Graphical abstract
Keywords: Transcatheter mitral valve replacement, Paravalvular leak, Hemolysis, Transcatheter aortic valve replacement
Highlights
-
•
PVL after TMVR with a Tendyne device can result in severe intravascular hemolysis.
-
•
SAM of the mitral valve can cause anterior PVL following Tendyne TMVR.
-
•
TAVR can relieve LVOT obstruction caused by SAM after Tendyne TMVR.
-
•
Pre-, peri-, and postprocedural imaging is key in decision making.
Introduction
Mitral regurgitation (MR) is a leading cause of valvular heart disease,1 but up to half of patients with moderate to severe MR are not referred for conventional mitral valve surgery, because of advancing age or multiple comorbidities.2 Transcatheter mitral valve replacement (TMVR) with the Tendyne device (Abbott Vascular, Santa Clara, CA) is an alternative therapy for a subset of patients with significant MR at prohibitive operative risk because it does not require cardiopulmonary bypass or sternotomy.3, 4, 5 Potential complications of TMVR devices include fixation of the native anterior mitral valve leaflet (AMVL) in the left ventricular outflow tract (LVOT) in systole, which may result in systolic anterior motion (SAM) of the AMVL and potential LVOT obstruction and paravalvular leak (PVL).6 We report a case of profound intravascular hemolysis causing acute kidney injury because of PVL and LVOT obstruction following Tendyne TMVR. Although PVL closure was attempted, definitive treatment was achieved with transcatheter aortic valve replacement (TAVR), which corrected both PVL and LVOT obstruction, with complete resolution of hemolysis and improvement of renal function.
Case Presentation
A 76-year-old man presented with exertional breathlessness. He had previously undergone coronary artery bypass grafting in 2004 and had a patent left internal mammary arterial graft to the left anterior descending coronary artery, a patent radial graft to the circumflex artery, and an occluded saphenous vein graft to the right coronary artery. He had hypertension and previous transitional cell carcinoma of the bladder (treated with intravesical mitomycin under annual surveillance). On examination, he weighed 75 kg, his body mass index was 25 kg/m2, he was in New York Heart Association functional class II/III and sinus rhythm (heart rate 58 beats/min), and his blood pressure was 166/56 mm Hg; venous pressure was not elevated, heart sounds were dual, and he had a pansystolic murmur, loudest in the mitral region, associated with soft aortic diastolic murmur. He had no peripheral edema, and his lung fields were clear. Twelve-lead electrocardiography confirmed sinus rhythm with first-degree atrioventricular block (PR interval 286 msec), incomplete left bundle branch block (QRS duration 109 msec), and normal cardiac axis.
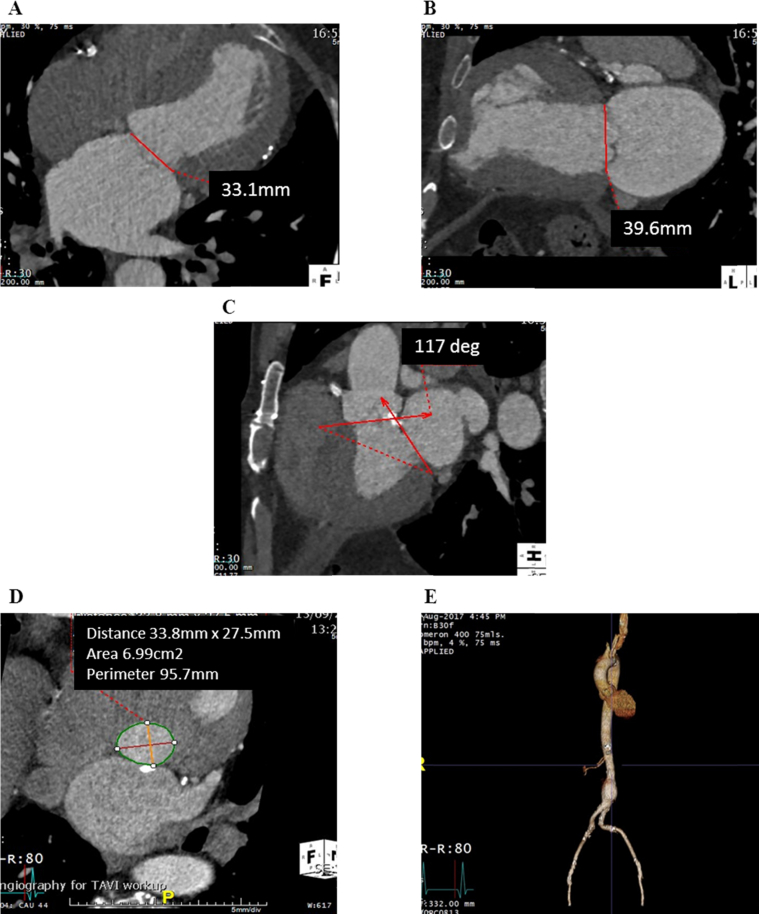
Transthoracic echocardiography (TTE) documented a dilated left ventricle (end-diastolic dimension 6.7 cm) with a left ventricular (LV) ejection fraction of 65%, apical septal infarct, severe MR, mild aortic stenosis, and significant aortic regurgitation (AR). Real-time three-dimensional (3D) transesophageal echocardiographic assessment of the mitral valve confirmed severe MR due to a restricted posterior mitral valve leaflet and chordal rupture of the lateral aspect of A2 and the medial aspect of A3 (Videos 1 and 2). Multislice computed tomographic imaging reported mildly calcified aortic valve leaflets (calcium score 1,370 Agatston units), aortic annular perimeter of 95 mm, an infrarenal abdominal aortic aneurysm (measuring 35 × 35 × 69 mm), and focal plaques of calcified atheromatous disease throughout the thoracoabdominal aorta but no significant luminal aortic stenosis (Figure 1).
Figure 1.
Preprocedural multislice computed tomographic assessment. The mitral annulus measured 33.1 mm in anteroposterior diameter (A) and 39.6 mm in intercommissural diameter (B), with an aortomitral angle of 117° (C). The aortic annular circumference was 95.7 mm (D), and an infrarenal aneurysm was noted (E).
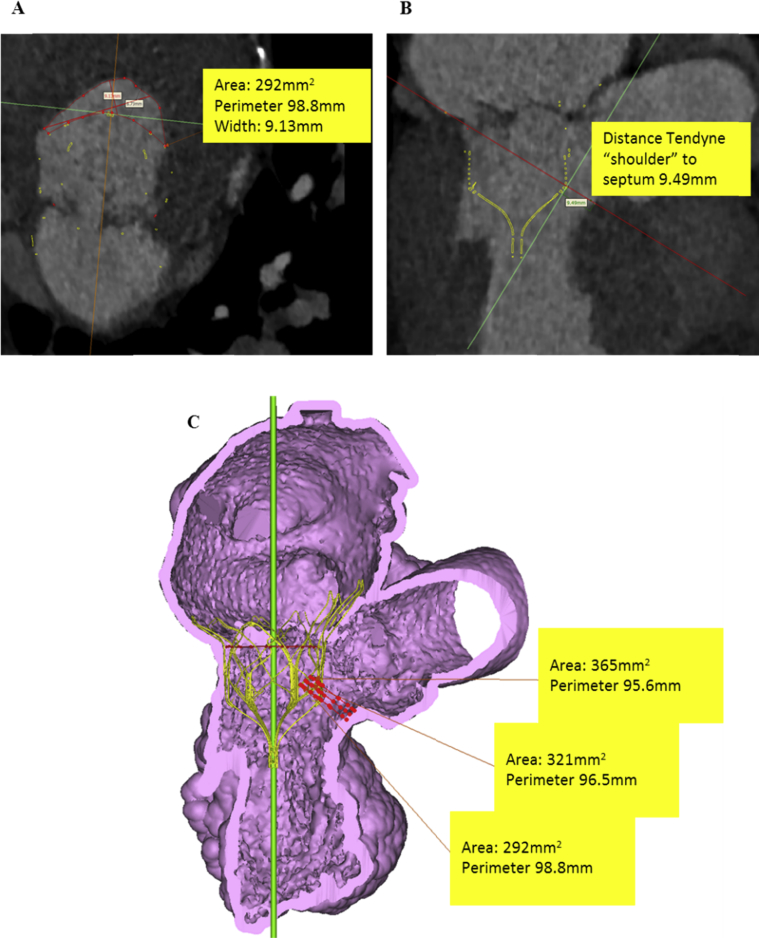
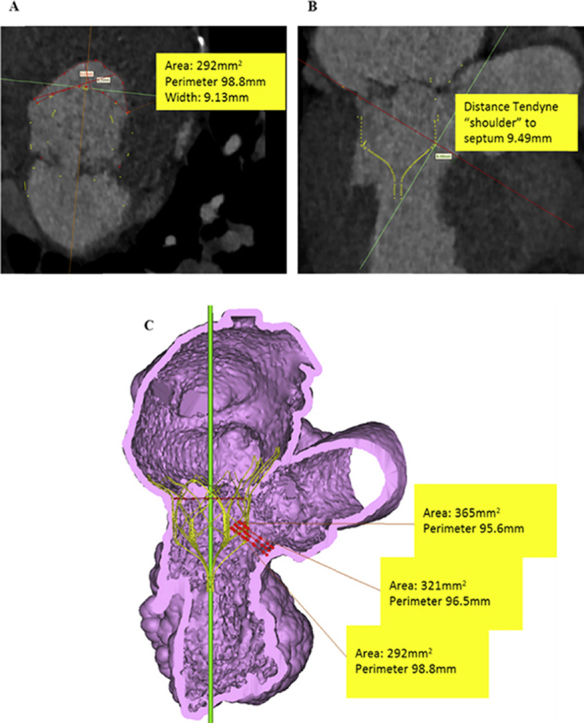
The case was discussed at a structural heart multidisciplinary meeting. The patient was deemed high risk for conventional mitral and aortic valve replacement because of patent retrosternal arterial grafts and a European System for Cardiac Operative Risk Evaluation II score of 9.26%. His dominant valvular lesion was felt to be MR, and potential transcatheter options were discussed: the MitraClip device was not available at the time of assessment, and he was anatomically unsuitable for any other Conformité Européenne–marked device available. Postprocessing simulation of a Tendyne device within the mitral annulus on the basis of multislice computed tomographic images predicted a neo-LVOT > 250 mm2 (Figure 2), and the patient was accepted for TMVR with a Tendyne device.
Figure 2.
Assessment of predicted LVOT dimensions with simulated valve superimposed on multislice computed tomographic images. Neo-LVOT area was 292 mm2 (A), with the smallest clearance from the shoulder of the device to the septal wall of 9.49 mm (B). On volume-rendered computed tomography, the smallest predicted LVOT area was 292 mm2(C). At end-systole, a neo-LVOT area of 250 mm2 and clearance distance from the shoulder of device to the septum of 5 mm suggested a low possibility of LVOT obstruction after Tendyne implantation. Septal bump, aortomitral angle, length and shape of outflow, and anterior mitral leaflet characteristics and dynamics are other important factors.
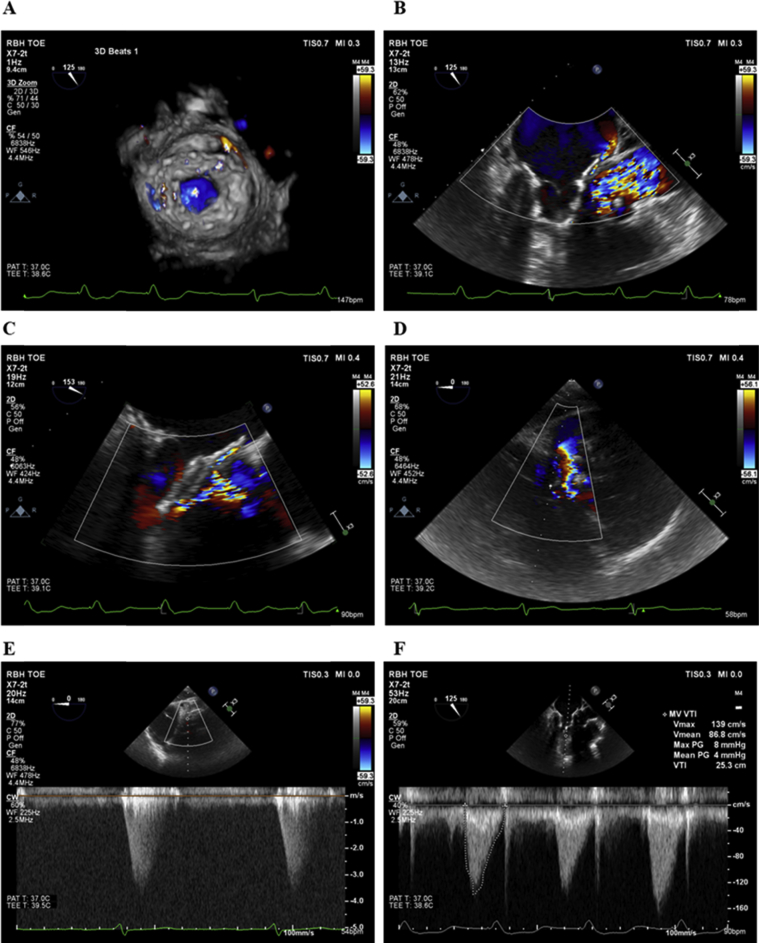
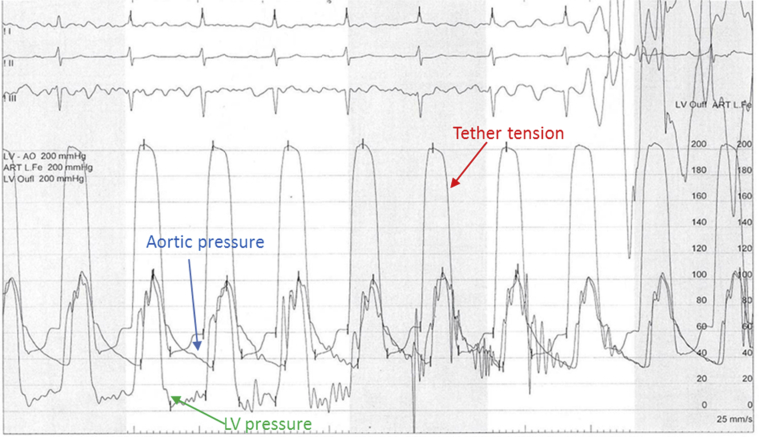
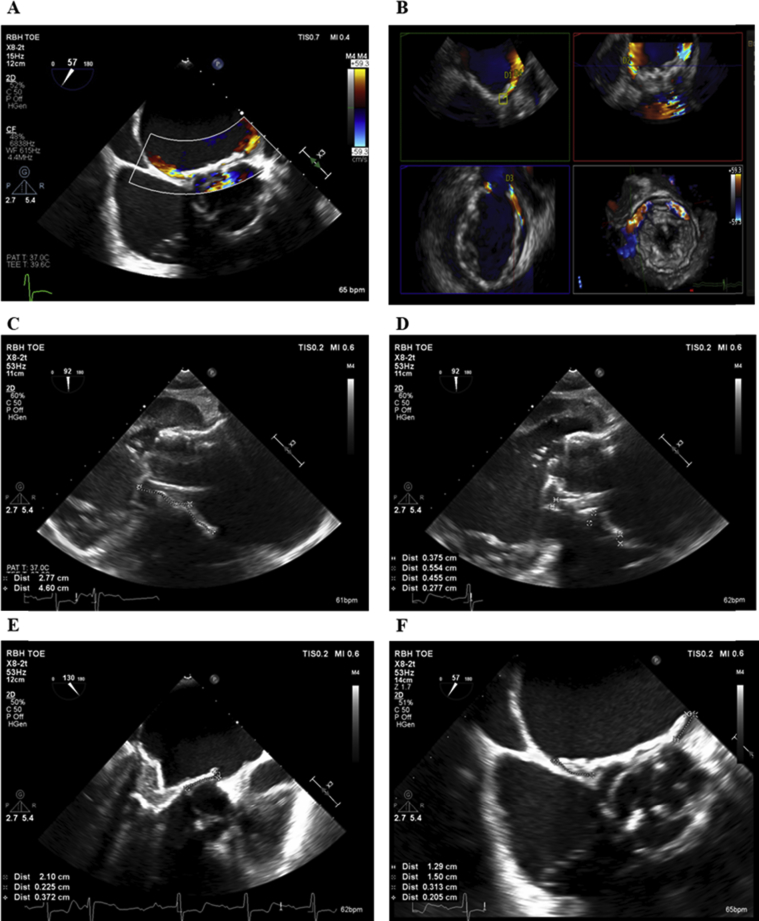
The patient gave informed consent for TMVR with a Tendyne device. The procedure was performed through a small left anterior thoracotomy under general anesthesia (full procedural details were reported previously7). The LV apex was exposed and noted to be extremely friable, with bleeding from epicardial veins. A Tendyne device was implanted under continuous real-time 3D transesophageal echocardiographic guidance (Video 3), with the valve positioned in a good location on both transesophageal echocardiography (TEE; Video 4) and fluoroscopy (Video 5). Mild PVL was noted (Figure 3), as was SAM from the mobile native AMVL, which resulted in a dynamic periprocedural LVOT gradient of 58 mm Hg on TEE. However, at a tether tension of 203 mm Hg, LV pressure was 95/37 mm Hg and aortic pressure was 108/34 mm Hg, resulting in a pull-back gradient on fluoroscopy of 13 mm Hg (Figure 4). Thus, we elected not to retrieve the Tendyne valve at that time.
Figure 3.
Periprocedural TEE. Mild anterolateral PVL (A, B) with flow acceleration in LVOT (C, D), peak LVOT gradient of 58 mm Hg (E), and mean transvalvular mitral gradient of 4 mm Hg (F).
Figure 4.
Hemodynamics at end of procedure. At a tether tension of 200 mm Hg, there was no significant pull-back gradient from the LVOT to the aorta. Tether tension is the pressure exerted on the apical pin when pulling the Tendyne device down toward the LV apex by the Tendyne tether device before final apical pin fixation.
The patient was hemodynamically stable off all inotropes periprocedurally and postoperatively and remained well on the ward. He was discharged on day 8 with hemoglobin of 146 g/L (normal range, 134–166 g/L), urea of 8.0 mmol/L (normal range, 2.5–7.8 mmol/L), creatinine of 93 μmol/L (normal range, 60–120 μmol/L), estimated glomerular filtration rate of 68 mL/min/1.73 m2 (normal range, 60 mL/min/1.73 m2), bilirubin of 17 μmol/L (normal range, 0–20 μmol/L), liver alanine transaminase of 19 IU/L (normal range, 8–40 IU/L), and albumin of 42 g/L (normal range, 35–50 g/L). No PVL was noted on predischarge TTE, and the peak LVOT gradient was reported as 25 mm Hg.
The patient presented again 2 weeks after discharge feeling nonspecifically unwell. On examination, he was anemic and jaundiced. He was on no known hepatotoxic medications. TTE documented a well-seated Tendyne device with trivial PVL, a mobile AMVL resulting in SAM (Video 6), an LVOT gradient of 26 mm Hg, and moderate to severe AR. Laboratory investigations reported anemia and deranged liver function but normal renal function: hemoglobin 86 g/L, urea 7.0 mmol/L, creatinine 76 μmol/L, estimated glomerular filtration rate 86 mL/min/1.73 m2, bilirubin 55 μmol/L, alanine transaminase 119 IU/L, and albumin 32 g/L. Further investigations reported a haptoglobin level of <0.3 g/L (normal range, 0.6–2.9 g/L) and a grossly elevated lactate dehydrogenase level of 7,635 IU (normal range, 266–500 IU/L). Three-dimensional TEE reported a well-positioned Tendyne device that had not altered in position or rotation since the final periprocedural images, and there was no qualitative change in the Tendyne tether tension. However, the Tendyne appeared to “seesaw” across a bar of calcification in the center of the anterior annulus, and two PVL jets were easily identified either side of this calcification, one anteromedially and another anterolaterally (Video 7). Although the mobile AMVL and SAM were visualized (Video 8), the peak LVOT gradient on TEE was only 15 mm Hg. Cardiac computed tomography was performed (Figure 5), which confirmed appropriate positioning of the Tendyne device. The patient remained hemodynamically stable and was initially treated medically with packed red cells and β-blockade.8
Figure 5.
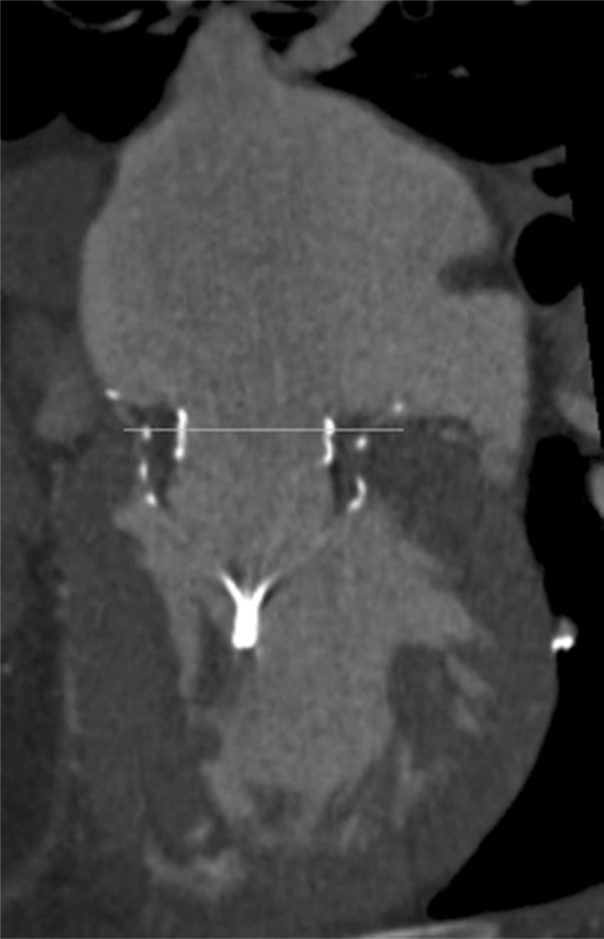
Postprocedural multislice computed tomography documenting well-seated intra-annular position of Tendyne device (medial and lateral aspects of the device positioned at the level of the mitral annulus with no rocking or movement of the device).
However, 2 days later, the patient developed very rapid acute kidney injury, with urea and creatinine rising from normal to 29 mmol/L and 463 μmol/L, respectively, accompanied by a significant reduction in estimated glomerular filtration rate. Autoantibody, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura renal screens were all negative, and renal ultrasound demonstrated normal kidney echo texture and size, no hydronephrosis, and a collapsed urinary bladder containing a catheter. Random urinary albumin was grossly elevated at 524 mg/L (normal range, 0–19 mg/L), as was the urine albumin/creatinine ratio at 476 mg/mol (normal range, 0–3.0 mg/mmol). The etiology of the patient's acute kidney injury was assumed secondary to intravascular hemolysis (plasma [i.e., free] hemoglobin level 2.9 g/L [normal range, <0.3 g/L]), and the patient was transferred to intensive care unit for renal support with hemofiltration.
The case was again discussed among the structural heart team. Initial consideration was given to retensioning the Tendyne device, but this option was rejected, in part because the LV apex had been very friable with LV bleeding at the time of Tendyne surgery and in part because the Tendyne was felt to be in a good position within the mitral annulus, and further tensioning of the tether might make the Tendyne device more vertical and worsen the PVL and LVOT gradient. Percutaneous PVL closure with device(s) was suggested as an alternative therapeutic option, but there was concern that this might cause lifting of the Tendyne device and subsequent worsening of the PVL. Following Tendyne TMVR, the native mitral valve leaflet is fixed in an “open” position (i.e., the “SAM” position) and thus can obstruct the LVOT. In addition, because the PVL jets were mostly anterior, SAM itself could have been accentuating the PVL. Alternatively, the AR jet, directed straight onto the anterior aspect of the Tendyne device prosthesis, might have been accentuating SAM. Potential treatment with LVOT placement of a TAVR, which might have the dual benefit of relieving LVOT obstruction caused by SAM while relieving the AR while alleviating the SAM was considered. However, there were potential issues with TAVR, including a large aortic annulus (95 mm), relatively noncalcified aortic valve leaflets, and problematic femoral access. Alcohol septal ablation, offered to patients with septal hypertrophy who develop SAM and LVOT obstruction after TMVR, was discussed. Finally, consideration was given performing a LAMPOON (laceration of the anterior mitral leaflet to prevent LVOT obstruction) procedure, whereby the AMVL could be cauterized using a transseptal and retrograde aortic approach,9 potentially reducing the buildup of high LV pressure behind the AMVL contributing to the PVL.
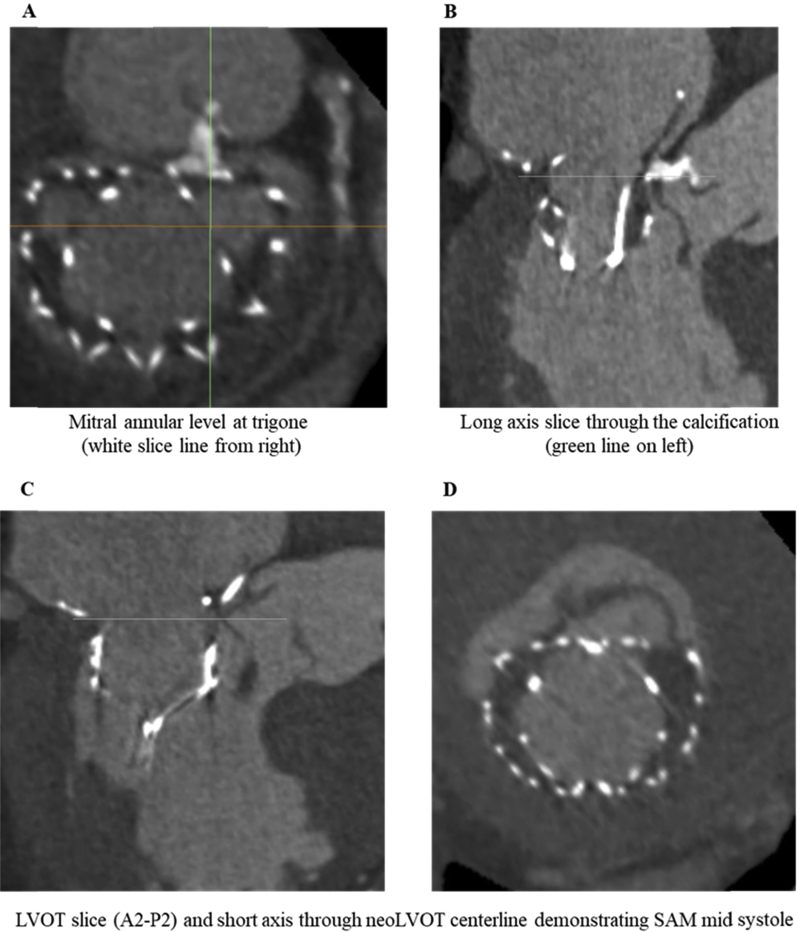
The postprocedural real-time 3D transesophageal echocardiographic and multislice computed tomographic images were re-reviewed. Seating depth of the Tendyne valve was deemed appropriate, with the level of the mitral annulus at the point of transition between the atrial cuff and sealing body of the valve circumferentially (Figure 4). However, a very focal area of calcification at A2 (midanterior aspect of the AMVL), 1.2 cm in length, and noted on preprocedural 3D TEE (Video 2), was found to protrude 4 mm into the bioprosthetic Tendyne valve between the A2 (anterior) and A3 (anteromedial) region of the anterior mitral annulus (Figure 6), causing a 4-mm gap between the anterior mitral annulus and Tendyne valve.
Figure 6.
Postprocedural multislice computed tomography. Defined area of calcification noted in A2/A3 region of mitral valve, approximating to the D-shaped portion of the Tendyne device (A). SAM (B–D) with contrast connection in conduit between left ventricle and left atrium.
In the first instance, we elected to attempt to address the PVL with closure devices under general anesthesia, which we believed was the least invasive therapy. A transseptal puncture was performed using a BRK needle (St. Jude Medical, St. Paul, MN) under continuous real-time 3D TEE, and transesophageal echocardiographic measurements were made to size the Amplatzer device of choice for both PVL sites (Figure 7).
Figure 7.
Real-time 3D TEE to locate and size the PVLs. Two jets were identified, one anterolateral and one anteromedial (A, B). The anterolateral defect measured 2.77 mm in length (C), with the tunnel width between device and annulus/left atrial wall device 2.77 to 5.54 mm (D). The anteromedial defect measured 2.1 cm in length (E), with a tunnel width of 0.31 mm (F).
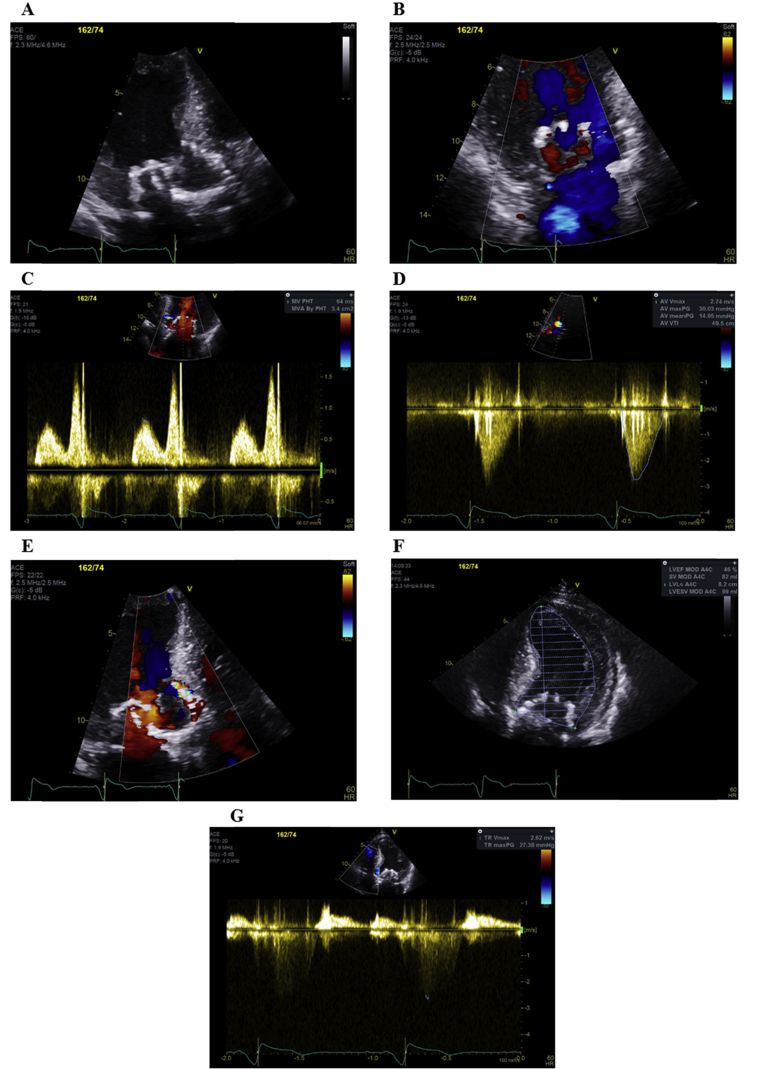
Real-time 3D TEE was then used to direct placement of an Agilis Steerable introducer (St. Jude Medical) to the anterolateral PVL (Videos 9 and 10). A 12-mm Amplatzer Vascular Plug II device (St. Jude Medical) was deployed in the correct position (Video 11). However, the Amplatzer device resulted in “lifting” of the Tendyne device (Videos 12 and 13), worsening of the PVL, and distortion of the central mitral valve orifice (Video 14) and was removed with a 15-mm goose-necked snare. Instead, a 34-mm Evolut R TAVR device (Medtronic, Minneapolis, MN) was positioned deliberately low in the LVOT under rapid LV pacing (Videos 15 and 16). The patient had three periarrest episodes with ventricular tachycardia at points when the underdeployed TAVR was obstructing cardiac output, but eventual deployment of the TAVR device in the correct position resulted in instantaneous removal of SAM and resolution of PVL. Correction of PVL removed the intracardiac hemolysis driving acute renal failure, such that plasma hemoglobin fell dramatically (from 2.9 to 0.3 g/L) within the next 24 hours. The patient required less frequent and finally no hemofiltration; on discharge, the urea had decreased to 15 mmol/L and creatinine had fallen to 266 μmol/L. A biventricular pacemaker was required for complete heart block after transcatheter aortic valve implantation. On discharge (4 weeks after re-presentation), TTE reported a well-seated Tendyne with no PVL, a well-seated TAVR with peak gradient of 28 mm Hg, and mild to moderate PVL, LV ejection fraction of 48%, and normal right ventricular size and function with right ventricular systolic pressure of 27 mm Hg (Figure 8). The patient's acute kidney injury continued to improve, and 8 weeks after TAVR, urea was 12 mmol/L, creatinine 172 μmol/L, and glomerular filtration rate 33 mL/min/1.73 m2.
Figure 8.
On TTE, the Tendyne TMVR was well seated (A) with no PVL (B) or mitral stenosis (C), the TAVR device was well-seated (A) with a peak gradient of 30 mm Hg (D) and mild to moderate PVL (E), LV ejection fraction of 48% (F), and right ventricular systolic pressure of 27 mm Hg (G).
Discussion
Acute kidney injury may occur with hemoglobin breakdown products, because hemoglobin proteins are toxic to tubular epithelial cells, causing acute tubular necrosis.10 Biochemical diagnosis of hemolysis is based on normocytic anemia, raised plasma hemoglobin, raised lactate dehydrogenase, elevated unconjugated bilirubin, reduced haptoglobin (a protein that binds to free hemoglobin released in active hemolysis), raised reticulocyte count (>2%), and red blood cell fragments and polychromasia on a blood film examination.
PVL is an uncommon but potentially serious complication following valve replacement surgery, resulting from incomplete apposition of the prosthetic sewing ring to the native annulus, leading to isolated or multiple blood flow jets through the communication between the two heart chambers related to the valve. The prevalence of mitral PVL following conventional mitral valve surgery is 7% to 17%11 but may be as high as 20% after TMVR.6 Shear stress placed on red blood cells passing through the abnormal channel and associated high-velocity jets across a nonendothelialized surface cause intravascular hemolytic anemia, which may be clinically subacute6 or result in profound hemolysis.12 Resolution of severe hemolysis has been reported after successful PVL closure.13, 14
In the present case, we attempted PVL closure15 even though we thought this might prove technically difficult because of the unique Tendyne device shape (we believed that this was a lower risk procedure than TAVR). Although challenging, we successfully placed a guidewire and catheter across the defect with deployment of the closure device with the aid of continuous real-time 3D TEE. However, placement of the Amplazter device resulted in lifting of the Tendyne, with worsening of the PVL. This may be because specific closure devices for PVL following Tendyne TMVR have yet to be developed. We went on to deploy a TAVR device low in the LVOT, which successfully reduced the high-velocity jet across the PVL by relieving LVOT obstruction caused by SAM of the AMVL. Our group had previous experience of treating SAM causing LVOT obstruction in a patient who had undergone Tendyne TMVR by inserting a cardiopulmonary stent into the LVOT,6 but that patient had no aortic valve disease, and we have not identified a case in the literature in which LVOT obstruction after any TMVR device was treated with a TAVR device. Removal of SAM resolved the PVL and reversed the patient's hemolysis within 24 hours, while relieving the patient of his AR.
Conclusions
We present a complex case of acute kidney injury after TMVR, caused by massive intravascular hemolysis, driven by the presence of two small but significant PVLs, and accentuated by SAM of the residual AMVL. The heart team required cardiologists, cardiac surgeons, and cardiac imagers experienced in TMVR therapy, transseptal puncture, PVL closure, and TAVR therapy. Successful placement of a transcatheter aortic valve device removed SAM of the AMVL and obliterated the high-velocity jets across the PVLs, which in turn resolved the drive for severe intravascular hemolysis and resulted in recovery of our patient's renal function.
Acknowledgments
We acknowledge Tom Vilkama and Neil Moat, who provided clinical advice on the management of this patient.
Footnotes
Conflicts of interest: Dr. Duncan, Dr. Quarto, and Dr. Yadav have received honoraria from Abbott. All other authors report no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2019.02.005.
Supplementary Data
Long axis two-dimensional TEE of the mitral valve showing chordal rupture of the AMVL and restriction of the posterior mitral valve leaflet.
Real-time 3D TEE, en face view of the mitral valve from the left atrium, confirming chordal rupture of the lateral aspect of A2 and the medial aspect of A3 and restricted posterior mitral valve leaflet.
Continuous real-time X-plane imaging on TEE showing deployment of the Tendyne in the mitral valve annulus.
Real-time 3D TEE, en face view, of the Tendyne device from the left atrium, confirming correct clocking of the outer frame of the Tendyne device and central orifice with three mobile neo–mitral valve leaflets.
Correct Tendyne positioning on fluoroscopy.
A well-seated Tendyne device but SAM of the mitral valve into the LVOT on TTE.
Real-time 3D TEE, en face view, of the Tendyne device from the left atrium showing two jets of PVL (one anteromedially and another anterolaterally).
Long-axis two-dimensional TEE of the Tendyne device showing a mobile AMVL with SAM into the LVOT
Real-time 3D TEE used to direct placement of an Agilis Steerable introducer to the anterolateral PVL.
Placement of an Agilis Steerable introducer to the anterolateral PVL on fluoroscopy.
Amplatzer Vascular Plug deployed in the anterior-lateral aspect of the Tendyne device on fluoroscopy.
Anterior “lifting” of the Tendyne device on continuous real-time X-plane imaging after placement of the Amplatzer plug.
Real-time 3D TEE en face view of the Tendyne device showing widening of the gap between the Tendyne device and the mitral annulus after placement of the Amplatzer plug.
Long-axis two-dimensional TEE of the Tendyne device showing worsening of the PVL and distortion of the central mitral valve orifice shown by increased transvalvular color-flow turbulence.
Long-axis two-dimensional TEE showing low deployment of a TAVR device to “pin back” the AMVL and prevent SAM of the AMVL.
Position of the TAVR device confirmed against the anterior aspect of the Tendyne device on fluoroscopy.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Mirabel M., Iung B., Baron G., Messika-Zeitoun D., Détaint D., Vanoverschelde J.L. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365. doi: 10.1093/eurheartj/ehm001. [DOI] [PubMed] [Google Scholar]
- 3.Perpetua E.M., Reisman M. The Tendyne transcatheter mitral valve implantation system. EuroIntervention. 2015;11(suppl W):W78–W79. doi: 10.4244/EIJV11SWA23. [DOI] [PubMed] [Google Scholar]
- 4.Moat N.E., Duncan A., Quarto C. Transcatheter mitral valve implantation: Tendyne. EuroIntervention. 2016;12(suppl Y):Y75–Y77. doi: 10.4244/EIJV12SYA20. [DOI] [PubMed] [Google Scholar]
- 5.Muller D.W.M., Farivar R.S., Jansz P., Bae R., Walters D., Clarke A. Tendyne Global Feasibility Trial Investigators. Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J Am Coll Cardiol. 2017;69:381–391. doi: 10.1016/j.jacc.2016.10.068. [DOI] [PubMed] [Google Scholar]
- 6.Duncan A., Daqa A., Yeh J., Davies S., Uebing A., Quarto C. Transcatheter mitral valve replacement: long-term outcomes of first-in-man experience with an apically tethered device- a case series from a single centre. EuroIntervention. 2017;13:e1047–e1057. doi: 10.4244/EIJ-D-17-00154. [DOI] [PubMed] [Google Scholar]
- 7.Quarto C., Davies S., Duncan A., Lindsay A., Lutter G., Lozonschi L. Transcatheter mitral valve implantation: 30-day outcome of first-in-man experience with an apically tethered device. Innovations (Phila) 2016;11:174–178. doi: 10.1097/IMI.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 8.Okita Y., Miki S., Kusuhara K., Ueda Y., Tahata T., Yamanaka K. Propranolol for intractable hemolysis after open heart operation. Ann Thorac Surg. 1991;52:1158–1160. doi: 10.1016/0003-4975(91)91301-b. [DOI] [PubMed] [Google Scholar]
- 9.Khan J.M., Lederman R.J., Devireddy C.M., Clements S.D., Jr., Kamioka N., Yousef A. LAMPOON to facilitate Tendyne transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2018;11:2014–2017. doi: 10.1016/j.jcin.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian Q., Nath K.A., Wu Y., Daoud T.M., Sethi S. Hemolysis and acute kidney failure. Am J Kidney Dis. 2010;56:780–784. doi: 10.1053/j.ajkd.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammermeister K., Sethi G.K., Henderson W.G., Grover F.L., Oprian C., Rahimtoola S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the veterans affairs randomised trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 12.Shapira Y., Vaturi M., Sagie A. Hemolysis associated with prosthetic heart valves: a review. Cardiol Rev. 2009;17:121–124. doi: 10.1097/CRD.0b013e31819f1a83. [DOI] [PubMed] [Google Scholar]
- 13.Kiefer T.L., Vavalle J., Hurwitz L.M., Hughes G.C., Harrison J.K. Resolution of severe hemolysis and paravalvular aortic regurgitation employing an Amplatzer Vascular Plug 4: the importance of detailed pre-procedural planning using CT angiography. Cardiovasc Interv Ther. 2017;32:48–52. doi: 10.1007/s12928-015-0363-z. [DOI] [PubMed] [Google Scholar]
- 14.Panaich S.S., Maor E., Reddy G., Raphael C.E., Cabalka A., Hagler D.J. Effect of percutaneous paravalvular leak closure on hemolysis. Catheter Cardiovasc Interv. 2019;93:713–719. doi: 10.1002/ccd.27917. [DOI] [PubMed] [Google Scholar]
- 15.Taramasso M., Maisano F., Pozzoli A., Alfieri O., Meier B., Nietlispach F. Catheter-based treatment of paravalvular leaks. EuroIntervention. 2016;12(suppl X):X55–X60. doi: 10.4244/EIJV12SXA11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long axis two-dimensional TEE of the mitral valve showing chordal rupture of the AMVL and restriction of the posterior mitral valve leaflet.
Real-time 3D TEE, en face view of the mitral valve from the left atrium, confirming chordal rupture of the lateral aspect of A2 and the medial aspect of A3 and restricted posterior mitral valve leaflet.
Continuous real-time X-plane imaging on TEE showing deployment of the Tendyne in the mitral valve annulus.
Real-time 3D TEE, en face view, of the Tendyne device from the left atrium, confirming correct clocking of the outer frame of the Tendyne device and central orifice with three mobile neo–mitral valve leaflets.
Correct Tendyne positioning on fluoroscopy.
A well-seated Tendyne device but SAM of the mitral valve into the LVOT on TTE.
Real-time 3D TEE, en face view, of the Tendyne device from the left atrium showing two jets of PVL (one anteromedially and another anterolaterally).
Long-axis two-dimensional TEE of the Tendyne device showing a mobile AMVL with SAM into the LVOT
Real-time 3D TEE used to direct placement of an Agilis Steerable introducer to the anterolateral PVL.
Placement of an Agilis Steerable introducer to the anterolateral PVL on fluoroscopy.
Amplatzer Vascular Plug deployed in the anterior-lateral aspect of the Tendyne device on fluoroscopy.
Anterior “lifting” of the Tendyne device on continuous real-time X-plane imaging after placement of the Amplatzer plug.
Real-time 3D TEE en face view of the Tendyne device showing widening of the gap between the Tendyne device and the mitral annulus after placement of the Amplatzer plug.
Long-axis two-dimensional TEE of the Tendyne device showing worsening of the PVL and distortion of the central mitral valve orifice shown by increased transvalvular color-flow turbulence.
Long-axis two-dimensional TEE showing low deployment of a TAVR device to “pin back” the AMVL and prevent SAM of the AMVL.
Position of the TAVR device confirmed against the anterior aspect of the Tendyne device on fluoroscopy.