as the development of new tumor at the ipsilateral lungs or ipsilateral hilar and mediastinal lymph nodes, and distant metastasis which included the recurrence in the supraclavicular lymph nodes, contralateral hilar lymph nodes, and separate tumor nodules in contralateral lobes were not included as local recurrences. And, lesions suspicious for local recurrence which appeared simultaneously or after the appearance of regional recurrence or distant metastasis were also not included in the local recurrence.
Surgical strategy and technique
After a thorough preoperative planning through chest CT scan, video-assisted thoracoscopic surgery (VATS) was performed under general anesthesia with single lung ventilation and the patient in the lateral decubitus position. Depending on the preoperative chest X-ray or chest CT findings, three thoracoports were made with 1.0 to 2.0 cm incisions. After locating the tumor and evaluating the surgical margin through manual palpation under the 10 mm thoracoscopic guidance, non-anatomical wedge resection of lung including metastatic nodule was performed using the Endo staplers. In cases where wedge resection is not sufficient to achieve a complete resection of the tumor or if the tumor is too deeply located near hilar bronchus or vessels, we decide intraoperatively to perform segmentectomy or lobectomy. Completeness of resection was confirmed with intraoperative frozen biopsy. Twenty-four or 28 Fr chest tube was inserted and wound was closed in a routine manner.
Groups and outcome measurements
Patient characteristics and perioperative results were recorded for the locally recurred and non-recurred patients. And, the possible associating factors were analyzed through univariable and multivariable analysis.
To focus on the correlation between the resection margin and the local recurrence, the patients were divided into two subgroups based on the pulmonary resection margin reported on the pathologic reports. Group 1 included those with resection margin width between 1 to 10 mm or shorter than the tumor size. Group 2 included those with resection margin width greater than 10 mm or at least greater than the tumor size.
The following parameters were recorded and included in the statistical analysis: age, sex, size and depth of the metastatic nodule, number of resected pulmonary metastatic nodules, disease-free interval (DFI) between primary cancer resection and first identification of pulmonary metastasis, visceral pleura involvement, thoracic lymph node involvement, surgical approach (VATS or open thoracotomy), preoperative serum carcinoembryonic antigen (CEA) level, use of post-colorectal surgery chemotherapy, complication, mortality, postoperative follow period, and tumor recurrence.
The Institutional Review Board of the Korea University Anam Hospital approved this study and waived the requirement for informed consent (IRB Number: 2018AN0354).
Statistical analysis
Continuous and binary variables of group 1 and group 2 such as age, sex, size and depth of the metastatic nodule, number of resected pulmonary metastatic nodules, DFI between primary cancer resection and identification of pulmonary metastasis, visceral pleura involvement, thoracic lymph node involvement, surgical approach (VATS or open thoracotomy), preoperative serum CEA level, use of post-colorectal surgery chemotherapy, complication, mortality, postoperative follow period, and tumor recurrence were analyzed and compared to evaluate their influence on patient survival and recurrence after pulmonary metastasectomy. Univariable data analysis included independent sample t-tests or the Mann-Whitney test for quantitative variables and the χ2 test or Fisher’s exact test for binary data. The Kaplan-Meier method was used to plot patient recurrence curves. Multivariable analysis included Cox regression analysis using the stepwise method, and it included a total of nine factors such as the resection margin, tumor size, visceral pleura involvement, DFI, serum CEA level, tumor depth larger than 23 mm, distant metastasis, age, chemotherapy to evaluate the hazard ratios of each risk factors.
Data were analyzed using IBM SPSS Statistics Version 20 (IBM SPSS Software, Armonk, NY, USA) to evaluate the correlations between the preoperative factors and the postoperative tumor recurrence. Data are reported as means ± standard errors of the mean. A value of P <0.05 was considered statistically significant.
Results
Patient characteristics
Among the 186 pulmonary metastasectomy [153 (82.3%) wedge resections, 25 (13.4%) lobectomies, 8 (4.3%) segmentectomies] patients between 2007 and 2017, 65 patients who were diagnosed for their first detected single pulmonary metastasis and operated with wedge resection were included in this study. The median age of the patients was 58 years (range, 34–81 years) and 46 (70.8%) were male. All the patients were operated by unilateral VATS, and colorectum was proven to be the primary site on pathology. None of the patients showed mediastinal lymph node metastasis on preoperative chest CT scan and PET scan. Post-colorectal surgery adjuvant chemotherapy was administered to 51 (78.5%) patients. The median DFI between the primary colorectal cancer and first identification of pulmonary metastasis was 9 months (range, 0–110 months). And the median postoperative follow up period was 33 months (range, 7–112 months). The median preoperative serum CEA level was 1.0 ng/mL (range, 0–15 ng/mL), and the median tumor size and depth on preoperative CT image were 1.1 cm (range, 0.4–3.5 cm) and 1.6 cm (range, 0.5–4.4 cm), respectively. Complete tumor resection was achieved in all cases, and the overall median resection margin width was 0.5 cm (range, 0.1–2.5 cm). There was no postoperative mortality and only one (1.5%) morbidity, where an additional chest tube insertion was needed due to the postoperative subcutaneous emphysema in a patient with severe emphysematous lung. Data on baseline characteristics of each group are shown in Table 1.
Table 1. Patient characteristics.
| Variables | Group 1 | Group 2 | Total | P value |
|---|---|---|---|---|
| N (%) | 53 (81.5) | 12 (18.5) | 65 | |
| Age (year) | 59.30±10.52 | 57.00±12.40 | 58.88±10.82 | 0.510 |
| Sex (F: M), (%) | 16 (30.2):37 (69.8) | 3 (25.0):9 (75.0) | 19 (29.0):46 (71.0) | 1.000 |
| Resection margin (mm) | 4.09±1.97 | 11.33±4.74 | 5.43±3.88 | < 0.001 |
| Metastatic nodule size (largest diameter, mm) | 13.15±6.73 | 9.83±3.88 | 12.54±6.41 | 0.106 |
| Metastatic nodule depth (deepest length from periphery, mm) | 19.19±8.83 | 13.33±6.99 | 18.11±8.78 | 0.036 |
| Disease-free interval (month) | 18.75±20.94 | 9.17±12.28 | 16.98±19.9 | 0.133 |
| Serum CEA (ng/mL) | 2.25±2.39 | 2.75±2.77 | 2.34±2.45 | 0.524 |
| VATS (%) | 53 (100.0) | 12 (100.0) | 65 | – |
| Visceral pleura involvement (%) | 12 (22.6) | 6 (50.0) | 18 | 0.077 |
| Chemotherapy (%) | ||||
| Preoperative | 34 (64.2) | 10 (83.3) | 44 | 0.309 |
| Postoperative | 40 (75.5) | 11 (91.7) | 51 | 0.436 |
| Radiotherapy (%) | ||||
| Preoperative | 8 (15.1) | 2 (16.7) | 10 | 1.000 |
| Postoperative | 6 (11.3) | 2 (16.7) | 8 | 0.634 |
Group 1: patients with resection margin length between 1 to 10 mm or shorter than the tumor size; Group 2: patients with resection margin length greater than 10 mm or at least greater than the tumor size. F, female; M, male; CEA, carcinoembryonic antigen; VATS, video-assisted thoracoscopic surgery.
Recurrence and related factors
Post-metastasectomy intrathoracic tumor recurrence including local, regional, and distant multiple pulmonary metastasis occurred in 27 (41.5%) patients. The overall local recurrence rates were 12/65 (18.5%), 17 (26.2%) patients showed up with contralateral pulmonary metastasis, and 10 (15.4%) patients among them had multiple pulmonary nodules suggesting systemic hematogenous spreading.
While the median resection margin width of the two subgroups were different as expected (group 1: 0.4 cm, group 2: 1.0 cm, P<0.001), other variables such as tumor size, DFI, serum CEA level, visceral pleural invasion, use of post-colorectal surgery chemotherapy and radiotherapy did not show any significant difference. Only the median tumor depth of group 1 was larger compared to group 2 (16 vs. 12 mm, P=0.036). However, there was no difference between the two groups regarding the local recurrence rates, and distant metastasis (Table 2). Also, the 3-year local recurrence-free survival rates (76.7% vs. 80.8%, P=0.756) did not show any significant difference between the two groups (Figure 2).
Table 2. Post-metastasectomy results.
| Variables | Group 1 | Group 2 | Total | P value |
|---|---|---|---|---|
| N (%) | 53 (81.5) | 12 (18.5) | 65 | |
| Post-metastasectomy recur (intrathoracic), (%) | 18 (34.0) | 6 (50.0) | 24 (36.9) | 0.335 |
| Post-metastasectomy recur (intra + extra), (%) | 19 (35.8) | 8 (66.7) | 27 (41.5) | 0.061 |
| Local recur (%) | 10 (18.9) | 2 (16.7) | 12 (18.5) | 1.000 |
| Regional recur (%) | 16 (30.2) | 4 (33.3) | 20 (30.8) | 1.000 |
| Distant metastasis (intrathoracic) (%) | 13 (24.5) | 4 (33.3) | 17 (26.2) | 0.717 |
| Distant metastasis (extrathoracic) (%) | 23 (43.4) | 7 (58.3) | 30 (46.2) | 0.523 |
| Postop. CT F/U (month) | 38.06±31.94 | 47.92±33.33 | 39.88±32.17 | 0.365 |
| F/U period (month) | 41.32±30.76 | 49.83±33.59 | 42.89±31.21 | 0.398 |
| Complete resection (%) | 53 (100.0) | 12 (100.0) | 65 (100.0) | – |
| Reoperation (%) | 9 (17.0) | 4 (33.3) | 13 (20.0) | 0.237 |
| Complication (%) | 1 (1.9) | 0 | 1 (1.5) | 1.000 |
| 30-day in-hospital mortality | 0 | 0 | 0 | – |
CT, computed tomography; F/U, follow up.
Figure 2.
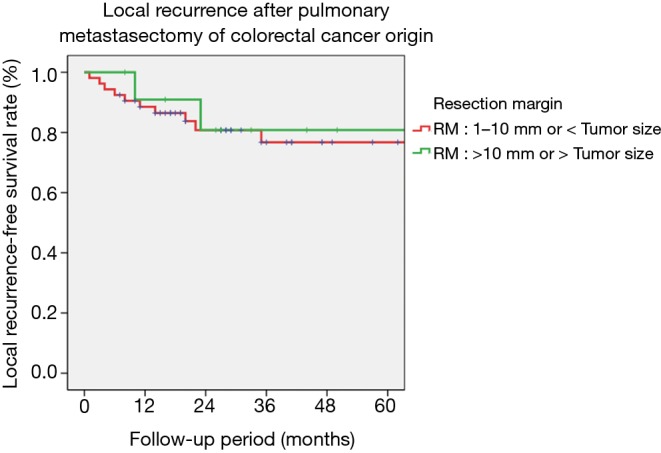
Local recurrence after pulmonary metastasectomy. The 3-year local recurrence-free survival rates (76.7% vs. 80.8%, P=0.756) did not show any significant difference between the two groups.
Focusing on the recurred patients, none of the evaluated factors such as the resection margin width, tumor size, DFI, and preoperative serum CEA level seemed to show any significant relevance with both the local recurrence in univariable analysis. Only the tumor depth, although statistically insignificant, seemed to show a weak borderline correlation with the local recurrence (P=0.091).
After adjusting for age, sex, resection margin, tumor size, depth, serum CEA level, visceral pleural invasion, use of adjuvant therapies by multivariable analysis, tumor depth greater than 23 mm was the only significant positive factors for prediction of local recurrence (Table 2).
Discussion
The benefits of pulmonary metastasectomy of colorectal cancer has been mentioned in many literatures (1,3,18-21). However, about 16% of patients were reported to go through more than one lung resection due to recurrent pulmonary metastasis (12). Therefore, in order to preserve the lung function for a possible additional lung resection in the future, wedge resection is considered as a reasonable approach for pulmonary metastasectomy by many thoracic surgeons (1).
The local recurrence at the surgical margins may suggest a failure in previous operation, and it still remains a problem to be solved in limited resections such as wedge resection. Complete tumor resection without malignancy positive surgical margin is considered as one of the most important factors for preventing the local recurrence (4,18). However, according to the previous studies, the reported rates of local recurrence after pulmonary metastasectomy even in the pathologically proven complete resection cases, were between 9% and 28% (22,23). Unlike in primary lung cancers which many studies have evaluated the influence of resection margin width on the local recurrence, there is still lack of data regarding the sufficient resection margin of pulmonary metastasectomy (24,25). While the exact reasons for the local recurrence still remains unclear, some have proposed that the existence of satellite tumor cells and aerogenous spread of floating cancer cell clusters surrounding the metastatic nodule might affect the recurrence. And therefore, they suggested a safety distance of more than 5 to 20 mm in order to completely remove the tumor and the peritumoral tissues (13,15-17). Considering the complexity of achieving a resection margin width greater than 2 cm in reality, and also the recommendations of a resection margin greater than 1cm or at least greater than the maximum tumor diameter in previous studies (25,26), we have chosen the cut-off value of resection margin width as 1cm or at least greater than the maximum tumor diameter. And then, we divided the patients into two subgroups to analyze the influence of resection margin width on the local recurrence.
Previous studies reported several independent prognostic factors that might affect the results of pulmonary metastasectomy. The mostly discussed factors included number of lesions, tumor size, lymph node involvement, DFI, primary tumor status, complete resection, and serum CEA levels (11,12).
In this study, in order to focus on the effect of surgical margin on the local recurrence, we excluded those patients with multiple metastatic lung nodules or lymph node metastasis. Only the patients with single metastatic nodule and no lymph node metastasis were included. Thereby, we assumed that the systemic effects of tumor spreading on the local recurrence might have been minimized. Along with the resection margin, we also analyzed other parameters such as the tumor size and tumor depth, which we considered that might influence on the resection margin.
According to our results, the resection margin width did not influence on the local recurrence of pulmonary metastasectomy once complete resection has been achieved. The subgroup 2, which had a larger resection margin compared to group 1 (1.0 vs. 0.4 cm, P<0.001), did not show any superiority in their local recurrence rate (16.7% vs. 18.9%, P=1.000). Also, the 3-year local recurrence-free survival rates (80.8% vs. 76.7%, P=0.756) did not differ between the two groups.
While other factors including the tumor size did not affect on the local recurrence, the tumor depth which was measured by the preoperative chest CT scan was an independent negative predictive factor for local recurrence. The local recurrence rate showed a tendency to increase together with the increase of tumor depth. Based on the receiver operating characteristics (ROC) curve analysis (P=0.0247), the hazard ratio of local recurrence showed a significant increase at the cut-off point of 23 mm. In multivariable analysis, central tumors with a depth greater than 23 mm showed a higher probability of local recurrence compared to the more peripherally located tumors (Table 3).
Table 3. Significant factors for predicting local recurrence after pulmonary metastasectomy in univariable and multivariable analysis.
| Variables | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| No. of patients | Local recurrence (%) | P value | RR | P value | 95% CI | ||
| Tumor depth | |||||||
| >23 mm | 15 | 5/15 (33.3) | 0.091 | 12.920 | 0.001 | 2.704 | |
| ≤23 mm | 50 | 7/50 (14.0) | 61.734 | ||||
RR, risk ratio.
Our study has several limitations. (I) This study was retrospective and included only a small number of patients in a single center; (II) the absence of lymph node metastasis was confirmed only by the radiologic images such as the preoperative chest CT scan and the PET CT scan. The microscopic lymph node metastasis may have not been completely excluded from the study; (III) the thoracoscopic wedge resection procedure is a very simple method for pulmonary resection, but also its result may depend on the surgeon’s technical ability; (IV) considering the 3D-dimension of the lung, the tumor depth measured either by the axial or coronal view may not reflect the exact shortest distance from the lung external surface. However, although our method of tumor depth measurement may not be accurate, it may reflect the real preoperative planning and be helpful in the clinical practice because most of the preoperative planning of wedge resection by the thoracic surgeons is mainly dependent on the chest CT scan. Gathering all the above limitations, caution is required while interpreting the results regarding the effect of resection margin width and tumor depth on the local recurrence. And, further prospective study with larger cohort should be performed for further validation of our results.
However, to our knowledge, this is the first study to demonstrate the relationship between the surgical resection margin, tumor depth on preoperative CT image, and the local recurrence after thoracoscopic pulmonary metastasectomy. Despite its small number of patients, the involved patients are those with single first detected metastatic nodules without radiologic lymph node metastasis which may have enabled us to minimize the effect of other confounding factors on the results of local recurrence. And finally, our results may be informative to the thoracic surgeons or oncologists when deciding their surgical procedure through CT images, and also when informing the patients about the prognosis regarding the local recurrence after pulmonary metastasectomy.
In conclusion, balance between adequate resection for preventing recurrence and limited resection to preserve pulmonary function is essential in pulmonary metastasectomy of colorectal cancer. Wedge resection is a widely accepted surgical procedure, and our results may suggest that the extent of resection margin in pulmonary metastasectomy does not seem to affect significantly on the local recurrence if complete resection is accomplished. Therefore, pulmonary metastasectomy through wedge resection should not be precluded from any patient who is expected to show a narrow resection margin, but extra caution when choosing the surgical procedure for thoracoscopic pulmonary metastasectomy or consideration of larger lung resection such as segmentectomy might be needed in cases of a more deeply located tumor, especially with a tumor depth greater than 23 mm on preoperative CT image.
Acknowledgments
None.
Ethical Statement: The Institutional Review Board of the Korea University Anam Hospital approved this study and waived the requirement for informed consent (IRB Number: 2018AN0354).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Caristo JM, Tian DH, Yan TD. Pulmonary metastasectomy: a cross sectional survey. J Thorac Dis 2018;10:3757-66. 10.21037/jtd.2018.05.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rama N, Monteiro A, Bernardo JE, et al. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardiothorac Surg 2009;35:444-9. 10.1016/j.ejcts.2008.10.047 [DOI] [PubMed] [Google Scholar]
- 3.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Annals of Thoracic Surgery 2007;84:324-38. 10.1016/j.athoracsur.2007.02.093 [DOI] [PubMed] [Google Scholar]
- 4.Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2017;51:504-10. [DOI] [PubMed] [Google Scholar]
- 5.Blackmon SH, Stephens EH, Correa AM, et al. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg 2012;94:1802-9. 10.1016/j.athoracsur.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. 10.1245/s10434-012-2726-3 [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Hanaoka N, Sato K, et al. Prognostic factors of pulmonary metastasectomy for colorectal carcinomas. World J Surg 2009;33:505-11. 10.1007/s00268-008-9875-3 [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Kim S, Namgung M, et al. The prognostic importance of the number of metastases in pulmonary metastasectomy of colorectal cancer. World J Surg Oncol 2015;13:222. 10.1186/s12957-015-0621-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iida T, Nomori H, Shiba M, et al. Prognostic factors after pulmonary metastasectomy for colorectal cancer and rationale for determining surgical indications: a retrospective analysis. Ann Surg 2013;257:1059-64. 10.1097/SLA.0b013e31826eda3b [DOI] [PubMed] [Google Scholar]
- 10.Rena O, Casadio C, Viano F, et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg 2002;21:906-12. 10.1016/S1010-7940(02)00088-X [DOI] [PubMed] [Google Scholar]
- 11.Tsitsias T, Toufektzian L, Routledge T, et al. Are there recognized prognostic factors for patients undergoing pulmonary metastasectomy for colorectal carcinoma? Interact Cardiovasc Thorac Surg 2016;23:962-9. 10.1093/icvts/ivw273 [DOI] [PubMed] [Google Scholar]
- 12.Nanji S, Karim S, Tang E, et al. Pulmonary Metastasectomy for Colorectal Cancer: Predictors of Survival in Routine Surgical Practice. Ann Thorac Surg 2018;105:1605-12. 10.1016/j.athoracsur.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 13.Higashiyama M, Tokunaga T, Nakagiri T, et al. Pulmonary metastasectomy: outcomes and issues according to the type of surgical resection. Gen Thorac Cardiovasc Surg 2015;63:320-30. 10.1007/s11748-015-0544-9 [DOI] [PubMed] [Google Scholar]
- 14.Hernández J, Molins L, Fibla JJ, et al. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann Oncol 2016;27:850-5. 10.1093/annonc/mdw064 [DOI] [PubMed] [Google Scholar]
- 15.Welter S, Theegarten D, Trarbach T, et al. Safety distance in the resection of colorectal lung metastases: a prospective evaluation of satellite tumor cells with immunohistochemistry. J Thorac Cardiovasc Surg 2011;141:1218-22. 10.1016/j.jtcvs.2010.08.089 [DOI] [PubMed] [Google Scholar]
- 16.Rusch VW. Pulmonary Metastasectomy: Current Indications. Chest 1995;107:322S-331S. 10.1378/chest.107.6_Supplement.322S [DOI] [PubMed] [Google Scholar]
- 17.Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. 10.1093/ejcts/ezx063 [DOI] [PubMed] [Google Scholar]
- 18.Dickinson KJ, Blackmon SH. Results of Pulmonary Resection: Colorectal Carcinoma. Thorac Surg Clin 2016;26:41-7. 10.1016/j.thorsurg.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Kiyoshima M, Kitahara M, et al. Long-Term Outcomes After Surgical Resection of Pulmonary Metastases From Colorectal Cancer. Annals of Thoracic Surgery 2015;99:435-40. 10.1016/j.athoracsur.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 20.Zheng Y, Fernando HC. Surgical and nonresectional therapies for pulmonary metastasis. Surg Clin North Am 2010;90:1041-51. 10.1016/j.suc.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 21.Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238-44. 10.1016/j.athoracsur.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 22.Higashiyama M, Kodama K, Takami K, et al. Intraoperative lavage cytologic analysis of surgical margins as a predictor of local recurrence in pulmonary metastasectomy. Archives of Surgery 2002;137:469-74. 10.1001/archsurg.137.4.469 [DOI] [PubMed] [Google Scholar]
- 23.Shiono S, Ishii G, Nagai K, et al. Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg 2005;80:1040-5. 10.1016/j.athoracsur.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 24.Moon Y, Lee KY, Moon SW, et al. Sublobar Resection Margin Width Does Not Affect Recurrence of Clinical N0 Non-small Cell Lung Cancer Presenting as GGO-Predominant Nodule of 3 cm or Less. World J Surg 2017;41:472-9. 10.1007/s00268-016-3743-3 [DOI] [PubMed] [Google Scholar]
- 25.Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. 10.1016/S0003-4975(03)01511-X [DOI] [PubMed] [Google Scholar]
- 26.Allen MS, Pairolero PC. Inadequacy, mortality, and thoracoscopy. Ann Thorac Surg 1995;59:6. 10.1016/0003-4975(94)00920-3 [DOI] [PubMed] [Google Scholar]