Abstract
Although skeletal muscle is highly regenerative following injury or disease, endogenous self-regeneration is severely impaired in conditions of volume traumatic muscle loss. Consequently, tissue engineering approach is a promising approach to regenerate skeletal muscle. Biological scaffolds serve as not only structural support for the promotion of cellular ingrowth, but they also impart potent modulatory signaling cues that may be beneficial for tissue regeneration. In this work, the progress of tissue engineering approaches for skeletal muscle engineering and regeneration is overviewed, with a focus on the techniques to create biomimetic engineered tissue using extracellular cues. These factors include mechanical and electrical stimulation, geometric patterning, and delivery of growth factors or other bioactive molecules. We further describe the progress of evaluating the therapeutic efficacy of these approaches in preclinical models of muscle injury.
Keywords: skeletal muscle regeneration, biomaterials, spatial patterning, volumetric muscle loss, Skeletal Muscle Tissue Engineering
Table of Contents:
Skeletal muscle tissue engineering approaches are reviewed, with a focus on the effects of biomaterials, soluble factors, mechanical factors that modulate skeletal muscle morphogenesis and function. The therapeutic efficacy of engineered muscle in preclinical settings of muscle injury is then described.
1. Introduction
Skeletal muscle is an abundant tissue type that is responsible for locomotion and movement. Physiologically, skeletal muscle has a natural ability to regenerate following injury. However, once a critical mass of muscle becomes damaged, endogenous self-repair becomes severely impaired, resulting in the loss of muscle functional capacity. Severe muscle trauma that results in volumetric muscle loss (VML) is commonly seen in patients suffering from combat and blast injuries, motor vehicle accidents, occupational machine and sports injuries, and gunshot wounds. The commonality among these conditions is severe trauma that results in the formation of non-functional fibrous tissue and degeneration of fatty muscle.[1] Current clinical treatments include engraftment of autologous local muscle flaps known as free functional muscle transfer (FFMT). However, when suitable muscle flaps are limited, FFMT treatment can lead to complications such as donor site morbidity and often necessitates coupling the procedure with extensive physical rehabilitation. Despite FFMT serving as the current standard of care, this approach does not carry a guarantee of full restoration of muscle function to pre-injury conditions.[2] Towards the clinical goal of long-term functional integration and recovery, tissue engineering and regenerative medicine offer a promising therapeutic approach to engineer patient-specific musculoskeletal replacements that can augment muscle function, stimulate regeneration, and improve quality of life.
As described by Cezar and Mooney[3], there are two basic approaches for the engineering of skeletal muscle. In the in vitro tissue engineering approach, tissue constructs are developed with structural and functional properties similar to that of the native tissue prior to in vivo transplantation. A requisite for developing skeletal muscle in vitro is the differentiation of skeletal muscle myoblasts or muscle precursors into multi-nucleated myotubes. The second approach is in situ tissue engineering, which utilizes the properties of the engineered construct to serve as a supportive niche for the delivery of cells and/or inductive factors for later remodeling by the host environment. A number of bioengineering techniques aim to develop biomimetic engineered skeletal muscle by mimicking the microenvironmental cues experienced by the native muscle. These cues include mechanical stimulation, electrical stimulation, and biochemical signaling by growth factors and other biomolecules. In this work, we will discuss the progress of scaffold-based tissue engineering, focusing on the role of microenvironmental factors in modulating skeletal muscle structure, function, regeneration, and neurovascularization (Figure 1).
Figure 1.
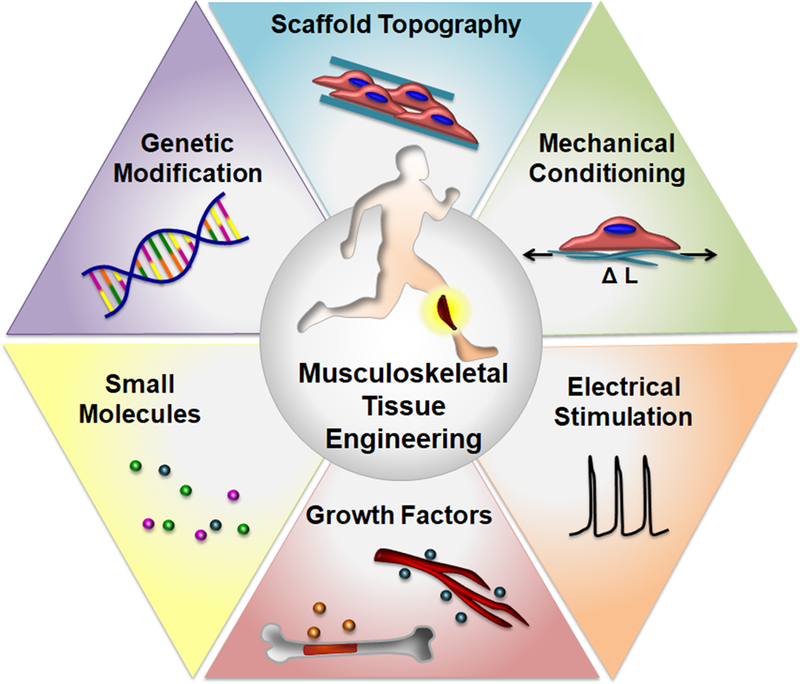
Overview of bioengineering approaches for skeletal muscle tissue engineering
2. Skeletal Muscle Organization and Regeneration
Skeletal muscle is hierarchically organized and composed of laterally integrated myofibers, vasculature, and nerves.[4] Parallel-aligned myofibers are bundled together to form fascicles that are each surrounded by a dense network of branched capillaries that support the high metabolic demands of skeletal muscle.[5] Muscle macro-architecture arises from the organization of fascicles into a muscle tissue unit held together by connective tissue. Sparingly interspersed among a large number of muscle fibers are skeletomotor neurons that function in synchronicity to induce contraction through coupling of neuromuscular junctions. At a minimum, these three critical components and their corresponding highly oriented structures should be recapitulated to mimic the physiological structure of muscle.
Endogenous repair of skeletal muscle occurs through the execution of a coordinated healing response that involves three phases. During the initial 24 hours, ruptured myofibers, torn blood vessels and severed nerves lead to rapid necrosis and activation of local mononuclear inflammatory cells such as neutrophils.[6, 7] This phase is also associated with increased cytokine production, including that of tumor necrosis factor-α (TNF-α), insulin-like growth factor (IGF) and several interleukins.[8] During the repair and fibroplasia phase (1 to 5 days after injury), the ruptured myofibers and necrotic tissue undergo phagocytosis by circulating monocytes. Concomitantly, resident multipotent myogenic stem cells known as satellite cells,[9] which reside between the basal lamina and sarcolemma, are activated by M1 (pro-inflammatory) macrophages with the aid of M2 (anti-inflammatory) macrophages. Upon activation, the satellite cells form muscle progenitor cells that turn into myoblasts.[10] Fusion of the individual myoblasts gives rise to new multi-nucleated myofibers.[6, 11] During this phase, the tissue experiences the invasion of blood vessels and nerves, while fibroblasts form scar tissue to bridge the gap between the ruptured myofibers. In the remodeling phase (>5 days after injury), the scar tissue in regions of small volume injuries is replaced with newly formed myofibers that close the myofiber stumps. In contrast, for large injuries, the tissue experiences a transformation of local fibroblasts into myofibroblasts, leading to contraction of the granulation scar tissue. In the case of VML, the rate of scar tissue formation outpaces the rate of myoblast differentiation and maturation such that a thick non-functional scar obstructs the fusion of the myofiber stumps.[12] The molecular pathways involved in the formation of new muscle involve myogenic regulatory factors such as MyoD, myogenin, and the paired box (Pax) transcription factors of Pax3 and Pax7.[13] Terminal differentiation into contractile units is concomitant with the expression of sarcomeric proteins such as skeletal muscle myosin heavy chain (MHC).[14] The expression of MHC isoforms is often a measure of in vitro myotube differentiation and efficiency. Understanding the phases of muscle regeneration and the molecular expression pathways may provide insight for mimicking physiologically relevant interactions and pathways for regenerating functional skeletal muscle.
In addition to genetic pathways, biophysical and biochemical cues from the extracellular matrix (ECM) play a directive role in endogenous cell-mediated muscle regeneration. Skeletal muscle is composed of three layers of connective tissues. The innermost layer is the endomysium that surrounds individual muscle fibers and is largely made of collagen type III. The intermediate layer is the perimysium that surrounds the fascicles and is primarily composed of collagen type I. The epimysium is the outermost layer that surrounds the entire muscle and contains the extracellular fluids.[15] Additionally, the basement membrane which is situated between muscle fibers can be subdivided into an outer reticular lamina (collagen types I and III and fibronectin) and the inner basal lamina (collagen type IV).[16] Two transmembrane receptors, dystrophin-associated glycoprotein complex (DGC) and the α7β1 integrin, are largely responsible for the transmission of lateral mechanical force in skeletal muscle.[17] In muscle development, injury and repair, the ECM plays an important role in dynamically regulating endogenous cell activity.[18] Following mechanical loading, the production of collagen types I, III, and IV increases concomitant with an increase in MMP-2 and MMP-9 enzymes that target the degradation and turnover of ECM components.[19, 20] This ECM remodeling has been linked to an accumulation of Pax7+ muscle satellite cells in type II fibers where intracellular MMP-2 expression is localized.[21] MMP-2 activity has also been implicated in stretch-induced activation of these satellite cells as a function of nitric oxide (NO) production.[22] The ECM itself plays an active role in the activation of resident muscle stem cells. When skeletal muscle is injured, the ECM located close to dormant satellite cells is induced by NO-activated MMP-2 to release hepatocyte growth factor (HGF), thereby activating these dormant stem cells to enter the cell cycle and undergo myogenesis and the process of muscle repair and regeneration.[22, 23]
3. Biomechanical and Biochemical Factors in Skeletal Muscle Tissue Engineering
3.1. Biomaterials for Skeletal Muscle Tissue Engineering
Biomaterials with tunable characteristics are important tools for in vitro tissue engineering and in vivo regeneration. Biomimetic scaffolds for musculoskeletal engineering aim to recapitulate the major cellular and tissue functions by modulating cellular attachment, survival, organization, and differentiation. Among the commonly used biomaterials include ECM derivatives such as collagen,[16, 24, 25] the most abundant structural protein in skeletal muscle,[26] fibrin,[27, 28] gelatin,[29] polysaccharides such as hyaluronic acid (HA),[30, 31] chitosan,[32, 33] keratin,[34] alginate,[35], and decellularized matrices. [36–38] Natural biomaterials have the advantage of generally being biocompatible and primed for enzymatic degradation, while possessing functional groups that allow for easy conjugation with small molecules and growth factors. Additionally, several structural properties such as porosity, topography, and stiffness/rigidity can be modulated. These materials can be molded to fit unique volumes, making them ideal for conforming to the complex defect shapes of muscle injuries. However, they are limited by batch-to-batch variability in chemical composition, and some components may pose issues with immunogenicity.[39]
Beside naturally derived ECMs, synthetic polymers such as poly-L-lactic acid (PLLA), poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL) are commonly used for musculoskeletal tissue engineering.[40–42] The mechanical properties and chemical composition (e.g., degradation rate, stiffness/rigidity) generally can be more precisely controlled in synthetic polymers, compared to naturally-derived biomaterials, and some synthetic polymers can also be made electrically conductive.[43, 44] However, some disadvantages include challenges in cellular attachment, the potential degradation into byproducts that impedes regeneration, and the potential formation of fibrous capsules due to an inflammatory response.[45]
The ideal biomaterial should meet the following criteria when being considered as scaffolds for musculoskeletal engineering: 1) matches the compliance of the native tissue to withstand unidirectional stress without fatigue or failure; 2) biodegrades at a rate that matches the rate of regeneration of the tissue so as to provide continued support throughout the repair process, as well as to allow gradual resorption; 3) possesses 3D spatial organization and porosity that enables infiltration and integration with the host tissue; and 4) can be reproducibly generated with ease in handling. Depending on the severity, biophysical characteristics, and anatomical location of the injury, the treatment strategy and therapeutic approach may vary.
3.2. Modulation of Scaffolds Using Bioactive Molecules
3.2.1. Growth Factors
Growth factors are soluble signaling polypeptides that regulate cellular growth, proliferation, viability, migration, and differentiation. Growth factors, drugs, and other bioactive molecules can be linked or embedded into scaffolds and delivered to the targeted tissue.[3, 46] Growth factors, when delivered systemically or locally, face the challenge of rapid degradation or loss of bioactivity. To prolong their retention and therapeutic contribution, growth factors can be immobilized to a scaffold-based delivery system using covalent bonding, physical entrapment, or surface adsorption depending on the physicochemical properties of both the growth factor and the substrate.[47–49] Chemical conjugation of growth factors to the scaffold provides controllable release kinetics of the growth factor, which is largely influenced by the degradation rate of the substrate. However, covalent coupling and other chemical factors in the microenvironment can alter these release kinetics as well as the biological function of some growth factors. One strategy is the use of physical encapsulation, which protects growth factors from enzymatic degradation and the harsh biological environment[50]. Several studies have introduced successful chemical conjugation techniques that preserve the biological function of growth factors, but the immobilized factors may induce cellular interactions that differ from their soluble counterparts.[50–52] In addition, due to poor myogenic cell survival and function after transplantation, scaffolds can co-deliver cells along with growth factors to maintain the viability of transplanted cells and also provide signaling cues for cellular infiltration or outgrowth (Table 1).[46, 52–56]
Table 1.
Components of bioactive tissue-engineered skeletal muscle constructs
| Approach | Advantages | Disadvantages | Bioactive Molecules |
Delivery Technique |
Major Findings | References |
|---|---|---|---|---|---|---|
| Growth Factors | Potential for controlled and sustained release and targeted delivery | Covalent conjugation can change the biological function of growth factors, physical entrapment results in rapid release | IGF-I/VEGF | Encapsulation in alginate hydrogels | 95% recovery of injured ischemic muscle and increased vascularization following 7-week implantation in mice | [73, 159] |
| SDF-1 | Encapsulation in alginate microspheres embedded in collagen-based scaffold | Stimulation of circulating progenitor cell (CPCs immobilization from bone marrow, increases the recruitment of bone marrow-derived cells and local angiogenic CXCR4+ cells and restoration of perfusion after 2 weeks in ischemic hindlimb muscle of mouse model | [160] | |||
| HGF/FGF2, HGF | Physical entrapment of two growth factors in alginate hydrogels for myoblast cell transplantation, bioconjugation to alginate hydrogels | Increased cell viability and outward migration of myoblasts, HGF release enhanced tissue blood perfusion and maturation of vascular network in ischemic hindlimb muscle of mice after 9 days | [161, 162] | |||
| SDF-1, IGF-I, SDF-1/IGF-I | Conjugation to PEGylated fibrin gel matrix | Conjugated SDF-1/PEG-Fib did not improve maximal force recovery after 2 weeks in tourniquet-induced ischemia/reperfusion injury (TK-I/R) of skeletal mice muscle, while dual delivery of IGF-I and SDF-I significantly improved functional recovery of muscle and revascularization Conjugation of IGF-I to PEG increases the function recovery and regeneration rate of muscles compared to recombinant protein | [163, 164] | |||
| Genetic substances | Higher efficacy compared to direct administrat ion, potential of local and sustained release of therapeutic agents | Low transfection efficiency of nonviral vectors, safety concerns of viral vectors, need to delivery to targeted cells | IGF-I/GFP encoding plasmid | Delivery by PEG Scaffolds | Scaffolds showed the ability to deliver IGF-I and GFP genes to C2C12 cells in vitro. The release level of genetic substances are controlled by changing the pore structure of scaffolds | [90] |
| FGF-2-encoding plasmid | Delivery using collagen/gelatin scaffold | Delivered FGF-2 transgenes significantly enhanced the myogenesis in rat muscle wounds | [91] | |||
| beta-Galactosidase transgene | Adenoviral vectors in fibrin scaffoldor | Fibrin containing adenoviral vector increased transfection at day 7 in rabbit ear wound compared to alone antiviral vectors. While in day 14, no difference in transfection was observed between alone viral vector and scaffold. | [165] | |||
| mRNA encoding HGF | Gene delivery from scaffold | Bioluminescence imaging of firefly luciferase could be detected for at least 7 days HGF mRNA release induced significant capillary formation near the site of ablation | [94] | |||
Abbreviations: IGF: Insulin-like growth factor; VEGF: Vascular endothelial growth factor; HGF: Hepatocyte growth factor; FGF: Fibroblast growth factor; AAV: Adeno-associated virus; SDF-1: Stromal cell-derived factor-1
During embryonic development and generation of skeletal muscle, numerous growth factors such as HGF and IGF are released in regular temporal and spatial patterns and modulate different myogenic signaling pathways such as Wnt, Notch, and sonic Hedgehog (Shh).[57–60] In adult skeletal muscle tissues, various growth factors regulate tissue hemostasis and regeneration by modulating the activation and differentiation of quiescent satellite cells and myoblasts.[60] HGF recruits and activates quiescent satellite cells following muscle damage through c-met receptors expressed on these cells.[61] Microthread fibrin scaffolds cross-linked using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and absorbed with HGF were shown to enhance the growth of C2C12 mouse myoblasts and significantly recovered injured muscle force by 200% , 60 days after injury, due to the sustained release of HGF. [62] In another example, fibroblast growth factors (FGFs) induced the activation and expansion of satellite cells by binding to cellular FGF receptors and promoting activation of signaling pathways such as MAPK/ERK or STATs.[63–65] Collagen matrices functionalized with FGF-2 showed an increase in the proliferation of C2C12 cells after 5 and 10 days in vitro, compared to the absence of growth factor.[66] In addition to HGF and FGF, IGF is another key growth factor that affects skeletal muscle growth and satellite cell proliferation by binding to IGF cell receptors (I or II) and activates the IGF-I/Akt signaling pathway.[67, 68] Hammers et al. used bi-functional succinimidylglutarate polyethylene glycol (PEG) to conjugate IGF-I to fibrin gel scaffolds. Degradation of fibrin matrices and subsequent controlled release of IGF-I markedly improved myoblast functional activity and force recovery of skeletal muscle by 1.5 fold compared to a saline injection control.[69]
Growth factor combinations targeting regeneration of predominately non-muscle components have also been examined for their ability to improve the functional restoration of injured muscle.. Growth factors that participate in angiogenesis (e.g., vascular endothelial growth factor (VEGF)) and innervation (e.g., nerve growth factor (NGF) and glial derived neurotrophic factor (GDNF)) also are essential for developing a functional tissue.[70–74] Liao et al. used co-axial electrospun fibers to encapsulate angiogenic growth factors including VEGF-A and platelet-derived growth factor-BB (PDGF-BB) in the core of electrospun polyurethane fibers that resulted in their sustained local delivery. [75] Designing a scaffold to allow for the controlled release of a mixture of growth factors in dynamic spatiotemporal patterns can improve the therapeutic potential of these factors to restore function to damaged skeletal muscle tissue. [73, 76] Examples of studies using growth factors for skeletal muscle tissue engineering are summarized in Table 1. There are many advantages to protein-based growth factor delivery including the convenience as an off-the-shelf approach. However, potential limitations include complications of immunogenicity, short half-lives, low biostability, and a high manufacturing cost.[77, 78]
3.2.2. Gene Activated Matrices
As an alternative to growth factor protein delivery, gene delivery enables in situ synthesis of growth factors and biomolecules of interest within the site of injured muscle tissue. In addition, the local synthesis of growth factors along with post-translational modification results in higher bioactivity, compared to the delivery of exogenous recombinant growth factors.[79, 80] Viral and non-viral vectors can be engineered to safely transfer genetic substances (e.g., cDNA) into the nucleus of targeted cells. Viruses, owing to their inherent ability to translocate their genomes into host cells, have a higher efficiency of gene delivery, compared to plasmid-based approaches.[81, 82] However, viruses can induce immune reactions, which may cause damaging results and restrict repeated dosing.[83, 84] Alternatively, non-viral vectors such as liposomes and synthetic particles, which show less immunogenicity compared to viruses, have been developed but were initially associated with reduced efficiency. To increase the efficiency of gene transfer in non-viral vectors, physical stimuli such as electroporation can be applied.[85, 86] Although genes can be delivered in vivo to the target tissue, the lack of spatiotemporal control of the gene delivery process is a major limitation of this approach. Another approach is the ex vivo genetic modification of cells that are subsequently implanted. Detailed description of gene delivery techniques for tissue regeneration can be found in other published literature.[87]
To regenerate functional muscle by gene therapy, the genes encoding growth factors or biomolecules involved with muscle generation can be targeted. Gene therapy can be performed by using scaffolds to safely deliver viral or non-viral vectors encoding targeted genes.[88] Scaffolds control the release profile of transgenes and regulate the sustainability and efficiency of gene delivery. For example, the release of lentiviral vectors from alginate hydrogels can be modulated by the molecular weight of alginate. Viral vectors loaded in alginate showed the ability of sustained transduction up to two months in murine models.[89]
Since angiogenesis plays a key role in muscle healing by promoting revascularization, scaffold-based delivery of plasmids encoding angiogenic growth factors has been a major interest to the regenerative medicine community. Porous single-component and bi-component scaffolds formed from 5-ethyl-5-(hydroxymethyl)-β,β-dimethyl-1,3-dioxane-2-ethanol diacrylate (EHD) and polyethylene glycol (PEG) were utilized for the delivery of a plasmid encoding IGF-I and GFP fused proteins to C2C12 cells in vitro. The rate of plasmid release was controllable upon changing the surface pore structure of the scaffold and the successful delivery of this plasmid induced IGF-I and GFP expression by the C2C12s.[90] In another example, FGF-2-encoding plasmids and adenoviral vectors delivered from collagen–gelatin admixtures into the quadriceps of a rat muscle defect model induced a significantly higher arteriole density in the injured muscle. FGF-2 transgene expression also induced skeletal muscle regeneration by 20-fold, based on the expression of myotube markers and CD56, while FGF-2 protein delivery did not show an equivalent response.[91] IGF-I gene delivery either by injection, electroporation, or transplantation of transduced myoblasts was also shown to promoted muscle regeneration in muscle injury mice models by promoting an increase in the twitch force amplitude (Table 1).[92, 93]
Besides delivery of plasmids and viruses, delivery of chemically modified mRNA from scaffolds was recently described. It was demonstrated that anisotropic nanofibrillar scaffolds could transiently release chemically modified HGF mRNA to induce angiogenesis into the ablated tibialis anterior muscle of mice.[94] Using the delivery of firefly luciferase chemically modified mRNA as a reference control group, the transfection time course could be tracked by bioluminescence imaging. The results demonstrated that transfection of firefly luciferase chemically modified mRNA could be detected in vivo for at least one week, and that delivery of HGF chemically modified mRNA from the scaffold significantly promoted neovascularization by increasing the capillary density near the site of ablation by approximately 30% after 14 days.
With the increasing convergence of biomaterials technology with gene delivery, it is likely that new developments will emerge in the near future; however, no clinical studies have been reported to date that use scaffold-mediated gene delivery for muscle regeneration. The limited knowledge of the effective time course and level of transgene expression from biological scaffolds, along with the cost, safety, immunogenicity and transfection efficiency, are among the major bottlenecks of this approach for skeletal muscle regeneration.
3.2.3. Small Molecules
Another important category of bioactive molecules used for skeletal muscle tissue engineering are small molecules. Small molecules are bioactive organic compounds, whose molecular size does not exceed 1000 Da. Unlike growth factors, small molecules do not induce an unwanted host immune response, owning to their small size. They can be synthesized with controlled physical, chemical, and biological properties and have a lower manufacturing cost compared to recombinant growth factors. The emergence of high-throughput screening technologies has enabled the discovery of small molecules that control cell behavior while also activating signaling pathways related to skeletal tissue regeneration.[95] These bioactive small molecules can serve as alternative therapeutics to growth factors and genetic manipulation, with additional advantages such as improved safety and cell permeability. For instance, retinoic acid is a small molecule that actively participates in AMPK-p38 MAPK signaling pathway and glucose metabolism for muscle cells, as well asplays a major role in differentiation of progenitor muscle cells.[95, 96] Other examples of potential small bioactive molecules for skeletal muscle regeneration include BIO (a glycogen synthase-3 kinase inhibitor),[66] SB203580 (a p38 MAP kinase inhibitor),[66] and 2-acetyl-4(5)- tetrahydroxybutlimidazole (a S1P Lyase inhibitor).[97] The detailed mechanisms of these molecules can be found in other reviews.[98, 99] Although small molecules have been largely studied in vitro or when directly injected in vivo, recent studies are beginning to explore the use of ECM as a delivery system for small molecules. For example, CEP03, a small molecule derivative of (ω-[2-carboxyethyl]pyrrole) protein adducts, was injected into ischemic murine muscle when encapsulated within Matrigel, a basement membrane extract derived from Engelbreth-Holm-Swarm (EHS) sarcoma that is rich in ECMs.[100] In comparison to treatment with Matrigel alone, treatment with CEP03-releasing Matrigel improved blood perfusion recovery to the ischemic muscle by nearly 100%, and increased the capillary density by 6-fold. As the benefits of delivering small molecules in a supportive biomaterial niche become increasingly apparent, it is likely that scaffold-mediated delivery of therapeutic small molecules will develop into a more widely used approach in the future.
3.3. The Roles of Mechanical and Electrical Stimulation in Skeletal Muscle Tissue Engineering
The first recognized generation of engineered skeletal muscle in vitro was demonstrated by Strohman et al., which consisted of a monolayer sheet of skeletal myoblasts that could be detached to form contracting 3D “myooids.”[101, 102] Since then, enhancing the physiologic function and relevance of engineered skeletal muscle has evolved with the incorporation of mechanical and electrical stimulation.. The main advantage of mechanical and electrical stimulation in vitro for skeletal muscle tissue engineering is the ability to mimic the physical simulation of stretch and electrical coupling in muscle. However, disadvantages tinclude the dependence on potentially sophisticated equipment as well as limitations in the kinds of scaffold materials that can be amenable to electrical conduction or mechanical stretch.
As a natural consequence of our daily movement, skeletal muscle undergoes continuous cyclic stretch and relaxation. Mechanical stretching plays an essential role in maintaining the physiological structure and function of these tissues such that exercise promotes the proliferation of muscle stem cells and increases muscle force production. In contrast, the lack of sufficient mobility leads to muscle atrophy.[103] In order to mimic the in vivo microenvironment, mechanical stimulation can be applied to cells in engineered tissues. Cells sense mechanical stresses through mechanotransduction pathways that relay these signals from cell membranes through the cytoskeleton to the nucleus and modulate gene regulation. [104] At the molecular level, many studies have shown that mechanical stimulation of myoblasts could alter the expression level of multiple transcription factors including nuclear factor-kappa B (NF-κB), MyoD, Myf5, and myogenin.[105] These molecules act during myogenic differentiation, in part by inducing the expression of MHC. Strain-induced activity changes in focal adhesion kinases (FAK) and Rho-GTPases also influence myoblast fusion and myogenesis.[106, 107] By modulating mechanical stimulation parameters (e.g., static vs dynamic mode, frequency, duration, magnitude, uniaxial vs biaxial strains), differential cellular responses can be induced.[108] For example, Akimoto et al. showed that applying a 20% mechanical stretch for 24 hours reduced the expression level of myogenic regulatory factors such as MyoD and myogenin in C2C12 cells by almost 40% and 70%, respectively,[109] suggesting that mechanical stretch inhibits the differentiation of C2C12 cells from forming myotubes. In contrast, other studies have shown an induction of myogenesis in C2C12 myoblasts when stimulated by uniaxial stretching ranging from 10–17%, compared to static control samples.[110, 111] The difference in findings may be attributed to differences in the magnitude of strain, the confluency of the cells, and potential differences in the degree of cellular adhesion on the deformable substrates during stretch. Beside the strain level, cells subjected to uniaxial vs biaxial stretch also manifest differential responses in cellular alignment, expression of myogenic factors and degree of differentiation.[110]
The effects of mechanical stimulation on the function of myoblasts in 3D biomaterials has highlighted the value of physical preconditioning in enhancing myogenic differentiation and maturation. [110] Li et al. showed that applying 40% static strain for 10 hours per day for 10 days applied to C2C12 myoblasts embedded in gelatin methacrylate (GelMA) hydrogels, induced the expression of myogenic factors including MyoD, myogenin, and MHC, which differed from findings using 2D substrates.[112] In another study, C2C12 cells seeded in fibrin hydrogels were placed in a bioreactor and subjected to a daily stretch regimen consisting of 10% strain for 6 hours, followed by 18 hours of 3% strain, for a total of 3 days. Mechanical stimulation induced cellular reorganization into aligned and elongated cells that were larger by 20–30%, compared to static control samples.[113] Machingal et. al. studied the contractility of engineered muscle generated from rat myoblasts on acellular matrices of porcine bladder. They employed a one-week stimulation regimen of 10% stretch repeated 15 times for a period of 5 min, followed by 55 min of rest. The resultant tissue-engineered constructs were able to generate force that was 72% that of native maximal muscle force after 2 months in in a VML model, while the muscle treated with the scaffold alone (as control) demonstrated 50% comparative native muscle force.[36] In a similar study, primary human muscle precursor cells were seeded onto acellular porcine bladder tissue scaffolds and subjected to the same regimen of repetitive strain for 5 days. The contractility of the engineered skeletal muscle was 10% that of the native muscle at 4 weeks after subcutaneous implantation. Moreover they showed that the highest contractile force was generated by the tissue-engineered skeletal muscle made from an acellular scaffold. [114] These studies together suggest a benefit of static strain on contractile function and/or phenotypic maturation.
In addition to the beneficial effect of repetitive static stretching, dynamic uniaxial stretch on primary human myoblasts seeded in collagen/matrigel matrices also showed significant enhancement of the dimensions of formed myofibers (12% increase in myofiber diameter and 40% increase in myofiber area percent) and created a more elastic structure.[115] The change of elasticity may be due to differences in the level of protein synthesis or collagen crosslinking as a result of stretching.[116] The pattern of mechanical stretching included 5% strain for the first 2 days, followed by 10% strain for the next 2 days, and then 15% strain for 4 more days. Stretching was applied in three sets consisting of 5 stretch/relaxation in each set with 30 seconds of rest per stretch/relaxation cycle as well as 28 minutes of rest between each set.[115] Studies that utilize mechanical stimulation for tissue-engineered skeletal muscle structures have demonstrated improvements in the differentiation, maturation, alignment, and contractility of the tissue-engineered muscle. However, tissue-engineered structures have not still been developed with contractility that matches the force generated by native muscle. Accordingly, systematic studies that investigate the effect of stretch in conjunction with other muscle regeneration approaches are needed to better identify optimal dynamic culture conditions and bioreactor designs..
Besides mechanical stimulation, electrical cues also influence skeletal muscle function. Cell membranes contain ion channels and pumping of ions generates an electrical potential within the membrane. Electrical fields can alter the level of intracellular calcium content, and the signals are transduced through different signaling pathways such as the calcium/calmodulin pathway.[117] Electrical impulses applied to myotubes from nearby motor neurons play a major role in the regulation and function of muscular tissues.[118] Electrical stimulation can induce myogenic differentiation and increase muscular forces during in vivo experiments.[119] However, finding optimal electrical conditions, including voltage type, amplitude, and pulse frequency, as well as the mode of electrical stimulation are extremely critical for exploring electrical stimulation in tissue-engineered muscles.[117] Hashimoto et al. demonstrated that an electric pulse of 0.1V for 3 days accelerated the differentiation of C2C12 cells, whereas voltages higher than 8V did not support cellular attachment onto the substrate.[120] Ito et al. examined the effects of electrical parameters, including voltage level (0.1–0.5 V/mm), pulse width (2–10ms), frequency (0.5–2 Hz) and duration (4, 7, 10 and 14 days) on the force generated by C2C12 cells in a tissue-engineered structures. They found a 4.5 fold increase in contractile force generated by cells when were cultured for 14 days under continuous electrical pulses of 0.3V/mm amplitude, 4ms width, and 1Hz frequency, compared to non-stimulated samples.[121] Shown in Table 2 are examples of published works in electrical stimulation for regenerative skeletal muscle engineering. Together, these studies illustrate the importance of mechanical and electrical stimulation in modulating the function of engineered skeletal muscle.
Table 2.
Examples of the application of electrical stimulation for developing in vitro tissue-engineered skeletal muscles
| Electrical stimulation parameters (voltage, pulse width, duration, frequency) |
Scaffold | Cell sources |
Outputs | References | |
|---|---|---|---|---|---|
| 1.25, 2.5, 5V/mm 0.1ms, 7days | 3D rapid prototyped polycarbonate bioreactor | C2C12 cells | Enhanced maturation and force | [166] | |
| 0.1–0.5V/mm, 2–10ms, 4, 7, 10 and 14 days, 0.5–2Hz | Collagen/matrigel | C2C12 cells | Increased force production | [121] | |
| 0.0564V/mm, pulse widths of 0.5–250ms, and frequency of 0.5–10 Hz, 1 and 14 days duration | polyglycolic acid (PGA) mesh | Adult rabbit skeletal myoblasts | Improved proliferation of skeletal myoblasts but differentiation did not change | [167] | |
| 40V, 40Hz, 1.20ms pulse, 2s train duration | Acellular muscles | C2C12 cells | contractile force production | [168] | |
| Electrical pulse (amplitude 22 mA, frequency 1 Hz, and duration 2 ms), 48hrs duration | microstructured methacrylated gelatin | C2C12 cells | Improved myoblast alignment and increased diameter of myofibrils | [169] | |
| Square pulse of 70 mV/cm amplitude for 3 ms with frequency of 33 mHz | micropatterned poly-(L-lactic acid) (PLA) membranes | Muscle precursor cell (MPC) | Combined electrical stimuli and micropatterning increase the skeletal muscle cell differentiation and enhance the formation of contractile alignment myotubes | [170] | |
| Electrical pulse of 70mV/cm amplitude for 2ms and frequency 1Hz | fibrin gels | C2C12 myotubes | The contractile force of myotube depends on the electrical stimulation | [171] | |
| Bipolar rectangular pulse of 3V, 4V and 4.5V magnitude for 12.5hrs and frequency of 1Hz | Micropatterned gelatin methacryloyl (GelMA) | C2C12 myotubes | Myotube maturation increased under applied voltage 4V and myotubes contracts upon applying voltage >4V | [172] | |
| Rectangular pulses of 2V, 5V and 7V, frequency of 1 and 2Hz for 2ms duration | Collagen | C2C12 cells | Enhanced contractile properties in the constructs stimulated by the 1Hz/5V and 2Hz/5V | [173] | |
| Continuous pulses of 0.3 V/mm amplitude and frequency of 1 Hz for 4ms | Collagen type I and Matrigel | C2C12 cells | Increased contractile force by 4.5 fold after 2 weeks | [121] | |
| Electrical pulse with amplitude of 22 mA, frequency 1 Hz, and duration 2ms for 48hrs | microgrooved methacrylated gelatin (GelMa) hydrogels | C2C12 cells | Enhancing alignment of myotubes | [29] | |
3.4. The Role of Spatial Patterning Cues in Engineering Skeletal Muscle
In order to engineer biomimetic skeletal muscle that resembles the highly anisotropic organization of muscle fibers, a number of techniques have been developed to create spatially patterned cells and tissue constructs. Micropatterning by photolithographic methods can create a range of micron-sized grooves that support the parallel alignment of myoblasts. It has been shown that the physical characteristics of these grooves influence the efficiency of myoblast fusion. In particular, parallel microgrooves that were 5–12 μm in width and 2–6 μm in depth were associated with greater myotube alignment, compared to shallower nano-scale grooves.[122] We have previously shown that parallel microgrooves that were 10 μm in width and 3 μm in depth could induce the generation of parallel-aligned multi-nucleated myotubes that were 0.7 mm in length.[123] While many studies utilize spatial patterning to organize myogenic cells, identification of the mechanism through which cells sense and respond to topography is not well understood. Some groups have probed these interactions to determine that sharp-edge features are not required for contact guidance of myoblast alignment using sinusoidal grooved micropatterns (0.1–10 μm) on polydimethylsiloxane.[124] For example, it was shown that a wave period of 6 μm was optimal for differentiation of myoblasts.[125] Coupling of micropatterned grooves with hydrogels further demonstrated that myotube alignment was dependent upon the geometry and aspect ratios of the topographically constrained cell-laden hydrogels, comprised of fibrin and matrigel with rat myocytes and myoblasts. This work highlights the potential of utilizing micropatterning in combination with supportive ECM components.[126]
Although micropatterning has proven to be efficient in providing contact guidance to align cells, in vivo ECM protein environments exhibit nanoscale features, such as collagen fibrils which have diameters in the hundreds of nanometers.[127] Recapitulation of these nano-scale features to mimic the native muscle niche may stimulate certain cytoskeletal-responsive pathways to enhance cell alignment and differentiation. Electrospinning of fibrous meshes is a versatile method of creating spatially patterned scaffolds made from a variety of natural and synthetic polymers that can be spun towards a collecting unit into meshes that range in fiber thickness and orientation. Using a combination of chitosan, polycaprolactone and poly(vinyl pyrrolidone), an electrospun tube was constructed with aligned fibers on the order of 70–200 nm in diameter and were shown to align C2C12 mouse myoblasts to form highly aligned myotubes rich in MHC [128]. In another study to utilize nanoscale features, Dungan et al. fabricated cellulose nanowhiskers (10–15 nm in diameter) to align myoblasts along the parallel direction of the whiskers, demonstrating that cells can sense and respond to features on this small scale.[129]
Generating 3D scaffolds with topographical cues can be accomplished using a wide range of bioengineering techniques. Fabrication approaches for 3D uniaxially patterned scaffolds include electrospinning, extrusion, freeze-drying, and phase separation approaches. We previously engineered skeletal muscle using C2C12 cells cultured in uniaxially patterned electrospun scaffolds and demonstrated nearly 3-fold longer myotubes, compared to scaffolds with randomly oriented fibrous structure.[123] Besides electrospinning, extrusion is also another effective approach for creating spatially oriented fibers. Using a shear-mediated extrusion approach in which acidic monomeric collagen I solution was extruded at high velocity into a pH neutral buffer, strip-like scaffolds composed of oriented nanofibrils could be generated.[130] When seeded with primary human endothelial cells and implanted into murine ischemic hindlimb muscle, treatment with cell-seeded aligned scaffolds increased the recovery of blood perfusion to the ischemic muscle by nearly 50%, compared to cell-seeded non-patterned scaffolds, suggesting that spatial patterning cues can modulate the angiogenic function of endothelial cells in the ischemic muscle.[131] In another example, fibrin microthread scaffold prepared by an extrusion technique demonstrated promising results as a scaffold and cell delivery vehicle for muscle regeneration in the mouse muscle defect model. Implanted fibrin microthreads containing human muscle progenitor cells improved the engraftment of transplanted progenitor cells, while significantly enhancing native muscle regeneration and reducing collagen tissue formation, compared to untreated controls. In addition, contractile force measurement showed the complete force recovery was achieved following 4 months duration in implantation.[132] Besides extrusion-based approaches, the generation of tubular pores in chitosan scaffolds by freeze-drying enabled anisotropic myotubes to grow to 50 μm in length and could mimic the mechanical properties of native tissue.[32] In another study, macromolecular collagen I scaffolds composed of parallel-aligned pores 20–50 μm in width were used to generate aligned C2C12 myotubes that induced muscle regeneration and force production in vivo.[133] Some limitations of current spatial patterning approaches include the need for specialized equipment for some approaches, and complications in scaling up to clinically relevant dimensions. Together, these examples highlight the role of spatially patterned scaffolds in enhancing muscle formation and regeneration.
4. Neurovascularization of Engineered Skeletal Muscle
Traumatic musculoskeletal injuries are concomitant with the loss of blood supply and denervation to the damaged tissues. Rapid and efficient revascularization of damaged tissue is paramount to prevention of additional tissue necrosis. Tissue engineering strategies that improve revascularization of the tissue are a vital contributor in achieving successful reintegration with the host and regeneration of damaged tissue. There are two primary approaches for improving vascularization in the region of injury: 1) in vitro culture of an endothelial cell population on a scaffold that can act as angiogenic donor cells that hook up with the host vasculature to form a hybrid vascular network[134, 135] or 2) delivery of angiogenic growth factors or transplantation of cells that, through secretion of paracrine factors, can stimulate endogenous neovascularization.[71, 136] For example, Levenberg et al. combined myoblasts, embryonic fibroblasts, and endothelial cells with a 3D porous polymer (50:50 PLLA:PLGA) scaffold to demonstrate the formation of a stabilized endothelial network with expression of vasculogenic and angiogenic factors such as VEGF and PDGF.[137] These tri-cultured vascularized constructs showed improved vascular densities following transplantation as a subcutaneous implant, and intra-muscular implant in the quadriceps muscle, and as a tissue replacement for an anterior abdominal muscle. Using a similar combination of cells with Surgisis scaffolds, this group later demonstrated a correlation between the extent of pre-vascularization to in vivo grafting efficacy, such that extended in vitro incubations that allowed greater development of a vascular bed, resulted in improved anastomosis and vascular integration following transplantation.[138] In other approaches, constructs have been designed to activate an endogenous host response to stimulate angiogenesis in vivo. By transplanting either a pre-differentiated myogenic population in a molded hydrogel/cell mixture or undifferentiated myoblasts under the dorsal skin of mice, Juhas et al. showed rapid vascularization of the previously avascular constructs and moreover, demonstrated the importance of pre-differentiation of the myogenic population which was associated with improved myofiber maturation and function as well as significantly more neovessel in growth.[139]
Besides vascularization, innervation of muscle is also important for long-term function of engineered skeletal muscle. During muscle injury, muscle fibers can become denervated, resulting in muscle atrophy and loss of function due to the inability of muscle myofibers to mature in the absence of innervation.[140] Therefore, the formation of neuromuscular junctions (NMJs) containing mature acetylcholine receptors (AChR) in transplanted engineered therapeutics is necessary to ensure adequate muscle myofiber regeneration and transmission of electrical stimuli to generate the appropriate force contractions.[141, 142] The importance of functional neuro-reintegration was highlighted by Larkin et al. in which 3D skeletal muscle constructs co-cultured with fetal nerve explants generated greater twitch and tetanic force in vitro, as well as exhibited a greater content of adult MHC isoforms, when compared with constructs cultured without nerve components.[143] Similarly, Dhawan et al. demonstrated that implantation of rat myoblasts in a fibrin gel around the femoral vessels and transected femoral nerve produced five times greater force contractions upon electrical stimulation when the construct was neurotized.[144] Together, these studies highlight the importance of neurovascularization in modulating the function and integration of engineered musculoskeletal tissues.
5. Preclinical Assessment of Engineered Skeletal Muscle for Muscle Regeneration
The ultimate goal of skeletal muscle tissue engineering is in vivo transplantation as a therapeutic treatment to restore injured or diseased muscle. Owing to the experimental progress of engineered skeletal muscle constructs in vitro, many constructs have been tested pre-clinically with promising results of safety and efficacy. One area of pre-clinical translation is in the field of VML. Acellular biological scaffolds have been tested in small and large animal models of VML. For example, decellularized small intestinal submucosa (SIS) scaffolds have been tested in a canine model of musculotendinous VML. At six months after transplantation, the site of the scaffold implant was characterized by vascularized and innervated muscle, with significantly improved contractile response that was nearly 50% of that of uninjured musculotendinous junction.[145] In a similar study in a rat model of VML, delivery of SIS scaffolds led to a complete recovery in maximal contractile force compared to native tissue after 26 weeks.[146] These therapeutic benefits may be due to the ability of the scaffolds to provide structural support as well as promote cellular ingrowth. However, conflicting findings in which decellularized scaffolds had no significant therapeutic benefit have also been reported.[147, 148] These differences in findings can be attributed in part to potential differences in the preparation of scaffold materials, the surgical model of VML, species, and age of the animals.
5.1. Preclinical Studies of Cell-Based Engineered Skeletal Muscle Transplantation
Combining the delivery of scaffolds with cells is a highly attractive strategy because of the increased combinatorial capacity and potential for functional therapeutic benefit. The incorporation of myogenic cells into supportive scaffolds has led to greater force generation and muscle regeneration at the site of traumatic muscle loss.[36, 149] To better understand the role of in vitro cellular phenotype and culture conditions that influence the in vivo efficacy of mouse muscle-derived cells seeded on bladder acellular matrix, the authors compared constructs seeded short-term with muscle-derived cells (MDCs) to generate myoblasts, or constructs with prolonged MDC culture in a bioreactor.[149] When implanted into the ablated mouse latissimus dorsi muscle, functional assessment of maximum tetanic force generation after one month demonstrated significantly higher values compared to the control ablated muscle group. However, at longer time points of 2 months, the authors found that only a third treatment group consisting of cell-seeded scaffolds with prolonged bioreactor conditioning as well as a second application of MDCs showed sustained functional benefit.
Besides culturing of myogenic cells alone within scaffolds, the addition of endothelial cells and other support cells was shown to promote higher force production in the ablated mouse muscle, compared to treatment with scaffolds that lacked endothelial cells. [150] To demonstrate the importance of endothelial cells in muscle regeneration, electrospun fibrin scaffolds seeded with human endothelial cells were implanted into the ablated mouse tibialis anterior muscle.[151] After 10 days following transplantation, human vessels were found to anastomose to murine vessels, based on histological staining of human- and mouse-specific vessels. In a mouse subcutaneous implant model, delivery of bladder acellular matrix seeded with murine muscle progenitor cells and human umbilical vein endothelial cells resulted in significantly improved vascularization, muscle formation, and innervation, compared to scaffolds seeded with muscle progenitor cells alone.[152]
5.2. Preclinical Studies of Preconditioned Tissue-engineered Skeletal Muscle
Preconditioning of engineered skeletal muscle or therapeutic cells has been found to augment the therapeutic impact. When bladder acellular matrix seeded with rat muscle progenitors were pre-treated over the course of one week with 10% stretch for the first five minutes of each hour, the authors reported that implantation of the pre-treated tissue constructs into the ablated mouse latissimus dorsi muscle led to significant recovery of force generation after 2 months, when compared to the no treatment control group.[36] In a similar study by the same authors, the effect of strain pretreatment on bladder acellular matrix seeded with rat muscle progenitors was evaluated in a rat tibialis anterior ablation model.[37] The authors reported a large variability in treatment response, with positive responders showing 61% improvement in function, whereas negative responders show no improvement, compared to non-repaired control animals. The variability in response was attributed in part to differential immune response.
We previously demonstrate that in vivo mechanical conditioning using voluntary caged wheel exercise could enhance the regenerative qualities of spatially patterned collagen scaffolds when implanted into the ablated tibialis anterior muscle in mice.[153] By allowing the animals to undergo voluntary exercise for 2 weeks after implantation of spatially patterned collagen scaffolds, histological analysis showed 30% higher perfused vascular density and a significant increase in the number of neuromuscular junctions, compared to implantation of spatially patterned scaffolds without exercise intervention. Besides the application of stretch, compression has also been examined for treatment of muscle regeneration. The application of cyclic compression to biphasic alginate-based ferrogels after implantation into mouse tibialis anterior muscle with myotoxin injury using magnetic actuation (5 minutes at 1 Hz every 12 hours) increased the contractile force and decreased fibrosis and inflammation in the regenerated muscle, compared to sham treatment controls after 2 weeks.[154]
In addition to mechanical conditioning, spatial patterning has been employed to control cellular organization prior to transplantation. When spatially patterned fibrin microfiber bundles seeded with C2C12 myoblasts were implanted into the site of the ablated mouse tibialis anterior muscle, both cell-seeded and the acellular scaffolds could recover full muscle contractile function in 4 weeks.[151] In another study, electrospun uniaxially aligned fibrin hydrogel microfibers were seeded with human adipose-derived stem cells (ASCs) and implanted in VML mice model. Following 1 and 3-month implantation, seeded scaffolds contained significantly more muscle cells and 4 times higher volume retention, compared to acellular fibers. In addition, aligned fibrin microfiber scaffolds were well integrated with native tissue and elicited little fibrosis formation. The findings of this study indicate ACS-seeded aligned fibrin microfibers could be a potential treatment for VML.[155] In another study, fibrin hydrogels with uniaxially patterned endothelial networks increased blood perfusion recovery to ischemic muscle, compared to the sham control group. The pre-endothelialized patterned patch resulted in the formation of muscle fibers that better approximated the size distribution of healthy fibers, compared to the sham control group. [156] Examples of various approaches that have been tested in the preclinical setting for treatment of volumetric muscle loss are illustrated in Table 3.
Table 3.
Examples of in vitro tissue engineering approaches for treatment of volumetric muscle loss
| Treatment Category |
Target Tissue for Ablation |
Treatment | Major Findings | Reference |
|---|---|---|---|---|
| Acellular Scaffolds | Canine gastrocnemius muscle and Achilles tendon bundle | Porcine small intestinal submucosa (SIS) decellularized scaffold for 6 months | Vascularized and innervated skeletal muscle had formed at the implantation site Scaffold-treated group had a contractile force that was 48% of the contralateral musculotendinous junction | [145] |
| Rat abdominal muscle | Porcine SIS decellularized scaffold for 26 weeks | Complete recovery in maximal contractile force to native tissue | [146] | |
| Rat tibialis anterior muscle | Porcine urinary bladder matrix | Limited muscle formation and scaffold-mediated fibrosis | [148] | |
| Mouse tibialis anterior muscle | Porcine urinary bladder matrix or nanofibrillar rat collagen scaffold | No benefit of decellularized matrix or collagen scaffolds on muscle or vascular regeneration | [153] | |
| Cell-Seeded Scaffolds | Mouse latissimus dorsi muscle | Muscle-derived cells (MDCs) seeded on porcine urinary bladder matrix | Higher max tetanic force compared to the control ablated muscle group at 1 month At 2 months, cell-seeded scaffolds with prolonged bioreactor conditioning + second application of MDCs showed sustained functional benefit | [149] |
| Mouse tibialis anterior muscle | Muscle stem cells, endothelial cells (ECs) and muscle resident cells in decellularized muscle scaffold | Addition of ECs and other support cells promoted higher force production in the ablated muscle, compared to scaffolds without ECs | [150] | |
| Mouse latissimus dorsi muscle | Rat muscle progenitors in bladder acellular matrix with stretch pretreatment | Significant recovery of force generation after 2 months, when compared to the no treatment control group | [36] | |
| Rat tibialis anterior muscle | Rat muscle progenitors in bladder acellular matrix with stretch pretreatment | Large variability in functional response, with positive responders showing 61% improvement in function, compared to non-repaired animals The variability was partially attributed to differential immune response | [37] | |
6. Conclusion and Future Directions
Although multiple techniques to promote skeletal muscle regeneration have been presented, the approach with the greatest near-term potential for clinical use is decellularized scaffolds, largely because of their off-the-shelf capacity. Also, the pathway for FDA approval is less challenging for acellular scaffolds than for scaffolds seeded with cells or other bioactive molecules. Emerging studies using of decellularized scaffolds in clinical studies have shown promising results. For example, Sicari et al implanted acellular porcine bladder ECM into sites of muscle injury in patients and demonstrated de novo formation of skeletal muscle.[38] Although tissue engineering approaches to treat muscle injury or diseases remains promising, a number of challenges will need to be resolved to enable clinical practice. Since skeletal muscle injuries and diseases are complex, with large variability in the spatial geometry of ablated muscle, the development of smaller modular-sized tissue constructs may enable the flexibility for clinicians to tailor to the unique spatial requirements of each patient’s muscle injury. Scalability is a major challenge, as most studies are performed in rodents, with limited studies that have been investigated using porcine models of muscle injury.[157, 158] Since cell-based approaches may require autologous cells to obviate immune rejection, the potential manufacturing costs may be a deterrent. In the advent of CRISPR gene editing technology, it may be foreseeable that the development of allogenic cells without immunogenic concerns. Although there remain numerous challenges to be overcome, tissue engineering-based approaches to skeletal muscle engineering and regeneration remains highly promising.
Acknowledgements
This research was funded in part from the Alliance for Regenerative Rehabilitation Research & Training (AR3T), which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Neurological Disorders and Stroke (NINDS), and National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under Award Number P2CHD086843. This study was supported by grants to N.F.H. from the US National Institutes of Health (R01 HL127113, R01 HL142718), National Science Foundation (1829534), and the Department of Veterans Affairs (1I01BX002310 and 1I01BX004259).
Biography

Ngan F. Huang, PhD
Dr. Huang is an assistant professor of Cardiothoracic Surgery at Stanford University and principle investigator at the Center for Tissue Regeneration, Repair, and Restoration at Veterans Affairs Palo Alto Health Care System. Dr. Huang completed her BS in Chemical Engineering from the Massachusetts Institute of Technology, followed by a PhD in bioengineering from the University of California Berkeley and University of California San Francisco Joint Program in Bioengineering. Her research laboratory focuses on the role of chemical and mechanical interactions between the extracellular matrix and pluripotent stem cells that regulate cardiovascular differentiation and tissue regeneration
Footnotes
Conflict of Interests: The authors do not hold any financial or non-financial interests that could be perceived as being a conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Defense, or the Department of Veteran Affairs.
Contributor Information
Karina H. Nakayama, Department of Cardiothoracic Surgery, Stanford University, Stanford, CA, 94305 Veterans Affairs Palo Alto Health Care System, 3801 Miranda Avenue, Palo Alto, CA, 94304; The Stanford Cardiovascular Institute, Stanford University, Stanford, CA, 94305.
Mahdis Shayan, Department of Cardiothoracic Surgery, Stanford University, Stanford, CA, 94305; Veterans Affairs Palo Alto Health Care System, 3801 Miranda Avenue, Palo Alto, CA, 94304; The Stanford Cardiovascular Institute, Stanford University, Stanford, CA, 94305.
Ngan F. Huang, Department of Cardiothoracic Surgery, Stanford University, Stanford, CA, 94305; Veterans Affairs Palo Alto Health Care System, 3801 Miranda Avenue, Palo Alto, CA, 94304; The Stanford Cardiovascular Institute, Stanford University, Stanford, CA, 94305; Stanford University, 300 Pasteur Drive, MC 5407, Stanford, CA 94305-5407, USA.
References
- 1.Winkler T, von Roth P, Radojewski P, Urbanski A, Hahn S, Preininger B, Duda GN, Perka C, Tissue Eng Regen Med. 2012, 6 Suppl 3,s60. [DOI] [PubMed] [Google Scholar]
- 2.Mertens JP, Sugg KB, Lee JD, Larkin LM, Regen Med. 2014, 9,89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cezar CA, Mooney DJ, Adv Drug Deliv Rev. 2015, 84,188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke RE, Tsairis P, J Physiol. 1973, 234,749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrovidov S, Hosseini V, Ahadian S, Fujie T, Parthiban SP, Ramalingam M, Bae H, Kaji H, hosseini A Khadem, Tissue Eng Part B Rev. 2014, 20,403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarvinen TA, Jarvinen M, Kalimo H, Muscles Ligaments Tendons J. 2013, 3,337. [PMC free article] [PubMed] [Google Scholar]
- 7.Toumi H, Best TM, Br J Sports Med. 2003, 37,284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philippou A, Maridaki M, Theos A, Koutsilieris M, Adv Clin Chem. 2012, 58,49. [DOI] [PubMed] [Google Scholar]
- 9.Mauro A, J Biophys Biochem Cytol. 1961, 9,493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldrin L, Muntoni F, Morgan JE, J Histochem Cytochem. 2010, 58,941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tidball JG, Villalta SA, Am J Physiol Regul Integr Comp Physiol. 2010, 298,R1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner NJ, Badylak SF, Cell Tissue Res. 2012, 347,759. [DOI] [PubMed] [Google Scholar]
- 13.Hawke TJ, Garry DJ, J Appl Physiol (1985). 2001, 91,534. [DOI] [PubMed] [Google Scholar]
- 14.Chal J, Pourquie O, Development. 2017, 144,2104. [DOI] [PubMed] [Google Scholar]
- 15.Gillies AR, Lieber RL, Muscle Nerve. 2011, 44,318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AS, Passey S, Greensmith L, Mudera V, Lewis MP, J Cell Biochem. 2012, 113,1044. [DOI] [PubMed] [Google Scholar]
- 17.Thomas K, Engler AJ, Meyer GA, Connect Tissue Res. 2015, 56,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg K, Boppart MD, J Appl Physiol (1985). 2016, 121,1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE, Pflugers Arch. 1999, 437,857. [DOI] [PubMed] [Google Scholar]
- 20.Carmeli E, Moas M, Lennon S, Powers SK, Exp Physiol. 2005, 90,613. [DOI] [PubMed] [Google Scholar]
- 21.Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Van Loon LJ, Med Sci Sports Exerc. 2013, 45,230. [DOI] [PubMed] [Google Scholar]
- 22.Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE, Int J Biochem Cell Biol. 2008, 40,2183. [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Tatsumi R, Kikuiri T, Okamoto S, Nonoshita S, Mizunoya W, Ikeuchi Y, Shimokawa H, Sunagawa K, Allen RE, Muscle Nerve. 2006, 34,313. [DOI] [PubMed] [Google Scholar]
- 24.Bian W, Bursac N, Biomaterials. 2009, 30,1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Holden K, Zhu J, Pan H, Li Y, J Biomed Biotechnol. 2011, 2011,812135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glowacki J, Mizuno S, Biopolymers. 2008, 89,338. [DOI] [PubMed] [Google Scholar]
- 27.Huang YC, Dennis RG, Larkin L, Baar K, J Appl Physiol (1985). 2005, 98,706. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, Sasaki J, Alsberg E, Egusa H, Yatani H, Sohmura T, PLoS One. 2007, 2,e1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, Ramalingam M, Khademhosseini A, Tissue Eng Part A. 2012, 18,2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Highley CB, Prestwich GD, Burdick JA, Curr Opin Biotechnol. 2016, 40,35. [DOI] [PubMed] [Google Scholar]
- 31.Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C, Vitiello L, Elvassore N, De Coppi P, FASEB J. 2011, 25,2296. [DOI] [PubMed] [Google Scholar]
- 32.Jana S, Cooper A, Zhang M, Adv Healthc Mater. 2013, 2,557. [DOI] [PubMed] [Google Scholar]
- 33.Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL, Acta Biomater. 2006, 2,313. [DOI] [PubMed] [Google Scholar]
- 34.Passipieri JA, Baker HB, Siriwardane M, Ellenburg MD, Vadhavkar M, Saul JM, Tomblyn S, Burnett L, Christ GJ, Tissue Eng Part A. 2017, 23,556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ, Nat Mater. 2016, 15,326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo JJ, Christ GJ, Tissue Eng Part A. 2011, 17,2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ, Tissue Eng Part A. 2014, 20,705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EH, Dziki JL, Fisher LE, Brown S, Badylak SF, Sci Transl Med. 2014, 6,234ra58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GN, Biomaterials. 2015, 53,502. [DOI] [PubMed] [Google Scholar]
- 40.Green JJ, Elisseeff JH, Nature. 2016, 540,386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ, Biomaterials. 2008, 29,2899. [DOI] [PubMed] [Google Scholar]
- 42.Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF, Adv Drug Deliv Rev. 2015, 84,208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jun I, Jeong S, Shin H, Biomaterials. 2009, 30,2038. [DOI] [PubMed] [Google Scholar]
- 44.Ku SH, Lee SH, Park CB, Biomaterials. 2012, 33,6098. [DOI] [PubMed] [Google Scholar]
- 45.Maleiner B, Tomasch J, Heher P, Spadiut O, Runzler D, Fuchs C, Front Physiol. 2018, 9,1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GN, Biomaterials. 2015, 53,502. [DOI] [PubMed] [Google Scholar]
- 47.Babensee JE, McIntire LV, Mikos AG, J Pharmaceutical research. 2000, 17,497. [DOI] [PubMed] [Google Scholar]
- 48.Chen RR, Mooney DJ, Pharm Res. 2003, 20,1103. [DOI] [PubMed] [Google Scholar]
- 49.Jha AK, Mathur A, Svedlund FL, Ye J, Yeghiazarians Y, Healy KE, J Control Release. 2015, 209,308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajimiri M, Shahverdi S, Kamalinia G, Dinarvand R, J Biomed Mater Res A. 2015, 103,819. [DOI] [PubMed] [Google Scholar]
- 51.Zbinden A, Browne S, Altiok EI, Svedlund FL, Jackson WM, Healy KE, Biomater Sci. 2018, 6,1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Silva EA, Mooney DJ, J R Soc Interface. 2011, 8,153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Discher DE, Mooney DJ, Zandstra PW, Science. 2009, 324,1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ, J Biomaterials. 2011, 32,8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorrez L, Shansky J, Wang L, Fast L, VandenDriessche T, Chuah M, Mooney D, Vandenburgh H, Biomaterials. 2008, 29,75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill E, Boontheekul T, Mooney DJ, Tissue Eng. 2006, 12,1295. [DOI] [PubMed] [Google Scholar]
- 57.Mohammed RH, Anderton H, Brameld JM, Sweetman D, PloS One. 2017, 12,e0185775 [Google Scholar]
- 58.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA, Trends Cell Biol. 2012, 22,602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsivitse S, Int J Biol Sci. 2010, 6,268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syverud BC, VanDusen KW, Larkin LM, Cells Tissues Organs. 2016, 202,169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webster MT, Fan CM, PLoS One. 2013, 8,e81757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grasman JM, Do DM, Page RL, Pins GD, Biomaterials. 2015, 72,49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karimi T, Moeinzadeh S, Jabbari E. Growth factors for musculoskeletal tissue engineering Regenerative engineering of musculoskeletal tissues and interfaces. Elsevier; 2015:43. [Google Scholar]
- 64.So W-K, Cheung TH. Molecular regulation of cellular quiescence: A perspective from adult stem cells and its niches Cellular quiescence. Springer; 2018:1. [DOI] [PubMed] [Google Scholar]
- 65.Pawlikowski B, Vogler TO, Gadek K, Olwin BB, Dev Dyn. 2017, 246,359. [DOI] [PubMed] [Google Scholar]
- 66.Yun YR, Lee S, Jeon E, Kang W, Kim KH, Kim HW, Jang JH, Biotechnol Lett. 2012, 34,771. [DOI] [PubMed] [Google Scholar]
- 67.Ansari S, Chen C, Xu X, Annabi N, Zadeh HH, Wu BM, Khademhosseini A, Shi, Moshaverinia, Ann Biomed Eng. 2016, 44,1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Machida S, Booth FW, Proc Nutr Soc. 2004, 63,337. [DOI] [PubMed] [Google Scholar]
- 69.Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ, Biotechnol Bioeng. 2012, 109,1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC, Am J Pathol. 2003, 163,1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lovett M, Lee K, Edwards A, Kaplan DL, Tissue Eng Part B Rev. 2009, 15,353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tajdaran K, Gordon T, Wood MD, Shoichet MS, Borschel GH, Acta Biomater. 2016, 29,62. [DOI] [PubMed] [Google Scholar]
- 73.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ, Proceedings of the National Academy of Sciences. 2010, 107,3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, Ho N, Cezar C, McCann C, Anderson E, Koullias J, Tapia JC, Vandenburgh H, Lichtman JW, Mooney DJ, Mol Ther. 2014, 22,1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao IC, Leong KW, Biomaterials. 2011, 32,1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richardson TP, Peters MC, Ennett AB, Mooney DJ, Nat Biotechnol. 2001, 19,1029. [DOI] [PubMed] [Google Scholar]
- 77.Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W, Pharm Res. 2004, 21,897. [DOI] [PubMed] [Google Scholar]
- 78.Buchtova M, Chaloupkova R, Zakrzewska M, Vesela I, Cela P, Barathova J, Gudernova I, Zajickova R, Trantirek L, Martin J, Kostas M, Otlewski J, Damborsky J, Kozubik A, Wiedlocha A, Krejci P, Cell Mol Life Sci. 2015, 72,2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osamor VC, Chinedu SN, Azuh DE, Iweala EJ, Ogunlana OO, Drug Des Devel Ther. 2016, 10,861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fakruddin M, Mohammad Mazumdar R, Bin Mannan KS, Chowdhury A, Hossain MN, ISRN Biotechnol. 2013, 2013,590587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kay MA, Nat Rev Genet. 2011, 12,316. [DOI] [PubMed] [Google Scholar]
- 82.Nayerossadat N, Maedeh T, Ali PA, Adv Biomed Res. 2012, 1,27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans CH, Huard J, Nat Rev Rheumatol. 2015, 11,234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madrigal JL, Stilhano R, Silva EA, Tissue Eng Part B Rev. 2017, 23,347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wells DJ, Gene Ther. 2004, 11,1363. [DOI] [PubMed] [Google Scholar]
- 86.Alsaggar M, Liu D, Adv Genet. 2015, 89,1. [DOI] [PubMed] [Google Scholar]
- 87.Ramamoorth M, Narvekar A, J Clin Diagn Res. 2015, 9,GE01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato M, Ito A, Kawabe Y, Nagamori E, Kamihira M, J Biosci Bioeng. 2011, 112,273. [DOI] [PubMed] [Google Scholar]
- 89.Stilhano RS, Madrigal JL, Wong K, Williams PA, Martin PK, Yamaguchi FS, Samoto VY, Han SW, Silva EA, J Control Release. 2016, 237,42. [DOI] [PubMed] [Google Scholar]
- 90.Falco EE, Wang MO, Thompson JA, Chetta JM, Yoon DM, Li EZ, Kulkami MM, Shah S, Pandit A, Roth JS, Fisher JP, Pharm Res. 2011, 28,1306. [DOI] [PubMed] [Google Scholar]
- 91.Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, Pierce GF, Mol Ther. 2002, 5,517. [DOI] [PubMed] [Google Scholar]
- 92.Ikeda K, Ito A, Sato M, Kawabe Y, Kamihira M, Regenerative Therapy. 2016, 3,38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato M, Ito A, Kawabe Y, Nagamori E, Kamihira M, Journal of bioscience. 2011, 112,273. [DOI] [PubMed] [Google Scholar]
- 94.Zaitseva TS,Alcazar C, Zamani M, Hou L, Sawamura S, Yakubov E, Hopkins M, Woo YJ, Paukshto MV, Huang NF, Tissue Eng Part A. 2018, 10.1089/ten.TEA.2017.0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vandenburgh H, Shansky J, Benesch‐Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G, nerve, Muscle 2008, 37,438. [DOI] [PubMed] [Google Scholar]
- 96.Haddad M El, Notarnicola C, Evano B, Khatib N El, Blaquière M, Bonnieu A, Tajbakhsh S, Hugon G, Vernus B, Mercier J, J Cell Mol Life Sci. 2017, 74,1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ieronimakis N, Pantoja M, Hays AL, Dosey TL, Qi J, Fischer KA, Hoofnagle AN, Sadilek M, Chamberlain JS, Ruohola-Baker H, Reyes M, Skelet Muscle. 2013, 3,20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lo KW, Jiang T, Gagnon KA, Nelson C, Laurencin CT, Trends Biotechnol. 2014, 32,74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lo KW, Ashe KM, Kan HM, Laurencin CT, Regen Med. 2012, 7,535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hou L, Yang G, Tang S, Alcazar C, Joshi P, Strassberg Z, Kim M, Kawamura M, Woo YJ, Shrager J, Ding S, Huang NF, J Am Heart Assoc. 2018, 7,e009234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strohman RC, Bayne E, Spector D, Obinata T, Micou-Eastwood J, Maniotis A, In Vitro Cell Dev Biol. 1990, 26,201. [DOI] [PubMed] [Google Scholar]
- 102.Dennis RG, Kosnik PE 2nd, In Vitro Cell Dev Biol Anim. 2000, 36,327. [DOI] [PubMed] [Google Scholar]
- 103.Michael K, Biol Res Nurs. 2000, 2,117. [DOI] [PubMed] [Google Scholar]
- 104.Li EW, McKee-Muir OC, Gilbert PM. Cellular biomechanics in skeletal muscle regeneration Current topics in developmental biology. Elsevier; 2018:125. [DOI] [PubMed] [Google Scholar]
- 105.Bakkar N, Guttridge DC, Physiol Rev. 2010, 90,495. [DOI] [PubMed] [Google Scholar]
- 106.Kumar A, Murphy R, Robinson P, Wei L, Boriek AM, FASEB J. 2004, 18,1524. [DOI] [PubMed] [Google Scholar]
- 107.Tidball JG, J Appl Physiol (1985). 2005, 98,1900. [DOI] [PubMed] [Google Scholar]
- 108.Shin JW, Mooney DJ, Cell Stem Cell. 2016, 18,16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akimoto T, Ushida T, Miyaki S, Tateishi T, Fukubayashi T, Mat Sci Eng C. 2001, 17,75 [Google Scholar]
- 110.Somers SM, Spector AA, DiGirolamo DJ, Grayson WL, Tissue Eng Part B Rev. 2017, 23,362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pennisi CP, Olesen CG, de Zee M, Rasmussen J, Zachar V, Tissue Eng Part A. 2011, 17,2543. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Huang G, Gao B, Li M, Genin GM, Lu TJ, Xu F, J NPG Asia Materials. 2016, 8,e238 [Google Scholar]
- 113.Heher P, Maleiner B, Prüller J, Teuschl AH, Kollmitzer J, Monforte X, Wolbank S, Redl H, Rünzler D, Fuchs C, Acta Biomater. 2015, 24,251. [DOI] [PubMed] [Google Scholar]
- 114.Moon DG, Christ G, Stitzel JD, Atala A, Yoo J, Tissue Eng Part A. 2008, 14,473. [DOI] [PubMed] [Google Scholar]
- 115.Powell CA, Smiley BL, Mills J, Vandenburgh HH, Am J Physiol Cell Physiol. 2002, 283,C1557. [DOI] [PubMed] [Google Scholar]
- 116.Vandenburgh HH, Hatfaludy S, Karlisch P, Shansky J, Am J Physiol Cell Physiol. 1989, 256,C674. [DOI] [PubMed] [Google Scholar]
- 117.Balint R, Cassidy NJ, Cartmell SH, J Tissue Engineering Part B: Reviews. 2012, 19,48. [DOI] [PubMed] [Google Scholar]
- 118.Ross JJ, Duxson MJ, Harris AJ, Development. 1987, 100,395. [DOI] [PubMed] [Google Scholar]
- 119.Wan Q, Yeung SS, Cheung KK, Au SW, Lam WW, Li YH, Dai ZQ, Yeung EW, Am J Phys Med Rehabil. 2016, 95,28. [DOI] [PubMed] [Google Scholar]
- 120.Yamada E, Hashimoto S, Tachibana K, Okada M, Yamasaki K, Kondo H, Imoto K, Mochizuki S, Fujisato T, Ohsuga M. Effect of electric stimulation on adhesion and proliferation of cultured muscle cells. Proc. 12th world multi-conference on systemics cybernetics and informatics. 2008;2:124 [Google Scholar]
- 121.Ito A, Yamamoto Y, Sato M, Ikeda K, Yamamoto M, Fujita H, Nagamori E, Kawabe Y, Kamihira M, Sci Rep. 2014, 4,4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Evans DJ, Britland S, Wigmore PM, Dev Genes Evol. 1999, 209,438. [DOI] [PubMed] [Google Scholar]
- 123.Huang NF, Patel S, Thakar RG, Wu J, Hsiao BS, Chu B, Lee RJ, Li S, Nano Lett. 2006, 6,537. [DOI] [PubMed] [Google Scholar]
- 124.Jiang X, Takayama S, Qian X, Ostuni E, Wu H, Bowden N, LeDuc P, Ingber DE, Whitesides GM, Langmuir. 2002, 18,3273 [Google Scholar]
- 125.Lam MT, Sim S, Zhu X, Takayama S, Biomaterials. 2006, 27,4340. [DOI] [PubMed] [Google Scholar]
- 126.Bian W, Liau B, Badie N, Bursac N, Nat Protoc. 2009, 4,1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bozec L, van der Heijden G, Horton M, Biophys J. 2007, 92,70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jana S, Zhang MQ, J Mater Chem B. 2013, 1,2575. [DOI] [PubMed] [Google Scholar]
- 129.Dugan JM, Gough JE, Eichhorn SJ, Biomacromolecules. 2010, 11,2498. [DOI] [PubMed] [Google Scholar]
- 130.Lai ES, Huang NF, Cooke JP, Fuller GG, Regen Med. 2012, 7,649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakayama KH, Hong G, Lee JC, Patel J, Edwards B, Zaitseva TS, Paukshto MV, Dai H, Cooke JP, Woo YJ, Huang NF, ACS Nano. 2015, 9,6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Page RL, Malcuit C, Vilner L, Vojtic I, Shaw S, Hedblom E, Hu J, Pins GD, Rolle MW, Dominko T, Tissue Eng Part A. 2011, 17,2629. [DOI] [PubMed] [Google Scholar]
- 133.Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H, J Cell Mol Med. 2008, 12,1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang W, Wray LS, Rnjak-Kovacina J, Xu L, Zou D, Wang S, Zhang M, Dong J, Li G, Kaplan DL, Jiang X, Biomaterials. 2015, 56,68. [DOI] [PubMed] [Google Scholar]
- 135.Verseijden F, Posthumus-van Sluijs SJ, van Neck JW, Hofer SO, Hovius SE, van Osch GJ, J Tissue Eng Regen Med. 2012, 6,169. [DOI] [PubMed] [Google Scholar]
- 136.Laschke MW, Menger MD, Biotechnol Adv. 2016, 34,112. [DOI] [PubMed] [Google Scholar]
- 137.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R, Nat Biotechnol. 2005, 23,879. [DOI] [PubMed] [Google Scholar]
- 138.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S, Proc Natl Acad Sci U S A. 2011, 108,14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Juhas M, Engelmayr GC Jr, Fontanella AN, Palmer GM, Bursac N, Proc Natl Acad Sci U S A. 2014, 111,5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bach AD, Beier JP, Stark GB, Cell Tissue Res. 2003, 314,263. [DOI] [PubMed] [Google Scholar]
- 141.Ko IK, Lee BK, Lee SJ, Andersson KE, Atala A, Yoo JJ, Biomaterials. 2013, 34,3246. [DOI] [PubMed] [Google Scholar]
- 142.Wang L, Shansky J, Vandenburgh H, Mol Neurobiol. 2013, 48,397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larkin LM, Van der Meulen JH, Dennis RG, Kennedy JB, In Vitro Cell Dev Biol Anim. 2006, 42,75. [DOI] [PubMed] [Google Scholar]
- 144.Dhawan V, Lytle IF, Dow DE, Huang YC, Brown DL, Tissue Eng. 2007, 13,2813. [DOI] [PubMed] [Google Scholar]
- 145.Turner NJ, Yates AJ Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, Badylak SF, Tissue Eng Part A. 2010, 16,3309. [DOI] [PubMed] [Google Scholar]
- 146.Valentin JE, Turner NJ, Gilbert TW, Badylak SF, Biomaterials. 2010, 31,7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aurora A, Roe JL, Corona BT, Walters TJ, Biomaterials. 2015, 67,393. [DOI] [PubMed] [Google Scholar]
- 148.Aurora A, Corona BT, Walters TJ, Cells Tissues Organs. 2016, 202,189. [DOI] [PubMed] [Google Scholar]
- 149.Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, Zhao W, Christ GJ, Tissue Eng Part A. 2012, 18,1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Quarta M, Cromie M, Chacon R, Blonigan J, Garcia V, Akimenko I, Hamer M, Paine P, Stok M, Shrager JB, Rando TA, Nat Commun. 2017, 8,15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gilbert-Honick J, Iyer SR, Somers SM, Lovering RM, Wagner K, Mao HQ, Grayson WL, Biomaterials. 2018, 164,70. [DOI] [PubMed] [Google Scholar]
- 152.Criswell TL, Corona BT, Wang Z, Zhou Y, Niu G, Xu Y, Christ GJ, Soker S, Biomaterials. 2013, 34,140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakayama KH, Alcazar C, Yang G, Quarta M, Paine P, Doan L, Davies A, Rando TA, Huang NF, NPJ Regen Med. 2018, 3,16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cezar CA, Roche ET, Vandenburgh HH, Duda GN, Walsh CJ, Mooney DJ, Proc Natl Acad Sci U S A. 2016, 113,1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gilbert-Honick J, Ginn B, Zhang Y, Salehi S, Wagner KR, Mao HQ, Grayson WL, Cell Transplant. 2018, 963689718805370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, Chen CS, Nat Biomed Eng. 2017, 1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ward CL, Pollot BE, Goldman SM, Greising SM, Wenke JC, Corona BT, J Orthop Trauma. 2016, 30,e396. [DOI] [PubMed] [Google Scholar]
- 158.Greising SM, Rivera JC, Goldman SM, Watts A, Aguilar CA, Corona BT, Sci Rep. 2017, 7,13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mooney DJ, Borselli C, Vandenburgh H, Shvartsman D, Storrie H, Lichtman J. Enhancement of skeletal muscle stem cell engraftment by dual delivery of vegf and igf-1. Google Patents. 2017 [Google Scholar]
- 160.Kuraitis D, Zhang P, Zhang Y, Padavan D, McEwan K, Sofrenovic T, McKee D, Zhang J, Griffith M, Cao X, Eur Cell Mater. 2011, 22,e23. [DOI] [PubMed] [Google Scholar]
- 161.Hill E, Boontheekul T, Mooney DJ, Tissue Eng 2006, 12,1295. [DOI] [PubMed] [Google Scholar]
- 162.Ruvinov E, Leor J, Cohen S, Biomaterials. 2010, 31,4573. [DOI] [PubMed] [Google Scholar]
- 163.Rybalko VY, Pham CB, Hsieh P-L, Hammers DW, Merscham-Banda M, Suggs LJ, Farrar RP, Biomater Sci. 2015, 3,1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martins KJ, Gehrig SM, Naim T, Saenger S, Baum D, Metzger F, Lynch GS, Growth Horm IGF Res. 2013, 23,128. [DOI] [PubMed] [Google Scholar]
- 165.Breen A, Dockery P, O’Brien T, Pandit A, J Biomed Mater Res A. 2009, 89,876. [DOI] [PubMed] [Google Scholar]
- 166.Donnelly K, Khodabukus A, Philp A, Deldicque L, Dennis RG, Baar K, Tissue Eng Part C. 2010, 16,711. [DOI] [PubMed] [Google Scholar]
- 167.Pedrotty DM, Koh J, Davis BH, Taylor DA, Wolf P, Niklason LE, Am J Physiol Heart Circ Physiol. 2005, 288,H1620. [DOI] [PubMed] [Google Scholar]
- 168.Borschel GH, Dennis RG, Kuzon WM Jr.Plast Reconstr Surg. 2004, 113,595. [DOI] [PubMed] [Google Scholar]
- 169.Hosseini V Ahadian S,Ostrovidov S, Camci-Unal G, Chen S, Kaji H, Ramalingam M, Khademhosseini A, Tissue Eng Part A. 2012, 18,2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Flaibani M, Boldrin L, Cimetta E, Piccoli M, De Coppi P, Elvassore N, Tissue Eng Pt A. 2009, 15,2447. [DOI] [PubMed] [Google Scholar]
- 171.Nagamine K Kawashima T, Ishibashi T, Kaji H, Kanzaki M, Nishizawa M, Biotechnol Bioeng. 2010, 105,1161. [DOI] [PubMed] [Google Scholar]
- 172.Sadeghian R Banan Ebrahimi M , Salehi S, J Tissue Eng Regen Med. 2018, 12,912. [DOI] [PubMed] [Google Scholar]
- 173.Park H Bhalla R, Saigal R, Radisic M, Watson N, Langer R, Vunjak-Novakovic G, J Tissue Eng Regen Med. 2008, 2,279. [DOI] [PMC free article] [PubMed] [Google Scholar]


