Abstract
Context
Increased testosterone (T) levels are a cardinal feature of polycystic ovary syndrome (PCOS). Female relatives of affected women, including premenarchal daughters, have elevated T levels supporting a genetic susceptibility to this phenotype. Girls with obesity (OB-g) also have increased T levels throughout puberty, which may indicate risk for PCOS.
Objective
We tested the hypothesis that premenarchal daughters of women affected with PCOS (PCOS-d) have distinctive phenotypic features compared with OB-g.
Design, Setting, and Participants
Forty-eight PCOS-d, 30 OB-g, and 22 normal weight (NW-g) premenarchal girls were studied. Mothers of OB-g and NW-g had no evidence for PCOS.
Main Outcome Measures
Reproductive hormones were measured.
Results
Body mass index differed by design, was highest in OB-g, followed by PCOS-d (P > 0.001). PCOS-d and OB-g had similar increases in free T levels compared with NW-g (PCOS-d vs NW-g, P = 0.01; OB-g vs NW-g, P = 0.0001). Sex hormone binding globulin levels were lowest in OB-g and lower in PCOS-d than in NW-g (PCOS-d vs NW-g, P = 0.005; OB-g vs NW-g, P < 0.0001; PCOS-d vs OB-g, P < 0.0001). Anti-Müllerian hormone (AMH) levels in PCOS-d were significantly increased compared with OB-g, who tended to have lower AMH levels than NW-g (PCOS-d vs OB-g, P < 0.0001; PCOS-d vs NW-g, P = 0.10).
Conclusions
Despite similarly elevated free T levels, PCOS-d had increased AMH levels compared with OB-g. This finding suggests that OB-g lack alterations in ovarian folliculogenesis, a key reproductive feature of PCOS. Causal mechanisms may differ in PCOS-d or OB-g, or elevated T in OB-g may not be an early marker for PCOS.
We found similar degrees of hyperandrogenemia in premenarchal daughters of women with PCOS and girls with obesity but differing anti-Müllerian hormone levels, suggesting ovarian folliculogenesis differs in these groups.
Hyperandrogenemia is a cardinal reproductive phenotype of polycystic ovary syndrome (PCOS) and may play a causal role in disease pathogenesis (1). Approximately 40% of reproductive-age sisters of affected women have elevated total or bioavailable testosterone (T) levels (1). Furthermore, male (2) as well as nonreproductive-age female [i.e., postmenopausal and premenarchal (3, 4)] first-degree relatives have hyperandrogenemia, suggesting a genetic susceptibility to this phenotype. In animal models, including nonhuman primates (5–7), androgen exposure in utero (5, 8), neonatally (9), or peripubertally (7) can result in phenocopies of PCOS. Accordingly, we hypothesized that genetic variation resulting in hyperandrogenemia during key developmental windows programs the phenotypic features of PCOS (4, 10). Consistent with this hypothesis, we found that daughters of women affected with PCOS (PCOS-d) have evidence of increased global 5α-reductase activity in early childhood (10) and elevated T levels beginning in early puberty (4). In addition to these early differences in androgen production and metabolism, PCOS-d exhibit other reproductive and metabolic phenotypes, including elevations of anti-Müllerian hormone (AMH) levels over the course of childhood, as well as early metabolic and β-cell dysfunction by the peripubertal years (4, 11, 12).
Girls with obesity (OB-g) also have increased total and free T levels during the pubertal transition (13). Because of the proposed central role of androgens in the pathogenesis of PCOS (14), it has been hypothesized that hyperandrogenemic OB-g will develop PCOS after puberty (15, 16). Thus, elevated T levels may be an early biomarker for PCOS (16). However, no longitudinal studies have been performed to test this hypothesis.
An important distinguishing feature between PCOS-d and OB-g is their differing genetic risk for PCOS. All mothers of PCOS-d are genetically affected by definition. Accordingly, a substantial percentage of PCOS-d will inherit one or more PCOS susceptibility variants (17–19) from their affected mothers (20). In contrast, the prevalence of PCOS susceptibility loci in OB-g and their mothers is expected to reflect the background 7% to 15% population prevalence of PCOS (21). Therefore, the mechanisms for early hyperandrogenemia and the risk for future development of PCOS may differ in these distinct groups. We performed this study to test the hypothesis that premenarchal reproductive phenotypes in OB-g differ from those in PCOS-d.
Methods
Premenarchal PCOS-d (n = 48), OB-g (n = 30), and normal weight control girls (n = 22) aged 8 to 12 years with Tanner breast stages I to III were studied. OB-g had the additional inclusion criterion of body mass index (BMI) ≥97th percentile, and normal weight girls (NW-g) had a criterion of BMI <85th percentile; PCOS-d with any BMI were included. All girls were in good health and were not taking any medications known to alter reproductive hormone metabolism or glucose homeostasis for at least 1 month before the study. PCOS-d had a mother who fulfilled National Institutes of Health criteria for PCOS [hyperandrogenism and oligomenorrhea with the exclusion of other reproductive disorders (21)] as confirmed by us before the current study or by their personal physician. Mothers of OB-g and NW-g had regular menses every 27 to 35 days as well as no history of reproductive disorders and no signs or symptoms of androgen excess by validated questionnaire (1). Participants were recruited through outreach to women who previously participated in our studies of PCOS; control adult women were recruited through letters sent to eligible patients at our medical centers, as well as by advertisements in local media and online.
PCOS-d, OB-g, and NW-g were studied at the Ann & Robert H. Lurie Children’s Hospital, and additional PCOS-d were studied at the Milton S. Hershey Medical Center at Penn State College of Medicine. The institutional review boards of the Feinberg School of Medicine, Northwestern University, Ann & Robert H. Lurie Children’s Hospital, and Penn State College of Medicine approved this study. Written informed consent was obtained from a parent of each girl, and written assent was obtained from each girl aged 12 years or older prior to participation. Clinical and biochemical data from some of the study subjects have been previously reported (4, 22).
A physical examination, including Tanner breast staging determined by visualization and palpation, was performed by a single pediatric endocrinologist at the Ann & Robert H. Lurie Children’s Hospital and by a trained study coordinator at the Hershey Medical Center at Penn State. A fasting early morning blood sample was collected for measurement of AMH, T, sex hormone binding globulin (SHBG), ultra-sensitive estradiol, dehydroepiandrosterone sulfate (DHEAS), and androstenedione levels.
Assays
Androstenedione, ultra-sensitive estradiol, AMH, SHBG, and DHEAS levels were measured at the University of Virginia Ligand Core Laboratory (Charlottesville, VA). Androstenedione was measured by using the Siemens Diagnostics (Los Angeles, CA) RIA system [sensitivity, 0.1 ng/mL; intra-assay coefficient of variation (CV), 4.9%; interassay CV, 7.0%], ultra-sensitive estradiol was measured using the Siemens Diagnostics RIA system (sensitivity, 10 pg/mL; intra-assay CV, 6.3%; interassay CV, 8.1%), and AMH was measured using the Beckman Coulter (Brea, CA) two-site ELISA System (sensitivity, 0.16 ng/mL; intra-assay CV, 3.9%; interassay CV, 6.2%) (23). SHBG and DHEAS levels were measured by the Siemens Diagnostics chemiluminescence system (SHBG sensitivity, 2 nmol/L; intra-assay CV, 2.7%; interassay CV, 5.2%; and DHEAS sensitivity, 150 ng/mL; intra-assay CV, 5.4%; interassay CV, 6.5%). T levels were analyzed by liquid chromatography-tandem mass spectrometry (Brigham Research Assay Core, Boston, MA) (sensitivity, 2 ng/dL; intra-assay CV, 9.0% at 16 ng/dL; interassay CV, 15.8% at 12 ng/dL) (12). Free T was calculated as reported (24).
Statistical analysis
Data were log-transformed or square root transformed when necessary to achieve homogeneity of variance. Pearson correlation was performed to assess the association of the potential confounders of age and Tanner breast stage with all end points. For variables that significantly correlated with age and/or Tanner breast stage, analysis of covariance adjusting for these parameters was applied. Otherwise, unadjusted ANOVA was used to assess differences in end point variables between the groups. Tukey post hoc testing was used to determine which groups differed significantly. Categorical variables were compared by Fisher’s exact test. We performed a subgroup analysis in PCOS-d with obesity and OB-g of comparable BMIs to control for the independent effect of obesity on major end points. To isolate a cohort of PCOS-d and OB-g with comparable BMIs, PCOS-d with the lowest BMI and OB-g with the highest BMI were excluded in a stepwise fashion until we achieved a cohort with comparable BMIs (n = 27; limited to participants with BMI z scores of 1.7 to 2.6). For this analysis, Student t tests were applied. Normative ranges for end point variables were defined by Tanner breast stage‒specific thresholds of ±2 SD in the l-g. Statistical analyses were performed with SAS 9.4 (SAS Institute, Inc., Cary, NC). Data are reported as mean ± SD, with the α level set at 0.05.
Results
BMI differed between the groups by design, being highest in OB-g (Table 1). Both OB-g and PCOS-d were heavier than NW-g (Table 1). There were minimal differences in age between the groups (P = 0.05), but with a trend toward younger age in PCOS-d than in OB-g and NW-g (Table 1). Consistent with their younger age, the percentage of girls with Tanner stage I and II breast (P = 0.01) and pubic hair (P = 0.01) assessments was higher in PCOS-d than in OB-g and NW-g (Table 1). The groups also varied by race and ethnicity, with a higher prevalence of Hispanic and black girls in the OB-g group than in the NW-g and PCOS-d groups (P < 0.0001).
Table 1.
Baseline Clinical Characteristics and Reproductive Hormones
| PCOS-d (n = 48) | OB-g (n = 30) | NW-g (n = 22) | P | PCOS-d vs OB-g | PCOS-d vs NW-g | OB-g vs NW-g | |
|---|---|---|---|---|---|---|---|
| Age, y | 9.7 ± 1.3a (8.0–12.3) | 10.3 ± 1.1 (8.3–12.3) | 10.3 ± 1.1 (8.3–12.7) | 0.05 | 0.09 | 0.14 | 0.99 |
| BMI percentile | 80 ± 23 (16–99) | 99 ± 1 (97–99) | 50 ± 19 (19–84) | NA | NA | NA | NA |
| BMI z score | 1.1 ± 0.9 (−1.0 to 2.5) | 2.4 ± 0.2 (1.9–2.9) | 0.0 ± 0.5 (−0.9 to 1.0) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Tanner breast stage | 44% Ib | 33% I | 32% I | 0.01c | NA | NA | NA |
| 40% II | 13% II | 27% II | |||||
| 16% III | 54% III | 41% III | |||||
| Tanner pubic hair stage | 67% I | 33% I | 32% I | 0.01c | NA | NA | NA |
| 29% II | 33% II | 45% II | |||||
| 2% III | 33% III | 18% III | |||||
| 2% IV | 5% IV | ||||||
| Total T,d ng/dL | 8 ± 6 (1–30) | 9 ± 6 (1–22) | 9 ± 8 (2–33) | 0.53 | NA | NA | NA |
| SHBG,e nmol/L | 54 ± 31 (9–180) | 22 ± 10 (7–54) | 71 ± 22 (38–121) | <0.0001 | <0.0001 | 0.005 | <0.0001 |
| Free T,f ng/dL | 0.13 ± 0.12 (0.01–0.58) | 0.22 ± 0.15 (0.03–0.58) | 0.10 ± 0.10 (0.02–0.40) | 0.0002 | 0.19 | 0.01 | 0.0001 |
| AMH,g ng/mL | 3.28 ± 2.34 (0.13–13.00) | 1.22 ± 0.72 (0.20–3.03) | 2.08 ± 0.94 (0.79–4.52) | <0.0001 | <0.0001 | 0.10 | 0.07 |
| DHEAS,h ng/mL | 493 ± 358 (91–1730) | 635 ± 349 (179–1555) | 549 ± 362 (145–1770) | 0.34 | NA | NA | NA |
| Androstenedione,i ng/mL | 0.47 ± 0.37 (0.13–1.27) | 0.57 ± 0.37 (0.11–1.77) | 0.50 ± 0.41 (0.11–1.78) | 0.52 | NA | NA | NA |
| Estradiol,j pg/mL | 13 ± 11 (4–59) | 20 ± 14 (7–76) | 14 ± 10 (5–43) | 0.09 | NA | NA | NA |
P values listed from analysis of covariance correcting for age and Tanner breast stage unless noted otherwise. BMI percentiles reported for descriptive purposes only. Statistical assessment for differences between the groups performed only on BMI z score. To convert DHEAS from ng/mL to nmol/L, multiply by 2.714; androstenedione from ng/dL to nmol/L, multiply by 0.0349; and estradiol from pg/mL to pmol/L, multiply by 3.671.
Abbreviation: NA, not applicable.
Mean ± SD (range).
Percentage of subjects included in each group is noted.
The categorical variables Tanner breast and pubic hair stages were analyzed by Fisher’s exact test.
Total T levels missing in three PCOS-d.
SHBG levels missing in two PCOS-d.
Free T levels missing in four PCOS-d.
AMH levels missing in nine PCOS-d, seven OB-g, and four NW-g.
DHEAS levels missing in six PCOS-d and two NW-g.
Androstenedione levels missing in 28 PCOS-d.
Estradiol levels missing in 12 PCOS-d, six OB-g, and four NW-g.
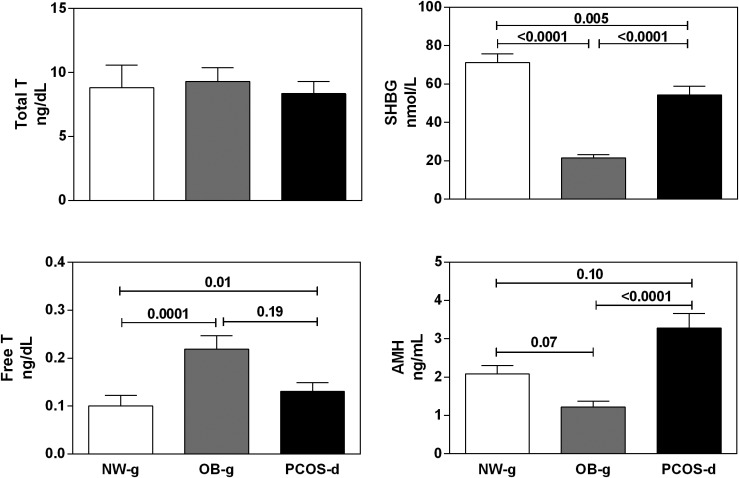
Total T levels did not differ between the groups (P = 0.53) (Fig. 1). SHBG levels were lowest in OB-g compared with PCOS-d (P < 0.0001) and NW-g (P < 0.0001) and were also lower in PCOS-d than in NW-g (P = 0.005) (Fig. 1). Free T levels were similarly increased in PCOS-d (P = 0.01) and in OB-g (P = 0.0001) compared with NW-g (Fig. 1). In total, 39% of PCOS-d and 38% of OB-g had elevated free T levels. DHEAS (P = 0.34) and androstenedione levels (P = 0.52) (Table 1) did not differ between the groups. Estradiol levels were also similar between the groups (P = 0.09) (Table 1) and were strongly correlated with Tanner breast stage (r: −0.51; P < 0.0001). AMH levels were significantly higher in PCOS-d than in OB-g (P < 0.0001); there was a trend toward higher AMH levels in PCOS-d compared with NW-g (P = 0.10) (Fig. 1). Thirty-one percent of PCOS-d with elevated AMH levels also had elevated free T levels.
Figure 1.
Reproductive hormone levels in PCOS-d and OB-g. Although total T levels did not differ between PCOS-d, OB-g, and NW-g (upper left panel, ANCOVA P = 0.53), PCOS-d and OB-g did have similarly decreased SHBG levels compared with NW-g (upper right panel, ANCOVA P < 0.0001; NW-g vs OB-g, P < 0.0001; PCOS-d vs OB-g, P < 0.0001; PCOS-d vs NW-g, P = 0.005). Accordingly, PCOS-d and OB-g had significant increases in free T levels compared with NW-g (lower left panel, ANCOVA P = 0.002; NW-g vs OB-g, P = 0.0001; PCOS-d vs OB-g, P = 0.19; and PCOS-d vs NW-g, P = 0.01). AMH levels also differed between the groups, with PCOS-d having significantly increased AMH levels compared with OB-g and a trend toward increased AMH levels compared with NW-g, which did not reach statistical significance (lower right panel, ANCOVA P < 0.0001; NW-g vs OB-g, P = 0.07; PCOS-d vs OB-g, P < 0.0001; and PCOS-d vs NW-g, P = 0.10). Unadjusted means and SEM are pictured; statistical analyses were adjusted for differences in age and Tanner breast stage. ANCOVA, analysis of covariance.
In the subgroup analysis in PCOS-d with obesity and OB-g, BMI z score (P = 0.08), age (P = 0.10), and Tanner breast stage (P = 0.29) did not differ. AMH levels (P = 0.03) remained increased in PCOS-d. SHBG levels remained lower in OB-g (P = 0.03), whereas free T levels tended to be higher in OB-g (P = 0.09).
Discussion
We found that peripubertal PCOS-d and OB-g have distinct reproductive phenotypes. Although their elevations in free T were similar to those in NW-g, AMH levels were significantly higher in PCOS-d than in OB-g. It is possible that obesity contributed to the lower AMH levels in OB-g, as a negative correlation between BMI and AMH level has been reported in some (25, 26), but not all (27), studies in adult women, including those with PCOS (28). However, the significant increase in AMH levels in PCOS-d compared with OB-g persisted in subgroups of comparable BMIs. The lack of elevated AMH levels in OB-g suggests that they do not have alterations in ovarian folliculogenesis characteristic of PCOS (29).
Small preantral and antral ovarian follicles up to 6 mm in size are the primary source of circulating AMH in women and girls (30, 31). Polycystic ovaries have a twofold to threefold increase in small preantral follicles (32, 33), and increased circulating AMH levels are a key feature of the PCOS reproductive phenotype (21, 26, 28, 34). Even before menarche, circulating AMH levels correlate with the number of small and medium ovarian follicles (35). Previous studies in PCOS-d found increased AMH levels during infancy, childhood (11), and puberty (36), suggesting differences in ovarian folliculogenesis may begin early in development in these girls.
Recent studies have suggested that AMH may play a role in the development of PCOS. We found that ∼3% of women with PCOS have mutations in AMH that decrease the bioactivity of the encoded protein; this may contribute to PCOS by decreasing AMH-mediated suppression of testosterone production (37). It is also possible that AMH plays a role in the pathogenesis of PCOS through neuroendocrine mechanisms. The AMH receptor is expressed in the hypothalamus on GnRH neurons in mice and humans (38). AMH increases GnRH-dependent LH secretion (38). Prenatal administration of high doses of AMH in pregnant mice induced GnRH-mediated hyperandrogenism and aromatase blockade in the mothers, resulting in intrauterine androgen exposure and ultimately a PCOS reproductive phenotype in their female offspring (39).
There is considerable evidence that androgens play a central role in the development of PCOS. Androgen excess during critical developmental windows produces phenocopies of PCOS in animal models (5–7). In humans, androgens antagonize the ability of estradiol and progesterone to slow the GnRH pulses contributing to the disordered gonadotropin secretion characteristic of PCOS (14). Hyperandrogenemia is a consistent reproductive phenotype in male as well as female relatives of women with PCOS (1, 2, 4). In the daughters of affected women, T elevations begin before menarche, and there is evidence for global increases in 5α-reductase in childhood, which could enhance the local conversion of T to its more potent metabolite dihydrotestosterone (10). Therefore, it is biologically plausible that girls with elevated free T levels will develop PCOS (40). However, no prospective studies have tested this hypothesis.
Our findings suggest that there may be additional phenotypic features in girls at increased genetic risk for PCOS (i.e., PCOS-d) because they have inherited one or more PCOS susceptibility alleles from their affected mothers (21). However, a subset of PCOS-d will not have inherited maternal PCOS susceptibility alleles. Accordingly, the PCOS-d cohort represents a mix of genetically affected and unaffected individuals. Nevertheless, the increased mean AMH levels in PCOS-d suggests that at least some of these girls have the changes in folliculogenesis characteristic of PCOS, in addition to elevated free T levels. Longitudinal studies are needed to determine whether these girls develop PCOS.
Our study had several limitations. Because our primary objective was to compare early reproductive phenotypes in two distinct putative PCOS risk groups, premenarchal PCOS-d and OB-g, BMI differed between our study groups by design. Differences in AMH levels remained significant in our subgroup analysis of OB-g and PCOS-d with obesity and comparable BMIs; however, we lacked adequate power to determine whether reproductive phenotypes differed in normal weight PCOS-d and NW-g of comparable BMIs. However, previous investigators reported a distinct reproductive phenotype of elevated AMH levels during infancy and childhood (11) and elevated total T levels by late puberty in normal weight PCOS-d compared with control girls of similar weight (12). Further, we did not have adequate power to perform separate analyses by individual Tanner stage. Nevertheless, we did account for the independent impact of pubertal stage by including Tanner breast stage as a covariate in our analyses. In addition, our subjects were not stratified by race or ethnicity because there was no evidence for racial or ethnic differences in circulating reproductive hormone levels, including AMH (41, 42) and androgen levels (43).
In summary, we have shown that despite similar increases in free T levels, premenarchal PCOS-d and OB-g have distinct reproductive phenotypes. In contrast to OB-g, PCOS-d have elevated AMH levels, a biomarker for altered follicular development. The distinct phenotype in PCOS-d may reflect increased genetic risk for PCOS compared with OB-g. It remains possible that both groups are at increased risk for PCOS by different causal mechanisms.
Acknowledgments
Financial Support: This research was supported by grants P50 HD044405 and R01 HD085227 (to A.D.), K12 HD055884 (to L.C.T.), and K23 HD090274 (to L.C.T.) from the Eunice Kennedy Shriver National Institute of Child Health and Development. Some hormone assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by grant no. U54 HD28934 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Research reported in this publication was also supported in part by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant no. UL1TR000150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors report no potential conflicts of interest relevant to this article.
Glossary
Abbreviations:
- AMH
anti-Müllerian hormone
- BMI
body mass index
- CV
coefficient of variation
- DHEAS
dehydroepiandrosterone sulfate
- NW-g
normal weight girls
- OB-g
girls with obesity
- PCOS
polycystic ovary syndrome
- PCOS-d
daughters of women affected with PCOS
- SHBG
sex hormone binding globulin
- T
testosterone
References
- 1. Legro RS, Driscoll D, Strauss JF III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95(25):14956–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(5):2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sam S, Legro RS, Essah PA, Apridonidze T, Dunaif A. Evidence for metabolic and reproductive phenotypes in mothers of women with polycystic ovary syndrome. Proc Natl Acad Sci USA. 2006;103(18):7030–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torchen LC, Fogel NR, Brickman WJ, Paparodis R, Dunaif A. Persistent apparent pancreatic β-cell defects in premenarchal PCOS relatives. J Clin Endocrinol Metab. 2014;99(10):3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. [DOI] [PubMed] [Google Scholar]
- 6. Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77(1):167–172. [DOI] [PubMed] [Google Scholar]
- 7. McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab. 2000;85(3):1206–1210. [DOI] [PubMed] [Google Scholar]
- 9. Pinilla L, Trimiño E, Garnelo P, Bellido C, Aguilar R, Gaytán F, Aguilar E. Changes in pituitary secretion during the early postnatal period and anovulatory syndrome induced by neonatal oestrogen or androgen in rats. J Reprod Fertil. 1993;97(1):13–20. [DOI] [PubMed] [Google Scholar]
- 10. Torchen LC, Idkowiak J, Fogel NR, O’Neil DM, Shackleton CH, Arlt W, Dunaif A. Evidence for increased 5α-reductase activity during early childhood in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(5):2069–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sir-Petermann T, Codner E, Maliqueo M, Echiburú B, Hitschfeld C, Crisosto N, Pérez-Bravo F, Recabarren SE, Cassorla F. Increased anti-Müllerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(8):3105–3109. [DOI] [PubMed] [Google Scholar]
- 12. Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(6):1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. [DOI] [PubMed] [Google Scholar]
- 15. Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(3):787–796. [DOI] [PubMed] [Google Scholar]
- 16. Anderson AD, Solorzano CM, McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32(03):202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55–59. [DOI] [PubMed] [Google Scholar]
- 18. Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JSE, Ong KK, Perry JRB. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6(1):8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A; Reproductive Medicine Network. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations [published correction appears in Nat Commun. 2016;7:10762].Nat Commun. 2015;6(1):7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1-2):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Legro RS, Kunselman AR, Stetter CM, Gnatuk CL, Estes SJ, Brindle E, Vesper HW, Botelho JC, Lee PA, Dodson WC. Normal pubertal development in daughters of women with PCOS: a controlled study. J Clin Endocrinol Metab. 2017;102(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR, Baker V, Usadi R, Seungdamrong A, Bates GW, Rosen RM, Haisonleder D, Krawetz SA, Barnhart K, Trussell JC, Jin Y, Santoro N, Eisenberg E, Zhang H; National Institute of Child Health and Human Development (NICHD) Reproductive Medicine Network. Assessment of multiple intrauterine gestations from ovarian stimulation (AMIGOS) trial: baseline characteristics. Fertil Steril. 2015;103(4):962–973.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 25. Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF III. Association of anti-Mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87(1):101–106. [DOI] [PubMed] [Google Scholar]
- 26. Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D. Elevated serum level of anti-Mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. [DOI] [PubMed] [Google Scholar]
- 27. Skałba P, Cygal A, Madej P, Dąbkowska-Huć A, Sikora J, Martirosian G, Romanik M, Olszanecka-Glinianowicz M. Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2011;158(2):254–259. [DOI] [PubMed] [Google Scholar]
- 28. Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Anti-Mullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296(2):E238–E243. [DOI] [PubMed] [Google Scholar]
- 29. Sopher AB, Grigoriev G, Laura D, Cameo T, Lerner JP, Chang RJ, McMahon DJ, Oberfield SE. Anti-Mullerian hormone may be a useful adjunct in the diagnosis of polycystic ovary syndrome in nonobese adolescents. J Pediatr Endocrinol Metab. 2014;27(11-12):1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK, Yding Andersen C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19(8):519–527. [DOI] [PubMed] [Google Scholar]
- 31. Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240–245. [DOI] [PubMed] [Google Scholar]
- 32. Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37(2):59–77. [DOI] [PubMed] [Google Scholar]
- 33. Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. [DOI] [PubMed] [Google Scholar]
- 34. Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau-Jonard S. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–3129. [DOI] [PubMed] [Google Scholar]
- 35. Hagen CP, Mouritsen A, Mieritz MG, Tinggaard J, Wohlfart-Veje C, Fallentin E, Brocks V, Sundberg K, Jensen LN, Anderson RA, Juul A, Main KM. Circulating AMH reflects ovarian morphology by magnetic resonance imaging and 3D ultrasound in 121 healthy girls. J Clin Endocrinol Metab. 2015;100(3):880–890. [DOI] [PubMed] [Google Scholar]
- 36. Crisosto N, Codner E, Maliqueo M, Echiburú B, Sánchez F, Cassorla F, Sir-Petermann T. Anti-Müllerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(7):2739–2743. [DOI] [PubMed] [Google Scholar]
- 37. Gorsic LK, Kosova G, Werstein B, Sisk R, Legro RS, Hayes MG, Teixeira JM, Dunaif A, Urbanek M. Pathogenic anti-Müllerian hormone variants in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(8):2862–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, Pigny P, Prescott M, Campbell R, Herbison AE, Prevot V, Giacobini P. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7(1):10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tata B, Mimouni NEH, Barbotin A-L, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ibáñez L, Ong KK, López-Bermejo A, Dunger DB, de Zegher F. Hyperinsulinaemic androgen excess in adolescent girls. Nat Rev Endocrinol. 2014;10(8):499–508. [DOI] [PubMed] [Google Scholar]
- 41. Bhide P, Gudi A, Shah A, Homburg R. Serum anti-Mullerian hormone levels across different ethnic groups: a cross-sectional study. BJOG. 2015;122(12):1625–1629. [DOI] [PubMed] [Google Scholar]
- 42. Elchuri SV, Patterson BC, Brown MR, Buchanan I, Mertens AC, Meacham LR. Anti-Mullerian hormone levels in American girls by age and race/ethnicity. J Pediatr Endocrinol Metab. 2015;28(1-2):189–193. [DOI] [PubMed] [Google Scholar]
- 43. Hannon TS, Arslanian SA. Differences in stimulated androgen levels in black and white obese adolescent females. J Pediatr Adolesc Gynecol. 2012;25(1):82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]