During pregnancy, Plasmodium falciparum-infected erythrocytes (IE) accumulate in the intervillous spaces of the placenta by binding to chondroitin sulfate A (CSA) and elicit inflammatory responses that are associated with poor pregnancy outcomes. Primigravidae lack immunity to IE that sequester in the placenta and thus are susceptible to placental malaria (PM).
KEYWORDS: antibody, Plasmodium falciparum, binding inhibition, malaria, opsonizing phagocytosis, pregnancy
ABSTRACT
During pregnancy, Plasmodium falciparum-infected erythrocytes (IE) accumulate in the intervillous spaces of the placenta by binding to chondroitin sulfate A (CSA) and elicit inflammatory responses that are associated with poor pregnancy outcomes. Primigravidae lack immunity to IE that sequester in the placenta and thus are susceptible to placental malaria (PM). Women become resistant to PM over successive pregnancies as antibodies to placental IE are acquired. Here, we assayed plasma collected at delivery from Malian and Tanzanian women of different parities for total antibody levels against recombinant VAR2CSA antigens (FCR3 allele), and for surface reactivity and binding inhibition and opsonizing functional activities against IE using two CSA-binding laboratory isolates (FCR3 and NF54). Overall, antibody reactivity to VAR2CSA recombinant proteins and to CSA-binding IE was higher in multigravidae than in primigravidae. However, plasma from Malian gravid women reacted more strongly with FCR3 whereas Tanzanian plasma preferentially reacted with NF54. Further, acquisition of functional antibodies was variant dependent: binding inhibition of P. falciparum strain NF54 (P < 0.001) but not of the strain FCR3 increased significantly with parity, while only opsonizing activity against FCR3 (P < 0.001) increased significantly with parity. In addition, opsonizing and binding inhibition activities of plasma of multigravidae were significantly correlated in assays of FCR3 (r = 0.4, P = 0.01) but not of NF54 isolates; functional activities did not correlate in plasma from primigravidae. These data suggest that IE surface-expressed epitopes involved in each functional activity differ among P. falciparum strains. Consequently, geographic bias in circulating strains may impact antibody functions. Our study has implications for the development of PM vaccines aiming to achieve broad protection against various parasite strains.
INTRODUCTION
In areas of malaria endemicity, pregnant women are at high risk of Plasmodium falciparum infection resulting in placental malaria (PM), which is characterized by the sequestration of infected erythrocytes (IE) in the intervillous spaces. PM results in inflammatory responses and adverse outcomes, such as maternal anemia, low birth weight (LBW), and prematurity, and increases maternal and perinatal mortality (1–4). First-time mothers are most susceptible to PM, which is explained by the absence of preexisting immunity against IE that bind chondroitin sulfate A (CSA) and sequester in the placenta. In contrast, multigravidae have acquired this immunity over successive pregnancies and can better control PM (5–10).
Women acquire antibodies against placental parasites over successive pregnancies, and these can vary in function (8, 11). High serum reactivity to placental IE, commonly found in protected multigravidae, has been associated with increased hemoglobin levels, birth weight, and gestational age (7–9, 12). Serum IgG that either blocks IE binding to CSA or opsonizes IE for phagocytosis has been related to reduced risks of infection and of LBW, as well as increased hemoglobin levels, birth weight, and gestational age (5, 13, 14). These relationships between antibody functions and improved outcomes strongly suggest that naturally acquired functional antibodies protect against PM, and therefore the target antigens can be exploited for vaccination strategies. However, the antibody effector mechanism that primarily confers protection against PM remains unclear (2, 15).
VAR2CSA (∼350 kDa), a member of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family, is the immunodominant variant IE surface antigen expressed by placental parasites (12, 16–18). Several lines of evidence suggest that VAR2CSA is the major surface protein expressed by placental parasites (10, 17), the principal ligand for CSA binding (19), and the main target of antibodies acquired during PM (17), supporting this protein as the leading vaccine candidate to prevent PM. Thus far, the design of VAR2CSA-based vaccines has focused on identifying subunit constructs comprising VAR2CSA domains or fragments from representative allelic variants that are capable of inducing potent antibodies, similar to those acquired by multigravidae, to offer broad protection against PM (20–23). Although VAR2CSA-specific opsonizing antibodies also appear to contribute to protection, their clinical significance has received less attention (5, 14). Consequently, a deeper understanding of naturally acquired protective or functional antibodies in pregnant women is needed to inform vaccine design.
Protective antibodies found in multigravidae may target conserved epitopes shared by multiple variants of a parasite or may represent the combined effect of specific reactivities against multiple parasite variants (24). The latter hypothesis has been recently demonstrated in a report showing that naturally acquired host immune defenses to different P. falciparum antigens act in an allele-specific manner to block specific parasite haplotypes from establishing blood-stage infections in infants and children, suggesting that vaccines targeting polymorphic parasite antigens must account for allele-specific immunity (25). Many studies have demonstrated that VAR2CSA displays extensive diversity between parasites/alleles, contains conserved interspersed regions shared between alleles (26–28), and is targeted by cross-reactive antibodies acquired during PM (29–32). However, the differential susceptibility of different CSA-binding parasites to binding inhibition and opsonizing antibody functions has not previously been established. Two VAR2CSA-based vaccine candidates based on N-terminal fragments from FCR3 and 3D7 parasite variants recently entered clinical trials (ClinicalTrials.gov registration numbers NCT02647489 and NCT02658253); hence, understanding functional activities of naturally acquired antibodies against these variants can inform the interpretation of clinical trial results.
Data on the geographical differences in the binding inhibition and opsonizing functions of naturally acquired antibodies in PM against CSA-binding parasites and the differential susceptibility of parasite variants to these antibody functions are undocumented. In this study, we explored the impact of parasite variant, as well as of maternal parity and geographical origin, on PM-specific antibody functions.
RESULTS
Plasma reactivities to recombinant VAR2CSA antigens are similar in Mali and Tanzania.
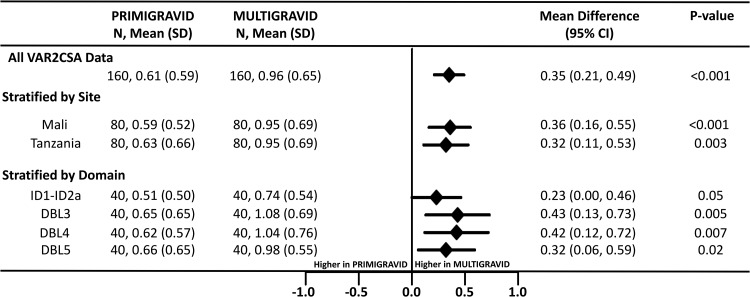
Plasma antibodies were assessed for their reactivity to ID1-DBL2X-ID2a, DBL3, DBL4, and DBL5 recombinant domains of the FCR3 VAR2CSA (VAR2CSAFCR3) by enzyme-linked immunosorbent assay (ELISA) and were significantly higher in multigravidae than in primigravidae (P < 0.001) (Fig. 1). This parity-dependent reactivity was consistent across individual VAR2CSA domain antigens and between sites. Reactivities to the P. falciparum merozoite antigen apical membrane antigen-1 (AMA-1), an asexual stage merozoite protein, were similar in primigravidae and multigravidae (see Fig. S1 in the supplemental material), suggesting a comparable exposure to malaria infection in these geographical areas. Overall, plasma IgG reactivities to VAR2CSAFCR3 antigens were similar between the two sites, suggesting similar levels of exposure to VAR2CSA-expressing parasites (Fig. S2).
FIG 1.
Reactivity of plasma IgG to ID1-DBL2X-ID2a, DBL3, DBL4, and DBL5 recombinant domains of VAR2CSAFCR3 by ELISA. The forest plot shows mean differences and 95% confidence intervals (CI) for the levels of IgG binding to all VAR2CSA antigens between primigravidae and multigravidae with analysis stratified by site and VAR2CSA domains. Levels of antibodies in optical density units were compared. N, combined number of samples assessed on all VAR2CSA recombinants or individually; SD, standard deviation.
Plasma IgG reactivity to native VAR2CSA increases with parity but depends on parasite variant and antibody subclass.
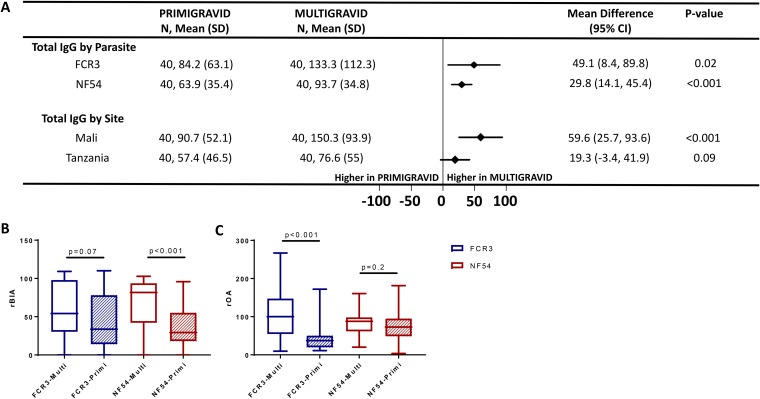
Following the selection of IE that bind CSA, both FCR3 and NF54 parasites expressed VAR2CSA on the IE surface (Fig. S3). The levels of surface reactivity of naturally acquired immunoglobulins to both parasites were analyzed by parity and study site (Fig. 2A and Fig. S4 and S5). IE reactivity was significantly higher in multigravidae than in primigravidae (P = 0.02 for FCR3 and P < 0.001 for NF54) (Fig. 2A), and this difference was greater in pregnant women from Mali (P < 0.001). The higher reactivity of IgG in multigravidae to native VAR2CSA is consistent with the higher reactivity to VAR2CSA recombinant proteins and supports previous evidence that VAR2CSA is the major antigen targeted by PM-specific immunity.
FIG 2.
Reactivity and function of plasma antibodies to CSA-binding parasites by parity. (A) The forest plot shows the mean difference with 95% confidence interval (CI) for total IgG binding to CSA-adhering parasites by parity, and data represent the relative median fluorescence intensity. N, number of samples in each group; SD, standard deviation. rBIA (B) and rOA (C) against FCR3 and NF54-CSA between primigravidae (hatched box) and multigravidae (open box) are shown. Horizontal lines indicate medians, boxes indicate interquartile ranges, and error bars indicate ranges. A Mann-Whitney test was performed for significance, and P values are reported.
The reactivity of plasma IgA, IgM, and isotypes IgG1 and IgG3 to parasites was also examined by flow cytometry. IgG3 binding to both parasites was significantly higher in multigravidae (P = 0.04 for FCR3 and P < 0.001 for NF54) (Fig. S5). While the binding of IgG1, IgA, and IgM antibodies to NF54 IE was greater in multigravidae, their binding to FCR3 IE was not significantly related to parity (Fig. S5).
Plasma binding inhibition and opsonizing activities increase with parity.
Overall, levels of binding inhibition activity (BIA) against CSA-binding NF54 and FCR3 IE were significantly higher in plasma from multigravidae than from primigravidae at both study sites (P < 0.001). After stratifying the analysis by parity and parasite variant, greater BIA in multigravidae was observed against both isolates, but the difference was significant only for NF54 (P < 0.001) (Fig. 2B). Stratified analysis by site and parasite variant revealed that multigravidae in Tanzania had higher BIA against both parasites, while those in Mali had significantly higher BIA against NF54 but not FCR3 parasites (Fig. S6A and S7A and B).
We next explored plasma opsonizing activity (OA) against NF54 and FCR3 IE in malaria-exposed pregnant women. Overall, OA was significantly higher in multigravidae than in primigravidae (P < 0.001). However, when the analysis was stratified by parity and parasite variant, multigravidae displayed significantly stronger OA than primigravidae only against FCR3 (P < 0.001) (Fig. 2C). This pattern was observed at both study sites (Fig.S6B and S7C and D) and notably was the opposite of the pattern seen for BIA against the parasite variants (see above).
Levels of plasma functional activities are parasite variant dependent.
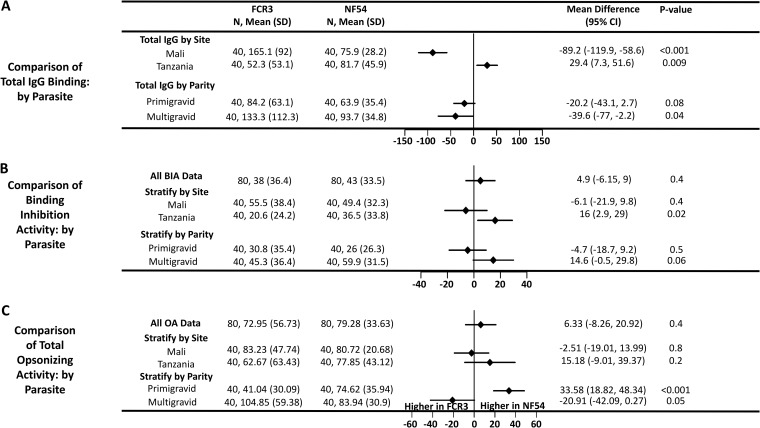
Because plasma functional activity depended on parasite variant and study site, we compared the plasma antibody reactivity to the IE surface of both parasite variants as measured by flow cytometry. Total-IgG reactive to CSA-binding IE revealed a parasite variant bias toward FCR3 in Mali and toward NF54 in Tanzania. Indeed, total-IgG reactivity to FCR3 was significantly higher than that to NF54 IE in Mali (P < 0.001) (Fig. 3A), while total-IgG reactivity to NF54 was higher than that to FCR3 in Tanzania (P = 0.009) (Fig. 3A). After stratifying the analysis by parity, pregnant women from both study sites had greater IgG reactivity to FCR3 than to NF54, but the difference was not statistically significant in primigravidae (Fig. 3A). Moreover, plasma IgG1 and IgG3 as well as plasma IgA, regardless of parity, bound preferentially to FCR3, whereas IgM showed significantly higher binding to NF54 than to FCR3 (Fig. S8).
FIG 3.
Reactivity and function of plasma antibodies from pregnant women by parasite variant and stratified by site and parity. The forest plot shows mean differences with 95% confidence intervals (CI) for relative MFI of total IgG binding to CSA-adhering parasites (A), rBIA (B), and rOA (C) against FCR3 and NF54 isolates. N, number of samples.
Overall, the binding of IE to CSA was inhibited by the plasma sets to similar degrees for both parasite variants (Fig. 3B). However, when the analysis was stratified by site, plasma samples from Tanzania showed stronger BIA against NF54 than against FCR3 (P = 0.02), largely explained by the activity in plasma of multigravidae (Fig. S9A).
The parasite variants were also opsonized to similar degrees by the two plasma sets (P = 0.4) (Fig. 3C). However, while plasma of primigravidae demonstrated higher OA against NF54 than against FCR3 (P < 0.001) (Fig. 3C), plasma of multigravidae demonstrated higher OA against FCR3 than against NF54, albeit this difference was not statistically significant (P = 0.05). Notably, these patterns were seen at both study sites (Fig. S9B).
Binding inhibition and opsonizing activities correlate in plasma of multigravidae but not of primigravidae.
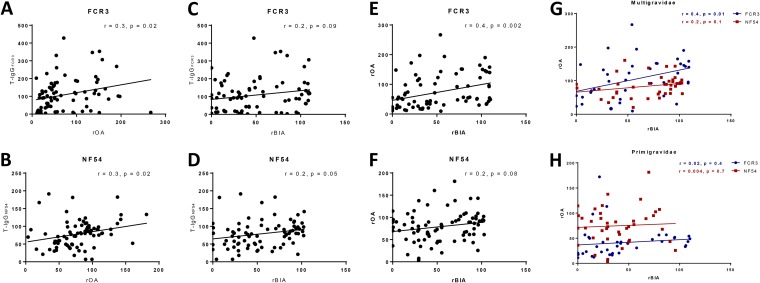
Relationship between OA, BIA, and the total-IgG binding to the parasites was explored. The OA function of antibodies against both parasites was significantly associated with the level of IE-bound total IgG (Fig. 4A and B). Conversely, the level of parasite-specific total IgG was related to BIA function against NF54 IE (P = 0.05) but less related to that against FCR3 (Fig. 4C and D). We then examined the relationship between BIA and OA functions. Among all pregnant women, these two functions were correlated in assays of both parasites but significantly only for FCR3 (P < 0.002) (Fig. 4E and F). When the analysis was stratified by parity, this relationship between BIA and OA of FCR3 was significant for multigravidae (P = 0.01) (Fig. 4G) but not primigravidae (Fig. 4H). For NF54, BIA and OA were not significantly related for either group. Notably, many multigravidae with relatively low levels of OA against NF54 had high BIA against the same parasite.
FIG 4.
Comparison of the binding inhibition and opsonizing activities of plasma by parasite variant and parity. Relationships between OA (A and B) and BIA (C and D) and the level of total-IgG (T-IgG) binding to FCR3 and NF54 parasites are shown. The relationship between BIA and OA functions against FCR3 (E) and NF54 (F) is shown. (G and H) Stratified analysis by comparing activities of plasma from multigravidae and primigravidae against FCR3 and NF54 parasites was carried out. Pearson’s (r) coefficient of correlation and P values are reported for each comparison.
Parity, site, and seroreactivity profiles differ in their associations with variant-dependent plasma functional activity.
We performed a multivariate analysis to determine factors associated with BIA and OA against each parasite variant (Table 1). Parity was consistently associated with both antibody functions, particularly the BIA, irrespective of parasite variant; multigravidae displayed significantly greater activity against both parasite variants. Generally, Tanzanian women had lower BIA levels against the FCR3 variant than Malian women, and elevated BIA against NF54 was significantly associated with higher levels of antibodies specific to the DBL3 domain of VAR2CSAFCR3 (DBL3FCR3) (P = 0.03). However, these DBL3FCR3-specific antibodies failed to predict the inhibition of the homologous parasite, suggesting that additional antibodies with other VAR2CSA specificities might be required to inhibit FCR3 binding to CSA. Unexpectedly, only IgA binding to FCR3 was positively associated with a high level of BIA against FCR3.
TABLE 1.
Multivariate analysis of factors associated with BIA and OA
| Antibody source or type | Binding inhibition activity |
Opsonizing activity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCR3 |
NF54 |
FCR3 |
NF54 |
|||||||||
| Coefficient | SEa | P value | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | |
| Mali | 46.7 | 10.3 | <0.001 | 10.7 | 6.6 | 0.1 | 14.3 | 13.9 | 0.3 | 9.03 | 8.2 | 0.3 |
| Primigravidae | −21.4 | 7.9 | 0.008 | −26.8 | 6.8 | <0.001 | −53.6 | 10.8 | <0.001 | −3.03 | 8.3 | 0.7 |
| DBL3_VAR2CSA | −6.3 | 9.9 | 0.5 | 17.7 | 8.1 | 0.03 | −0.7 | 13.08 | 0.9 | −4.6 | 10.04 | 0.7 |
| ID1-ID2a_VAR2CSA | 6.3 | 9.3 | 0.5 | −14.7 | 7.6 | 0.065 | 1.1 | 12.2 | 0.9 | −1.2 | 9.1 | 0.9 |
| DBL4_VAR2CSA | −2.03 | 8.4 | 0.8 | −8.7 | 7.3 | 0.244 | 10.8 | 11.5 | 0.3 | 10.1 | 9.0 | 0.3 |
| DBL5_VAR2CSA | 10.3 | 10.1 | 0.3 | 12.1 | 8.4 | 0.17 | 17.5 | 13.8 | 0.2 | 2.8 | 10.2 | 0.8 |
| IgA_FCR3 | 0.08 | 0.03 | 0.008 | NDb | ||||||||
| IgG1_FCR3 | −0.04 | 0.05 | 0.4 | −0.2 | 0.06 | 0.008 | ||||||
| IgG3_FCR3 | −0.05 | 0.03 | 0.1 | −0.01 | 0.03 | 0.7 | ||||||
| IgM_FCR3 | −0.05 | 0.1 | 0.7 | ND | ||||||||
| Total IgG_FCR3 | −0.1 | 0.0 | 0.06 | 0.005 | 0.08 | 0.9 | ||||||
| IgA_NF54 | −0.005 | 0.1 | 0.9 | ND | ||||||||
| IgG1_NF54 | 0.1 | 0.2 | 0.6 | −0.3 | 0.2 | 0.1 | ||||||
| IgG3_NF54 | −0.07 | 0.1 | 0.6 | 0.008 | 0.2 | 0.9 | ||||||
| IgM_NF54 | −0.01 | 0.07 | 0.9 | ND | ||||||||
| Total IgG_NF54 | −0.004 | 0.2 | 0.9 | 0.4 | 0.2 | 0.03 | ||||||
SE, standard error.
ND, not done.
While OA against the FCR3 parasite was highly associated with parity (P < 0.001), OA against NF54 parasites showed no significant association. Instead, OA against NF54 was associated with NF54 total-IgG reactivity (P = 0.02), and OA against FCR3 parasites was inversely associated with IgG1 reactivity against FCR3 (IgG1FCR3) (P = 0.008).
Surface displays of VAR2CSA differ between parasite variants.
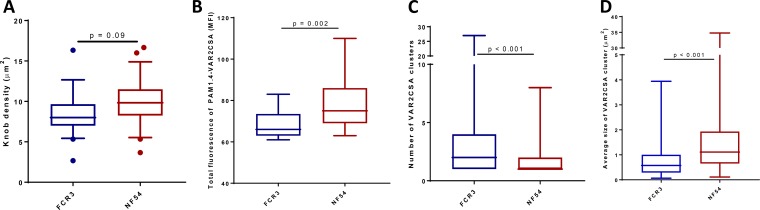
We observed that the function of PM-specific antibodies can depend on the parasite variant. We hypothesized that the variant-dependent nature of functional antibodies might be related to the differential display of antigens on the IE surface. We therefore examined IE surface expression of VAR2CSA by both parasites using imaging. The densities of knobs on the IE surface did not differ between isolates, based on scanning electron microscopy (SEM) (Fig. 5A). However, based on fluorescence of IE surface-bound PAM1.4 measured in Airyscan microscopy, VAR2CSA was more abundant on NF54 than on FCR3 IE (P = 0.002) (Fig. 5B). This finding was confirmed by total internal reflection fluorescence (TIRF) microscopy (Fig. S10).
FIG 5.
Knob density and surface display of VAR2CSA by FCR3 and NF54 isolates. (A) Knob density on VAR2CSA-expressing FCR3 and NF54 parasites. (B) Total fluorescence of PAM1.4-labeled VAR2CSA measured by Airyscan microscopy. (C and D) Number and size of VAR2CSA clusters identified on CSA-binding FCR3 and NF54 parasites. Horizontal lines indicate medians, boxes indicate interquartile ranges, and error bars indicate ranges. A Mann-Whitney test was performed for significance, and P values are reported.
A cluster-based analysis was performed from the TIRF data to investigate whether the topologies of PAM1.4 surface-stained VAR2CSA were similar between isolates. FCR3 IE appeared to have more clusters of VAR2CSA with multiple discrete foci of surface-stained VAR2CSA than NF54 IE wherein a single large VAR2CSA cluster was often observed (Fig. 5C and D). In summary, the surface displays of VAR2CSA stained by PAM1.4 differed between the two isolates despite their similar knob topologies.
VAR2CSA sequences from Mali and Tanzania exhibit a bias in circulating FCR3- and NF54-like parasites.
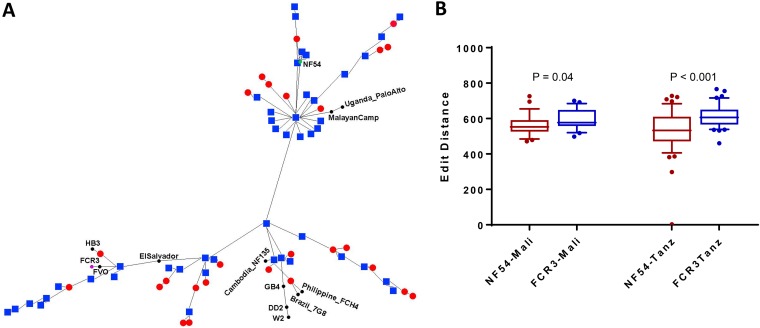
To investigate whether there is a bias in the geographical distribution of parasites similar to FCR3 and NF54, VAR2CSA sequences originating from Mali and Tanzania were analyzed. The phylogenetic tree highlighted the distance between the VAR2CSA sequences from Mali and Tanzania and different lab strains, including FCR3 and NF54 (Fig. 6A). Based on phylogenetic distances, FCR3 and NF54 alleles represent relatively distal points on their respective branches of the phylogenetic tree while another subset of isolates branched off the tree at a point roughly equidistant to FCR3 and NF54. Among the sequences that clustered in the NF54 or FCR3 branch, the Malian isolates were equally likely to be in either branch (9 NF54-like versus 9 in FCR3-like) while Tanzanian isolates were more frequently observed in the NF54 branch (21 NF54-like versus 15 in FCR3-like). Based on the edit distance values, while NF54-like parasites were more prevalent at both study sites, the bias to NF54 and away from FCR3 is stronger in Tanzania than in Mali, with a greater difference in the edit distances and higher significance (Fig. 6B).
FIG 6.
Distribution of FCR3- and 3D7-like strains in Mali and Tanzania. (A) A phylogenetic tree based on sequence alignment of full-length extracellular VAR2CSA sequence highlighting the distance of the VAR2CSA sequences from Mali (red dots) and Tanzania (blue squares) to FCR3 and NF54. Eleven sequences from other lab strains are also included. (B) Edit distance values of NTS-DBL6 of VAR2CSA sequences originating from Mali and Tanzania are plotted to assess the similarities of the sequences to NF54 and FCR3. Horizontal lines indicate medians, boxes indicate interquartile ranges, and error bars indicate the 10 to 90% range. A Mann-Whitney test was performed for significance, and P values are reported.
DISCUSSION
A vaccine that can induce antibodies with broadly neutralizing activity is key to ensuring its efficacy, but this threshold has been challenging to attain for placental malaria vaccine development. Observations that multigravidae living in areas of malaria endemicity acquire strain-transcendent antibody activity (7–9) has supported the feasibility of a vaccine such as VAR2CSA against PM. In this study, we confirm that antibodies in plasma from multigravidae bind VAR2CSA recombinant proteins at significantly higher levels than those of primigravidae, as previously reported (33). Moreover, the high reactivity of IgG to native VAR2CSA among multigravidae, in line with the IgG reactivity to recombinant VAR2CSA antigens, supports VAR2CSA as the major IE surface antigen involved in PM pathology. Indeed, the levels of IgG binding to the recombinant VAR2CSA antigens were positively correlated to the level of total IgG that bound to surface antigens of FCR3 and NF54 IE (see Fig. S11 in the supplemental material). Parity-dependent immunity to VAR2CSA was also observed in assays against native protein expressed by different CSA-binding parasites, including IE surface reactivity as well as functions measured by binding inhibition and opsonizing assays.
Antibodies that block adhesion of P. falciparum IE to CSA have been associated with protection (6, 34) while OA has been associated with reduced risk of maternal anemia and LBW (5, 15). Here, the parity-related functional antibody activity appeared to be significantly shaped by the parasite variant. Prior studies have described variant-specific BIA and OA of naturally acquired antibodies in pregnant women (16, 35) although related data assessing these two functions of antibodies in the same samples are absent. Plasma BIA in multigravidae was significantly higher against NF54 parasites, and plasma OA was significantly higher against FCR3 parasites than in primigravidae. These findings underscore the importance of parasite variant selection in evaluating these two functions. NF54 and FCR3 share 79% identity and 85% similarity in VAR2CSA amino acid sequences, differing at 553/2,678 amino acid positions in the extracellular domain. Further studies are required to identify VAR2CSA epitopes shared by multiple variants of parasite that are targeted by antibodies capable of engendering both functional activities in PM. Additional data on more isolates, including field isolates, would also be valuable. For this study, we have focused on the parasite lines carrying VAR2CSA alleles included in the leading VAR2CSA vaccines as these data will be pertinent to interpreting immunogenicity results for those vaccines.
Differential display of VAR2CSA on the IE surface could also influence high-avidity, cross-linking antibody reactions (36) and thereby impact antibody function. We observed a higher surface density of PAM1.4-stained VAR2CSA on NF54 while FCR3 displayed a greater number of VAR2CSA clusters on the IE surface. This topological difference in surface-expressed VAR2CSA by the two parasites was independent of the densities of IE surface knobs, which were similar between the isolates (37). PAM1.4 is a human monoclonal antibody which is specific for an unknown conformational epitope in full-length VAR2CSA (38–40). It is therefore possible that differential folding of VAR2CSA on NF54 and FCR3 may lead to a difference in PAM1.4 epitopes displayed by the two parasites. The lower PAM1.4-stained VAR2CSA density on FCR3 might also be explained by the presence of other parasite surface antigens on the knobs (36). A previous study demonstrated that the fractions of the PfEMP1 pool exported to the membrane differed greatly between parasite strains expressing the same PfEMP1 (41). Here, our data suggest that surface density or change in the tertiary structure of VAR2CSA might impact BIA and OA functions of antibodies. However, this hypothesis requires additional investigation of antibodies responsible for each of these functions with regard to the distribution of VAR2CSA by different parasite lines.
While ELISA titers of plasma samples from Mali and Tanzania showed similar reactivities to multiple recombinant FCR3 allele VAR2CSA antigens, Malian plasma had greater reactivity to FCR3 IE surface than Tanzanian plasma (mean of 165.1 for Malian plasma versus mean of 52.3 for Tanzanian plasma; P < 0.0001), suggesting potential differences in epitopes displayed by recombinant and native antigens. Tanzanian plasma samples consistently showed higher IE surface reactivity, BIA, and OA to NF54 parasites, while Malian samples favored FCR3 (IE surface reactivity) or showed no bias (BIA and OA). These observations suggest that parasites similar to FCR3 might be more prevalent in Mali and that those like NF54 might be more prevalent in Tanzania; however, our data indicate that parasites from both sites have greater similarity to NF54 (Fig. 6). Further, a recent study on the global diversity of VAR2CSA did not find any evidence of genetically different clades in East and West Africa (42). Nonetheless, the higher reactivity of antibodies from Malian pregnant women to FCR3 than to NF54 detected in this study was in line with recent reports from Mali showing that antibody levels were significantly higher against DBL4FCR3 than against DBL43D7 in all gravidity groups (13). It is also possible that antibodies against FCR3-like parasites have greater durability, as was reported from Malawi and Papua New Guinea, where total antibodies against CS2 isolate (isogenic of FCR3) remained relatively stable despite declining malaria transmission (43).
Many studies have underlined the predominance of IgG in naturally acquired antibodies associated with protection against adverse pregnancy outcomes (6, 9). Cytophilic IgG, IgG1, and IgG3 are the main subclasses involved in acquired immunity to placental parasites (44). In vitro studies have provided evidence that IgG1 and IgG3 can mediate OA (45) by targeting VAR2CSA (35, 46), suggesting that these antibodies may also play a major role in protection against PM. In general, IgG1 or IgG3 reactivities were not positively related to OA or to BIA, but a more detailed understanding of antibody specificities may be needed to identify such relationships. Our data confirm parity-dependent levels of these IgG isotypes and for the first time indicate a greater reactivity of IgG1 and IgG3 as well as IgA to the FCR3 isolate than to NF54, a pattern unrelated to parity (Fig. S8). Conversely, the higher reactivity of IgM to the NF54 isolate independent of parity indicates that the patterns of PM-related antigen recognition by these antibody types differ.
The role of IgA in malaria immunity is unclear although it has been suggested that IgA targeting FcαRI (CD89) could offer greater potential for therapeutic antibodies. FcαRI is believed to be the most potent Fc receptor at triggering lysis of antibody-targeted tumor cells as well as phagocytosis of antibody-coated pathogens via neutrophils, eosinophils, most monocytes/macrophages, and other myeloid lineage cells (47–49). Maternal serum IgA binding to P. falciparum antigens has been reported but at a lower level than that of secretory IgA in breastmilk (50). This study is the first documented investigation to examine PM-specific IgA and its association with BIA: IgA levels correlated to BIA against FCR3 parasites. However, the correlation was modest, and further investigations on a larger sample size can better define any role for IgA in PM immunity.
One limitation of this work is the lack of antibody reactivity data to VAR2CSA recombinant proteins generated from NF54 sequence. Nevertheless, relatively low sequence diversity within the DBL3, DBL4, and DBL5 domains of VAR2CSA has been reported (18, 26). In this study, high levels of antibody reactivity to recombinant DBL3FCR3 were associated with greater BIA against NF54 but not against the FCR3 isolate. This observation is consistent with our previous report showing that anti-DBL3 antibodies purified from multigravidae had no BIA against the homologous isolate yet disrupted binding of a heterologous parasite to CSA (16). This unexpected failure of BIA against the homologous FCR3 parasite may be explained by allele-specific nonfunctional anti-DBL3FCR3 antibodies that impede the binding of functional antibodies (51), but this hypothesis requires additional study. Another limitation is the small size of samples used in this study, resulting in some borderline significant observations. A calculation of the power estimates based on the data reported in this study was performed and is reported in Table S1. Thus, further studies on a larger sample size are needed to refine various biological trends observed in this work. Finally, we cannot exclude the possibility that IE surface antigens other than VAR2CSA may contribute to antibody reactivity or function against CSA-binding parasites. However, there are limited data to support roles for other IE surface antigens in PM, and VAR2CSA is known to be the immunodominant antigen targeted by parity-specific antibodies (19).
To inform the design of future PM-specific vaccines, these findings reinforce VAR2CSA as a potential candidate by demonstrating that multigravidae develop greater functional activities to parasites with a placenta-binding phenotype. In addition, our investigation sheds light on the relationships between the parasite variant and the binding inhibition and opsonizing function of antibodies as well as the geographical bias in those antibodies. To our knowledge, this is the first study to simultaneously measure binding inhibition and opsonizing activities within the same plasma samples and show that the balance of binding inhibition versus opsonizing activities can be biased by the parasite variant and geographical location. Understanding these issues is crucial for vaccine design with respect to selecting alleles for inclusion in future vaccines and determining whether vaccine antigens may need to differ between regions. While pregnant women may benefit from other malaria vaccines such as those that target sporozoites to prevent infection, similar issues of antigenic diversity may pertain to these as well.
MATERIALS AND METHODS
Study populations.
One set of samples used in this study was collected from women participating in the Mother-Offspring Malaria Studies (MOMS) project conducted in northeastern Tanzania. Details of the project have been reported elsewhere (52). Briefly, pregnant women between the ages of 18 and 45 years who delivered at the Muheza Designated District Hospital, Tanga region, were recruited to participate between September 2002 and October 2005. The U.S. National Institutes of Health (NIH) International Clinical Studies Review Committee of the Division of Microbiology and Infectious Diseases approved the study procedures, and the Institutional Review Boards of the Seattle Biomedical Research Institute and the National Institute for Medical Research in Tanzania provided ethical clearance.
A second set of samples was collected from pregnant women participating in the immunoepidemiology (IMEP) study conducted in Ouelessebougou (a region of intense seasonal malaria transmission), Mali. A detailed description of the IMEP study has been previously reported (13). This study protocol was approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (USA), and by the Ethical Review Committee of the Faculty of Medicine and Pharmacology in Bamako, Mali.
All participating mothers provided informed consent before their enrollment in these studies. For this work, 80 (Tanzania, n = 40; Mali, n = 40) women with no evidence of placental malaria (determined by blood smears) were selected (20 multigravidae and 20 primigravidae in each setting).
P. falciparum isolates and cell cultures.
P. falciparum lines FCR3 and NF54 selected to bind CSA salt from bovine trachea (Sigma) by repeated panning, as previously described (53), and expressing VAR2CSA on the IE surface were maintained in culture for assays. THP-1 monocyte-like cells were cultured as previously described (54) and used to quantify opsonizing phagocytic antibodies.
Antibody binding to VAR2CSA recombinant antigens.
Plasma IgG reactivity to several VAR2CSA recombinant antigens based on the FCR3 allele were assessed by ELISA as previously described (16). Briefly, Escherichia coli-produced ID1-DBL2X-ID2a (16, 55) and DBL3X, DBL4ε, and DBL5ε (16, 55) recombinant proteins from the FCR3 strain were coated at 100 ng per well in flat-bottom 96-well plates (Immulon 4; Dynex Technology, Inc., Chantilly, VA). Plates were incubated at 4°C overnight and blocked with 5% (wt/vol) skim milk (Difco, Detroit, MI) in Tris-buffered saline (BioFluids, Camarillo, CA) for 2 h at room temperature (RT). Plasma (diluted 1:500) was added in duplicate to antigen-coated wells and incubated for 2 h at RT, followed by washes. Samples were then incubated with anti-human IgG (H+L) secondary antibody (KPL, Inc., Gaithersburg, MD) conjugated with alkaline phosphatase for 2 h at RT and washed, followed by a further 20-min incubation with substrate (p-nitrophenyl phosphate, Sigma 104 substrate; Sigma-Aldrich, St. Louis, MO) in the dark at RT. Absorbance at 405 nm was read using a SpectraMax 340PC microplate reader (Molecular Devices Co., Sunnyvale, CA).
Antibody binding to surface antigens of infected erythrocytes.
Flow cytometry experiments were performed to assess the capacity of naturally acquired IgG1, IgG3, IgA, IgM, and total IgG to recognize the native protein expressed on the surface of FCR3- and NF54-parasitized IE surfaces. For each synchronized parasite culture, mature trophozoites/schizonts were magnetically enriched using CS magnetically activated cell sorting (CS-MACS) columns (Miltenyi Biotec, Auburn, CA) and resuspended in phosphate-buffered saline (PBS) containing 2% fetal bovine serum. Cells (2.5 × 106) were incubated in a 96-well U-bottom plate (Corning) with individual plasma samples (1:20 final dilution). After plates were washed, IE were resuspended and split into five replicate wells, and the subclasses and isotypes of bound antibodies were detected by incubation with mouse anti-human specific to IgG1, IgG3, IgA, IgM, and total IgG (Invitrogen), followed by Alexa-Fluor-conjugated goat anti-mouse IgG (Invitrogen) staining. IE were labeled with 0.1% SYBR green (Life Technologies). Data were acquired using an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo, version 10, software (Tree Star, Inc.). Median fluorescence intensity (MFI) was converted into relative fluorescence intensity as described previously (56), using plasma pooled from hyperimmune multigravidae and U.S. naive individuals as positive and negative controls, respectively.
Binding inhibition activity of plasma antibodies to CSA-binding isolates.
One function of antibodies acquired by malaria-exposed pregnant women was defined as their ability to inhibit adhesion of IE to CSA, assessed by an in vitro binding inhibition assay as previously described (16). Briefly, CSA (10 μg/ml) was adsorbed as spots on a 100- by 15-mm petri dish (Falcon 351029) by overnight incubation at 4°C in a humid chamber and then blocked with 3% bovine serum albumin (BSA)-PBS. Mature trophozoite/schizont-stage IE were magnetically enriched, adjusted to 20% parasitemia at 0.5% hematocrit, and incubated with plasma (1:5 dilution). Cells were added to CSA-coated spots in duplicate and incubated for 15 min at RT. Nonadherent IE were washed away on a shaker washing system, and bound IE were immediately fixed, stained, and quantified by microscopy. The percentage of inhibition was determined relative to the blank spot without plasma to measure the antibody binding inhibition activity (BIA). Plasma samples pooled from multigravidae and U.S. naive individuals were used as positive and negative controls, respectively.
Plasma opsonizing activity against CSA-binding isolates.
A second function of antibodies acquired by malaria-exposed pregnant women was defined as their ability to opsonize for phagocytosis of CSA-binding IE in vitro. Enriched trophozoite/schizont P. falciparum IE were stained with ethidium bromide (EtBr), resuspended at 1.67 × 107/ml, and opsonized with plasma samples (dilution 1:10) for 1 h. After a washing step, phagocytosis was performed by incubating IE with THP-1 cells (ATCC) (1 effector to 10 target cells) for 40 min in a 5% CO2 humidified incubator at 37°C. Phagocytosis was stopped by centrifugation at 4°C for 5 min. Nonphagocytosed IE were lysed, and cells were resuspended in 2% paraformaldehyde. Samples were analyzed by Accuri (BD) flow cytometry, and opsonizing antibodies were expressed as the percentage of THP-1 cells that were EtBr positive. All available samples were tested in duplicate, and experiments included plasma pooled from multigravidae (positive controls) and a pool of U.S. naive sera and no serum (negative controls).
Microscopy experiments.
Late-trophozoite and schizont stages were magnetically isolated from synchronized cultures of FCR3 and NF54 parasites and washed with 1× PBS (pH 7.4). For scanning electron microscopy (SEM), IE were fixed with 2.5% glutaraldehyde, 3% paraformaldehyde, 0.05 M phosphate buffer, and 4% sucrose and then postfixed with 1.0% osmium tetroxide in 0.1 M sodium cacodylate buffer. Specimens were dehydrated with a graded ethanol series, critical-point dried under CO2 in a Bal-Tec model cpd 030 drier (Balzers, Liechtenstein), mounted on aluminum studs, and sputter coated with approximately 75 Å of iridium in a model IBSe ion beam sputter coater (South Bay Technologies, San Clemente, CA) prior to viewing at 5 kV in a Hitachi SU-8000 field emission scanning electron microscope (Hitachi, Tokyo, Japan). For each isolate, images of about 20 single IE were captured at the same magnification, and the knob densities were determined manually.
Late-trophozoite- and schizont-enriched IE from both isolates were also stained with PAM1.4 (VAR2CSA-specific antibody [38]), followed by Alexa-Fluor 647-conjugated anti-human IgG and 4′,6′-diamidino-2-phenylindole (DAPI; for nucleic acid) labeling. Confocal fluorescence microscopy was performed on the stained cells using a Zeiss LSM 880 microscope equipped with Airyscan detectors. Airyscan processing of the z-stack images was executed with Zen, version 2.3, and the generated images were analyzed using Imaris, version 8.0. Surfaces relevant to nucleic acid content and PAM1.4-bound VAR2CSA were generated, and the intensity of the channel corresponding to each surface area was quantified.
Total internal reflection fluorescence (TIRF) microscopy, recently described to study the interaction of appropriately labeled membrane-associated molecules (57), was also performed for single VAR2CSA molecule detection and cluster analysis. Briefly, DAPI- and PAM1.4-stained cells were applied to a LabTek II chamber that had been treated with polylysine for 1 h, and images were captured using a Nikon Ti2 microscope with a 100×/1.49 numerical aperture (NA) TIRF objective. We wrote an algorithm in MATLAB to process the TIRF images. Otsu’s method (58) was used to perform clustering-based analysis of the surface-expressed VAR2CSA molecules by both FCR3 and NF54 parasites.
Analysis of VAR2CSA sequences from Mali and Tanzania.
The distribution of VAR2CSA sequences with similarities to NF54 and FCR3 sequences was assessed in our in-house database of transcriptomic VAR2CSA sequences generated from parasites collected from pregnant and nonpregnant individuals and those publicly available online (public accession numbers for raw sequence data analyzed are contained in the NCBI Sequence Read Archive under accession numbers SRP007838, ERP003908, and ERP000190). A total of 71 N-terminal segment (NTS)-DBL6 fragments of VAR2CSA sequences from Mali (26) and Tanzania (34) have been analyzed. Sequences were aligned, and the edit distance score defined as the number of mismatched residues of amino acids using NF54 and FCR3 sequences as references was generated for each sequence. The lowest edit distance value against FCR3 and NF54 is used to qualify the sequence as FCR3- or NF54-like.
To estimate the distribution of the sequences in clades containing the FCR3 and NF54 strains, a phylogenetic tree of the NTS-DBL6 VAR2CSA sequences was generated using the Hamming distance method in SplitsTree4 (version 4.14.6) software. This method computes a minimum spanning network of edit distance between every pair of sequences (59). Sequences from 11 well-established lab strains were included in this analysis in addition to those of NF54 and FCR3.
Statistical analysis.
Statistical analysis was executed using GraphPad Prism (version 7), SAS (version 9.4), and the R program (version 3.2.2). The binding inhibition and opsonizing activities of antibody data were converted into relative binding inhibition activity (rBIA) and relative opsonizing activity (rOA) using the following equation: 100 × [(MFIsample – MFIpooled U.S naive samples)/(MFIpooled samples of multigravidae – MFIpooled U.S. naive samples)]. Reproducibility studies of both the binding inhibition and opsonizing assays have been previously described (60, 61). For the THP-1 cell-based opsonizing and phagocytosis assay, a low interassay variation with a correlation coefficient r2 of 0.9 has been reported (60), while a relatively large interassay variation of 33% was reported for a petri dish-based static binding inhibition assay (61). Continuous variables were compared by nonparametric Mann-Whitney tests using GraphPad Prism, version 7, for Windows (GraphPad Software, La Jolla, CA, USA). Forest plots were produced to visually assess the mean difference(s) and 95% confidence intervals (CI) of each parameter, and a P value was generated from a two-sample t test by the R function t.test. Analyses stratified by study site, parity, parasite variant, and antibody type were conducted. Relationships between the BIA and the OA capacities of the plasma samples in relation to parity and to parasite variant were determined by Pearson’s correlation test. Multivariate regression models were performed to investigate factors associated with these functions of plasma antibodies using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. J.L. is funded by the Francis Crick Institute, which receives its core funding from the UK Medical Research Council (FC10101), Cancer Research UK (FC001101), and Wellcome (FC001101). L.H. is funded by the Danish Medical Research Council (DFF-4183-00539), Danish Research Council for Development Research (17-02-KU), and Novo Nordisk Foundation (15OC0017654).
We thank Raja Guha for providing THP-1 cells, Jason Wendler for sharing assembled VAR2CSA sequence reads, and J. Patrick Gorres for assistance in editing and preparing the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00865-18.
REFERENCES
- 1.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Fried M, Duffy PE. 2017. Malaria during pregnancy. Cold Spring Harb Perspect Med 7:a025551. doi: 10.1101/cshperspect.a025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis 7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 4.Fried M, Duffy PE. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 5.Ataide R, Mwapasa V, Molyneux ME, Meshnick SR, Rogerson SJ. 2011. Antibodies that induce phagocytosis of malaria infected erythrocytes: effect of HIV infection and correlation with clinical outcomes. PLoS One 6:e22491. doi: 10.1371/journal.pone.0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy PE. 2003. Maternal immunization and malaria in pregnancy. Vaccine 21:3358–3361. doi: 10.1016/S0264-410X(03)00332-3. [DOI] [PubMed] [Google Scholar]
- 7.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. 1998. Maternal antibodies block malaria. Nature 395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 8.Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, Hviid L. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol 165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 9.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet 363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 10.Tuikue Ndam NG, Salanti A, Bertin G, Dahlback M, Fievet N, Turner L, Gaye A, Theander T, Deloron P. 2005. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J Infect Dis 192:331–335. doi: 10.1086/430933. [DOI] [PubMed] [Google Scholar]
- 11.Duffy PE, Fried M. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun 71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuikue Ndam NG, Fievet N, Bertin G, Cottrell G, Gaye A, Deloron P. 2004. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J Infect Dis 190:2001–2009. doi: 10.1086/425521. [DOI] [PubMed] [Google Scholar]
- 13.Fried M, Kurtis JD, Swihart B, Morrison R, Pond-Tor S, Barry A, Sidibe Y, Keita S, Mahamar A, Andemel N, Attaher O, Dembele AB, Cisse KB, Diarra BS, Kanoute MB, Narum DL, Dicko A, Duffy PE. 2018. Antibody levels to recombinant VAR2CSA domains vary with Plasmodium falciparum parasitaemia, gestational age, and gravidity, but do not predict pregnancy outcomes. Malar J 17:106. doi: 10.1186/s12936-018-2258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaworowski A, Fernandes LA, Yosaatmadja F, Feng G, Mwapasa V, Molyneux ME, Meshnick SR, Lewis J, Rogerson SJ. 2009. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin Vaccine Immunol 16:312–319. doi: 10.1128/CVI.00356-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teo A, Feng G, Brown GV, Beeson JG, Rogerson SJ. 2016. Functional antibodies and protection against blood-stage malaria. Trends Parasitol 32:887–898. doi: 10.1016/j.pt.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Doritchamou JY, Herrera R, Aebig JA, Morrison R, Nguyen V, Reiter K, Shimp RL, MacDonald NJ, Narum DL, Fried M, Duffy PE. 2016. VAR2CSA domain-specific analysis of naturally acquired functional antibodies to Plasmodium falciparum placental malaria. J Infect Dis 214:577–586. doi: 10.1093/infdis/jiw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med 200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol 49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Duffy MF, Maier AG, Byrne TJ, Marty AJ, Elliott SR, O'Neill MT, Payne PD, Rogerson SJ, Cowman AF, Crabb BS, Brown GV. 2006. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum. Mol Biochem Parasitol 148:117–124. doi: 10.1016/j.molbiopara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Bigey P, Gnidehou S, Doritchamou J, Quiviger M, Viwami F, Couturier A, Salanti A, Nielsen MA, Scherman D, Deloron P, Tuikue Ndam N. 2011. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J Infect Dis 204:1125–1133. doi: 10.1093/infdis/jir499. [DOI] [PubMed] [Google Scholar]
- 21.Clausen TM, Christoffersen S, Dahlbäck M, Langkilde AE, Jensen KE, Resende M, Agerbæk MØ, Andersen D, Berisha B, Ditlev SB, Pinto VV, Nielsen MA, Theander TG, Larsen S, Salanti A. 2012. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem 287:23332–23345. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doritchamou J, Bigey P, Nielsen MA, Gnidehou S, Ezinmegnon S, Burgain A, Massougbodji A, Deloron P, Salanti A, Ndam NT. 2013. Differential adhesion-inhibitory patterns of antibodies raised against two major variants of the NTS-DBL2X region of VAR2CSA. Vaccine 31:4516–4522. doi: 10.1016/j.vaccine.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava A, Gangnard S, Dechavanne S, Amirat F, Lewit Bentley A, Bentley GA, Gamain B. 2011. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS One 6:e20270. doi: 10.1371/journal.pone.0020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barfod L, Dobrilovic T, Magistrado P, Khunrae P, Viwami F, Bruun J, Dahlback M, Bernasconi NL, Fried M, John D, Duffy PE, Salanti A, Lanzavecchia A, Lim CT, Ndam NT, Higgins MK, Hviid L. 2010. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J Immunol 185:7553–7561. doi: 10.4049/jimmunol.1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Early AM, Lievens M, MacInnis BL, Ockenhouse CF, Volkman SK, Adjei S, Agbenyega T, Ansong D, Gondi S, Greenwood B, Hamel M, Odero C, Otieno K, Otieno W, Owusu-Agyei S, Asante KP, Sorgho H, Tina L, Tinto H, Valea I, Wirth DF, Neafsey DE. 2018. Host-mediated selection impacts the diversity of Plasmodium falciparum antigens within infections. Nat Commun 9:1381. doi: 10.1038/s41467-018-03807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bockhorst J, Lu F, Janes JH, Keebler J, Gamain B, Awadalla P, Su XZ, Samudrala R, Jojic N, Smith JD. 2007. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol Biochem Parasitol 155:103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Rajwani J, Klinger CM, Arango E, Arroyo MI, Sabbagh A, Maestre A, Dacks JB, Gnidehou S, Yanow SK. 2017. Genetic analysis of ID1-DBL2X predicts its validity as a vaccine candidate in Colombia and supports at least two independently introduced Plasmodium falciparum populations in the region. Infect Genet Evol 55:175–185. doi: 10.1016/j.meegid.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Trimnell AR, Kraemer SM, Mukherjee S, Phippard DJ, Janes JH, Flamoe E, Su XZ, Awadalla P, Smith JD. 2006. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol Biochem Parasitol 148:169–180. doi: 10.1016/j.molbiopara.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Avril M, Cartwright MM, Hathaway MJ, Hommel M, Elliott SR, Williamson K, Narum DL, Duffy PE, Fried M, Beeson JG, Smith JD. 2010. Immunization with VAR2CSA-DBL5 recombinant protein elicits broadly cross-reactive antibodies to placental Plasmodium falciparum-infected erythrocytes. Infect Immun 78:2248–2256. doi: 10.1128/IAI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avril M, Hathaway MJ, Srivastava A, Dechavanne S, Hommel M, Beeson JG, Smith JD, Gamain B. 2011. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLoS One 6:e16622. doi: 10.1371/journal.pone.0016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avril M, Kulasekara BR, Gose SO, Rowe C, Dahlback M, Duffy PE, Fried M, Salanti A, Misher L, Narum DL, Smith JD. 2008. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect Immun 76:1791–1800. doi: 10.1128/IAI.01470-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeson JG, Mann EJ, Byrne TJ, Caragounis A, Elliott SR, Brown GV, Rogerson SJ. 2006. Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies. J Infect Dis 193:721–730. doi: 10.1086/500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babakhanyan A, Fang R, Wey A, Salanti A, Sama G, Efundem C, Leke RJ, Chen JJ, Leke RG, Taylor DW. 2015. Comparison of the specificity of antibodies to VAR2CSA in Cameroonian multigravidae with and without placental malaria: a retrospective case-control study. Malar J 14:480. doi: 10.1186/s12936-015-1023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, Fievet N, Massougbodji A, Luty AJ, Deloron P. 2015. Protective antibodies against placental malaria and poor outcomes during pregnancy, Benin. Emerg Infect Dis 21:813–823. doi: 10.3201/eid2105.141626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hommel M, Chan JA, Umbers AJ, Langer C, Rogerson SJ, Smith JD, Beeson JG. 2018. Evaluating antibody functional activity and strain-specificity of vaccine candidates for malaria in pregnancy using in vitro phagocytosis assays. Parasit Vectors 11:69. doi: 10.1186/s13071-018-2653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joergensen LM, Salanti A, Dobrilovic T, Barfod L, Hassenkam T, Theander TG, Hviid L, Arnot DE. 2010. The kinetics of antibody binding to Plasmodium falciparum VAR2CSA PfEMP1 antigen and modelling of PfEMP1 antigen packing on the membrane knobs. Malar J 9:100. doi: 10.1186/1475-2875-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quadt KA, Barfod L, Andersen D, Bruun J, Gyan B, Hassenkam T, Ofori MF, Hviid L. 2012. The density of knobs on Plasmodium falciparum-infected erythrocytes depends on developmental age and varies among isolates. PLoS One 7:e45658. doi: 10.1371/journal.pone.0045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barfod L, Bernasconi NL, Dahlback M, Jarrossay D, Andersen PH, Salanti A, Ofori MF, Turner L, Resende M, Nielsen MA, Theander TG, Sallusto F, Lanzavecchia A, Hviid L. 2007. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol Microbiol 63:335–347. doi: 10.1111/j.1365-2958.2006.05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen MD, Quintana MDP, Ditlev SB, Bayarri-Olmos R, Ofori MF, Hviid L, Garred P. 2018. Evasion of classical complement pathway activation on Plasmodium falciparum-infected erythrocytes opsonized by PfEMP1-specific IgG. Front Immunol 9:3088. doi: 10.3389/fimmu.2018.03088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Perez M, Larsen MD, Bayarri-Olmos R, Ampomah P, Stevenson L, Arevalo-Herrera M, Herrera S, Hviid L. 2018. IgG Responses to the Plasmodium falciparum antigen VAR2CSA in Colombia are restricted to pregnancy and are not induced by exposure to Plasmodium vivax. Infect Immun 86:e00136-18. doi: 10.1128/IAI.00136-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horrocks P, Pinches RA, Chakravorty SJ, Papakrivos J, Christodoulou Z, Kyes SA, Urban BC, Ferguson DJ, Newbold CI. 2005. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J Cell Sci 118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 42.Benavente ED, Oresegun DR, de Sessions PF, Walker EM, Roper C, Dombrowski JG, de Souza RM, Marinho CRF, Sutherland CJ, Hibberd ML, Mohareb F, Baker DA, Clark TG, Campino S. 2018. Global genetic diversity of var2csa in Plasmodium falciparum with implications for malaria in pregnancy and vaccine development. Sci Rep 8:15429. doi: 10.1038/s41598-018-33767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo A, Hasang W, Randall LM, Feng G, Bell L, Unger H, Langer C, Beeson JG, Siba PM, Mueller I, Molyneux ME, Brown GV, Rogerson SJ. 2014. Decreasing malaria prevalence and its potential consequences for immunity in pregnant women. J Infect Dis 210:1444–1455. doi: 10.1093/infdis/jiu264. [DOI] [PubMed] [Google Scholar]
- 44.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. 2005. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect Immun 73:4112–4118. doi: 10.1128/IAI.73.7.4112-4118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tebo AE, Kremsner PG, Luty AJ. 2002. Fcγ receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin Exp Immunol 130:300–306. doi: 10.1046/j.1365-2249.2002.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert LH, Bullock JL, Cook ST, Miura K, Garboczi DN, Diakite M, Fairhurst RM, Singh K, Long CA. 2014. Antigen reversal identifies targets of opsonizing IgGs against pregnancy-associated malaria. Infect Immun 82:4842–4853. doi: 10.1128/IAI.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monteiro RC, Van De Winkel JG. 2003. IgA Fc receptors. Annu Rev Immunol 21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 48.Otten MA, Rudolph E, Dechant M, Tuk CW, Reijmers RM, Beelen RH, van de Winkel JG, van Egmond M. 2005. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol 174:5472–5480. doi: 10.4049/jimmunol.174.9.5472. [DOI] [PubMed] [Google Scholar]
- 49.Pleass RJ, Holder AA. 2005. Opinion: antibody-based therapies for malaria. Nat Rev Microbiol 3:893–899. doi: 10.1038/nrmicro1267. [DOI] [PubMed] [Google Scholar]
- 50.Kassim OO, Ako-Anai KA, Torimiro SE, Hollowell GP, Okoye VC, Martin SK. 2000. Inhibitory factors in breastmilk, maternal and infant sera against in vitro growth of Plasmodium falciparum malaria parasite. J Trop Pediatr 46:92–96. doi: 10.1093/tropej/46.2.92. [DOI] [PubMed] [Google Scholar]
- 51.Nwuba RI, Sodeinde O, Anumudu CI, Omosun YO, Odaibo AB, Holder AA, Nwagwu M. 2002. The human immune response to Plasmodium falciparum includes both antibodies that inhibit merozoite surface protein 1 secondary processing and blocking antibodies. Infect Immun 70:5328–5331. doi: 10.1128/IAI.70.9.5328-5331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X, Fried M, Duffy PE. 2005. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med 2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med 182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tippett E, Fernandes LA, Rogerson SJ, Jaworowski A. 2007. A novel flow cytometric phagocytosis assay of malaria-infected erythrocytes. J Immunol Methods 325:42–50. doi: 10.1016/j.jim.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Obiakor H, Avril M, Macdonald NJ, Srinivasan P, Reiter K, Anderson C, Holmes KL, Fried M, Duffy PE, Smith JD, Narum DL, Miller LH. 2013. Identification of VAR2CSA domain-specific inhibitory antibodies of the Plasmodium falciparum erythrocyte membrane protein 1 using a novel flow cytometry assay. Clin Vaccine Immunol 20:433–442. doi: 10.1128/CVI.00638-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brolin KJ, Persson KE, Wahlgren M, Rogerson SJ, Chen Q. 2010. Differential recognition of P. falciparum VAR2CSA domains by naturally acquired antibodies in pregnant women from a malaria endemic area. PLoS One 5:e9230. doi: 10.1371/journal.pone.0009230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sohn HW, Tolar P, Brzostowski J, Pierce SK. 2010. A method for analyzing protein-protein interactions in the plasma membrane of live B cells by fluorescence resonance energy transfer imaging as acquired by total internal reflection fluorescence microscopy. Methods Mol Biol 591:159–183. doi: 10.1007/978-1-60761-404-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue JH, Titterington DM. 2011. t-Tests, F-tests and Otsu's methods for image thresholding. IEEE Trans Image Process 20:2392–2396. doi: 10.1109/TIP.2011.2114358. [DOI] [PubMed] [Google Scholar]
- 59.Excoffier L, Smouse PE. 1994. Using allele frequencies and geographic subdivision to reconstruct gene trees within a species: molecular variance parsimony. Genetics 136:343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ataide R, Hasang W, Wilson DW, Beeson JG, Mwapasa V, Molyneux ME, Meshnick SR, Rogerson SJ. 2010. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS One 5:e10807. doi: 10.1371/journal.pone.0010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pehrson C, Heno KK, Adams Y, Resende M, Mathiesen L, Soegaard M, de Jongh WA, Theander TG, Salanti A, Nielsen MA. 2017. Comparison of functional assays used in the clinical development of a placental malaria vaccine. Vaccine 35:610–618. doi: 10.1016/j.vaccine.2016.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.