Abstract
Context
Accurate measures are needed for the prediction and diagnosis of type 1 diabetes (T1D) in at-risk persons.
Objective
The purpose of this study was to explore the value of continuous glucose monitoring (CGM) in predicting T1D onset.
Design and Setting
The Diabetes Autoimmunity Study in the Young (DAISY) prospectively follows children at increased risk for development of islet autoantibodies (islet autoantibody positive; Ab+) and T1D.
Participants
We analyzed 23 Ab+ participants with available longitudinal CGM data.
Main Outcome Measure
CGM metrics as glycemic predictors of progression to T1D.
Results
Of 23 Ab+ participants with a baseline CGM, 8 progressed to diabetes at a median age of 13.8 years during a median follow-up of 17.7 years (interquartile range, 14.6 to 22.0 years). Compared with nonprogressors, participants who progressed to diabetes had significantly increased baseline glycemic variability (SD, 29 vs 21 mg/dL; P = 0.047), daytime sensor average (122 vs 106 mg/dL; P = 0.02), and daytime sensor area under the curve (AUC, 470,370 vs 415,465; P = 0.047). They spent 24% of time at >140 mg/dL and 12% at >160 mg/dL compared with, respectively, 8% and 3% for nonprogressors (both P = 0.005). A receiver-operating characteristic curve analysis showed an AUC of 0.85 for percentage of time spent at >140 or 160 mg/dL. The cutoff of 18% time spent at >140 mg/dL had 75% sensitivity, 100% specificity, and a 100% positive predictive value for diabetes prediction, although these values could change because some nonprogressors may develop diabetes with longer follow-up.
Conclusions
Eighteen percent or greater CGM time spent at >140 mg/dL predicts progression to diabetes in Ab+ children.
In Ab+ children, CGM accurately predicted progression to clinical diabetes. .
Prospective studies, such as TrialNet, TEDDY (The Environmental Determinants of Diabetes in the Young), and DAISY (Diabetes Autoimmunity Study in the Young) have shown that children identified to be at risk through islet autoantibody testing and monitored with diabetes education rarely present with ketoacidosis (1–3). Islet autoantibody–positive (Ab+) persons benefit from timely recognition of the initial symptoms of diabetes and from a streamlined access to diabetes care. TrialNet and other studies (4, 5) have demonstrated a period of dysglycemia [i.e., impaired fasting glucose (100 to 125 mg/dL) or impaired glucose tolerance (IGT; 2-hour glucose 140 to 199 mg/dL)] that precedes type 1 diabetes (T1D) onset by several months or years. The ability to reliably identify dysglycemia and implement prevention or early treatment may help to preserve endogenous insulin secretion and prevent diabetes complications (6, 7). However, dysglycemia that precedes T1D poses diagnostic and therapeutic challenges (8–11).
Continuous glucose monitoring (CGM) reliably detects postprandial hyperglycemia, even when hemoglobin A1c (HbA1c) levels are within normal ranges (12). Importantly, CGM can detect dysglycemia before diagnosis of T1D in Ab+ children (13–15), although the number of participants in these previous studies was small.
In this study we analyzed various CGM metrics to define the best glycemic predictors of progression to diabetes in children known to be at high genetic risk and positive for islet autoantibodies. We found that in this population, ≥18% CGM time spent at >140 mg/dL predicts progression to clinical diabetes.
Material and Methods
Study population
Since 1993, DAISY has followed two cohorts of young children at increased risk of T1D, including siblings and offspring of patients with T1D and general population children with susceptibility HLA-DR/DQ genotypes identified through newborn screening. The details of screening and follow-up have been previously published (16). Autoantibodies to insulin, glutamic acid decarboxylase, IA-2, and ZnT8 were measured in the Immunogenetics Laboratory at the Barbara Davis Center, Aurora, Colorado, using previously described radiobinding assays (17). Since October 2011, DAISY offers CGM to all persons who have ever had multiple (≥2) positive results on testing for islet autoantibodies. All Ab+ DAISY participants who had at least one CGM were included in these analyses (n = 23). Participants had no acute illnesses at the time of CGM placement. Each participant and/or their parent provided informed consent. The Colorado Multiple Institutional Review Board approved all study protocols.
CGM
Participants were asked to complete a 5- to 7-day period of CGM. The Dexcom SEVEN Plus System (18) (before July 2014) or Dexcom G4 (starting 1 July 2014) was used (Dexcom Inc., San Diego, CA), and participants were instructed to avoid acetaminophen 1 day before and during the time of CGM. Participants could not see the real-time CGM reading but were given a One Touch Ultra (LifeScan Inc., Johnson & Johnson, Milpitas, CA) meter for blood glucose calibrations twice a day. For patient safety, the results were reviewed by a study physician (pediatric endocrinologist) at the end of monitoring.
Statistical analysis
Statistical analyses were performed by using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). The first 12 hours of CGM data were removed from the analyses. If >20% of data were missing on any given day, the data for that day were also excluded. Only CGM result with at least 96 hours of data were included. Measures of glycemic control included HbA1c (DCA2000, Siemens, Bayer Corp., Elkhart, IN), the overall mean of glucose values from blood glucose meter calibrations, overall sensor glucose (SG) values, percentage of time above various SG cutoffs, and area under the curve (AUC) of glucose calculated by the trapezoidal rule. Primary variables to characterize glycemic variability included glucose range, the overall SD, and the coefficient of variation. Receiver-operating characteristic (ROC) curves were generated to compare AUC of different CGM cutoffs for T1D prediction. We also performed ROC analyses for prediction of T1D onset using both baseline CGM and baseline HbA1c as predictors. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for diabetes prediction were calculated for different CGM cutoffs. A two-tailed P value with an α level for significance was set at 0.05.
From 69 longitudinal CGM data sets for those 23 participants, we tested whether different CGM cutoffs over time predicted risk for progression to T1D by using the joint longitudinal–survival model. Briefly, joint modeling accounts for potential correlation between the random subject effects of longitudinal measurements and the random subject effect in time-to-event data (19). The longitudinal model simultaneously considered all CGM data up to the end of follow-up, modeling it by using a linear line. A proportional hazards model with a piecewise constant baseline risk function was determined to be the best fit for the baseline hazard function. The longitudinal and survival models were linked by using a current value association structure (20). This allowed estimation of the correlation between CGM levels and risk for developing T1D. Although multiple additional computed measures have been proposed in this dynamically evolving analytical field, they do not appear to offer a particular advantage, and we limited the number of comparisons to minimize the chance of type I error.
Results
A total of 23 Ab+ participants with a baseline CGM were followed for development of T1D for a median of 25.6 months [interquartile range (IQR) 5.3 to 55.9 months], and 8 of these participants progressed to T1D at a median age of 13.8 years (IQR, 11.9 to 19.1 years). The characteristics of study participants at initial CGM visit are shown in Table 1. They were similar for Ab+ participants who progressed to T1D and those who did not progress to T1D, except for higher mean blood glucose levels (129 vs 104 mg/dL; P = 0.02) and a trend for higher HbA1c (5.8% vs 5.3%; P = 0.053) in progressors vs nonprogressors, respectively.
Table 1.
Demographic Characteristics of DAISY Participants at Initial CGM Visit
| Characteristic | Ab+ Progressors (n = 8) | Ab+ Nonprogressors (n = 15) | P Value |
|---|---|---|---|
| Mean age, y | 13.9 ± 3.8 | 16.6 ± 4.5 | 0.2 |
| Male, n (%) | 6 (75) | 5 (33) | 0.09 |
| White, n (%) | 8 (100) | 15 (100) | NA |
| HLA-DR3/4, n (%) | 2 (25) | 6 (40) | 0.70 |
| Mean missing CGM, % | 0.08 ± 0.05 | 0.06 ± 0.06 | 0.21 |
| Mean BMI z score | 0.00 ± 0.76 | 0.02 ± 0.84 | 0.97 |
| Mean HbA1c, % | 5.8 ± 0.4 | 5.3 ± 0.7 | 0.053 |
| Mean BG (glucometer), mg/dL | 129 ± 22 | 104 ± 10 | 0.02 |
| First-degree relatives with T1D, n (%) | 5 (62.5) | 6 (40) | 0.4 |
| Ab+ participants, single/multiple, n (%) | 0 (0)/8 (100) | 5 (33.33)/10 (66.67) | 0.12 |
| Impaired OGTT before any CGM, n (%) | 7 (87.5) | 2 (13.3) | 0.001 |
| Abnormal OGTT result before any CGM, n (%) | 3 (37.5) | 1 (6.67) | 0.10 |
Unless otherwise noted, values are the mean ± SD (continuous variables). Impaired and abnormal OGTT results are defined according to American Diabetes Association criteria.
Abbreviations: BG, blood glucose; BMI, body mass index; NA, not available.
Baseline CGM variables are summarized in Table 2. Compared with nonprogressors, participants who progressed to diabetes had significantly increased glycemic variability (SD, 29 vs 21 mg/dL; P = 0.047), daytime sensor average (122 vs 106 mg/dL; P = 0.02), and daytime sensor AUC (P = 0.047). The AUC during daytime, but not overall or during night, was greater in Ab+ progressors compared with nonprogressors. Ab+ progressors spent 24% of time at >140 mg/dL and 12% at >160 mg/dL compared with, respectively, 8% and 3% (both P = 0.005) in nonprogressors; time spent at >200 mg/dL was minimal and did not differ between groups (Table 2).
Table 2.
CGM Measures of Glycemic Control and Variability
| Variable | Ab+ Progressors | Ab+ Nonprogressors | P Value |
|---|---|---|---|
| Sensor glucose, mg/dL | 120 ± 16 | 106 ± 10 | 0.06 |
| Sensor daya glucose, mg/dL | 122 ± 16 | 106 ± 11 | 0.02 |
| Sensor night glucose, mg/dL | 113 ± 20 | 107 ± 14 | 0.36 |
| Maximum daya SG value, mg/dL | 218 ± 45 | 197 ± 53 | 0.30 |
| Maximum night SG value, mg/dL | 176 ± 35 | 160 ± 33 | 0.16 |
| SD, mg/dL | 29 ± 8 | 21 ± 7 | 0.047 |
| Coefficient of variation, mg/dL | 24 ± 6 | 19 ± 6 | 0.12 |
| Range, mg/dL | 160 ± 45 | 140 ± 57 | 0.38 |
| Time, % | |||
| At ≥120 mg/dL | 0.46 ± 0.22 | 0.24 ± 0.14 | 0.02 |
| At ≥140 mg/dL | 0.24 ± 0.17 | 0.08 ± 0.06 | 0.005 |
| At ≥160 mg/dL | 0.12 ± 0.11 | 0.03 ± 0.02 | 0.005 |
| At ≥180 mg/dL | 0.05 ± 0.06 | 0.01 ± 0.01 | 0.02 |
| At ≥200 mg/dL | 0.02 ± 0.04 | 0.01 ± 0.01 | 0.11 |
| AUC,b mg/min/dL | |||
| Overall | 610,255 ± 77,574 | 561,485 ± 50,699 | 0.13 |
| Daya | 470,370 ± 64,501 | 415,465 ± 38,474 | 0.047 |
| Night | 139,663 ± 26,207 | 145,734 ± 21,709 | 0.87 |
| Time to diagnosis/last visit (from initial CGM), y | 1.2 ± 1.3 | 3.0 ± 2.0 | 0.04 |
Values between 6 am and midnight.
Calculated by the trapezoidal rule.
ROC curves were generated to compare the AUCs of different baseline CGM cutoffs for T1D prediction. Performance of prediction by ROC analyses showed the highest AUC, of 0.85, for both percentage of time spent at >140 mg/dL and 160 mg/dL (Table 3). We also performed ROC analyses for prediction of T1D onset using both baseline CGM and baseline HbA1c as predictors. The model with CGM percentage of time at >140 mg/dL alone (AUC, 0.85) was very similar to the model with both HbA1c and CGM percentage of time at >140 mg/dL (AUC, 0.87), whereas HbA1c alone had a lower AUC, of 0.77; however, this was not statistically different (P = 0.15).
Table 3.
ROC Analyses for Prediction of T1D
| Percentage of Time | AUC (95% CI) | P Value |
|---|---|---|
| At >120 mg/dL | 0.80 (0.57–1.00) | 0.009 |
| At >140 mg/dL | 0.85 (0.62–1.00) | 0.003 |
| At >160 mg/dL | 0.85 (0.63–1.00) | 0.002 |
| At >180 mg/dL | 0.79 (0.58–1.00) | 0.008 |
| At >200 mg/dL | 0.70 (0.46–0.93) | 0.10 |
Sensitivity, specificity, PPV, and NPV for T1D prediction were calculated for different baseline CGM cutoffs. The cutoff of 18% time spent at >140 mg/dL had 100% specificity and 100% PPV with 75% sensitivity for diabetes prediction (Table 4). Among the 15 nonprogressors, 7 of the 55 CGM studies had percentage of time at >140 mg/dL exceeding 18%. Two participants had their third (last) CGM showing >18% of time at >140 mg/dL; in three other participants, one or two CGM studies (of the five to eight CGM studies they completed) showed >18% of time at >140 mg/dL (these abnormal CGM values were all completed in the middle of their follow-up— i.e., not the first or the last available CGM). Of note, some of the nonprogressors may likely become progressors with longer follow-up time.
Table 4.
Sensitivity, Specificity, PPV, and NPV for Different CGM Cutoffs
| Percentage of Time | Cutoff (%) | PPV | NPV | Sensitivity | Specificity |
|---|---|---|---|---|---|
| At >120 mg/dL | 36 | 0.714 | 0.812 | 0.625 | 0.867 |
| At >140 mg/dL | 18 | 1.000 | 0.883 | 0.750 | 1.000 |
| At >160 mg/dL | 6 | 0.833 | 0.824 | 0.625 | 0.933 |
| At >180 mg/dL | 2 | 0.714 | 0.813 | 0.625 | 0.867 |
| At >200 mg/dL | 0.2 | 0.546 | 0.813 | 0.750 | 0.667 |
In longitudinal analyses that included results of 69 CGMs from these 23 participants over a median follow-up of 18.5 months (IQR, 4.4 to 37.3 months), both percentage of time at >140 mg/dL and 160 mg/dL were significantly associated with risk for progression to T1D, with hazard ratios of 1.09 (95% CI, 1.02 to 1.17; P = 0.018) and 1.14 (95% CI, 1.02 to 1.27; P = 0.022), respectively. For 10% of time spent at >140 mg/dL and 160 mg/dL, the hazard ratios were 2.39 (95% CI, 1.16 to 4.90) and 3.68 (95% CI, 1.21 to 11.20), respectively. The other cutoffs had similar trends but did not reach statistical significance.
Longitudinal analyses showing CGM percentage of time at >140 mg/dL between progressors and nonprogressors over time are shown in Fig. 1. Time is shown as years after seroconversion (i.e., years after participants were confirmed to have persistent islet autoantibodies). The intrasubject coefficients of variation for CGM were 6.6% and 27.4% for nonprogressors and progressors, respectively.
Figure 1.
CGM percentage of time at >140 mg/dL over time between progressors and nonprogressors. Time is shown as years after seroconversion [i.e. years after participants were confirmed to have persistent islet autoantibodies (≥2 consecutive Ab+ visits)]. *A total of seven participants (three progressors and four nonprogressors) had CGM done only once at the time of these analyses.
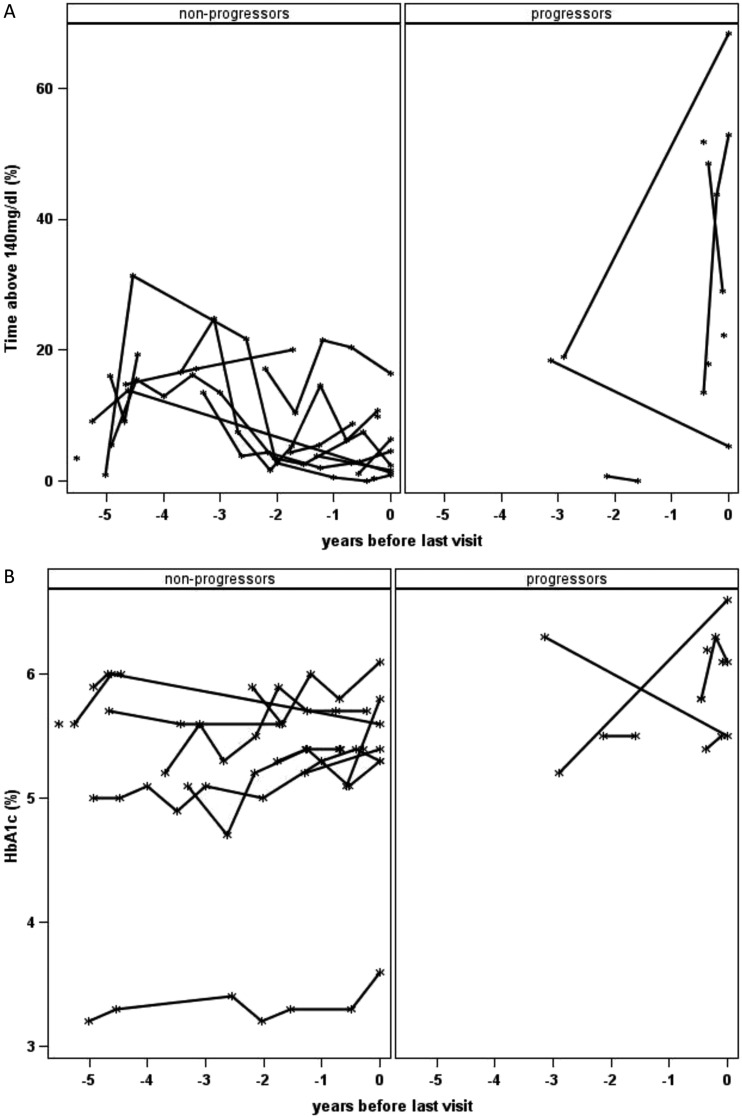
Figure 2 shows analyses of CGM percentage of time at >140 mg/dL (Fig. 2A) and HbA1c (Fig. 2B) between progressors and nonprogressors over time. Time is shown as years before last visit (i.e., years before last visit in DAISY for nonprogressors and years before T1D onset for progressors). Most participants (n=11) had two to three CGMs over time, and five participants had five or more CGMs over time. The median interval between CGMs was 0.53 years (IQR, 0.47 to 0.71 years). Unfortunately, because of the small sample size, we could not compare the prediction accuracy between overall HbA1c and CGM percentage of time at >140 mg/dL for progression to T1D.
Figure 2.
CGM percentage of time at >140 mg/dL (A) and HbA1c (B) over time between progressors and nonprogressors. Time is shown as years before last visit (i.e., years before last visit in DAISY for nonprogressors and years before T1D onset for progressors). Among progressors, the participant with <1% of time at >140 mg/dL on the graph was diagnosed 19 months after his last CGM, with an HbA1c of 6.9% (no CGM or OGTT was done close to diagnosis). Of note, the nonprogressor participant with a low HbA1c (∼3.5%) has a hemoglobinopathy. *A total of seven participants (three progressors and four nonprogressors) had CGM done only once at the time of these analyses, whereas six participants (three progressors and three nonprogressors) had only one HbA1c result at the time of these analyses.
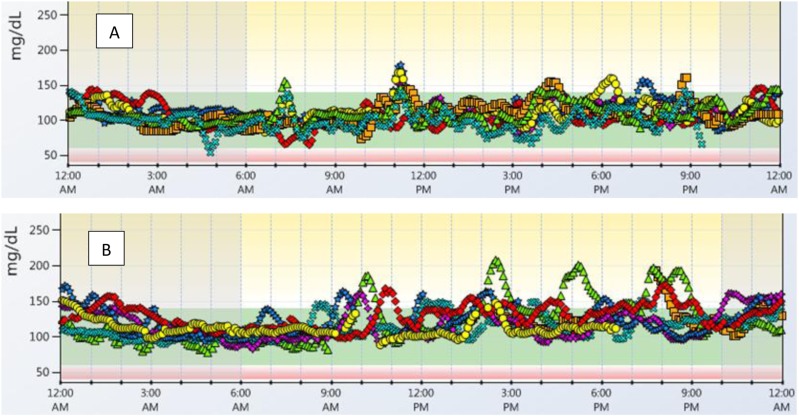
Some “typical” CGM profiles of DAISY participants with <10% of time at >140 mg/dL and >10% of time at >140 mg/dL are shown in Fig. 3.
Figure 3.
“Typical” CGM profiles of DAISY participants with <10% of time and >10% of time above 140 mg/dl. (A) CGM tracing with 4% of time at >140 mg/dL and 96% of time at target (60 to 140 mg/dL). (B) CGM tracing with 18% of time at >140 mg/dL and 82% of time at target (60 to 140 mg/dL). Each color/shape represents a different day.
Discussion
Only a few small studies have analyzed CGM data before diagnosis of T1D in Ab+ children (13–15). Our study analyzed longitudinal CGM data and defined best glycemic CGM predictors of progression to T1D in a group of participants at high risk for developing T1D. This study found that ≥18% of CGM time spent at >140 mg/dL predicts progression to clinical diabetes in Ab+ children with 100% specificity, a 100% PPV, and 75% sensitivity, and that both percentage of time at >140 mg/dL and 160 mg/dL were strongly associated with risk for progression to T1D. Of note, these values could change because some of the nonprogressors may likely develop diabetes with longer follow-up.
We have previously reported that CGM can detect early hyperglycemia in Ab+ children who are at risk for progression to diabetes, but we did not have enough participants to analyze which CGM variable(s) would be the best CGM-based predictor of progression to T1D (13). Other pilot studies to date include the Type 1 Diabetes Prediction and Prevention (DIPP) study, which analyzed CGM data from 10 asymptomatic children with multiple islet autoantibodies (cases) and 10 age- and sex-matched autoantibody-negative children (14). The authors found that the cases had higher mean values and higher variation in glucose levels during CGM compared with the controls, with time spent at ≥7.8 mmol/L of 5.8% in the cases compared with 0.4% in the controls (P = 0.04). Twenty-two Ab+ first-degree relatives of patients with T1D from the Belgian Diabetes Registry underwent CGM, a hyperglycemic clamp test, and an oral glucose tolerance test (OGTT) (15); two of these participants developed IGT and five progressed to T1D. The authors showed that CGM-derived glycemic variability measures and the glucose disposal rate, reflecting both insulin secretion and action, outperformed self-monitoring of blood glucose (SMBG) and first- or second-phase AUC C-peptide in identifying first-degree relatives with dysglycemia or diabetes. Although the number of participants in this study is still relatively small, we were able to perform ROC curves to compare AUC of different CGM cutoffs for T1D prediction and calculate sensitivity, specificity, PPV, and NPV for diabetes prediction; this analysis confirms that ≥18% of CGM time spent at >140 mg/dL predicts progression to clinical diabetes. Of note, Ab+ progressors had a higher frequency of impaired results on an OGTT as well as higher HbA1c and mean meter glucose values than Ab+ nonprogressors. In addition, this study analyzed longitudinal CGM data in Ab+ participants at risk for progression to T1D, showing that CGM can predict progression to T1D years before disease onset.
CGM shows the potential promise of an accurate, minimally invasive, nearly instantaneous measure to identify dysglycemia and diagnose diabetes in participants at risk for T1D. The OGTT, performed in clinical trials of diabetes prevention largely to formalize the diagnosis of clinical diabetes, is also valuable in predicting progression to T1D among participants with islet autoantibodies (4). Diabetes Prevention Trial-Type 1 (DPT-1) reported that a risk score based on age, body mass index, and OGTT indices (glucose and C-peptide values) accurately predicted diabetes risk over a short period in ICA-positive relatives (21, 22). The likelihood of progression to diabetes increased with mild fasting or after oral glucose load dysglycemia. Similarly, the combination of metabolic markers derived from OGTT improved accuracy in predicting progression to T1D in high-risk participants (23, 24). However, many families find the 2-hour OGTT too onerous. In addition, OGTTs represent a patient's response to an artificial sugar load, whereas CGM offers actual SG pattern for a patient over a few days in the real home environment. HbA1c is easier to measure but has limited sensitivity in children.
The DAISY study has demonstrated that Hb1Ac steadily increases within the normal range over a few years preceding diabetes onset and may therefore be a useful marker of progression to diabetes (5). Among children who have persistent islet autoimmunity, increase in HbA1c predicted increased risk for progression to T1D, with a hazard ratio of 4.8 for each 0.4% (SD) increase in HbA1c, independent of random glucose and number of autoantibodies. These findings have been confirmed by other studies (25). On the other hand, Vehik et al. (26) analyzed 1982 high-risk participants age <21 years from DPT-1, TrialNet, TEDDY, and Trial to Reduce IDDM in the Genetically at Risk (TRIGR) and found that, across the studies, HbA1c was only 8% to 42% sensitive and 64% to 95% specific in detecting IGT. Patients with T1D can have similar HbA1c but different glycemic variability on CGM, demonstrating the limitations of HbA1c as a sole measure of glycemic control.
Limitations of this study include a relatively small numbers of participants overall, as well as not enough participants completing both CGM and OGTT at the same time. Therefore, we were not able to directly compare the prediction accuracy of CGM vs OGTT. Because of the small sample size, we could not compare the prediction accuracy between overall HbA1c and CGM percentage of time at >140 mg/dL for progression to T1D. In addition, this study used both the Dexcom SEVEN Plus System (18) and the Dexcom G4 (available at the time of this study), which both require calibration and are not as accurate as current CGMs. We did not correct for multiple testing. These results should be confirmed in larger population of persons at risk for T1D with current CGM, such as Dexcom G6, offering the advantages of better accuracy (similar to SMBG) and factory calibration (i.e., avoiding calibration in participants not used to perform SMBG). As the accuracy of CGM devices improves with newly available devices (27, 28), CGM is likely to play an important role in both determining intermediate endpoints useful in the future design of T1D prevention clinical trials (29) as well as possible revisions to the current American Diabetes Association criteria for diagnosis of diabetes in children at high risk.
In conclusion, this study analyzed longitudinal CGM data and define best glycemic CGM predictors of progression to diabetes in a group of participants at high risk for developing T1D. Confirmation in larger prospective studies is needed to define CGM criteria useful for prediction and diagnosis of IGT and T1D in at-risk persons.
Acknowledgments
Financial Support: This research was supported by National Institutes of Health grants DK32493 (M.J.R.) and DK32083 (M.J.R.) and American Diabetes Association grant 1-14-CD-17 (A.K.S.).
Author Contributions: A.K.S. takes full responsibility for the contents of the article.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- Ab+
islet autoantibody positive
- AUC
area under the curve
- CGM
continuous glucose monitoring
- DAISY
Diabetes Autoimmunity Study in the Young
- DPT-1
Diabetes Prevention Trial-Type 1
- HbA1c
hemoglobin A1c
- IGT
impaired glucose tolerance
- IQR
interquartile range
- NPV
negative predictive value
- OGTT
oral glucose tolerance test
- PPV
positive predictive value
- ROC
receiver-operating characteristic curve
- SG
sensor glucose
- SMBG
self-monitoring of blood glucose
- T1D
type 1 diabetes
- TEDDY
The Environmental Determinants of Diabetes in the Young
References
- 1. Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M; DAISY study. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–1404. [DOI] [PubMed] [Google Scholar]
- 2. Triolo TM, Chase HP, Barker JM; DPT-1 Study Group. Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care. 2009;32(5):769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308–313. [DOI] [PubMed] [Google Scholar]
- 4. Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS; Diabetes Prevention Trial-Type 1 Study Group. Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2007;30(1):38–42. [DOI] [PubMed] [Google Scholar]
- 5. Stene LC, Barriga K, Hoffman M, Kean J, Klingensmith G, Norris JM, Erlich HA, Eisenbarth GS, Rewers M. Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes. 2006;7(5):247–253. [DOI] [PubMed] [Google Scholar]
- 6. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care. 2003;26(3):832–836. [DOI] [PubMed] [Google Scholar]
- 7. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care. 2017;40(9):1249–1255. [DOI] [PubMed] [Google Scholar]
- 8. Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonifacio E, Mathieu C, Nepom GT, Ziegler AG, Anhalt H, Haller MJ, Harrison LC, Hebrok M, Kushner JA, Norris JM, Peakman M, Powers AC, Todd JA, Atkinson MA. Rebranding asymptomatic type 1 diabetes: the case for autoimmune beta cell disorder as a pathological and diagnostic entity. Diabetologia. 2017;60(1):35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knip M, Selvenius J, Siljander H, Veijola R. Reclassification of asymptomatic beta cell autoimmunity: a critical perspective. Diabetologia. 2017;60(1):39–42. [DOI] [PubMed] [Google Scholar]
- 11. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017;66(2):241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klonoff DC, Buckingham B, Christiansen JS, Montori VM, Tamborlane WV, Vigersky RA, Wolpert H; Endocrine Society. Continuous glucose monitoring: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(10):2968–2979. [DOI] [PubMed] [Google Scholar]
- 13. Steck AK, Dong F, Taki I, Hoffman M, Klingensmith GJ, Rewers MJ. Early hyperglycemia detected by continuous glucose monitoring in children at risk for type 1 diabetes. Diabetes Care. 2014;37(7):2031–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helminen O, Pokka T, Tossavainen P, Ilonen J, Knip M, Veijola R. Continuous glucose monitoring and HbA1c in the evaluation of glucose metabolism in children at high risk for type 1 diabetes mellitus. Diabetes Res Clin Pract. 2016;120:89–96. [DOI] [PubMed] [Google Scholar]
- 15. Van Dalem A, Demeester S, Balti EV, Decochez K, Weets I, Vandemeulebroucke E, Van de Velde U, Walgraeve A, Seret N, De Block C, Ruige J, Gillard P, Keymeulen B, Pipeleers DG, Gorus FK; Belgian Diabetes Registry. Relationship between glycaemic variability and hyperglycaemic clamp-derived functional variables in (impending) type 1 diabetes. Diabetologia. 2015;58(12):2753–2764. [DOI] [PubMed] [Google Scholar]
- 16. Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS Jr, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich HA. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia. 1996;39(7):807–812. [DOI] [PubMed] [Google Scholar]
- 17. Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81(12):4264–4267. [DOI] [PubMed] [Google Scholar]
- 18. Garg SK, Smith J, Beatson C, Lopez-Baca B, Voelmle M, Gottlieb PA. Comparison of accuracy and safety of the SEVEN and the Navigator continuous glucose monitoring systems. Diabetes Technol Ther. 2009;11(2):65–72. [DOI] [PubMed] [Google Scholar]
- 19. Asar Ö, Ritchie J, Kalra PA, Diggle PJ. Joint modelling of repeated measurement and time-to-event data: an introductory tutorial. Int J Epidemiol. 2015;44(1):334–344. [DOI] [PubMed] [Google Scholar]
- 20. Mauff K, Steyerberg EW, Nijpels G, van der Heijden AAWA, Rizopoulos D. Extension of the association structure in joint models to include weighted cumulative effects. Stat Med. 2017;36(23):3746–3759. [DOI] [PubMed] [Google Scholar]
- 21. Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, Cuthbertson D, Lachin JM, Skyler JS; Diabetes Prevention Trial-Type 1 Study Group. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31(3):528–533. [DOI] [PubMed] [Google Scholar]
- 22. Sosenko JM, Skyler JS, Mahon J, Krischer JP, Beam CA, Boulware DC, Greenbaum CJ, Rafkin LE, Cowie C, Cuthbertson D, Palmer JP;. Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group. The application of the diabetes prevention trial-type 1 risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care. 2012;35(7):1552–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu P, Beam CA, Cuthbertson D, Sosenko JM, Skyler JS, Krischer JP; DPT-1 Study Group. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care. 2012;35(10):1975–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steck AK, Dong F, Frohnert BI, Waugh K, Hoffman M, Norris JM, Rewers MJ. Predicting progression to diabetes in islet autoantibody positive children. J Autoimmun. 2018;90:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helminen O, Aspholm S, Pokka T, Hautakangas MR, Haatanen N, Lempainen J, Ilonen J, Simell O, Knip M, Veijola R. HbA1c predicts time to diagnosis of type 1 diabetes in children at risk. Diabetes. 2015;64(5):1719–1727. [DOI] [PubMed] [Google Scholar]
- 26. Vehik K, Cuthbertson D, Boulware D, Beam CA, Rodriguez H, Legault L, Hyytinen M, Rewers J, Schatz DA, Krischer JP Performance of HbA1c as an early diagnostic indicator of type 1 diabetes in children and youth. Diabetes Care 2012;35(9):1821–1825. [DOI] [PMC free article] [PubMed]
- 27. Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther. 2018;20(6):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krischer JP; Type 1 Diabetes TrialNet Study Group. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia. 2013;56(9):1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]