Tissue-resident memory T cells (TRM cells) are a novel population of tissue-restricted antigen-specific T cells. TRM cells are induced by pathogens and promote host defense against secondary infections. Although TRM cells cannot be detected in circulation, they are the major memory CD4+ and CD8+ T-cell population in tissues in mice and humans.
KEYWORDS: CD4+, Citrobacter, colitis, T cells, tissue-resident memory
ABSTRACT
Tissue-resident memory T cells (TRM cells) are a novel population of tissue-restricted antigen-specific T cells. TRM cells are induced by pathogens and promote host defense against secondary infections. Although TRM cells cannot be detected in circulation, they are the major memory CD4+ and CD8+ T-cell population in tissues in mice and humans. Murine models of CD8+ TRM cells have shown that CD8+ TRM cells maintain tissue residency via CD69 and though tumor growth factor β-dependent induction of CD103. In contrast to CD8+ TRM cells, there are few models of CD4+ TRM cells. Thus, much less is known about the factors regulating the induction, maintenance, and host defense functions of CD4+ TRM cells. Citrobacter rodentium is known to induce IL-17+ and IL-22+ CD4+ T cells (Th17 and Th22 cells, respectively). Moreover, data from IL-22 reporter mice show that most IL-22+ cells in the colon 3 months after C. rodentium infection are CD4+ T cells. This collectively suggests that C. rodentium may induce CD4+ TRM cells. Here, we demonstrate that C. rodentium induces a population of IL-17A+ CD4+ T cells that are tissue restricted and antigen specific, thus meeting the criteria of CD4+ TRM cells. These cells expand and are a major source of IL-22 during secondary C. rodentium infection, even before the T-cell phase of the host response in primary infection. Finally, using FTY 720, which depletes circulating naive and effector T cells but not tissue-restricted T cells, we show that these CD4+ TRM cells can promote host defense.
INTRODUCTION
Tissue-resident memory T cells (TRM cells) are a recently described population of terminally differentiated tissue-restricted memory T cells (1). TRM cells are induced locally at sites of pathogen invasion and vaccinations and are maintained over the long term in the tissue. Although they are held in a resting state, TRM cells can rapidly produce cytokines upon stimulation (2). Indeed, murine models have shown that TRM cells have the capacity to clear secondary infections more efficiently than circulating T effector memory cells (TEM cells) (3, 4). These findings have led to the conjecture that TRM cells patrol peripheral tissues as the first line of adaptive immunity to secondary infections. Commensurate with this purported function, TRM cells are enriched at surfaces with high microbial burdens such as the skin, pulmonary tree, and genitourinary and gastrointestinal tracts (1).
Murine models of TRM cells, most of which have generated CD8+ TRM cells, have shown that cell ingress and egress from tissues are a critical control point for TRM cells. Specifically, CD8+ TRM cells do not bear lymph node homing markers CCR7 and CD62L but almost universally express CD69 and CD103, both of which are required for tissue residency (1). The C-type lectin receptor CD69 promotes tissue residency by negatively regulating the lymphocyte trafficking receptor sphingosine-1-phosphate receptor 1 (S1PR1), thus obstructing memory T-cell egress from tissues (5, 6). CD103 promotes retention of CD8+ TRM cells by an incompletely described mechanism that is presumed to involve binding to its ligand, E-cadherin, which is expressed on epithelial cells (7). Furthermore, tumor growth factor β (TGF-β) is required for induction of CD103 and CD69 by CD8+ TRM cells, implying that locally produced cytokines also regulate TRM tissue residency (7–9).
In contrast to CD8+ TRM cells, there is a relative paucity of data on CD4+ TRM cells. In particular, little is known about the generation, maintenance, and functional profile of CD4+ TRM cells in the intestine (10). CD4+ TRM cells are the major population of memory T cells in the intestine in healthy humans, outnumbering CD8+ TRM cells by a 2:1 margin in the colon (11). Furthermore, CD4+ Th17 cells have been heavily implicated in the pathogenesis of inflammatory bowel disease (IBD). Thus, given the abundance of CD4+ TRM cells in the human intestine, elucidating CD4+ TRM cell biology is likely to advance our understanding of intestinal disorders such as IBD.
We used the well-described Citrobacter rodentium model to investigate CD4+ TRM cells (12, 13). C. rodentium breaches the epithelial barrier with systemic spread, resulting in a transient infection characterized by diarrhea and weight loss. CD4+ Th17 cells are induced by C. rodentium and play a critical role in protective immunity to C. rodentium (14–19). However, much less is known about the long-term fate of C. rodentium-induced Th17 cells and their role during secondary infection. Here, we show that C. rodentium induces a long-lived population of CD4+ TRM cells. Many of these cells coproduce IL-17A and gamma interferon (IFN-γ) and variably express CD103 independently of intraepithelial or lamina propria residence. Moreover, these cells increase in number early with C. rodentium reinfection to produce IL-22, IL-17, and IFN-γ against secondary infections.
RESULTS
Citrobacter rodentium-induced alterations in the colonic CD4+ T-cell distribution are maintained over the long term.
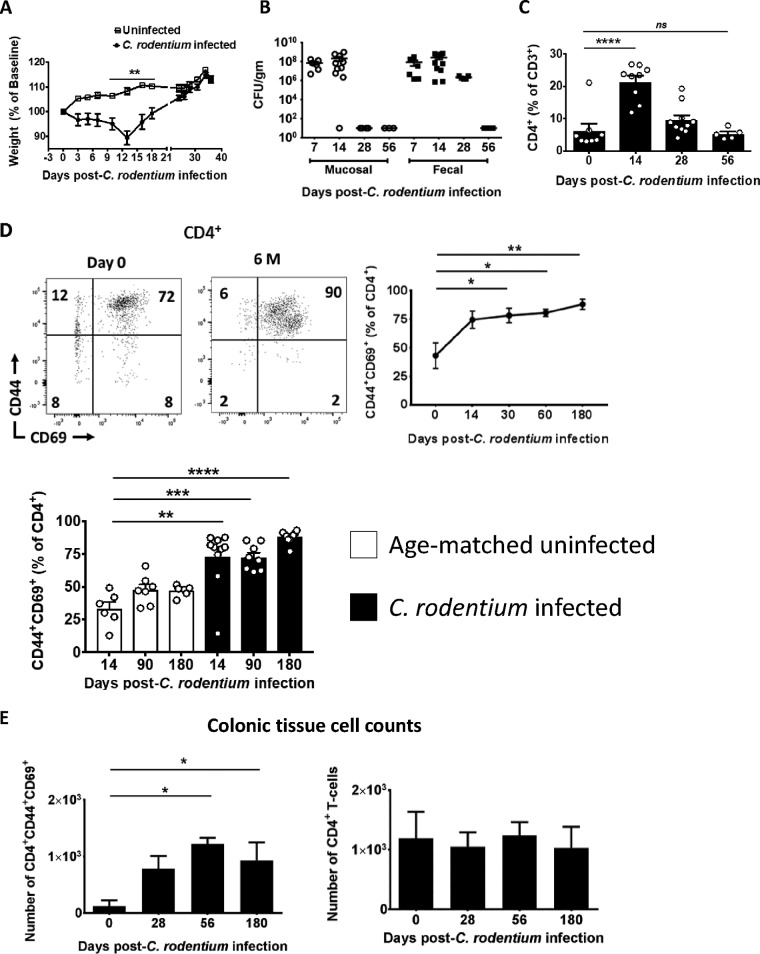
C. rodentium induces IL-17-producing CD4+ T cells (Th17 cells) by a mechanism that requires pathogen contact with the colonic epithelium (16). Immune-competent wild-type (WT) mice clear C. rodentium by 2 to 4 weeks. Consistent with this, colonic mucosal adherent C. rodentium was cleared by day 28 postinfection (p.i.) in our facility. This was followed by clearance of luminal bacteria (as assessed in fecal pellets) by day 35 p.i. (Fig. 1A and B). Concomitant with the clearance curve of C. rodentium, CD4+ T cells as a percentage of total CD3+ T cells peaked at day 14 before returning to baseline by day 56 p.i. (Fig. 1C). CD44 is expressed on activated T cells, CD69 is expressed by most TRM cells, and antigen-specific T cells that persist in nonlymphoid tissue in murine models of viral and parasitic infections maintain expression of the activation markers CD44 and CD69 (20, 21). Thus, in order to examine whether C. rodentium can induce CD4+ TRM cells, we assessed expression of CD69 on mucosal CD4+ T cells after C. rodentium infection. In this regard, C. rodentium infection induced sustained expression of CD44 and CD69 in a high fraction of colonic mucosal CD4+ T cells well after pathogen clearance, even 6 months p.i. (Fig. 1D, top right panel). Moreover, larger fractions of mucosal CD4+ T cells were CD44+ CD69+ after C. rodentium infection relative to age- and gender-matched uninfected control mice (Fig. 1D, bottom left panel). In addition, the total number of CD4+ CD44+ CD69+ T cells was elevated at late times after C. rodentium infection compared to baseline despite the stability of total CD4+ T cells (Fig. 1E). These data show that C. rodentium infection alters the mucosal T-cell distribution over the long term and suggest that C. rodentium may induce CD4+ TRM cells.
FIG 1.
C. rodentium-induced alterations in the colonic CD4+ T-cell distribution are maintained over the long term. (A) Weight change of C. rodentium-infected and uninfected control mice. WT mice were orally gavaged with C. rodentium, and weight as a percentage of baseline was determined. (B) WT mice eradicate mucosal and luminal C. rodentium within 5 weeks. Bacterial load in colonic mucosa and colonic fecal pellets, expressed as CFU/g of intestine or stool, was determined at the indicated times. (C) The fraction of CD4+ T cells as a percentage of CD3+ T cells peaks by day 14. The fraction of CD4+ T cells as a percentage of CD3+ cells was determined by flow cytometry in the colon tissue of C. rodentium-infected mice at the indicated times. (D) Most mucosal CD4+ T cells exhibit a surface phenotype consistent with TRM cells after C. rodentium infection. Mononuclear cells were isolated from the colon of C. rodentium-infected mice at the indicated time points, and expression of CD44 and CD69 was determined on live CD3+ CD4+ T cells (top left panel; representative plot) and is quantitated relative to uninfected 6-week-old mice at day 0 (top right panel) and relative to age- and gender-matched uninfected mice (bottom left panel). (E) The total number of CD4+ CD44+ CD69+ T cells in the colon increases after C. rodentium infection. The total number of CD4+ CD44+ CD69+ T cells (left panel) and CD4+ T cells (right panel) in the colon was determined at the indicated times using flow cytometry normalized to colon weight (cells/g). Each time point consists of 5 to 10 mice (A to C) or 6 to 10 mice (D and E). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, by parametric Student’s t test (A) or ANOVA (B to E).
C. rodentium-induced CD4+ T cells are consistent with tissue-resident memory T cells.
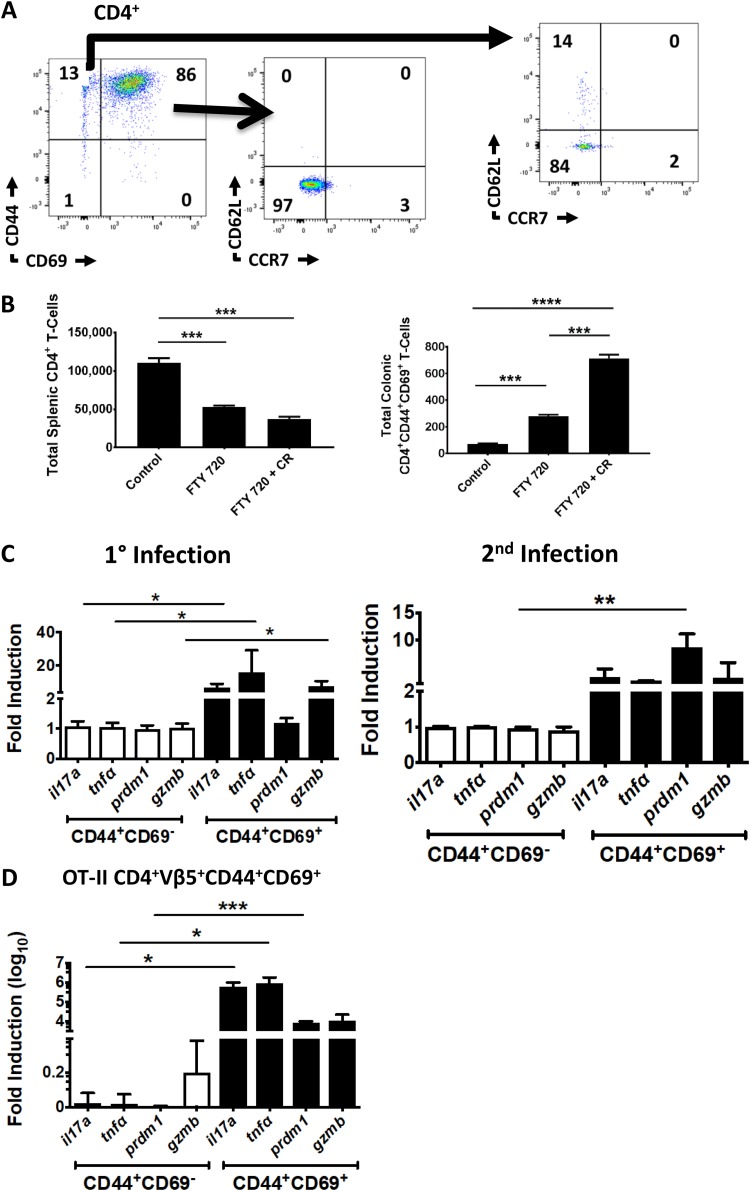
Tissue-resident memory T cells are nonrecirculating and antigen specific and are often identified as CD69+ CD62L− CCR7− T cells at mucosal and epithelial sites (1, 2, 10). Thus, we next sought to determine whether C. rodentium can induce tissue-restricted, antigen-specific CD4+ T cells. In order to evaluate this, we first determined expression of lymph node homing markers on C. rodentium-induced CD4+ CD44+ CD69+ T cells. Consistent with a tissue-restricted effector memory phenotype, colonic CD4+ CD44+ CD69+ T cells were CD62L− CCR7− (Fig. 2A) (22). In contrast, the CD4+ CD44+ CD69− fraction expressed CD62L and splenic T cells expressed both CD62L and CCR7 (Fig. 2A; see also Fig. S1A in the supplemental material). Next, we used the S1PR1 inhibitor FTY 720 to verify tissue residency of the CD4+ CD44+ CD69+ fraction. By inhibiting S1PR1, FTY 720 blocks egress of circulating lymphocytes (which express S1PR1) from lymph nodes and secondary lymphoid organs. This has the effect of redistributing circulating naive and effector memory T cells to these sites. In contrast, TRM cells do not express S1PR1 and are retained in tissue despite treatment with FTY 720 (3). Because of these properties, FTY 720 is commonly used to verify tissue residency in murine models of TRM cells (3, 6). As expected, the number of splenic T cells decreased with FTY 720 treatment (Fig. 2B, left panel). This is consistent with redistribution of circulating naive and TEM cells as these types of cells are in the intravascular space in the spleen (and are in equilibrium with the systemic circulation). Moreover, many splenic T cells in specific-pathogen-free (SPF) uninfected young B6 mice are naive. In contrast, there was an increase in the number of CD44+ CD69+ CD4+ T cells in the colon with FTY 720. This is consistent with sequestration of CD44+ CD69+ CD4+ T cells in the colonic mucosa (Fig. 2B, right panel). Furthermore, this effect was pronounced in mice previously infected with C. rodentium (Fig. 2B, right bars). These results indicate that C. rodentium-induced CD4+ CD44+ CD69+ T cells are tissue restricted.
FIG 2.
C. rodentium-induced CD4+ CD44+ CD69+ cells are nonrecirculating and contain an antigen-specific subset. (A) C. rodentium-induced CD4+ CD44+ CD69+ T cells do not express lymph node homing markers. Expression of the lymph node homing markers CD62L (L-selectin) and CCR7 was determined on CD4+ CD44+ CD69+ and CD4+ CD44+ CD69− mucosal T cells 8 weeks after C. rodentium infection. (B) C. rodentium-induced CD4+ CD44+ CD69+ T cells do not recirculate. The total number of CD4+ T cells in the spleen (left panel) and CD4+ CD44+ CD69+ T cells in the colon (right panel) was enumerated with flow cytometry in uninfected WT mice (control) and mice administered FTY 720 with or without prior C. rodentium (CR) infection. (C) Mucosal CD4+ CD44+ CD69+ T cells contain a C. rodentium antigen-specific population over the long term after C. rodentium infection. Expression of genes associated with C. rodentium-induced Th17 cells was determined in the CD69+ and CD69− fractions of FACS-purified CD4+ CD44+ mucosal T cells in WT mice 8 weeks after primary infection (left panel) or 10 days after secondary infection (right panel). (D) Antigen-specific Th17 cells are contained in the CD4+ CD44+ CD69+ fraction in OT-II mice. CD4+ Vβ5+ CD44+ CD69+ colonic T cells were FACS purified from OT-II mice 10 days after inoculation with OVA-expressing C. rodentium, and expression of the selected genes was determined. Data are normalized to GAPDH and presented as fold induction over CD4+ CD44+ CD69− fractions for panels C and D. The experiment in panel A is representative of 6 mice. Each time point in panel B consists of 9 mice. Experiments in panels C and D were performed twice with 3 mice per trial (6 mice total). *, P < 0.5; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P < 0.0001, by ANOVA.
Tissue-resident memory T cells are by definition antigen specific. However, it is problematic to isolate antigen-specific T cells in the C. rodentium model as the dominant epitopes(s) is unknown. We therefore used two separate approaches to assess antigen specificity. First, we determined the expression of Th17 cell-associated genes in the CD69+ and CD69− fractions of fluorescence-activated cell sorting (FACS)-purified CD4+ CD44+ T cells from mice 60 days after primary or 10 days after secondary C. rodentium infection (Fig. 2C). The CD44+ CD69+ CD4+ T-cell fractions consistently exhibited high expression of genes associated with C. rodentium-induced Th17 cells compared to the CD44+ CD69− fractions (Fig. 2C).
As a second method to verify antigen specificity, we utilized OT-II mice in conjunction with an ovalbumin-expressing strain of C. rodentium (OVA-C. rodentium) in order to verify that the CD4+ CD44+ CD69+ fraction contained antigen-specific T cells. This OVA-C. rodentium strain has been previously reported and exhibits similar in vivo pathogenicity as wild-type strains of C. rodentium (23, 24). Inoculation of mice with OVA-C. rodentium induced mucosal responses consistent with acute infection. As described above, CD3+ CD4+ Vβ5+ CD44+ CD69+ T cells from OT-II mice infected with OVA-C. rodentium expressed high levels of genes associated with C. rodentium-induced Th17 cells, whereas there was little induction of these genes in the corresponding CD4+ CD44+ CD69− fraction (Fig. 2D). Thus, these data collectively indicate that C. rodentium induces a subset of antigen-specific CD4+ T cells that are restricted to the colonic mucosa and are therefore consistent with CD4+ TRM cells.
C. rodentium-induced CD4 TRM cells exhibit a Th17 and Th1/17 phenotype.
C. rodentium is well known to induce Th17, Th22, and Th1 cells (14, 16). Th1 cells are redundant for host defense against C. rodentium but are thought to be a consequence of plasticity in the Th17 subset (“ex-Th17” cells) (25). We assessed changes in cytokine production of CD4+ TRM cells with time after C. rodentium infection in order to better characterize these cells.
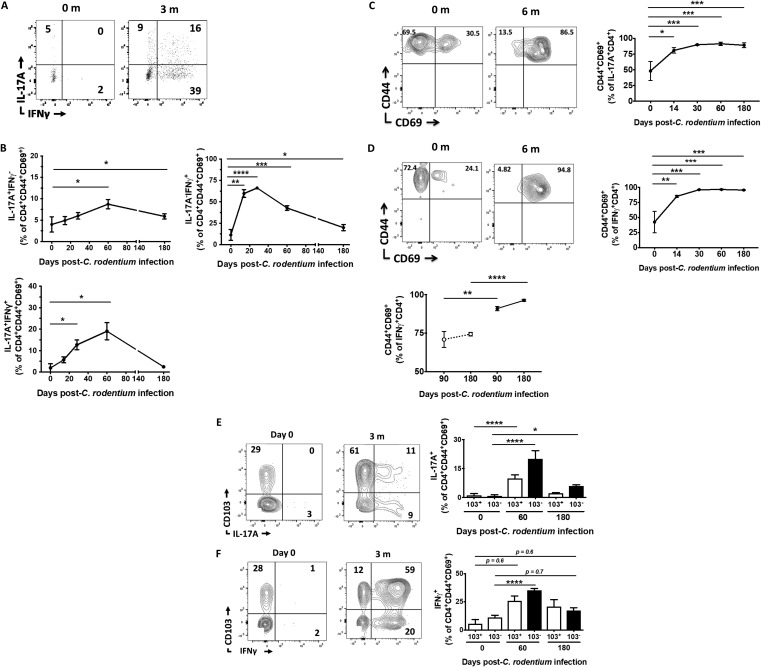
There was negligible expression of IL-17A and IFN-γ by CD4+ TRM cells at baseline, which is well reported in C57BL/6 mice from the Jackson Laboratory (Fig. 3A) (19). However, the fraction of IL-17A and IFN-γ single-positive CD4+ TRM cells increased substantially after C. rodentium infection, peaking at approximately 2 months p.i. and remaining elevated above baseline even at 6 months p.i. (Fig. 3B). There was also a parallel increase in IL-17A and IFN-γ double-producing CD4+ TRM cells, which is consistent with Th1/17 cells that form from “ex-Th17” cells at late time points after C. rodentium infection (Fig. 3A and B). In order to test whether the converse was also true, we determined the cellular phenotype of IL-17A- and IFN-γ-producing CD4+ T cells after C. rodentium infection. Consistent with a TRM paradigm, progressively higher fractions of IL-17A+ and IFN-γ+ CD4+ T cells expressed TRM markers after C. rodentium infection compared to baseline values in uninfected mice (Fig. 3C and D). Moreover, this pattern of CD44 and CD69 expression by IFN-γ+ CD4+ T cells persisted compared to age- and gender-matched uninfected control mice (Fig. 3D, bottom left panel). We could not compare expression of TRM markers in IL-17A+ CD4+ T cells from age- and gender-matched uninfected mice as there is very little IL-17A production in these mice in the absence of C. rodentium infection.
FIG 3.
C. rodentium-induced CD4+ TRM cells exhibit a Th1 and Th17 profile. (A) Mucosal CD4+ CD44+ CD69+ T cells exhibit a Th17 and Th1/17 signature over the long term after C. rodentium infection. Mucosal CD4+ CD44+ CD69+ T cells isolated from the colon of C. rodentium-infected mice at the indicated time points were stimulated with phorbol myristate acetate and ionomycin, and expression of IL-17A and of IFN-γ was determined. m, months. (B) Data from panel A are quantified and presented as IL-17A and IFN-γ single-positive (top left and right panels, respectively) and double-positive (bottom panel) CD4+ TRM cells. (C and D) Increasing fractions of IL-17A- (C) and IFN-γ-producing (D) CD4+ T cells express TRM markers after C. rodentium infection. Expression of CD44 and CD69 on IL-17A+ (C) and IFN-γ+ (D) mucosal CD4+ T cells from C. rodentium-infected mice was determined at baseline (0) and 6 months (6 m) relative to uninfected 6-week-old mice or relative to uninfected age-matched controls (D, bottom panel). The following gating schema was used: live lymphocytes→IL-17A+ (or IFN-γ+)→CD3+→CD4+→CD44 versus CD69. FACS plots (left panels) and quantitated data (right panels and bottom panel of panel D) are presented. (E and F) C. rodentium-induced CD4+ TRM cells produce IL-17 and IFN-γ independently of CD103 expression. Coordinated expression of CD103 and IL-17A (E) or CD103 and IFN-γ (F) was determined in CD4+ CD44+ CD69+ mucosal T cells at the indicated time points postinfection and quantified (right panels). Panel A is a representative plot, while 6 to 9 mice (B and C) or 6 to 11 mice (E and F) are presented for each time point. *, P < 0.5; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P < 0.0001, by ANOVA.
CD103 is expressed by nearly all CD8+ TRM cells and is necessary to maintain their tissue residency (21). In contrast, the role of CD103 in maintaining CD4+ TRM cells is uncertain as multiple murine and human studies show variable expression of CD103 by CD4+ TRM cells. We found that very low fractions of C. rodentium-induced CD4+ TRM cells expressed CD103 relative to their CD8+ TRM counterparts (Fig. S2A). The ligand of CD103, E-cadherin, is expressed by intestinal epithelial cells, but CD103 expression levels were similar between intraepithelial and lamina propria CD4+ TRM cells and did not vary with time (Fig. S2B and C). Interestingly, both CD103+ and CD103− CD4+ TRM cells produced IL-17A and IFN-γ (Fig. 3E and F). In contrast to CD4+ TRM cells, the majority of IL-17A- and IFN-γ-producing CD8+ CD44+ CD69+ T cells were in the CD103+ compartment (Fig. S2D). These data collectively indicate that C. rodentium-induced CD4+ TRM cells are the major source of mucosal CD4+ T-cell-derived IL-17A and IFN-γ late after C. rodentium infection and suggest that CD103 does not regulate their cytokine production.
C. rodentium-induced CD4 TRM cells are reactivated and are a source of IL-22 during secondary infections.
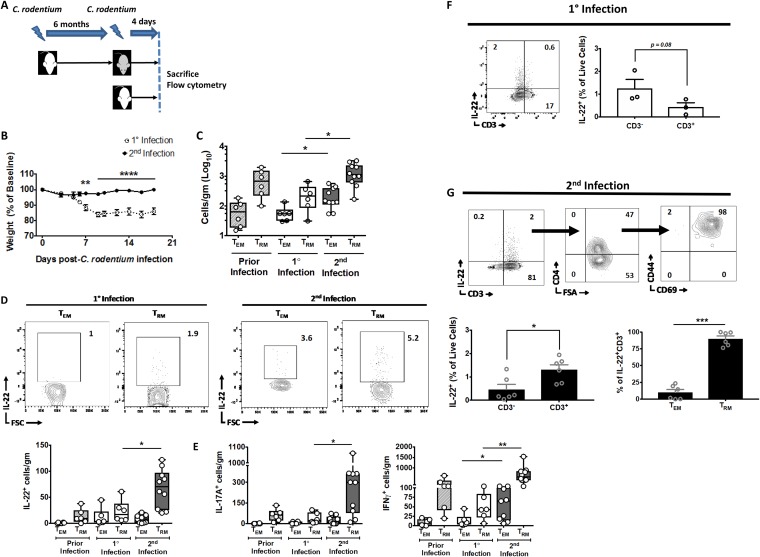
IL-22 mediates epithelial restitution and induction of antimicrobial peptides and is critical for immunity to C. rodentium (26). IL-22 is produced by innate lymphoid cells (ILCs) during the early phase and by IL-6- and IL-23-dependent and Th22 cells during the late phase of C. rodentium infection (14, 16, 17). Our data indicate that C. rodentium induces CD4+ TRM cells. Thus, given the putative role of TRM cells in immunity, we conjectured that C. rodentium-induced CD4+ TRM cells are a source of IL-22 during secondary infections. In order to test our conjecture, we set up an experimental system that allowed us to assess T-cell responses during the early phase of primary and secondary infection (Fig. 4A). T-cell responses to C. rodentium typically take 8 to 10 days in most experimental systems, including ours (Fig. 1C) (14, 17). Thus, we could assess whether CD4+ TRM cells expanded during secondary infection before the expected T-cell phase of the host response using this system.
FIG 4.
C. rodentium-induced CD4+ TRM cells are reactivated and are a source of IL-22 during secondary infections. (A) The experimental scheme. WT mice that had recovered from C. rodentium infection 6 months before were reinfected and sacrificed 4 days postinfection (dark gray). Age- and gender-matched mice that had primary infection served as controls (white). (B) Weight loss for mice postinfection. (C) CD4+ TRM cell compartment expands with secondary infection. The total number of colonic CD4+ CD44+ CD69− (TEM) and CD4+ CD44+ CD69+ (TRM) T cells was enumerated with flow cytometry 4 days after primary or secondary infection of mice per the schema in panel A. The number of TEM and TRM cells was also quantified in age-matched mice that had cleared C. rodentium infection 6 months previously (Prior Infection) as an additional control. (D and E) IL-22+ (D) and IL-17+ (left panel) and IFN-γ+ (right panel) (E) CD4+ TRM cell compartments expand with secondary infection. (D) Representative FACS plots (top panels) and quantified total numbers of IL-22+ (D) and IL-17A+ (E, left panel) and IFN-γ+ (E, right panel) CD4+ CD44+ CD69− (TEM) and CD4+ CD44+ CD69+ (TRM) T cells were enumerated with flow cytometry 4 days after primary or secondary infection of mice per the schema in panel A. The number of IL-22+, IL-17A+, and IFN-γ+ TEM and TRM cells was also quantified in age-matched mice that had cleared C. rodentium infection 6 months before (Prior Infection). (F) Most IL-22+ cells early during primary infection are CD3−. Coordinated expression of IL-22 and CD3 was determined in primary-infection mice day 4 postinfection. (G) T cells with a TRM phenotype are a source of IL-22 early during secondary infection. Sequential expression of CD4 (top row, middle panel) and CD44 and CD69 (top row, right panel) was determined on live IL-22+ CD3+ mucosal cells (top row, left panel). Cumulative data are quantified (bottom row). Data are expressed as cells per gram of colon (C to E). Experiments in panels B to E and G comprise 6 to 10 mice per group. The experiment in panel F is representative of an experiment with 3 mice (performed twice). *, P < 0.5; **, P ≤ 0.01; ***, P ≤ 0.001, by the parametric Student t test (B, F, and G) or ANOVA (C to E).
We sacrificed mice with primary and secondary infection at day 4 p.i., which is before the T-cell phase of the host response (Fig. 4A). As expected, mice with secondary infection did not demonstrate any significant illness compared to mice with primary infection (Fig. 4B). Interestingly, we found that CD4+ TRM cell numbers increased in the colonic mucosa in mice with secondary infection relative to those in primary infection as early as day 4 p.i., when there is no appreciable T-cell response in primary mice (Fig. 1C and Fig. 4C). Furthermore, these cells produced substantial amounts of IL-22 relative to mice with primary infection and to age-matched mice that had previously cleared C. rodentium but were not reinfected (Fig. 4D). Similarly, IL-17+ and IFN-γ+ CD4+ TRM cell numbers also increased during secondary infection compared to those during primary infection and in previously infected mice (Fig. 4E).
This demonstrates that IL-22+ CD4+ TRM cells in the intestinal mucosa increase in number during secondary infection but does not identify whether CD4+ TRM cells are a major source of mucosal IL-22 within the T-cell compartment with secondary infection. In order to address this, we examined the phenotype of IL-22+ cells during primary and secondary infection. We found a trend toward IL-22 production primarily by the CD3− compartment during primary infection (Fig. 4F). Although our data did not reach statistical significance, they are consistent with well-reported data demonstrating that CD3− ILCs are the primary source of IL-22 during primary infection. This pattern was reversed during secondary infection, where most IL-22+ cells were in the CD3+ compartment (Fig. 4G, top and bottom left panels). Furthermore, the majority of IL-22+ CD4+ T cells were in the TRM compartment with only sparing fractions in the effector memory (TEM) compartment (Fig. 4G, top and bottom right panels). Thus, these data demonstrate that C. rodentium-induced CD4+ TRM cells increase in number in the colonic mucosa early after secondary infection and are a source of IL-22.
C. rodentium-induced CD4+ TRM cells promote host defense.
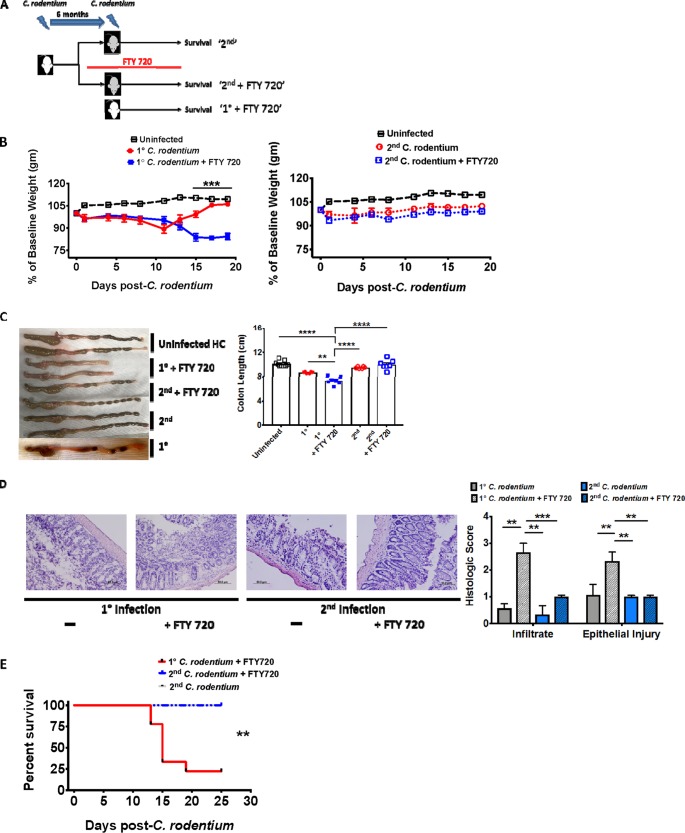
We next set up an experimental protocol designed to test the in vivo role of C. rodentium-induced CD4+ TRM cells in host defense. We once again utilized a primary and secondary infection system but also administered FTY 720 to a subset of mice before C. rodentium inoculation (Fig. 5A). We used this protocol on the reasoning that since FTY 720 restricts circulating T cells to lymph nodes but not TRM cells, mice with C. rodentium-induced CD4+ TRM cells would survive FTY 720 pretreatment. As a control, we pretreated previously uninfected mice (which would not possess C. rodentium-induced CD4+ TRM cells) with FTY 720 prior to primary infection. Since naive cells in these mice are circulating, this has the effect of removing naive CD4+ T cells from the systemic circulation, thus preventing a CD4+ T-cell response to C. rodentium primary infection.
FIG 5.
Circulating naive T cells are redundant for host defense during secondary C. rodentium infection. (A) The experimental scheme. WT mice that had recovered from primary C. rodentium infection 6 months previously were reinfected (gray) with or without FTY 720 and compared to primary-infection mice (white). FTY 720 was administered in primary-infection and a subset of secondary-infection mice 14 days prior to infection and continued for the duration of the experiment. (B, C, and D) Circulating naive T cells are redundant for host defense against secondary C. rodentium infection. (B) Weight loss in uninfected controls and primary-infection (left panel) and secondary-infection mice (right panel) with or without FTY 720. (C) Colon length in uninfected control and on day 15 after primary and secondary C. rodentium infection with or without FTY 720. (D) Hematoxylin-and-eosin-stained sections and quantified histologic score (bottom row) in primary-infection (left panel) and secondary-infection mice (right panel) with or without FTY 720 and primary-infection age- and gender-matched mice. (E) Noncirculating T cells promote host defense against secondary C. rodentium infections. Overall survival in primary-infection and secondary-infection mice with or without FTY 720. Experiments in panels B to E consist of 6 to 7 mice per group, repeated twice. Data from a representative experiment are shown in panels B to E. **, P < 0.01; ***, P ≤ 0.001; ****, P < 0.0001, by the parametric Student t test (B), ANOVA (C and D), or Kaplan-Meier method (E).
Consistent with prior publications, primary-infection mice pretreated with FTY 720 progressively lost weight even when mice with primary infection who were not pretreated began to recover (Fig. 5B, left panel) (27). In contrast, mice with secondary infection that were pretreated with FTY 720 maintained a stable weight, similarly to those with secondary infection without FTY 720 pretreatment and age- and gender-matched uninfected controls (Fig. 5B, right panel). The weight loss was associated with colonic shortening in primary-infection FTY 720-pretreated mice compared with their counterparts (Fig. 5C). Commensurate with the colonic shortening, primary-infection FTY 720-pretreated mice also exhibited substantially increased lymphocyte tissue infiltration and epithelial damage (Fig. 5D). In contrast to primary-infection FTY 720-pretreated mice, secondary-infection FTY 720-pretreated mice maintained colon length and epithelial integrity (Fig. 5C and D). Finally, consistent with the overall degree of pathology, survival was markedly impaired in primary-infection FTY 720-pretreated mice compared to secondary-infection FTY 720-pretreated and control (secondary infection, not treated with FTY 720) mice (Fig. 5E). Collectively, this indicates that C. rodentium induced CD4+ TRM cells to promote host defense against secondary infection.
DISCUSSION
We found that C. rodentium alters the distribution of mucosal CD4+ T cells in the colonic mucosa to favor the persistence of CD44+ CD69+ T cells. This population of CD4+ CD44+ CD69+ T cells does not express lymph node homing markers, is detached from the systemic circulation, and contains a subset of antigen-specific cells. These features are compatible with CD4+ TRM cells. The C. rodentium-induced population of CD4+ TRM cells is IL-17A+ (Th17 cells), and conversely, most IL-17A+ and IFNγ+ CD4+ T cells in the mucosa at late time points after C. rodentium infection express TRM markers. Finally, cells in the CD4+ CD44+ CD69+ T-cell compartment were increased upon secondary infection before the T-cell phase of the host response to C. rodentium and are a source of IL-22 (and IL-17 and IFN-γ). Thus, these CD4+ CD44+ CD69+ T cells are consistent with CD4+ TRM cells.
Tissue-resident memory T cells are the most abundant memory T-cell population in tissues in mice and humans and are defined by antigen specificity with little or no recirculation. Nearly all TRM cells express the activation marker CD69 and show the ability to rapidly produce cytokines (1, 2, 21, 28). The majority of published data pertain to CD8+ TRM cells, and there are comparatively few data on CD4+ TRM cells and even fewer data on intestinal CD4+ TRM cells (10). Moreover, Th17 cells, which are largely regulated in the intestine, are heavily implicated in autoimmunity in mice and humans (29). Thus, it is highly likely that determining the mechanism regulating the long-term maintenance of intestinal Th17 cells will lead to targeted therapies for conditions such as IBD. The intestinal microenvironment has unique properties compared to other lymphoid and nonlymphoid sites with distinct microbiota and expression patterns of adhesion molecules. Consistent with this, intranasal challenge with Listeria monocytogenes failed to induce CD8+ TRM cells compared to oral challenge, suggesting that microenvironmental cues impact TRM cell formation (9). Additionally, oral ovalbumin challenge results in antigen tolerance in OT-II mice compared to intravenous or intraperitoneal (systemic) challenge. Collectively, this highlights the importance of delineating the mechanisms regulating TRM cell formation specific to tissue sites.
Murine CD4+ and CD8+ TRM cells exhibit similar genetic signatures largely pertaining to genes encoding lymphocyte trafficking (7, 30). Despite these similarities, however, there are cell-, tissue-, and even species-specific differences in TRM cell biology. Most CD8+ TRM cells require IL-15 for tissue residency, but IL-15-dependent and independent CD4+ TRM cells have been described (31). Indeed, CD4+ TRM cells may rely on distinctly different mechanisms to maintain tissue residency. CD4+ TRM cells in murine skin and vaginal mucosa reside in clusters associated with antigen-presenting cells (APCs) (3, 32). In the vaginal mucosa, CD4+ TRM cells are dependent upon APC-produced CCL5 to maintain tissue residence, but whether this is also true for skin-resident CD4+ TRM cells is unknown. In contrast, murine CD103+ CD8+ TRM cells do not exhibit such a requirement for APC clusters, although there is some evidence that CD103− CD8+ TRM cells might (33).
Expression of CD103 is a hallmark of nearly all CD8+ TRM cells (1, 21, 28). Tumor growth factor β is necessary to induce and maintain CD103+ CD8+ TRM cells, likely via induction of CD103 (8, 21, 33). While CD103 is clearly necessary for the induction or maintenance of some CD8+ TRM cells, the functional significance of CD103 remains uncertain. Indeed, these models have not reported impaired immunity to secondary infection, only impaired generation or maintenance of CD8+ TRM cells (9, 33). In contrast to CD8+ TRM cells, expression of CD103 is highly variable in most murine models of CD4+ TRM cells (3, 31). This distinction in CD103 expression between CD4+ and CD8+ TRM cells appears to also be a feature of human TRM cells (11). We found that relatively few C. rodentium-induced CD4+ TRM cells expressed CD103 (see Fig. S2A in the supplemental material) (34). Moreover, we found that CD11c+ dendritic cell (DC)-produced TGF-β1 was redundant for the induction and maintenance of C. rodentium-induced CD4+ TRM cells (Fig. S3). Although TGF-β1 is produced by multiple cell types in the intestine, including DCs, other APCs, and epithelial cells, this was somewhat surprising considering the important role of CD11c+ DCs in promoting the induction of Th17 cells.
Interestingly, E-cadherin, the ligand for CD103, is highly expressed by epithelial cells, but we found no difference in expression of CD103 by C. rodentium-induced CD4+ TRM cells between the intraepithelial and lamina propria compartments (Fig. S3B and C). Moreover, there were no consistent differences in IL-17A or IFN-γ production between CD103+ and CD103− CD4+ TRM cells (Fig. 3E and F). Our data are therefore consistent with other models of CD4+ TRM cells and collectively suggest that CD103 neither impacts the function of CD4+ TRM cells nor is a major feature of these cells (3, 31). This, in turn, suggests that CD4+ TRM cells may patrol the intraepithelial and lamina propria compartments, in contrast to CD103+ CD8+ TRM cells, which appear to be enriched in the intraepithelial compartment.
Traditional models of TRM cells have utilized pathogens with known epitopes, which facilitates the tracking of antigen-specific T-cell responses. An important caveat of our approach is that these transgenic T-cell systems do not exist for C. rodentium infections. We circumvented this problem in several ways. First, we relied on CD69 expression as nearly all TRM cells express this marker (1, 5, 28). Second, we used FTY 720 to assess tissue restriction. FTY 720 is commonly used in murine models of TRM cells for this explicit purpose (3, 6). Third, we utilized FACS with OT-II mice and OVA-C. rodentium to examine antigen specificity. Finally, we used C57/BL6 mice from the Jackson Laboratory, which have very low levels of Th17 cells at baseline (35). Indeed, tracking Th17 cells in these mice alone has been used to demonstrate antigen specificity independent of other factors (19). Finally, although we demonstrated that mice with secondary infection pretreated with FTY 720 have preserved immunity relative to their primary-infection counterparts, it is important to note that the effect is most likely not due to mucosal TRM cells alone. This is because T-cell-dependent humoral responses are necessary for final eradication of C. rodentium, and it is very likely that T-follicular cells, which mediate T-cell-dependent antibody production, are very likely to be preserved with FTY 720 (15). Collectively, our data show that C. rodentium can induce CD4+ TRM cells that promote host defense, thus establishing this as a potentially useful model for CD4+ Th17 TRM cells.
MATERIALS AND METHODS
Mice.
Wild-type (WT) and OT-II mice were purchased from the Jackson Laboratory (Bar Harbor, ME). OT-II mice are well described and express transgenic α and β T-cell receptor chains that recognize residues 323 to 339 of chicken ovalbumin in the context of I-Ab (36). Dendritic cell-specific TGF-β1 knockout mice (CD11c TGF-β1fl/fl) were generated by crossing CD11c-cre mice with TGF-β1flox ex6 mice, both of which were purchased from the Jackson Laboratory (34). TGF-β1flox ex6 mice feature loxP sites flanking exon 6 of Tgfb1, resulting in deletion of Tgfb1 when crossed with cre-expressing tissues (37). The TGF-β1flox ex6 mice have been backcrossed onto inbred C57BL6/J strains at the Jackson Laboratory and exhibit spontaneous lung and salivary gland inflammation at 5 months of age (37). CD11c-cre and TGF-β1flox ex6 mice were crossed at the University of Michigan, and genotype was confirmed prior to use in experiments (34). CD11c is a pan-DC marker and is expressed by CD8− and CD8+ DCs, tissue-derived and lymph node DCs, and plasmacytoid DCs. All WT, OT-II, and CD11ccre TGF-βfl/fl mice for these experiments were housed in specific-pathogen-free facilities at the University of Michigan. This protocol was approved by the University of Michigan Animal Care and Use Committee.
C. rodentium infections.
Mice were age and gender matched for all experiments. Wild-type C. rodentium (ATCC 51459) from frozen stock was cultured in 5 ml of Luria-Bertani (LB) broth supplemented with ampicillin (100 μg/ml) overnight on a shaker at 200 rpm at 37°C. The concentration (CFU/ml) was assessed after the overnight incubation by a spectrophotometer. A volume sufficient to obtain 109 CFU was diluted in sterile PBS sufficient to achieve a 200-μl/ml oral inoculum. Wild-type, OT-II, and CD11ccre TGF-βfl/fl 5- to 8-week-old mice were inoculated by oral gavage with 109 CFU in 100 μl. Mice were monitored for clinical signs of C. rodentium colitis and sacrificed at the indicated time points. Ovalbumin-expressing C. rodentium was generously provided by Danyvid Olivares-Villagomez (Vanderbilt University) and was handled as described for WT C. rodentium (23). For rechallenge (secondary) infections, mice were allowed to clear primary infection and were reinoculated >4 weeks after primary infection.
FTY 720.
FTY 720 was mixed with drinking water and was administered to mice for 2 weeks before sacrifice for mucosal T-cell quantitation or prior to C. rodentium infection or reinfection.
Quantification of C. rodentium burden.
The stool was collected from the colon of infected mice and weighed prior to homogenization in PBS to an even suspension with a homogenizer. Serial dilutions of the homogenized stool were plated in triplicate on MacConkey agar plates and incubated for 72 h at 37°C. Fecal bacterial load was determined by enumerating colonies after the incubation period and was expressed per unit weight of stool (CFU/g). The bacterial load of mucosal adherent C. rodentium was assessed using a piece of colon tissue. Colon was washed with PBS to remove stool and then weighed, homogenized in PBS, and plated in serial dilution. CFU per unit weight was determined as described above.
Mucosal T-cell isolation.
Lamina propria mononuclear cells (LPMCs) were isolated from mouse colon tissues using previously described protocols with limited modification (38). Briefly, dissected mouse colon mucosa was incubated in wash buffer (calcium- and magnesium-free Hanks’ balanced salt solution containing 2.5% fetal bovine serum [FBS] and 1 mM dithiothreitol [Sigma-Aldrich]) to remove mucus. The mucosa was then incubated twice in the wash buffer containing 2 mM EDTA (Sigma-Aldrich) for 45 min at 37°C. Tissues were collected and incubated in prewarmed wash buffer containing 17.86 μg/ml Liberase (Roche Diagnostics) and 0.04 mg/ml DNase I (Sigma-Aldrich) and shaken at 200 rpm for 60 min at 37°C. The fraction was pelleted, resuspended in a 40% Percoll solution (GE Healthcare), and then layered on an 80% Percoll gradient before centrifugation at 1,000 × g for 20 min at room temperature with the brake off. Viable LPMCs were recovered from the 40% to 80% layer interface. Intraepithelial fractions were recovered after the EDTA incubation, while the remaining fraction (lamina propria) lymphocytes were recovered after the tissue digestion steps.
Flow cytometry.
Freshly isolated LMPCs were rested overnight in RPMI supplemented with glutamine, sodium pyruvate, antibiotics, and 10% FBS. Cells were then stimulated with 1× stimulation cocktail per kit instructions for 4 to 6 h (eBioscience) with Golgi Plug (BD Biosciences, Franklin Lakes, NJ) prior to flow cytometry. Flow cytometry was performed on the LSR II (BD Biosciences) or the FACS Aria II (BD Biosciences) cytometer and analyzed with FlowJo (Ashland, OR). The following antibodies were used for flow cytometry: CD44 BV 421 (BioLegend), CD103 PE-Dazzle 594 (BioLegend, San Diego, CA), IFN-γ allophycocyanin (BioLegend), CD4 fluorescein isothiocyanate (FITC) (Thermo Fisher, Waltham, MA), Fix Viability eFluor 780 (Thermo Fisher), CD69 phycoerythrin (PE)-Cy7 (BioLegend), IL-17A PE (BioLegend), CD3ε BV 510 (BioLegend), CD8β peridinin chlorophyll protein (PerCP)-eFluor 710 (eBioscience), CD62L PE (Miltenyi Biotec, Bergisch Gladbach, Germany), and CCR7 allophycocyanin (Miltenyi Biotec).
Cell sorting.
Freshly purified LPMCs from WT mice were labeled with Fix Viability eFluor 780, CD3ε, CD4 FITC, CD8β PerCP-eFluor 710, CD44 BV 421, and CD69 PE-Cy7, and live lymphocytes were FACS purified into CD4+ CD44+ CD69+ and CD4+ CD44+ CD69− fractions. Cells were recounted for viability before RNA isolation for qRT-PCR. Lymphocytes from OT-II mice were stained with the abovementioned panel excluding CD8β PerCP-eFluor 710 but including Vβ5.1/5.2 PerCP-eFluor 710.
Quantitative PCR.
Colon was homogenized in RLT buffer (RNeasy kit; Qiagen, Valencia, CA) with a tissue homogenizer. RNA was extracted with RNeasy kits, and cDNA was synthesized with iScript cDNA synthesis kits (Hercules, CA). Relative quantification of genes was determined by real-time PCR with SYBR green (Bio-Rad) normalized to GAPDH. Some data were additionally normalized to baseline conditions as noted in the figure legends. All primers were from Bio-Rad. Results were analyzed on a CFX Connect system (Bio-Rad).
Statistics.
Data were analyzed using the ANOVA or unpaired parametric Student t test as noted in the figure legends. We performed analysis to determine if distributions were skewed prior to parametric Student t test analysis. All data were analyzed using Prism (GraphPad, San Diego, CA).
Supplementary Material
ACKNOWLEDGMENTS
We have no conflict of interest to declare.
S.B. designed the study, drafted the manuscript, and analyzed all data. D.P., G.H., and M.Z. performed all experiments. M.Z., H.G., W.Z., P.D.R.H., R.W.S., N.K., and J.Y.K. helped plan the study and experiments and provided support for data analysis. All authors have reviewed the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00295-19.
REFERENCES
- 1.Mueller SN, Mackay LK. 2016. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 2.Park CO, Kupper TS. 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zens KD, Chen JK, Farber DL. 2016. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1:85832. doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 6.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 7.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Bevan MJ. 2013. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner DL, Farber DL. 2014. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol 5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. 2014. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 12:612–623. doi: 10.1038/nrmicro3315. [DOI] [PubMed] [Google Scholar]
- 13.Silberger DJ, Zindl CL, Weaver CT. 2017. Citrobacter rodentium: a model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal Immunol 10:1108–1117. doi: 10.1038/mi.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamada N, Sakamoto K, Seo SU, Zeng MY, Kim YG, Cascalho M, Vallance BA, Puente JL, Nunez G. 2015. Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe 17:617–627. doi: 10.1016/j.chom.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov II, Sugiyama T, Nunez G, Camp JG, Hattori M, Umesaki Y, Honda K. 2015. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. 2011. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlfors H, Morrison PJ, Duarte JH, Li Y, Biro J, Tolaini M, Di Meglio P, Potocnik AJ, Stockinger B. 2014. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol 193:4602–4613. doi: 10.4049/jimmunol.1401244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campisi L, Barbet G, Ding Y, Esplugues E, Flavell RA, Blander JM. 2016. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat Immunol 17:1084–1092. doi: 10.1038/ni.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinbach K, Vincenti I, Kreutzfeldt M, Page N, Muschaweckh A, Wagner I, Drexler I, Pinschewer D, Korn T, Merkler D. 2016. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med 213:1571–1587. doi: 10.1084/jem.20151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkel JM, Masopust D. 2014. Tissue-resident memory T cells. Immunity 41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaech SM, Hemby S, Kersh E, Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837–851. doi: 10.1016/S0092-8674(02)01139-X. [DOI] [PubMed] [Google Scholar]
- 23.Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. 2010. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect Immun 78:2653–2666. doi: 10.1128/IAI.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda A, Yoshida M, Shiomi H, Ikezawa S, Takagawa T, Tanaka H, Chinzei R, Ishida T, Morita Y, Kutsumi H, Inokuchi H, Wang S, Kobayashi K, Mizuno S, Nakamura A, Takai T, Blumberg RS, Azuma T. 2008. Fcgamma receptor regulation of Citrobacter rodentium infection. Infect Immun 76:1728–1737. doi: 10.1128/IAI.01493-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. 2015. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 112:7061–7066. doi: 10.1073/pnas.1415675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CT, Hall LJ, Hurley G, Quinlan A, MacSharry J, Shanahan F, Nally K, Melgar S. 2012. The sphingosine-1-phosphate analogue FTY720 impairs mucosal immunity and clearance of the enteric pathogen Citrobacter rodentium. Infect Immun 80:2712–2723. doi: 10.1128/IAI.06319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar J-P, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, Friedman AL, Shen Y, Farber DL. 2017. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romagnoli PA, Fu HH, Qiu Z, Khairallah C, Pham QM, Puddington L, Khanna KM, Lefrancois L, Sheridan BS. 2017. Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol 10:520–530. doi: 10.1038/mi.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, Haque A, Bedoui S, Heath WR, Mueller SN, Kupper TS, Gebhardt T, Carbone FR. 2016. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun 7:11514. doi: 10.1038/ncomms11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergsbaken T, Bevan MJ. 2015. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol 16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, El-Zaatari M, Eaton KA, Grasberger H, Kao JY. 2017. Dendritic cell-derived TGF-beta mediates the induction of Helicobacter-specific regulatory T-cell response essential for maintenance of immune tolerance in mice. Gastroenterology 152:S14. doi: 10.1016/S0016-5085(17)30423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov II, Frutos R. D. L., Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnden MJ, Allison J, Heath WR, Carbone FR. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 37.Rahmani W, Liu Y, Rosin NL, Kline A, Raharjo E, Yoon J, Stratton JA, Sinha S, Biernaskie J. 2018. Macrophages promote wound-induced hair follicle regeneration in a CX3CR1- and TGF-beta1-dependent manner. J Invest Dermatol 138:2111–2122. doi: 10.1016/j.jid.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, Nakai T, Hasegawa A, Inoue N, Watanabe N, Akagawa KS, Hibi T. 2005. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol 175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.