Abstract
Background:
Plasma-based cell-free DNA is an attractive biospecimen for assessing somatic mutations due to minimally-invasive real-time sampling. However, next generation sequencing (NGS) of cell-free DNA (cfDNA) may not be appropriate for all patients with advanced prostate cancer (PC).
Methods:
Blood was obtained from advanced PC patients for plasma-based sequencing. UW-OncoPlex, a ~2Mb multi-gene NGS panel performed in the CLIA/CAP environment, was optimized for detecting cfDNA mutations. Tumor tissue and germline samples were sequenced for comparative analyses. Multivariate logistic regression was performed to determine the clinical characteristic associated with the successful detection of somatic cfDNA alterations (i.e. detection of at least one clearly somatic PC mutation).
Results:
Plasma for cfDNA sequencing was obtained from 93 PC patients along with tumor tissue (N=67) and germline (N=93) controls. We included data from 76 patients (72 prostate adenocarcinoma; 4 variant histology PC) in the analysis. Somatic DNA aberrations were detected in 34 cfDNA samples from patients with prostate adenocarcinoma. High PSA level, high tumor volume, and castration-resistance were significantly associated with successful detection of somatic cfDNA alterations. Among samples with somatic mutations detected, the cfDNA assay detected 93/102 (91%) alterations found in tumor tissue, yielding a clustering-corrected sensitivity of 92% (95% confidence interval 88%–97%). All germline pathogenic variants present in lymphocyte DNA were also detected in cfDNA (N=12). Somatic mutations from cfDNA were detected in 30/33 (93%) instances when PSA was >10ng/ml.
Conclusions:
Disease burden, including a PSA >10ng/ml, is strongly associated with detecting somatic mutations from cfDNA specimens.
Keywords: cell-free DNA, castration-resistant prostate cancer, next-generation sequencing, precision medicine
Introduction
In order to develop the next generation of targeted prostate cancer therapies, it is essential that we understand the molecular events driving resistance to current therapies. Precision medicine has offered the promise that through understanding these molecular events, we may be able to tailor treatments accordingly.1 We first need to reliably identify and understand the key oncogenic drivers leading to progressive disease. Efforts to this end are already underway, with multiple large-scale efforts aimed at obtaining serial metastatic biopsies in patients with advanced castration-resistant prostate cancer (CRPC).2,3 While these efforts will undoubtedly produce a wealth of important data, the implementation of widespread metastatic biopsy protocols are fraught with difficulty. Biopsies are potentially morbid and costly, and in diseases that frequently involve skeletal spread (e.g. metastatic CRPC), the yield of bone biopsies is often disappointingly low. In addition, biopsy is typically directed at a single metastatic site, therefore not addressing the important issue of clonal heterogeneity.4
Recent advances in next-generation sequencing (NGS) technology, as well as the ability to capture circulating tumor cells (CTCs), have made it possible to glean valuable ‘omics’ data from simple blood draws. These so-called liquid tumor biopsies are relatively non-invasive and potentially allow for real-time assessment of the evolving landscape of molecular aberrations within subpopulations of tumor cells.5 A number of studies in men with advanced prostate cancer have shown that cell-free DNA (cfDNA) sequencing allows for the detection of somatic alterations, with results that are highly concordant with contemporary tumor tissue samples and thus providing valuable insights into mechanisms of response/resistance to available therapies.6–12 While there are still questions surrounding the clinical validity, reproducibility, and utility of many cfDNA-based assays, it is likely that blood draws will soon complement or even replace metastatic biopsies in a number of important settings – becoming a cornerstone in our ability to characterize an individual’s cancer and allowing us to effectively deploy precision medicine.13,14
While several groups have successfully used ultra-sensitive cfDNA NGS technology to interrogate genes known to be relevant to prostate cancer biology, this approach has several limitations, including: i) the use of limited gene panels (i.e. ~50–75 genes tested); and ii) high cost associated with ultra-sensitive NGS approaches. In addition, cfDNA NGS workflows require patient plasma samples to be processed in specialized labs in order to determine if the quantity of cfDNA is sufficient to yield reliable NGS results – potentially delaying genetic testing by days to weeks in patient who would be better served by tumor tissue sequencing. To overcome these issues, we utilized a more comprehensive (≥250 genes) NGS panel, UW-OncoPlex, which was modified for low-input DNA samples (i.e. cfDNA) while employing lower-cost standard ~500x coverage.6,7,15–19 We sought to determine if clinical characteristics could be used to define the patient population with a high probability for detecting somatic alterations from plasma samples, thereby allowing us to determine which patients to triage toward cfDNA vs. tumor tissue sequencing.
Materials and Methods
Patients
Patients with advanced prostate cancer (i.e. metastatic disease or rising PSA following definitive local therapy) were identified and recruited by their treating medical oncologist at the Seattle Cancer Care Alliance or University of Washington Medical Center (both in Seattle, WA). All subjects provided written informed consent prior to enrolling on this study. This study was performed in accordance with the declaration of Helsinki guidelines with approval at the University of Washington/Fred Hutchinson Cancer Consortium institutional review board.
Sequencing methods
All DNA extraction, sequencing and bioinformatics analyses were performed within the CLIA-certified CAP-accredited environment according to clinical protocols. Blood samples (2 × 10mL) were collected in Streck BCT tubes (STRECK, Omaha NE), and processed as per manufacturer’s instructions. Briefly, the tubes were centrifuged at low speed, the supernatant was transferred to another tube (being careful to not disturb the buffy coat), and supernatant was centrifuged again at high speed. The plasma supernatant (4mL) containing the ctDNA was extracted using the QIAsymphony DSP Circulating DNA Kit (cat no. 937556, Qiagen, Germantown, MD). After extraction, DNA was quantitated using the Agilent Tape Station (Agilent Technologies, Inc, Santa Clara, CA) to confirm concentration and presence of low molecular weight DNA.
cfDNA samples were not sheared prior to library prep due to inherently being in the desired size range and were processed using the Kapa HyperPrep kit (KK8504, Roche Sequencing, Pleasanton, CA) followed by hybridization with the UW-OncoPlex target baits, as previously described.19 The corresponding tumor or germline DNA samples (for each cfDNA) was processed using the same library prep protocol, except the initial shearing step was included. Resulting libraries were loaded on an Illumina sequencer, either the HiSeq 2500 or the NextSeq500 depending on number of samples in a library pool. Data were generated and preprocessed by the University of Washington NGS Laboratory and Analytics group.
Endpoint definitions
Cell-free DNA sequencing was designated as “successful” if at least one mutation that was clearly somatic was detected (i.e. not present in germline DNA or previously observed in somatic DNA from tumor material). If plasma DNA elution failed, that sample was excluded from this analysis. If no somatic mutations were detected in cfDNA and tumor tissue sequencing was unavailable or unsuccessful, the success of cfDNA sequencing was designated as “indeterminate”. In cases where both tumor tissue and cfDNA sequencing studies were negative (i.e. no somatic mutations detected), results were excluded from the analysis. This decision was made on the basis that: i) prior studies have shown that the majority of tumors from advanced prostate cancer patients possess somatic mutations and false negative tissue sequencing could not be ruled out; and ii) we could not confidently categorize the cfDNA sequencing as failed given the lack of somatic mutations detected in tumor tissue.20
Clinical data elements were abstracted from the clinical charts. The reported clinical, lab, and imaging parameters were those closest in time to when the plasma sample for cfDNA sequencing was drawn. High volume disease was defined as described in the CHAARTED clinical trial (i.e. visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis).21 PSA doubling time (PSAdt) was calculated as the log of 2 divided by the slope of the linear regression line of the log PSA vs. time (in months). A minimum of three PSA values were required to calculate PSAdt.
Statistical methods
Statistical analyses correlating clinical variables with successful sequencing status excluded prostate cancers displaying variant histology (e.g. small cell/neuroendocrine cases), as PSA and other clinical variables are less informative in these cases. Differences in clinical characteristics of samples from adenocarcinoma patients stratified by successful detection of somatic mutations in cfDNA were evaluated using Fisher’s exact tests for categorical characteristics or the Kruskal-Wallis test for continuous characteristics. Prognostic potential of clinical characteristics for successful sequencing were evaluated by multivariate logistic regression. Continuous variables were log-transformed to reduce skewness. Weakly informative Bayesian priors were used to account for numerical instability due to all metastatic prostate cancer patients having successful sequencing and near perfect discrimination of the PSA level.22 Because PSAdt could only be evaluated for a minority of patients, separate models were fit with and without this variable. Sensitivity of cfDNA sequencing to detect somatic mutations detected in tumor tissue was calculated using weighted estimation to account for clustered responses23. P-values <0.05 were considered statistically significant. Statistical analyses were performed using R packages arm (version 1.9–3) and effects (version 3.1–2).
Results
Blood was obtained for plasma cfDNA sequencing from 93 prostate cancer patients. Data from 76 patients (72 prostatic adenocarcinoma; 4 variant histology) were included in this analysis, with three patients having sequencing performed at multiple time points, including one patient with three blood draws (median time between draws: 5 months, range: 1 to 8 months) (Figure S1). Matched germline controls were available for all prostatic adenocarcinoma patients and tumor sequencing was available for 67 patients (median time between tissue and blood acquisitions: 6 months, range: −2.6 to 13.5 years). Average depth of sequencing in cfDNA and tumor tissue was 480x.
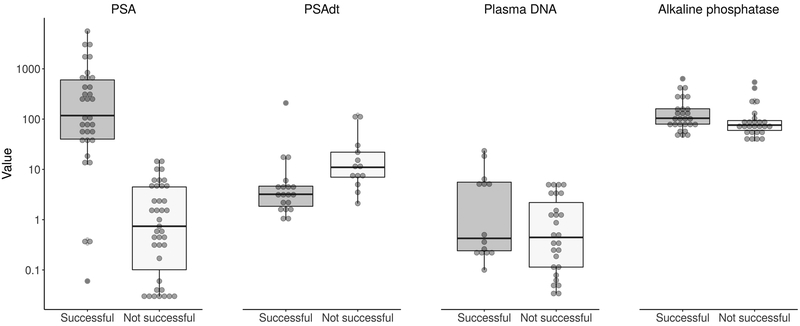
The clinical characteristics for the 72 patients (76 sequencing runs) with prostatic adenocarcinoma in whom it was possible to determine if cfDNA sequencing successfully detected somatic mutations are shown in Table 1. The success of cfDNA sequencing to detect somatic alterations was significantly associated with higher PSA level (P<0.001), shorter PSAdt (P=0.003) and higher baseline alkaline phosphatase level (P=0.036) (Figure 1). Overall, cfDNA sequencing successfully detected prostate cancer mutations in 30/33 (91%) instances when PSA was >10 ng/mL and 27/27 (100%) instances when PSA was >20 ng/mL. Patients with CRPC, metastatic disease at the time of blood draw, and high-volume disease as defined in the CHAARTED clinical trial were also more likely to have somatic alterations detected from cfDNA (all P<0.001) (Figure 2).21 Of the 4 patients with variant histology (small cell [N=3]; squamous cell [N=1]), only one subject with small cell histology failed to have somatic mutations detected in cfDNA.
Table 1.
Clinical characteristics of samples from adenocarcinoma patients stratified by success of cfDNA sequencing (i.e. detection of somatic alterations in plasma).
| Successful | Not successful | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | P* | ||
| Total samples | 34 | 100 | 42 | 100 | ||
| CRPC | No | 5 | 15 | 27 | 66 | <0.001 |
| Yes | 28 | 85 | 14 | 34 | ||
| Mets | None | 0 | 0 | 17 | 42 | <0.001 |
| Any | 34 | 100 | 24 | 59 | ||
| Lymph node† | 21 | 66 | 14 | 61 | 0.032 | |
| Bone† | 32 | 97 | 18 | 75 | <0.001 | |
| Lung† | 6 | 19 | 4 | 17 | 0.330 | |
| Liver† | 7 | 22 | 1 | 4 | 0.019 | |
| High volume† | 26 | 81 | 9 | 38 | <0.001 | |
| Gleason score | 6–7 | 9 | 30 | 8 | 33 | 1.0 |
| 8–10 | 21 | 70 | 16 | 67 | ||
| N | Median, Min-Max | N | Median, Min-Max | P* | ||
| PSA, ng/mL | 33 | 117.5, 0.1–5612.4 | 39 | 0.7, 0.0–15.2 | <0.001 | |
| PSAdt, months | 19 | 3.2, 1.0–209.0 | 13 | 11.0, 2.1–124.0 | 0.003 | |
| Alkaline phosphatase, U/L | 27 | 104, 43–635 | 26 | 76, 36–542 | 0.036 | |
| Plasma DNA, ng/μL | 14 | 0.4, 0.1–23.4 | 27 | 0.4, 0.0–5.2 | 0.174 | |
P-values for categorical characteristics are from Fisher’s exact tests and for continuous characteristics are from Kruskal-Wallis tests.
Among patients with any metastases before ctDNA blood draw.
Summary statistics exclude unknowns
Figure 1.
Boxplots of continuous characteristics by successful cfDNA sequencing status (i.e. detection of somatic alterations in plasma).
Figure 2.

Barplots of categorical characteristics by successful sequencing status (i.e. detection of somatic alterations in plasma). Gleason score is excluded given that this was not significant on univariate analysis. Note: Dark bars indicate percentage of patients with the given clinical characteristic.
Multivariate logistic regression demonstrated that PSA level prior to blood draw, CRPC, and high-volume disease burden were significantly associated with successfully detecting somatic mutations from cfDNA (Table 2). Note that PSAdt was omitted from the multivariate regression model because many (N=44) patients were missing data. Predictions for successfully detecting somatic mutations from cfDNA were unchanged when adjustment was also made for Gleason sum and alkaline phosphatase (P=0.67 and 0.65, respectively) but we present results without these adjustments because missing values for these measurements would reduce the total number of samples used to inform the model. Table S1 of the Supplemental Materials shows results among patients with evaluable PSAdt. While patients with hormone-sensitive, low-volume disease only had somatic mutations detected in plasma samples when PSA levels were high, most patients with castration-resistant or high-volume disease had detectable mutations in cfDNA samples regardless of PSA level. Figure 3 visualizes predicted probabilities of detecting somatic mutations in cfDNA using the fitted model but excluding metastatic prostate cancer because it was not significant. Figure S2 in the Supplemental Materials shows that the fitted model has reasonable discrimination and calibration.
Table 2.
Estimated multivariate logistic regression to predict detection of somatic alterations through cfDNA sequencing (N=69).
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Intercept | 0.0 | 0.0–0.3 | 0.007 |
| Log PSA | 2.0 | 1.4–2.9 | <0.001 |
| Castration-resistant PC | 6.5 | 1.0–41.5 | 0.050 |
| Metastatic PC | 2.7 | 0.1–69.9 | 0.550 |
| High volume PC | 6.3 | 1.3–31.9 | 0.026 |
Figure 3.

Predicted probability of successful sequencing (i.e. detection of somatic alterations in plasma) as determined using the multivariate logistic regression model excluding patients with confirmed metastases before the blood draw. Shaded areas indicate 95% confidence limits.
We evaluated the performance of plasma sequencing to identify somatic mutations in both cfDNA and tumor tissue (Figure 4 and Table S2). Mutations (rows) are sorted alphabetically and patient samples (columns) are sorted from high to low PSA. Patients with variant histologies or clinical features concerning for aggressive-variant prostate cancer (e.g. predominately visceral metastases, low PSA level compared to disease burden, short interval to developing CRPC) are presented on the right side of the figure.24 Disease burden (i.e. high- vs. low-volume) and whether the study was deemed successful are provided across the top of the figure. Detectable mutations include not only point mutations, but also copy number variations and genomic rearrangements (Figure S3).
Figure 4.
Identification of genomic alterations by cfDNA and tumor tissue sequencing. Each column represents an individual patient sample. Rows are each gene/type of mutation detected. Samples are stratified along the x-axis by PSA value. Note: four duplicate samples with unsuccessful sequencing are not presented in this figure. Excluded samples were from the same patient in whom Samples 54 (excluded sample PSA: 13.8 ng/ml and 1.7 ng/ml), 73 (excluded sample PSA: 15.2 ng/ml) and 78 (excluded sample PSA: 1.55) were obtained. Aggressive-variant (AV) prostate cancers were defined based on previously described clinical features (e.g. predominately visceral metastases, low PSA level compared to disease burden, short interval to developing CRPC).24
Overall, the cfDNA assay detected 93 of 102 (91%) somatic alterations detected in tumor tissue. Accounting for clustering of alterations within patients, sensitivity of the cfDNA assay for detecting prostate cancer mutations was 92% (95% confidence interval [CI] 88%–97%) and was highly consistent across patient strata (e.g. patients with high PSA levels). All germline variants were detected from cfDNA (N=12 pathogenic variants detected).
Discussion:
In our series of advanced prostate cancer patients, we have shown PSA level and/or tumor burden is highly associated with the ability to detect somatic alterations from plasma. Consequently, these widely available clinical parameters allow the treating physician to triage patients toward cell free vs. tumor tissue based sequencing – avoiding delays in determining eligibility for precision oncology treatment approaches (e.g. checkpoint blockade in patients with mismatch repair deficiency or PARP inhibitors in those with homologous recombination deficiency). In addition, we find that using a standard depth of coverage NGS panel produces highly similar results to tumor tissue sequencing in these carefully selected patients. Additional advantages of this approach, include: 1) minimizing costs by using standard depth of coverage (~500x), as opposed to ultra-sensitive methodology; 2) reliably determining pathogenic germline through plasma sequencing; and 3) utilizing a large set of genes (≥250) to provide a more comprehensive overview of the neoplastic genetic landscape in men with advanced prostate cancer. Based on this analysis, we now offer plasma-based sequencing to advanced prostate cancer patients with evidence of higher tumor burden and more aggressive disease.
We found that patient selection is critical to detecting somatic mutations in plasma. In our cohort of 72 patients with advanced prostatic adenocarcinoma, we were able to successfully detect somatic alterations in 34 cfDNA samples. This proportion was higher in patients with castration-resistant prostate cancer (CRPC), high-volume disease burden, and/or high PSA level. It is also worth noting that the majority of patients with clinically aggressive-variant or variant prostate cancer histologies (i.e. small cell, squamous cell) also had somatic mutations successfully detected in plasma. These findings are consistent with other studies that have found that, compared to patients with localized-disease, more advanced cancers are associated with increased sensitivity for detecting mutations in plasma samples.25,26 This suggests that had more stringent patient selection requirements been used (e.g. high PSA, high-volume disease), a larger proportion of patients may have had mutations detected in cfDNA. Of note, while prior studies have shown that clinical features of prostate cancer patients correlate with cfDNA fraction; this is the first study to correlate somatic NGS results with clinical features using a multivariate regression model.11 Furthermore, this is the first study to provide concrete guidelines utilizing widely available clinical parameters as a basis for who to offer plasma-based sequencing to.
An important finding from this study was that our NGS bioinformatics pipeline was able to accurately identify copy number variations. Overall, we detected 51 copy number changes in tumor tissue, of which 27 (53%) were also found in cfDNA. When limited to cases with a PSA >10 ng/mL, the proportion of patients with detectable copy number changes increases to 9/13 (69%). Because copy number variations (e.g. AR amplification, PTEN loss) represent some of the most frequent alterations found in prostate cancer, the ability to make these calls is of paramount importance.20 This is highlighted by the fact that AR amplification, loss of homologous recombination genes (e.g. BRCA1/2, ATM), and PTEN loss are all being developed as candidate predictive biomarkers for several targeted prostate cancer drugs.27–30 As such, non-invasive means to detect these alterations are essential to wide deployment of these drug development strategies. Moreover, because our method does not require specialized bioinformatics algorithms to make these calls, it can theoretically interface with other already established sequencing pipelines. Prospective therapeutic trials incorporating cfDNA NGS with UW-OncoPlex should be designed to formally evaluate its clinical utility.
The reproducibility of NGS assays across platforms has only been evaluated by a handful of studies, and the performance characteristics of most cfDNA NGS assays remains to be rigorously evaluated.13,14,31,32 Highlighting this deficiency, a previous study showed low rates of congruence between the Guardant360 (Guardant Health, Inc) and PlasmaSELECT (Personal Genome Diagnostics, Inc) NGS cfDNA assays.13 In their study, two plasma samples were obtained at the same timepoint and sent to each company for analysis. Out of 40 patients, only nine had at least partial congruence in sequencing results. Interestingly, both assays utilize ultra-sensitive NGS methods, with an average depth of coverage ≥8000x. While this would in theory increase sensitivity, it may be at the expense of a high clinical false-positive rate. It seems plausible that these assays are detecting low-level clones in plasma that, while analytically real, represent either dead-end passenger mutations or somatic mutations from other non-prostate cancer sources (e.g. age-related hematopoietic clones).16–18 If we assume that only concordant alterations represent true-positive mutational events, then a similar trend is observed whereby cases with a high PSA level, presumably reflecting a high tumor burden, are associated with an increased likelihood of successful sequencing. In this case, 3/4 (75%) cases with PSA >10 ng/mL had a positive sequencing test, whereas only 6/21 (29%; P=0.116, Fisher’s exact) with PSA ≤10 ng/mL were positive. An approach using PSA concentration as a metric for tumor burden selects for those patients with sufficient cfDNA for the detection of meaningful genomic alterations (e.g. copy number alterations, pathogenic non-synonymous mutations) using standard depth sequencing, while avoiding clinical false-positives seen in some commercial assays. Just as with traditional tissue biopsies, it is critical that an adequate diagnostic specimen be tested to obtain meaningful results.
A limitation of this study is that tumor tissue was not obtained contemporaneously with all plasma samples. As such, we are not able to definitively determine if alterations found in plasma only represent false-positives or new resistance mutations (e.g. AR amplification, AR p.L702H). However, by including these cases, the calculated sensitivity of UW-OncoPlex for detecting somatic alterations in cfDNA represents a conservative estimate of the true sensitivity of this assay. Supporting this is the observation that sensitivity is higher (analysis not shown) when sequencing is performed on blood drawn within 9 months of tumor tissue collection – likely decreasing the influence of evolving resistance mutations over time. These findings are consistent with those reported by Higgins and colleagues, who found that the concordance between cfDNA and tumor tissue sequencing was higher when plasma samples were obtained contemporaneously with tumor tissue.33 Importantly, many of the key genes being developed as biomarkers for precision oncology studies (e.g. homologous recombination genes) are typically found in primary prostate cancer samples.34 Future studies obtaining metastatic tissue concurrently with plasma for sequencing studies should be performed to confirm this assumption.
In conclusion, we have optimized a panel-based test assessing the status of both germline and somatic oncogenic events for use with minimal DNA input, and have successfully applied it to sequence prostate cancer patient cfDNA samples. Additional work is needed to further refine these approaches so that mutations can be determined from samples provided by patients with lower disease burden, but at this time confidence can be placed in any somatic alterations detected using this method. Importantly, we have found that cfDNA sequencing reliably reflects alterations found in tumor tissue from prostate cancer patients with higher tumor burden (i.e. PSA >10 ng/ml) and more aggressive disease – a finding we expect will extrapolate to other cfDNA NGS platforms – and should be considered as an alternative to tumor tissue sequencing in select patients.
Supplementary Material
Acknowledgements:
We thank the University of Washington Genetics and Solid Tumors Laboratory and NGS analytics Laboratory for help with DNA sequencing and data processing. We thank Christina Smith and Moon Chung for assistance with sample intake and DNA preparation.
Funding: This research was supported by the UW/FHCRC Institute for Prostate Cancer Research (IPCR); FHCRC Solid Tumor Translational Research Program; National Cancer Institute grants P30 CA015704, R50 CA221836 (RG) and R01 CA181605 (PSN); the Pacific Northwest Prostate Cancer SPORE CA097186; Prostate Cancer Foundation Young Investigator Awards (CCP, HHC, MTS); CDMRP Awards W81XWH-16–1-0484 (MTS), PC170510 and PC170503P2 (CCP), W81XWH-17–1-0380 (NDS) and W81XWH-15–1-0430 (PSN).
Disclosures: MTS is a paid consultant/advisor to Janssen and has received research funding to his institution from Janssen, AstraZeneca, Zenith, Pfizer, and Hoffmann-La Roche. HHC has received research funding to her institution from Clovis, Janssen and Inovio.
Footnotes
This is the peer reviewed version of the following article: Schweizer et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. The Prostate 2019 79;7:701–708, which has been published in final form at https://doi.org/10.1002/pros.23778.This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Use of Self-Archived Versions.
References:
- 1.Collins FS, Varmus H. A new initiative on precision medicine. The New England journal of medicine. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korpal M, Korn JM, Gao X, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer discovery. 2013;3(9):1030–1043. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery RB, Antonarakis ES, Hussain M, et al. A phase 1/2 open-label study of safety and antitumor activity of EPI-506, a novel AR N-terminal domain inhibitor, in men with metastatic castration-resistant prostate cancer (mCRPC) with progression after enzalutamide or abiraterone Paper presented at: American Society of Clinical Oncology Annual Meeting2015; Chicago, IL. [Google Scholar]
- 4.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366(10):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends in molecular medicine. 2010;16(9):398–406. [DOI] [PubMed] [Google Scholar]
- 6.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Science translational medicine. 2015;7(312):312re310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyatt AW, Azad AA, Volik SV, et al. Genomic Alterations in Cell-Free DNA and Enzalutamide Resistance in Castration-Resistant Prostate Cancer. JAMA oncology. 2016;2(12):1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodall J, Mateo J, Yuan W, et al. Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer discovery. 2017;7(9):1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quigley D, Alumkal JJ, Wyatt AW, et al. Analysis of Circulating Cell-Free DNA Identifies Multiclonal Heterogeneity of BRCA2 Reversion Mutations Associated with Resistance to PARP Inhibitors. Cancer discovery. 2017;7(9):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conteduca V, Wetterskog D, Sharabiani MTA, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Annals of oncology:official journal of the European Society for Medical Oncology /ESMO. 2017;28(7):1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer discovery. 2018. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt AW, Annala M, Aggarwal R, et al. Concordance of Circulating Tumor DNA and Matched Metastatic Tissue Biopsy in Prostate Cancer. Journal of the National Cancer Institute. 2017;109(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torga G, Pienta KJ. Patient-Paired Sample Congruence Between 2 Commercial Liquid Biopsy Tests. JAMA oncology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merker JD, Oxnard GR, Compton C, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review . Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2018:Jco2017768671. [DOI] [PubMed] [Google Scholar]
- 15.Annala M, Struss WJ, Warner EW, et al. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair-deficient Prostate Cancer. European urology. 2017;72(1):34–42. [DOI] [PubMed] [Google Scholar]
- 16.Lanman RB, Mortimer SA, Zill OA, et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PloS one. 2015;10(10):e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Personal Genome Diagnostics I. PlasmaSELECT-R. 2018; http://www.personalgenome.com/wp-content/uploads/2015/05/PGDx_PlasmaSelect-R_Product_Information.pdf.
- 18.Webb S The cancer bloodhounds. Nature biotechnology. 2016;34(11):1090–1094. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. The Journal of molecular diagnostics: JMD. 2014;16(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. The New England journal of medicine. 2015;373(8):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. New York, NY: Cambridge University Press; 2006. [Google Scholar]
- 23.Lee EW, Dubin N. Estimation and sample size considerations for clustered binary responses. Statistics in medicine. 1994;13(12):1241–1252. [DOI] [PubMed] [Google Scholar]
- 24.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer.Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(13):3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Science translational medicine. 2014;6(224):224ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nature reviews Clinical oncology. 2017;14(9):531–548. [DOI] [PubMed] [Google Scholar]
- 27.de Bono J, De Giorgi U, Massard C, et al. PTEN loss as a predictive biomarker for the Akt inhibitor ipatasertib combined with abiraterone acetate in patients with metastatic castration-resistant prostate cancer (mCRPC) Paper presented at: European Society for Medical Oncology (ESMO) Annual Congress2016; Copenhagen, Denmark. [Google Scholar]
- 28.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. The New England journal of medicine. 2015;373(18):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweizer MT, Yu EY. Persistent androgen receptor addiction in castration-resistant prostate cancer. Journal of hematology & oncology. 2015;8(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Science translational medicine. 2015;7(269):269ra262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung cancer (Amsterdam, Netherlands). 2015;90(3):509–515. [DOI] [PubMed] [Google Scholar]
- 32.Xu T, Kang X, You X, et al. Cross-Platform Comparison of Four Leading Technologies for Detecting EGFR Mutations in Circulating Tumor DNA from Non-Small Cell Lung Carcinoma Patient Plasma. Theranostics. 2017;7(6):1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins MJ, Jelovac D, Barnathan E, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(12):3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mateo J, Carreira S, Seed G, et al. Genomic profiling of primary prostate tumors from patients who develop metastatic castration-resistant prostate cancer (mCRPC) Paper presented at: ASCO Annual Meeting2018; Chicago, IL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.