Abstract
Background: The objective of this study was to evaluate potential sex-specific differences on episodic memory function and determine whether sex moderates the effects of acute exercise on episodic memory.
Methods: A randomized controlled intervention was employed. This experiment was conducted among young University students (mean age = 21 years). Both males (n=20) and females (n=20)completed two counterbalanced laboratory visits, with one visit involving a 15-minute bout of moderate-intensity exercise prior to the memory task. The control visit engaged in a time matched seated task. Memory function (including short-term memory, learning, and long-term memory) was assessed from the RAVLT (Rey Auditory Verbal Learning Test).
Results: We observed a significant main effect for time (P<0.001, ƞ2p= 0.77) and a marginally significant main effect for sex (P=0.06, ƞ2p= 0.09), but no time by sex by condition interaction(P=0.91, ƞ2p= 0.01). We also observed some suggestive evidence of a more beneficial effect of acute exercise on memory for females.
Conclusion: In conclusion, females outperformed males in verbal memory function. Additional research is needed to further evaluate whether sex moderates the effects of acute exercise on memory function.
Keywords: Cognition, Encoding, Learning, Memory, Physical activity
Introduction
Regular participation in physical activity has many health benefits, including a reduced risk of diabetes, cardiovascular disease and premature mortality,1-6 as well as enhancing psychological health.7,8 As such, from a health promotion perspective, promotion of physical activity is of critical importance for living a healthy lifestyle. In addition to the cardiometabolic benefits of physical activity, both acute and chronic physical activity participation demonstrate neurocognitive benefits.9,10 Promotion of physical activity is critical even among young adults, as cognitive decline may start to occur during the young adult years.11
Among young adults, experimental work from our laboratory has examined the effects of exercise on memory function, providing suggestive evidence that acute exercise may subserve episodic memory performance12-18 and may even attenuate a memory interference effect.19 We have previously detailed the dearth of research on this topic among this population.20 The mechanisms through which exercise may influence episodic memory function has also been extensively detailed by our group.21-24 Also, as we initially indicated elsewhere,25 very little research has evaluated the potential effects that biological sex may have on the relationship between acute exercise and memory function. Further, we recently detailed the literature on the role of sex on memory function, and how sex may moderate the effects of exercise on memory.26 However, to our knowledge, no study has specifically evaluated this potential exercise-induced sex-specific effect on episodic memory function.
As we have discussed elsewhere,26 in general, females outperform males on autobiographical memory (particularly with high retrieval support via verbal probing27), random word recall,28 story recall,29 auditory episodic memory,30 semantic memory (driven by superiority in fluency),31 and face recognition tasks.32,33 For race recognition tasks, females particularly have better recognition memory for female faces (and greater face perception).34,35 This may be a result of females being more familiar with female faces,36 which aligns with other work showing that recognition memory is superior for individuals who are of the same ethnic background as themselves.37 Females also have been shown to have greater scanning behavior at encoding,33 which may also contribute to their superior recognition memory. Females, however, do not outperform males for non-spatial memory, where males tend to perform better.26 In the context of exercise, there also appears to be a potential sex-specific effect on the response to exercise,38 which may subserve episodic memory function. For example, recent work suggests that older males (vs. older females) have a greater exercise-induced brain derived neurotrophic factor response.39
At this point, however, it is uncertain as to whether biological sex may moderate the effects of exercise on memory function. Thus, the purpose of this study was to twofold: 1) evaluate potential sex-specific differences on episodic memory function, and 2) evaluate whether biological sex moderates the effects of acute exercise on episodic memory function. We hypothesize that females (vs. males) will perform better on the non-exercise memory task, but there will be no differences in memory performance after acute exercise.
Material and Methods
Study design
A randomized controlled intervention was employed. This experiment was conducted among young University students in the authors’ laboratory. Data collection occurred between August and December of 2018. Both males and females completed two counterbalanced laboratory visits, with one visit involving a 15-minute bout of exercise prior to the memory task. The control visit engaged in a time-matched seated task. Each within-subject visit occurred around the same time of day (± 2 years) and occurred at least 24 hours after the first visit. Allocation concealment occurred by the lead researcher (L.J.) not looking at the allocation sheet until the participant arrived in the laboratory and completed the consent document. The random allocation sequence was generated by the principal investigator (P.L.). This study was approved by the authors’ institutional review board (#18-123) and participants provided written consent prior to participation.
Participants and procedures
In total, 40 participants were recruited (20 males and 20 females). There were no drop outs after randomization. This was based on a conservative a-priori power analysis in G*Power (v. 3.1.9.2), using a RM-ANOVA within-between interaction model. With inputs of 0.05 (α), 0.80 (1-β), 2 groups (male/female), and 7 repeated measurements (trials), a sample size per group of 17 was needed to obtain sufficient statistical power. Our evaluated sample of 40 (n=20 per group) is in alignment with other related experiments.14,16,40,41 Recruitment occurred via a convenience-based, non-probability sampling approach (classroom announcement and word-of-mouth). Participants included undergraduate and graduate students between the ages of 18 and 35 years.
Additionally, and identical to other studies,42 participants were excluded if they:
- Self-reported as a daily smoker43,44
- Self-reported being pregnant45
- Exercised within 5 hours of testing46
- Consumed caffeine within 3 hours of testing47
- Had a concussion or head trauma within the past 30 days48
- Took marijuana or other illegal drugs within the past 30 days49
- Were considered a daily alcohol user (>30 drinks/month for women; >60 drinks/month for men)50
Table 1 displays the demographic and behavioral characteristics of the sample. Participants, on average, were 20.8 (0.9) years of age. The sample was equally distributed across sex, with 50% male and 50% female participants. There were no significant BMI (P = 0.98) or race-ethnicity (P = 0.11) differences across the two sexes. However, males (224 min/wk) were significantly (P = 0.006) more active than females (107.4 min/wk of moderate-to-vigorous physical activity).
Table 1. Demographic and behavioral characteristics of the sample .
| Variable | Males | Females | P value |
| N | 20 | 20 | |
| Age, mean years | 20.95 (1.1) | 20.65 (0.81) | 0.34 |
| Race, % non-Hispanic white | 75.0 | 95.0 | 0.11 |
| BMI, mean kg/m2 | 26.20 (3.4) | 26.23 (4.8) | 0.98 |
| MVPA, mean min/wk | 224.0 (161.0) | 107.4 (72.1) | 0.006 |
BMI, body mass index; MVPA, moderate-to-vigorous physical activity.
Values in parentheses are standard deviations.
Independent samples t-test was used to make comparisons across the continuous variables (e.g., age). A chi-square test was used to make comparisons across the categorical variables (e.g., race-ethnicity).
Exercise protocol
The exercise bout (a single exercise session) involved exercising on a treadmill for 15 minutes. Participants exercised at approximately 70% of their estimated heart rate max (220-age), which corresponds with moderate-intensity exercise.51
Immediately after the bout of exercise, participants rested in a seated position for 5 minutes. During this resting period, they played on-line game of Sudoku (described below) to prevent boredom. After this resting period, they commenced the memory assessment, as described below. We have experimental evidence that playing Sudoku does not prime or enhance memory function.
Control protocol
For the control visit, and similar to other studies,52 participants completed a medium-level, on-line administered, Sudoku puzzle for 20-minutes. The website for this puzzle is located here: https://www.websudoku.com/.
Memory assessment
Identical to our related experimental work,14,16,41 short-term and long-term memory (retrospective memory) were assessed using the standardized Rey Auditory Verbal Learning Test (RAVLT).53 Participants listened to and immediately recalled a recording of a list of 15 words (List A) five times in a row (Trials 1-5). Each word list (example words were: drum, curtain, bell, coffee, school, etc) was recorded at a rate of approximately 1 word per second. Participants then were asked to listen to and immediately recall a list of 15 new words (List B). Immediately following the recall of List B, participants were asked to recall the words from List A (Trial 6). Following Trial 6, participants watch a 20-minute video clip of “The Office – Bloopers”. After this 20-minute video clip, participants were asked to recall as many words as possible from List A (Trial 7).
Additional assessments
Various demographic (e.g., BMI) and behavioral (i.e., habitual physical activity) assessments were completed to ensure that the groups were similar on these parameters. As a measure of habitual physical activity behavior, participants completed the Physical Activity Vital Signs Questionnaire to evaluate time spent per week in moderate-to-vigorous physical activity (MVPA).54 Height/weight (BMI; kg/m2) were measured to provide anthropometric characteristics of the sample. Lastly, before and at the end of the exercise and control conditions, heart rate (chest-strapped Polar monitor, F1 model) was assessed.
Statistical analysis
All statistical analyses were computed in Jasp (v. 0.9.1). For the physiological data (heart rate), a 2 (sex) x 2 (exercise vs. control) x 2 (rest vs. endpoint) repeated measures ANOVA was computed. For the memory data, 2 (sex) x 2 (exercise vs. control) x 7 (trials) repeated measures ANOVA was computed. Statistical significance was set at an alpha of 0.05. Partial eta-square (η2p) was calculated as an estimate of effect size.
Results
Table 2 displays the heart rate exercise responses. There was a significant main effect for time (F(1,38)=254.6, P < 0.001, ƞ2p=0.87), main effect for sex (F(1,38)=8.11, P = 0.007, ƞ2p=0.17), main effect for condition (F(1,38)=192.2, P < 0.001, ƞ2p=0.83), and time by condition interaction (F(1,38)=162.3, P < 0.001, ƞ2p=0.81). That is, heart rate was significant higher at the endpoint of exercise when compared to baseline, and heart rate was slightly higher for females (vs. males). Notably, there was no significant time by sex (F=0.35, P = 0.55, ƞ2p=0.01), sex by condition (F=0.62, P = 0.43, ƞ2p=0.01), or time by sex by condition (F=0.40, P = 0.52, ƞ2p=0.01) effects.
Table 2. Heart rate responses across the conditions .
| Variable | Males | Females | Test-Statistic | ||
| Exercise | Control | Exercise | Control | ||
| Baseline heart rate, mean bpm | 84.55 (13.11) | 72.65 (14.2) | 93.75 (11.3) | 83.4 (16.0) | F(time)=254.6, P<0.001, ƞ2p=0.87 F(sex)=8.11, P=0.007, ƞ2p=0.17 F(condition)=192.2, P<0.001, ƞ2p=0.83 F(time x sex)=0.35, P=0.55, ƞ2p=0.01 F(time x condition)=162.3, P<0.001, ƞ2p=0.81 F(sex x condition)=0.62, P=0.43, ƞ2p=0.01 F(time x sex x condition)=0.40, P=0.52, ƞ2p=0.01 |
| Endpoint heart rate, mean bpm | 130.6 (10.2) | 76.25 (16.3) | 136.0 (11.3) | 87.25 (18.4) | |
Bpm, beats per minute.
Values in parentheses are standard deviations.
For the physiological data (heart rate), a 2 (sex) x 2 (exercise vs. control) x 2 (rest vs. endpoint) repeated measures ANOVA was computed.
Table 3 displays the memory scores for both sexes and across the exercise and control periods. There was no main effect for condition (F(1,38)=0.66, P = 0.41, ƞ2p=0.02), but for females, more words were recalled for every trial during the exercise visit when compared to the control visit. We did not observe any interaction effects for time by sex (F(6,228)=0.76, P = 0.60, ƞ2p=0.02), time by condition (F(6,228)=0.53, P = 0.77, ƞ2p=0.01), sex by condition (F(1,228)=0.12, P = 0.72, ƞ2p=0.003), or time by sex by condition (F(6,228)=0.34, P = 0.91, ƞ2p=0.01). However, there was a significant main effect for time (F(6,228)=126.7, P < 0.001, ƞ2p=0.77) and a marginally significant main effect for sex (F(1,38)=3.76, P = 0.06, ƞ2p=0.09).
Table 3. Memory scores across the experimental conditions and by sex .
| Variable | Males | Females | Test-Statistic | ||
| Exercise | Control | Exercise | Control | ||
| Trial 1, mean # words | 5.80 (1.7) | 6.10 (1.6) | 7.20 (2.0) | 7.15 (2.0) | F(time)=126.7, P<0.001, ƞ2p=0.77 F(sex)=3.76, P=0.06, ƞ2p=0.09 F(condition)=0.66, P=0.41, ƞ2p=0.02 F(time x sex)=0.76, P=0.60, ƞ2p=0.02 F(time x condition)=0.53, P=0.77, ƞ2p=0.01 F(sex x condition)=0.12, P=0.72, ƞ2p=0.003 F(time x sex x condition)=0.34, P=0.91, ƞ2p=0.01 |
| Trial 2, mean # words | 8.70 (2.4) | 8.55 (1.7) | 10.20 (2.5) | 9.70 (2.0) | |
| Trial 3, mean # words | 10.55 (2.4) | 10.60 (2.0) | 11.70 (2.2) | 11.20 (1.9) | |
| Trial 4, mean # words | 12.05 (1.9) | 11.45 (2.1) | 12.60 (2.5) | 12.35 (1.8) | |
| Trial 5, mean # words | 12.20 (1.9) | 12.35 (1.9) | 13.00 (2.6) | 12.70 (2.4) | |
| Trial 6, mean # words | 10.70 (2.4) | 10.55 (2.4) | 11.85 (2.6) | 11.60 (2.3) | |
| Trial 7, mean # words | 10.35 (2.9) | 9.85 (3.0) | 11.70 (3.0) | 11.30 (2.4) | |
Values in parentheses are standard deviations
Trials 1-6 are the free-recall assessments of List A.
Trial 7 is the free-recall delayed assessment of List A, which occurred 20-minutes after Trial 6.
For the memory data, 2 (sex) x 2 (exercise vs. control) x 7 (trials) repeated measures ANOVA was computed.
As expected, there was a significant main for time (F(6,228)=126.7, P < 0.001, ƞ2p=0.77). The number of recalled words increased over the first 5 trials, then decreased throughout the delayed assessment period. Regarding sex differences, there was a marginally significant main effect for sex (F(1,38)=3.76, P = 0.06, ƞ2p=0.09). As shown in Figure 1, for both the control and exercise visits, females (vs. males) had higher memory scores for every trial.
Figure 1.
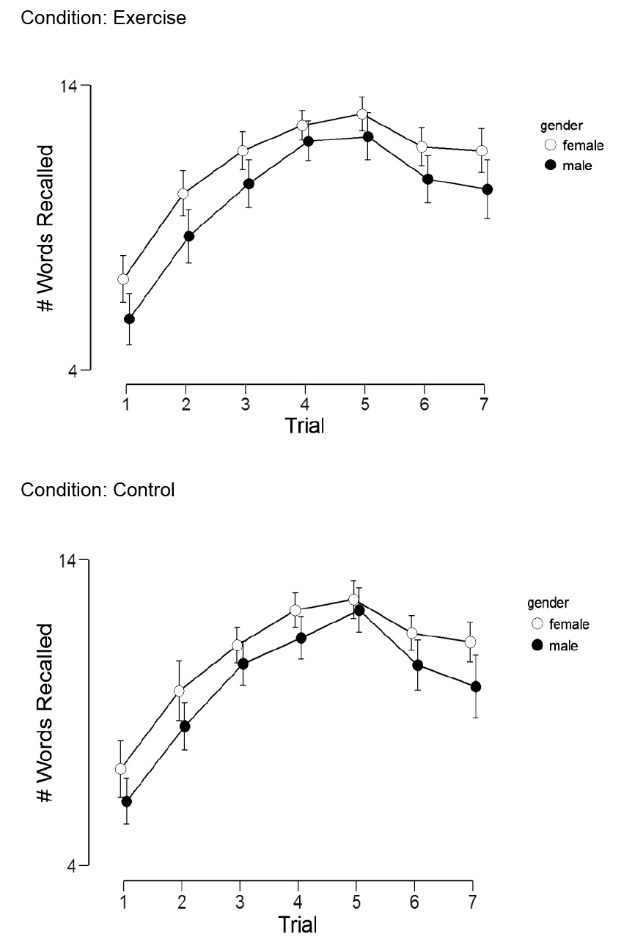
Memory scores across condition and sex. Error bars are 95% CI.
Discussion
Previous experimental work suggests that acute exercise may subserve episodic memory function. Additionally, research demonstrates that females (vs. males) tend to outperform males on most non-spatial memory tasks. However, to our knowledge, no study has specifically evaluated whether there is a sex-specific exercise-related memory effect, which was the purpose of this experiment. The main findings of this experiment are as follows. Females outperformed males across all the 7 episodic memory trials (i.e., the word recall for the 7 trials), suggesting a sex-specific effect on short-term memory, learning, and long-term memory. Additionally, and although not statistically significant, females (but not males) had higher memory scores after exercising, when compared to their non-exercise visit.
As stated in the Introduction section, and as we have thoroughly addressed elsewhere,26 females tend to outperform males across nearly all non-spatial memory tasks. Our findings from the present experiment are in alignment with this body of literature. Our results also provide some suggestive evidence of an exercise-induced benefit for females; however, these results were not statistically significant, and thus, this should be interpreted with caution. These null exercise findings are likely not a result of a statistical power issue, as we were powered to observe such an effect. Further, our sample size is larger or similar to other studies that have observed statistically significant effects.14,40
Although other work has demonstrated that acute moderate-intensity physical activity is effective in enhancing memory function,41 our null exercise findings may, in part, be a result of the exercise intensity stimulus. Unlike moderate-intensity exercise, recent work demonstrates that higher-intensity exercise may be more effective in enhancing memory function.55 Our recent experimental work also supports episodic memory benefits from high-intensity exercise.14 Thus, future work should evaluate whether there are sex-specific, high-intensity exercise effects on memory function, and whether this occurs for spatial- and non-spatial memory function. In addition to exercise intensity, it is possible that our null exercise-induced effects may be a result of the time period in which long-term memory was assessed (i.e., 20-minute delay). Although research demonstrates that acute exercise can improve memory function when assessed at this 20-minute delay period,14 it is possible that a longer delay period may be needed for exercise-induced memory stabilization effects. Lastly, it is always important to be mindful that acute exercise may not always have beneficial effects on memory, which aligns with review work showing that, on average, only 48% to 71% of studies on exercise and memory observe a significant association.20,56
Limitations of this study include the homogenous sample of young, healthy adults. Thus, future work on this topic should consider evaluating other populations, including older adults with and without memory impairment.57 Strengths of this study include the experimental design and study novelty.
In conclusion, our experiment provides evidence that young adult females outperform males in verbal memory function. We did not observe sufficient evidence that sex moderates the effects of exercise on verbal memory performance, including short-term memory, learning, or long-term memory. Despite these findings, it is of critical importance to promote regular participation in physical activity, among all age populations, as habitual physical activity can help reduce a multitude of cardiovascular and cognitive morbidities.
Ethical approval
This study was approved by the University of Mississippi’s ethics committee (#18-123).
Competing interests
The authors declare that they have no competing interests.
Funding
None.
Authors’ contributions
LJ was involved in study conceptualization, data collection and manuscript revising; PL was involved in study conceptualization, statistical analyses, and manuscript writing.
Citation: Johnson L, Loprinzi PD. The effects of acute exercise on episodic memory function among young university students: moderation considerations by biological sex. Health Promot Perspect. 2019;9(2):99-104. doi: 10.15171/hpp.2019.14.
References
- 1.Warburton DE, Bredin SS. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32(5):541–56. doi: 10.1097/hco.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 2.Loprinzi PD, Addoh O, Joyner C. Multimorbidity, mortality, and physical activity. Chronic Illn. 2016;12(4):272–80. doi: 10.1177/1742395316644306. [DOI] [PubMed] [Google Scholar]
- 3.Loprinzi PD, Davis RE. Psycho-socioeconomic bio-behavioral associations on all-cause mortality: cohort study. Health Promot Perspect. 2016;6(2):66–70. doi: 10.15171/hpp.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loprinzi PD. Effect of physical activity on mortality risk among Americans with retinopathy. Health Promot Perspect. 2016;6(3):171–3. doi: 10.15171/hpp.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loprinzi PD, Edwards MK, Sng E, Addoh O. Sedentary behavior and residual-specific mortality. Health Promot Perspect. 2016;6(4):196–201. doi: 10.15171/hpp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menai M, Brouard B, Vegreville M, Chieh A, Schmidt N, Oppert JM. et al. Cross-Sectional and longitudinal associations of objectively-measured physical activity on blood pressure: evaluation in 37 countries. Health Promot Perspect. 2017;7(4):190–6. doi: 10.15171/hpp.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frith E, Loprinzi PD. Experimental investigation of exercise-related hedonic responses to preferred and imposed media content. Health Promot Perspect. 2018;8(2):109–19. doi: 10.15171/hpp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards MK, Loprinzi PD. Experimental effects of brief, single bouts of walking and meditation on mood profile in young adults. Health Promot Perspect. 2018;8(3):171–8. doi: 10.15171/hpp.2018.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponce P, Loprinzi PD. A bi-directional model of exercise and episodic memory function. Med Hypotheses. 2018;117:3–6. doi: 10.1016/j.mehy.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Loprinzi PD, Herod SM, Cardinal BJ, Noakes TD. Physical activity and the brain: a review of this dynamic, bi-directional relationship. Brain Res. 2013;1539:95–104. doi: 10.1016/j.brainres.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30(4):507–14. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loprinzi PD, Kane CJ. Exercise and cognitive function: a randomized controlled trial examining acute exercise and free-living physical activity and sedentary effects. Mayo Clin Proc. 2015;90(4):450–60. doi: 10.1016/j.mayocp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Crush EA, Loprinzi PD. Dose-response effects of exercise duration and recovery on cognitive functioning. Percept Mot Skills. 2017;124(6):1164–93. doi: 10.1177/0031512517726920. [DOI] [PubMed] [Google Scholar]
- 14.Frith E, Sng E, Loprinzi PD. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur J Neurosci. 2017;46(10):2557–64. doi: 10.1111/ejn.13719. [DOI] [PubMed] [Google Scholar]
- 15.Loprinzi P, Blough J, Crawford L, Ryu S, Zou L, Li H. The temporal effects of acute exercise on episodic memory function: Systematic review with meta-analysis. Brain Sci. 2019;9(4):E87. doi: 10.3390/brainsci9040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haynes Iv JT, Frith E, Sng E, Loprinzi PD. Experimental effects of acute exercise on episodic memory function: considerations for the timing of exercise. Psychol Rep. 2018:33294118786688. [DOI] [PubMed]
- 17.Sng E, Frith E, Loprinzi PD. Experimental effects of acute exercise on episodic memory acquisition: Decomposition of multi-trial gains and losses. Physiol Behav. 2018;186:82–4. doi: 10.1016/j.physbeh.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 18. Siddiqui A, Loprinzi PD. Experimental investigation of the time course effects of acute exercise on false episodic memory. J Clin Med 2018;7(7). doi: 10.3390/jcm7070157. [DOI] [PMC free article] [PubMed]
- 19.Wingate S, Crawford L, Frith E, Loprinzi PD. Experimental investigation of the effects of acute exercise on memory interference. Health Promot Perspect. 2018;8(3):208–14. doi: 10.15171/hpp.2018.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loprinzi PD, Frith E, Edwards MK, Sng E, Ashpole N. The effects of exercise on memory function among young to middle-aged adults: systematic review and recommendations for future research. Am J Health Promot. 2018;32(3):691–704. doi: 10.1177/0890117117737409. [DOI] [PubMed] [Google Scholar]
- 21.Loprinzi PD, Edwards MK, Frith E. Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. Eur J Neurosci. 2017;46(5):2067–77. doi: 10.1111/ejn.13644. [DOI] [PubMed] [Google Scholar]
- 22.Loprinzi PD, Ponce P, Frith E. Hypothesized mechanisms through which acute exercise influences episodic memory. Physiol Int. 2018;105(4):285–97. doi: 10.1556/2060.105.2018.4.28. [DOI] [PubMed] [Google Scholar]
- 23.Loprinzi PD, Frith E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin Physiol Funct Imaging. 2019;39(1):9–14. doi: 10.1111/cpf.12522. [DOI] [PubMed] [Google Scholar]
- 24.Loprinzi PD. IGF-1 in exercise-induced enhancement of episodic memory. Acta Physiol (Oxf) 2019;226(1):e13154. doi: 10.1111/apha.13154. [DOI] [PubMed] [Google Scholar]
- 25.Loprinzi PD. An integrated model of acute exercise on memory function. Med Hypotheses. 2019;126:51–9. doi: 10.1016/j.mehy.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 26. Loprinzi PD, Frith E. The role of sex in memory function: Considerations and recommendations in the context of exercise. J Clin Med 2018;7(6). doi: 10.3390/jcm7060132. [DOI] [PMC free article] [PubMed]
- 27.Fuentes A, Desrocher M. The effects of gender on the retrieval of episodic and semantic components of autobiographical memory. Memory. 2013;21(6):619–32. doi: 10.1080/09658211.2012.744423. [DOI] [PubMed] [Google Scholar]
- 28.Herlitz A, Nilsson LG, Backman L. Gender differences in episodic memory. Mem Cognit. 1997;25(6):801–11. doi: 10.3758/bf03211324. [DOI] [PubMed] [Google Scholar]
- 29.Dixon RA, Wahlin A, Maitland SB, Hultsch DF, Hertzog C, Backman L. Episodic memory change in late adulthood: generalizability across samples and performance indices. Mem Cognit. 2004;32(5):768–78. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- 30.Pauls F, Petermann F, Lepach AC. Gender differences in episodic memory and visual working memory including the effects of age. Memory. 2013;21(7):857–74. doi: 10.1080/09658211.2013.765892. [DOI] [PubMed] [Google Scholar]
- 31.Maitland SB, Herlitz A, Nyberg L, Backman L, Nilsson LG. Selective sex differences in declarative memory. Mem Cognit. 2004;32(7):1160–9. doi: 10.3758/bf03196889. [DOI] [PubMed] [Google Scholar]
- 32.Herlitz A, Yonker JE. Sex differences in episodic memory: the influence of intelligence. J Clin Exp Neuropsychol. 2002;24(1):107–14. doi: 10.1076/jcen.24.1.107.970. [DOI] [PubMed] [Google Scholar]
- 33.Heisz JJ, Pottruff MM, Shore DI. Females scan more than males: a potential mechanism for sex differences in recognition memory. Psychol Sci. 2013;24(7):1157–63. doi: 10.1177/0956797612468281. [DOI] [PubMed] [Google Scholar]
- 34.Megreya AM, Bindemann M, Havard C. Sex differences in unfamiliar face identification: evidence from matching tasks. Acta Psychol (Amst) 2011;137(1):83–9. doi: 10.1016/j.actpsy.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Lewin C, Herlitz A. Sex differences in face recognition--women’s faces make the difference. Brain Cogn. 2002;50(1):121–8. doi: 10.1016/s0278-2626(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 36.Rehnman J, Herlitz A. Women remember more faces than men do. Acta Psychol (Amst) 2007;124(3):344–55. doi: 10.1016/j.actpsy.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Bothwell RK, Brigham JC, Malpass RS. Cross-racial identification. Pers Soc Psychol Bull. 1989;15(1):19–25. doi: 10.1177/0146167289151002. [DOI] [Google Scholar]
- 38.Sheel AW. Sex differences in the physiology of exercise: an integrative perspective. Exp Physiol. 2016;101(2):211–2. doi: 10.1113/ep085371. [DOI] [PubMed] [Google Scholar]
- 39.Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C. et al. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp Gerontol. 2015;70:144–9. doi: 10.1016/j.exger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Labban JD, Etnier JL. The effect of acute exercise on encoding and consolidation of long-term memory. J Sport Exerc Psychol. 2018;40(6):336–42. doi: 10.1123/jsep.2018-0072. [DOI] [PubMed] [Google Scholar]
- 41.Sng E, Frith E, Loprinzi PD. Temporal effects of acute walking exercise on learning and memory function. Am J Health Promot. 2018;32(7):1518–25. doi: 10.1177/0890117117749476. [DOI] [PubMed] [Google Scholar]
- 42. Yanes D, Loprinzi PD. Experimental effects of acute exercise on iconic memory, short-term episodic, and long-term episodic memory. J Clin Med 2018;7(6). doi: 10.3390/jcm7060146. [DOI] [PMC free article] [PubMed]
- 43.Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 2008;199(1):89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klaming R, Annese J, Veltman DJ, Comijs HC. Episodic memory function is affected by lifestyle factors: a 14-year follow-up study in an elderly population. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2017;24(5):528–42. doi: 10.1080/13825585.2016.1226746. [DOI] [PubMed] [Google Scholar]
- 45.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29(8):793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 46.Labban JD, Etnier JL. Effects of acute exercise on long-term memory. Res Q Exerc Sport. 2011;82(4):712–21. doi: 10.1080/02701367.2011.10599808. [DOI] [PubMed] [Google Scholar]
- 47.Sherman SM, Buckley TP, Baena E, Ryan L. Caffeine enhances memory performance in young adults during their non-optimal time of day. Front Psychol. 2016;7:1764. doi: 10.3389/fpsyg.2016.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wammes JD, Good TJ, Fernandes MA. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn. 2017;111:112–26. doi: 10.1016/j.bandc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Hindocha C, Freeman TP, Xia JX, Shaban NDC, Curran HV. Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: a placebo-controlled trial. Psychol Med. 2017;47(15):2708–19. doi: 10.1017/s0033291717001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41(8):1432–43. doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM. et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 52.McNerney MW, Radvansky GA. Mind racing: The influence of exercise on long-term memory consolidation. Memory. 2015;23(8):1140–51. doi: 10.1080/09658211.2014.962545. [DOI] [PubMed] [Google Scholar]
- 53.Rey A. The psychological examination in cases of traumatic encephalopathy. Arch Psychol. 1941;28:215–85. [Google Scholar]
- 54.Ball TJ, Joy EA, Gren LH, Shaw JM. Concurrent validity of a self-reported physical activity “Vital Sign” questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:E16. doi: 10.5888/pcd13.150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loprinzi PD. Intensity-specific effects of acute exercise on human memory function: considerations for the timing of exercise and the type of memory. Health Promot Perspect. 2018;8(4):255–62. doi: 10.15171/hpp.2018.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci Biobehav Rev. 2013;37(8):1645–66. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Loprinzi PD, Blough J, Ryu S, Kang M. Experimental effects of exercise on memory function among mild cognitive impairment: systematic review and meta-analysis. Phys Sportsmed. 2019;47(1):21–6. doi: 10.1080/00913847.2018.1527647. [DOI] [PubMed] [Google Scholar]


