Abstract
Background:
Meta-analyses have reported that the effects of cognitive remediation might go beyond improvement in cognition to include unexpected benefits for schizophrenia patients such as negative symptom reduction and improvements in functioning. In addition, some evidence indicated that these potentially beneficial effects are also present in the initial course of schizophrenia, but work in this area is still developing.
Method:
A RCT compared Cognitive Remediation (CR) to Healthy Behaviors Training (HBT) in 80 patients (78% male) with a mean age of 21.9 years and mean education of 12.3 years who had a first psychotic episode within two years of study entry. Participants were trained using CR programs or received HBT involving 50 sessions over 6 months and then booster sessions over the next 6 months. The SANS and BPRS were used to assess symptoms. The UCLA Social Attainment Survey assessed social functioning.
Results:
Using GLMM, improvements over 12 months were found favoring CR for SANS Expressive Symptoms (p<.01), which was composed of Affective Flattening (p<.01) and Alogia (p=.04), and for SANS Experiential Symptoms, composed of Avolition/Apathy (p=.04) and Anhedonia / Asociality (p<.01). CR was associated with improvements in social functioning (p=.05) as compared to HBT.
CONCLUSIONS:
We confirmed that the beneficial effects of CR appear to extend beyond cognition to improvements in negative symptoms and social functioning in early course schizophrenia patients. These results suggest that cognitive remediation might have an impact when the reduction of risk factors for chronicity is most critical for promoting recovery.
Introduction
Negative symptoms are considered a core feature of schizophrenia for several reasons (Marder and Galderisi, 2017). Negative symptoms have been identified in children at risk for schizophrenia, are found in clinical high risk populations, and are present in unaffected relatives of schizophrenia patients (Galderisi et al., 2016). Further, negative symptoms are often present long before a diagnosis of schizophrenia and are known to be part of the prodromal phase. Also, prevalence studies indicate that negative symptoms are common in first episode patients in that 33% had at least one symptom of moderate severity (Galderisi et al., 2013; Hovington et al., 2012) and even more common among schizophrenia patients with long-term illness, 57% had at least one symptom (Bobes et al., 2010). In addition, in first episode schizophrenia patients, negative symptoms at baseline predict negative symptoms at follow-up, have long-term stability, and are associated with lower levels of recovery (Austin et al., 2013; Bertelsen et al., 2009; Ergül and Üçok, 2015; Galderisi et al., 2013; Üçok and Ergül, 2014; Ventura et al., 2015). There is a well-established correlation between negative symptoms and poor school and work outcomes as well as between lower levels of social functioning even in first episode patients (Chang et al., 2013; Mezquida et al., 2017). In fact, negative symptoms have been found to be a mediator of the relationship between neurocognition and functional outcome both in first-episode patients and in patients with established illness (Lin et al., 2013; Ventura et al., 2009). Thus, there is little doubt that negative symptoms are predictors of poor function, impose a significant burden on patients, their families, and their caregivers, and so are important treatment targets.
Most clinicians would agree that in addition to being a core feature of schizophrenia, negative symptoms are difficult to treat (Aleman et al., 2016). One of the primary reasons is that negative symptoms do not typically respond to traditional pharmacological treatments for schizophrenia, which primarily target positive symptoms (Fusar-Poli et al., 2015; Mucci et al., 2016). Furthermore, negative symptoms tend to remain present after positive symptoms resolve and are moderately stable for long periods of time, even up to 8 years (Ventura et al., 2015). Although some studies have found that antipsychotic medication improves negative symptoms, this effect might be limited to secondary negative symptoms in acutely ill patients or only occur if positive symptoms remit (Aleman et al., 2016; Subotnik et al., 2014). However, antipsychotic medication might also worsen negative symptoms or the subjective experience of taking mediation (Austin et al., 2015; Awad and Voruganti, 2013). Clearly, any non-pharmacological interventions that could help improve negative symptoms, even moderately, would go a long way toward improving the patient outcomes.
Effects of cognitive remediation on symptoms and functioning in established schizophrenia patients have been reported in meta-analytic studies (McGurk et al., 2007; Wykes et al., 2011) indicating small to medium effects for symptoms (d=.28, d=.18, respectively) and small to medium effects for functioning (d=.38, d=.42, respectively). These findings suggested that cognitive remediation might have broader than expected effects on clinical outcomes not directly targeted (Medalia and Saperstein, 2013). In addition, a recent meta-analysis indicated that cognitive remediation had beneficial effects on negative symptoms where the effect size was small to moderate (g=.30) (Cella et al., 2017). Furthermore, a meta-analysis on first episode psychosis (FEP) patients showed that cognitive remediation (CR) produces small, but statistically significant improvements in secondary targets such as symptoms (d=0.19) and functioning (d=.18) (Revell et al., 2015). However, the authors report that CR’s effect on symptoms and functioning was larger in trials that occurred in a rehabilitation context with group interventions as compared to individual treatment. Apparently, more work needs to done on early course patients receiving cognitive remediation in the context of a psychiatric rehabilitation program.
The effects of Cognitive Remediation (CR) versus Healthy Behaviors Training (HBT) on cognition and role functioning in this RCT are being reported in another publication. The aim of the current analyses is to examine the potential benefits of cognitive remediation on secondary, non-targeted domains of negative symptoms and social functioning in the context of a psychiatric rehabilitation program. Software from the Brain Injury field and educational programs used in school settings made up the CR program. In this study, negative symptoms and social functioning were secondary targets, an active psychosocial intervention was chosen as a control condition in this study to allow for a rigorous comparison to cognitive remediation. The HBT involved a holistic approach to healthy behavior change that combined education in nutrition, stress management, and exercise. The HBT training was informed by the U.S. National Consensus Statement on Mental Health Recovery (SAMHSA, 2006) which emphasizes the development of holistic well-being in mental and physical domains.
Methods
Subjects
The sample consisted of 80 participants recruited from Los Angeles area psychiatric hospitals, clinics, and community psychiatrists (Table 1). All study participants received outpatient psychiatric treatment at the UCLA Aftercare Research Program (Nuechterlein et al., 1992; Nuechterlein et al., 2008; Nuechterlein et al., 2014). This study was approved by the UCLA Institutional Review Board and all participants gave written informed consent.
Table 1.
Demographic and Clinical Characteristics of the Study Sample (n = 80)
| Characteristic | Cognitive Remediation (n = 31) |
Healthy Behaviors Training (n = 49) |
Total (n = 80) |
|---|---|---|---|
| Age, mean (SD) | 21.5 (3.0) | 21.5 (4.0) | 21.5 (3.5) |
| Educational level, mean (SD) | 12.7 (1.8) | 12.2 (1.5) | 12.4 (1.6) |
| Parental educational level, mean (SD) | 13.9 (3.8) | 13.9 (3.7) | 13.9 (3.7) |
| Time since psychosis onset, mean (SD) in months | 7.2 (6.2) | 8.9 (7.1) | 8.0 (6.7) |
| Male sex | 74% | 83% | 78% |
| Marital status | |||
| Single | 92% | 97% | 95% |
| Married | 8% | 3% | 5% |
| Race | |||
| White | 48% | 51% | 50% |
| Asian | 10% | 12% | 11% |
| Native American | 8% | 10% | 9% |
| African American | 33% | 24% | 29% |
| Pacific Islander | 0% | 2% | 1% |
| Ethnicity | |||
| Hispanic | 38% | 44% | 41% |
| Non-Hispanic | 62% | 56% | 59% |
| Diagnosis | |||
| Schizophrenia | 51% | 61% | 56% |
| Schizoaffective disorder | 8% | 17% | 13% |
| Schizophreniform disorder | 41% | 22% | 31% |
Note: There were no statistically significant differences among the groups at p < .05
Entry criteria were: 1) a recent-onset of psychotic illness, with the beginning of the first major psychotic episode within the last 2 years; 2) a diagnosis by the DSM-IV of schizophrenia, schizoaffective disorder, depressed type, or schizophreniform disorder; 3) 18 to 45 years of age; 4) no evidence of a known neurological disorder; 5) no evidence of significant and habitual drug abuse or alcoholism in the 6 months prior to hospitalization and no evidence that the psychosis was accounted for by substance abuse; 6) no premorbid mental retardation. Additional details on recruitment and enrollment are available (Subotnik et al., 2015).
Procedures
All patients were enrolled in the UCLA Aftercare Research Program and were provided treatment with antipsychotic medication and regular visits with a psychiatrist. The patients were randomized to either Cognitive Remediation (CR) or Healthy Behaviors Training (HLT) and to Oral risperidone (Oral) or Long-acting Injectable (LAI) risperidone in a fully crossed 2×2 design. Randomization occurred after treatment with oral risperidone as the sole antipsychotic medication for at least 3 weeks and completion of a baseline assessment battery. In addition, each patient was provided with individual case management services (independent of condition) which included services aimed at promoting recovery and included psychoeducation for patients and family members. The case manager’s approach involved community outreach and services aimed a return to work or school and patients were provided ongoing support. The level of assistance offered for finding employment or returning to school varied depending on the patient’s individual needs.
Symptom Assessment
Scale for the Assessment of Negative Symptoms (SANS).
Raters who were trained to criterion levels of ICC = .75 or higher on either the Global Items or all SANS items (Ventura et al., 1993) administered the SANS, a 25-item measure that is widely used to assess two negative symptom domains: 1) Expressive Symptoms, which consisted of Affective Flattening (blunted affect) and Alogia, and 2) Experiential Symptoms, which consisted of Avolition / Apathy and Anhedonia / Asociality. The Attention subscale was not included in these analyses due to the strong correlational relationship to objectively measured cognition and low correlations with other negative symptoms (Blanchard et al., 1998). The Global SANS was administered every 2 weeks along with the BPRS and the full SANS every 3 months.
Brief Psychiatric Rating Scale (BPRS).
Each BPRS rater achieved a median Intraclass Correlation Coefficient (ICC) of .80 or higher across all items compared with the criterion ratings and participated in a quality assurance program (Ventura et al., 1993). Each patient was rated by a trained rater on an expanded version of the BPRS (Lukoff et al., 1986) from baseline to 12 months. We examined the 3 observable negative symptoms separately which consisted of Blunted Affect, Motor Retardation, and Emotional Withdrawal. The BPRS was administered every 2 weeks.
Functional Outcome Assessment
The UCLA Social Attainment Survey (SAS) was used to rate social functioning in 7 areas: 1) Same Sex Peer relationships, 2) Leadership in same-sex peer relationships, 3) Opposite Sex peer relationships, 4) Dating History, 5) Sexual Experience, 6) Outside Activities, and 7) Participation in Organizations. The SAS items were rated on a 1–5 point anchored scale every 3 months. For the SAS, two sub-factors were created using a rational approach, Platonic Factor (items 1 and 2) and Dating Factor (items 3–5) similarity of content. The SAS was administered every 3 months.
Randomized Conditions:
Oral Risperidone (Oral) versus Long-Acting Injectable (LAI) Risperidone
The medication treatment was managed by the Aftercare Program psychiatrists. After a brief, initial period of at least 3-weeks of treatment with oral risperidone as the only antipsychotic medication, participants were administered baseline assessments and then were randomly assigned to continued treatment with oral risperidone (Oral) or to long-acting injectable (LAI) risperidone (Subotnik et al., 2015). Following randomization, study psychiatrists were able to prescribe an alternative second-generation antipsychotic medication if there was an inadequate response to risperidone or if a change in medication was indicated due to intolerable side effects. Patients randomly assigned to the LAI risperidone group received an initial injection of 25 mg open label. An initial dosage level of 25 mg of injectable risperidone was selected based on published treatment recommendations (Kane et al., 1998; Kane et al., 2013; Marder et al., 2003). Higher dosages of injectable risperidone were administered if a higher maintenance dosage was required to treat psychotic symptoms. The 12.5 mg dose was used if side-effects of the 25 mg dosage are intolerable. After the LAI was started, the daily dosage of oral risperidone was titrated to zero over the subsequent days or weeks. Most patients required less than one week to titrate to zero mg of oral risperidone.
Cognitive Remediation Program
Patients were also randomly assigned to receive Cognitive Remediation (CR) with the intention of improving cognitive functioning in the CR group or Healthy Behaviors Training (HBT) to improve healthy lifestyle habits in the HBT group. The Cognitive Remediation Program included computerized cognitive training and a Bridging Group to facilitate generalization in the context of a psychiatric rehabilitation program, the UCLA Aftercare Program, a clinical research and treatment program which specializes in patients who are in the early course of schizophrenia. The computerized sessions involved 2 hours per week of in-clinic cognitive remediation (CR) for a 6-month period, followed by 1 hour per week of CR during months 7–9 and then 1 hour every other week during months 10–12. The program used 23 computer-based software programs adapted from the brain injury rehabilitation field (Bracy, 1994) as part of Neurocognitive Enhancement Therapy (NET) (Bell et al., 2007) and educational software for school children and adolescents as part of Neuropsychological Educational Approach to Remediation (Medalia and Revheim, 1999). This approach combined aspects of NET and NEAR to address both bottom-up and top-down approaches to cognitive remediation. The cognitive training exercises contained increasing levels of difficulty and were administered in a computer lab setting in groups at the clinic. Trained cognitive coaches provided the cognitive training using a manualized approach guided by the principles of NEAR (Medalia et al., 2009). Cognitive coaches worked with the patients in the learning lab to help reinforce the active use of positive cognitive strategies and/or suggest strategies to improve cognitive skills, and track patient’s progress. Additional details of the CR program are reported separately (Nuechterlein et al., 2014).
Bridging Group
Patients also participated in a one-hour Bridging Group aimed at making connections between the abilities learned in cognitive training and functional performance such as work or school (Bell et al., 2001; Medalia et al., 2009). They attended weekly for months 1–9 and then every other week in months 10–12. Bridging involved a review of the training in the cognitive remediation lab, e.g., successful strategies, sharing strategies, and peer mentoring. Reviews of the recent work and school activity of individual participants were provided with feedback from the Work Behavior Inventory (a structured assessment of work involvement) (Bryson et al., 1997). The Work Behavior Inventory is a standardized work performance assessment instrument specifically designed for individuals with severe mental illness. The focus of the Bridging Group was on patient goal setting, i.e., short-term and long-term goals, and applying cognitive skills in daily life. The Bridging Group also included role plays and patient presentations of work or school activities, and interests or hobbies.
Healthy Behaviors Training
The Healthy Behaviors Training (HBT) program was developed to serve as an active control condition that was not specifically targeting negative symptoms or social functioning. However, the HBT was considered an active intervention that was useful for improving a patient’s quality of life. The HBT focused on increasing positive lifestyle habits and overall well-being. The 12-month HBT program was a manual-driven didactic program that covered three topic areas: 1) nutrition and health, 2) stress management, and 3) physical exercise. The HBT program was designed to provide an equal amount of group facilitator intervention time as compared to the cognitive training program (Gretchen-Doorly et al., 2012; Gretchen-Doorly et al., 2009). Thus, HBT involved 3 hours of training per week for the first 6 months of the intervention, then was decreased to 2 hours per week for months 7–9 and 2 hours every other week for months 10–12. Each module included a group activity which promoted positive lifestyle mastery-building experiences. Additional details about how the HBT was developed are available (Gretchen-Doorly et al., 2009).
Data Analysis Plan
A Generalized Linear Mixed Model (GLMM) approach was used to examine the effects of Cognitive Remediation (CR) compared to Healthy Behavior Training (HBT) on the change of negative symptoms and social functioning over time. A statistically significant between-subject interaction term (group × time) indicates significant differences in the degree of change over time in one group (intervention) as compared to the other group (active control). For all analyses the medication group (oral vs injectable antipsychotic medication) was also included in the model in addition to the main effect of interest (CR vs. HBT), and the interaction of the medication group effect and psychosocial treatment effect were included in the model, resulting in a full factorial 2×2 design. Tables are used to report the statistical results of the GLMM and figures highlight primary findings on changes in slope for symptoms and functioning.
Results
Negative Symptom Analyses:
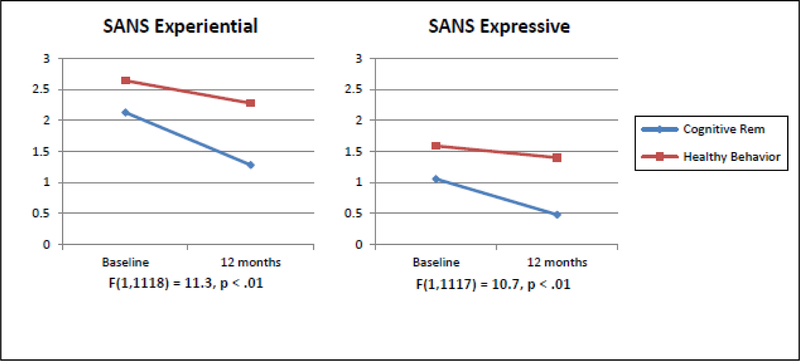
There were no statistically significant differences at p < .05 in the study outcome measures at baseline between patients receiving Cognitive Remediation (CR) as compared to Healthy Behaviors Training (HBT) (Table 2). Using GLMM there were no statistically significant differences in the slope for Oral Risperidone (Oral) vs Long Acting Injectable Medication (LAIs) over time for either the SANS or BPRS (Table 3). However, we did find a change from baseline to 12 months for CR as compared to HBT indicating a significant decrease for SANS measured negative symptoms. Specifically, improvement occurred for SANS Expressive Symptoms (p<.01), as well as the component items Global Affective Flattening (p<.01) and Alogia (p=.04) favoring CR (Figure 1). On the BPRS, Blunted Affect also showed clinical improvement (p=.03) favoring CR over HBT (Table 1), but Motor Retardation and Emotional Withdrawal were not individually affected. In addition, a decrease in trajectory was found indicating clinical improvement for CR as compared to HBT for SANS Experiential Negative Symptoms (p<.01), which included Avolition/Apathy (p=.04) and Anhedonia / Asociality (p<.01) (Figure 1). For SANS Global Apathy, we found a significant 3-way interaction with CR-HBT × Oral-LAI × Time indicating that greater improvement in Apathy was seen for patients assigned to both CR and Oral medication (Figure 2).
Table 2.
Means, Standard Deviations, and ANOVA Results for the Primary Study Symptom Outcome Variables
| n | Mean | SD | F | p | ||
|---|---|---|---|---|---|---|
| SANS Expressive |
Cognitive Training |
38 | 1.14 | 1.1 | 3.39 | .07 |
| Healthy Behaviors |
40 | 1.60 | 1.1 | |||
| Total | 78 | 1.38 | 1.1 | |||
| SANS Experiential |
Cognitive Training |
38 | 2.30 | 1.3 | 3.56 | .06 |
| Healthy Behaviors |
40 | 2.84 | 1.2 | |||
| Total | 78 | 2.57 | 1.3 | |||
| Anhedonia | Cognitive Training |
38 | 1.87 | 1.4 | 2.98 | .09 |
| Healthy Behaviors |
40 | 2.43 | 1.4 | |||
| Total | 78 | 2.15 | 1.4 | |||
| Apathy | Cognitive Training |
38 | 2.74 | 1.5 | 2.49 | .12 |
| Healthy Behaviors |
40 | 3.25 | 1.4 | |||
| Total | 78 | 3.00 | 1.5 | |||
| Alogia | Cognitive Training |
38 | 1.55 | 1.2 | 2.68 | .11 |
| Healthy Behaviors |
40 | 1.43 | 1.2 | |||
| Total | 78 | 1.21 | 1.2 | |||
| Affect | Cognitive Training |
38 | 1.32 | 1.2 | 2.86 | .10 |
| Healthy Behaviors |
40 | 1.78 | 1.2 | |||
| Total | 78 | 1.55 | 1.2 | |||
| BPRS-Blunted | Cognitive Training |
39 | 2.13 | 1.1 | 2.75 | .10 |
| Healthy Behaviors |
41 | 2.54 | 1.1 | |||
| Total | 80 | 2.34 | 1.1 | |||
| BPRS-Motor Retardation |
Cognitive Training |
39 | 1.56 | .8 | 3.02 | .09 |
| Healthy Behaviors |
41 | 1.95 | 1.1 | |||
| Total | 80 | 1.76 | 1.0 | |||
| BPRS-Emotional Withdrawal | Cognitive Training |
39 | 1.49 | .9 | .76 | .39 |
| Healthy Behaviors |
41 | 1.68 | 1.1 | |||
| Total | 80 | 1.59 | 1.0 | |||
| SAS Total | Cognitive Training |
38 | 2.71 | .9 | 3.48 | .07 |
| Healthy Behaviors |
40 | 2.33 | .9 | |||
| Total | 78 | 2.51 | .9 | |||
| SAS Dating | Cognitive Training |
38 | 2.29 | 1.2 | .28 | .60 |
| Healthy Behaviors |
40 | 2.14 | 1.3 | |||
| Total | 78 | 2.21 | 1.2 |
BPRS – Brief Psychiatric Rating Scale, SANS – Scale for the Assessment of Negative Symptoms, SAS – Social Attainment Survey. Note: There were no statistically significant differences among the groups at p < .05
Table 3.
General Liner Mixed Model Analyses Showing the Effects of Medication Type, Oral vs Injectable, and Intervention Type, Cognitive Training vs Healthy Behaviors Training on SANS and BPRS Negative Symptoms
| SANS Expressive |
SANS Experiential |
Anhedonia | Apathy | Alogia | Affect | BPRS-Blunted | BPRS-Motor Retardation |
BPRS-Emotional Withdrawal |
|
|---|---|---|---|---|---|---|---|---|---|
| CT/HBT | F(1,88)=6.7, p=.01 | F(1,89)=4.1, p=.05 | F(1,91)=3.4, p=.07 | F(1,93)=3.1, p=.08 | F(1,93)=6.5, p=.01 | F(1,89)=5.8, p=.02 | F(1,90)=5.6, p=.02 | F(1,104)= 4.0, p=.05 | F(1,111)= 2.9, p=.09 |
| Medication (med) | F(1,88)=.2, p=.70 | F(1,89)=1.3, p=.27 | F(1,91)=.5, p=.49 | F(1,93)=3.1, p=.08 | F(1,93)=.9, p=.34 | F(1,89)=0.0, p=.84 | F(1,90)=0.0, p=.96 | F(1,104)= 0.2, p=.64 | F(1,111)= 0.6, p=.44 |
| Time | F(1,1117)= 42.6, p<.01 | F(1,1118)= 71.6, p<.01 | F(1,1119)= 46.0, p<.01 | F(1.93)=47.6, p<.01 | F(1,1121)= 20.5, p<.01 | F(1,1118)= 46.7, p<.01 | F(1,1125)= 24.3, p<.01 | F(1,1132)= 33.0, p<.01 | F(1,1136)= 3.0, p=.08 |
| Medication*time | F(1,1117)= 1.2, p=.27 | F(1,1118)= .45, p=.50 | F(1,1119)= .76, p=.38 | F(1,1120)= .1, p=.76 | F(1,1121)= .1, p=.81 | F(1,1118)= 3.0, p=.08 | F(1,1125)= 0.0, p=.97 | F(1,1132)= 2.4, p=.12 | F(1,1136)= 0.1, p=.79 |
| CT/HBT*time | F(1,1117)= 10.7, p<.01 | F(1,1118)= 11.3, p<.01 | F(1,1119)= 12.4, p<.01 | F(1,1120)= 4.3, p=.04 | F(1,1121)= 4.4, p=.04 | F(1,1118)= 13.3, p<.01 | F(1,1125)= 4.7, p=.03 | F(1,1132)= 0.0, p=.95 | F(1,1136)= .01, p=.78 |
| CT/HBT*med | F(1,88)=.38, p=.54 | F(1,89)=3.7, p=.06 | F(1,91)=1.2, p=.28 | F(1,93)=5.3, p=.02 | F(1,93)=.3, p=.62 | F(1,89)=0.5, p=.50 | F(1,90)=.06, p=.80 | F(1,104)= 2.5, p=.12 | F(1,110)= 3.7, p=.06 |
| CT/HBT*med*time | F(1,1117)= .18, p=.67 | F(1,1118)= .55, p=.46 | F(1,1119)= 1.6, p=.20 | F(1,1120)= 4.4, p=.04 | F(1,1121)= .30, p=.58 | F(1,1118)= 1.7, p=.19 | F(1,1125)= 0.0, p=.83 | F(1,1132)= 0.1, p=.80 | F(1,1136)= 1.2, p=.28 |
FIGURE 1.
Plots of the Slopes over Time Showing the Effects of Cognitive Training vs Healthy Behavior Training for SANS Experiential and SANS Expressive Symptoms from Baseline to 12 months
FIGURE 2.
Plots of the Slopes over Time Showing the Effects of Oral vs Injectable Cognitive vs Time for SANS Apathy from Baseline to 12 months
Social Functioning Analyses:
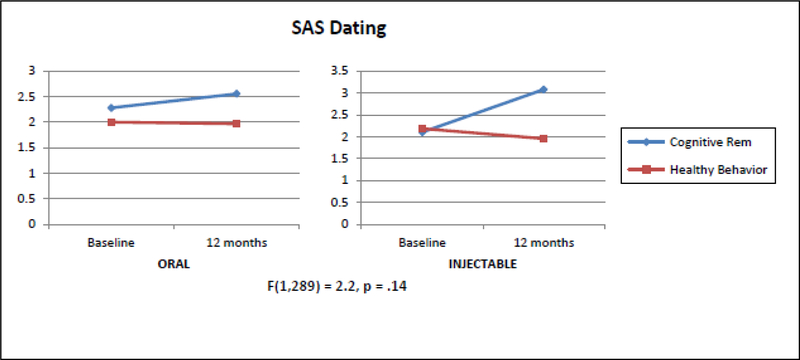
Using GLMM there were no statistically significant differences in the slope for Oral Risperidone (Oral) vs Long Acting Injectable (LAIs) Risperidone time for either the SAS Total or the SAS Dating factor (Table 4). Using GLMM, a significant increase (indicating clinical improvement) in social functioning favoring CR on the UCLA Social Attainment Survey total (p=.05) (Table 3) (Figure 2). This effect seems to be mostly driven by the items that deal with dating including emotional involvement (p=.06) and opposite sex peer relations (p<.01) rather than items that assess platonic relationships (Table 2). For SAS Dating, we found a significant 3-way interaction with CR-HBT × Oral-LAI × Time indicating that greater improvement in Dating was seen for patients assigned to both CR and Injectable medication (Figure 4).
Table 4.
General Liner Mixed Model Analyses Showing the Effects of Medication Type, Oral vs Injectable, and Intervention Type, Cognitive Training vs Healthy Behaviors Training on SAS Social Functioning
| SAS Total | SAS Dating | |
|---|---|---|
| CT/HBT | F(1,112)=3.3, p=.07 | F(1,89)=.1, p=.72 |
| Medication (med) | F(1,112)=.45, p=.50 | F(1,89)=0.0, p=.99 |
| Time | F(1,222)=6.4, p=.01 | F(1,289)=8.8, p<.01 |
| Medication*time | F(1,222)=.87, p=.35 | F(1, 289)=2.2, p=.14 |
| CT/HBT*time | F(1,222)=3.7, p=.05 | F(1, 289)=20.5, p<.01 |
| CT/HBT* med | F(1,112)=.04, p=.85 | F(1,89)=.40, p=.51 |
| CT/HBT*med*time | F(1,222)=2.5, p=.11 | F(1, 289)=7.2, p<.01 |
FIGURE4.
Plots of the Slopes over Time Showing the Effects of Oral vs Injectable Cognitive vs Time for SAS Dating from Baseline to 12 months
Discussion
Our findings support and extend the work by Cella et al in established schizophrenia patients and Revell et al in FEP on symptoms and functional outcomes. The current study found that patients who were randomized to Cognitive Remediation (CR) as compared to Healthy Behaviors Training (HBT) showed significant improvements in negative symptoms over a 12-month period. Improvements favoring CR vs HBT were found in SANS Expressive Negative Symptoms, consisting of Flattened Affect and Alogia. These SANS findings were consistent with comparable improvement in BPRS Blunted Affect over 12 months. In addition, we found that patients randomized to CR as compared to HBT also showed a benefit for SANS Experiential Negative Symptoms. From baseline to 12 months, patients showed an improvement in Avolition / Apathy and Anhedonia / Asociality. Additional gains with CR as compared to HBT were seen in social functioning over the course of 12 months, as measured by the UCLA Social Attainment Survey. We also examined 2 types of medication administration, oral risperidone vs long acting injectable risperidone to determine their effects on the study outcome variables. There were no significant main effects for type of medication administration. There were only 2 significant 3-way interaction effects between CR vs HBT vs Medication administration type, Oral vs LAI. For the SANS global domain Apathy, patients who received CR and Oral risperidone experienced a greater reduction in negative symptoms as compared to HBT and LAIs. For SAS Dating, the significant 3-way interaction indicated that patients who received CR and injectable medication had greater improvement in this domain of social functioning.
One of the key implications of this study is that cognitive remediation seems to have broader, unexpected benefits for outcomes that were not specifically or directly targeted during the intervention, such as the reduction of negative symptoms and improvements in social functioning. Our findings are consistent with the meta-analytic findings on patients with established schizophrenia (Cella et al., 2017) and on FEP (Revell et al., 2015) indicating that cognitive training compared to control conditions showed small to medium effects on negative symptoms. One possible explanation for the modest effects, as suggested by Revell et al, is because of the reduced range of possible symptom improvement in FEP. But, given the relative paucity of effective treatment options for negative symptoms, our findings offer additional evidence that cognitive remediation can improve negative symptoms. Given the overwhelming number of studies showing the deleterious effects of negative symptoms for functioning and recovery from schizophrenia, even small to moderate effects on negative symptoms might have substantial treatment and recovery implications.
There are several mechanisms of action that could account for the improvements seen in negative symptoms in cognitive remediation programs. One such mechanism is based on the hypothesis offered by Cella and colleagues, and is in line with the findings of Wykes et al 2011, that cognitive remediation which occurred in a rehabilitation context strengthens both social and work outcomes. In fact, Cella et al report that an emphasis on helping patients to apply cognitive abilities in everyday life could explain why we see improvements in negative symptoms. Further, they found that in studies with better methodological rigor, there was also greater patient–facilitator contact. Those studies showed larger improvements in negative symptoms, which supports the idea that greater facilitator contact might be one key ingredient to overcoming negative symptoms. Similarly, Revell et al’s meta-analysis found that patients who received cognitive remediation in a group setting showed significantly larger improvements in symptoms than those in individual therapy. Although we controlled for the amount of time of facilitator contact between the two conditions, the type of facilitator contact differed. This orientation was adopted in our cognitive training bridging group which aimed to provide support and encouragement for improved functional outcomes and differed from the aim of the HBT control condition. In fact, our experience with the NET and NEAR approaches included a high ratio of coach-to-patient interaction because advancement through the cognitive training program levels was done manually for several programs and/or required close monitoring. This type of monitoring allowed for making specific suggestions, where indicated, of cognitive strategies to increase the likelihood of success performance and links to functional changes. Thus, the frequent contact with a facilitator or therapist during cognitive training could be one mechanism for the improvement in negative symptoms.
Another possible mechanism of action for the improvement of negative symptoms is that negative symptom improvement is secondary to the improvement in cognition effected by cognitive training. Cognitive abilities such as working memory, attention, and verbal memory have been linked to functional outcome and have also been correlated with negative symptoms (Harvey et al., 2006; Ventura et al., 2009). In one study, for schizophrenia patients who were receiving cognitive training vs computer games, an association was found between an improvement in cognition (speed of processing and cognitive control) and an improvement in quality of life outcomes (Fisher et al., 2010). This could explain why we would see improved negative symptom scores on measures such as the SANS and BPRS. Similar results were found in studies of cognitive training on a related construct, work functioning, suggesting that improving cognition removes an impediment to functioning (Lindenmayer et al., 2008; McGurk et al., 2010). These studies provide possible mechanisms of action for explaining how improvements in cognition might be associated with improvements in negative symptoms, and possibly social functioning.
Improvements in cognition coupled with reductions in negative symptoms might similarly be associated with improvements in social functioning. Being able to track conversations due to improved attention and focus might lead to improvement in social interactions by allowing patients to show a greater range of social responses. Improvements in mental activity might translate into more immediate responses for patients in social situations such as facial expressions and increased affective gestures. If improvement in cognition is associated with the patient’s ability to successfully perform everyday behaviors (Harvey et al., 2006) and decreasing negative symptoms such as asociality resulted in increased social engagement, then quantity and quality of social interactions should increase. Thus, the combination of improvements in cognition through cognitive training plus the emphasis on applying those behavioral skills in daily life, could have synergistic effects in the context of a psychosocial rehabilitation program that leads to improvements in social functioning (Medalia and Saperstein, 2013).
Future Directions:
A more intensive form of cognitive training or new combinations of cognitive Remediation and other cognition-boosting treatments might be needed to produce larger and more robust improvements in negative symptoms and social functioning. We are currently testing a combination of cognitive Remediation and aerobic exercise to determine whether we can further boost the impact of cognitive remediation (Nuechterlein et al., 2016).
Summary:
The beneficial effects of cognitive remediation, when combined with a bridging group to encourage generalization of training, extend to improvements in negative symptoms and social functioning in recent-onset schizophrenia patients. Although the effects were generally small, these results suggest that cognitive remediation has an impact in the early course of schizophrenia that reaches beyond cognition at a time that reduction of risk for chronicity is critical for promoting recovery. Future research should explore whether improvements in negative symptoms and social functioning are mediated by improvements in facilitator contact or cognition.
FIGURE 3.
Plots of the Slopes over Time Showing the Effects of Cognitive Training vs Healthy Behavior Training for SAS Social Functioning Symptoms and SAS Dating from Baseline to 12 months
Acknowledgement
This research was supported by National Institute of Mental Health grants MH37705 (Principal Investigator K.H.N.) and P50 MH066286 (Principal Investigator K.H.N.) and supplemental support from an investigator-initiated grant from Janssen Scientific Affairs, LLC.
The authors would like to thank the following research associates who worked on data collection and data management: Robin Kite, Jackie Hayata, and Lilian Medina.
Role of the Funding Source
The funding sources did not play a role in the study design, implementation, interpretation of results, preparation of manuscript drafts, or the publication of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Joseph Ventura, Ph.D., has received funding from Janssen Scientific Affairs, LLC, Posit Science Inc., and Genentech, Inc. He has served as a consultant to Posit Science, Inc., Lumosity Inc., and Boehringer-Ingelheim, GmbH.
Kenneth L. Subotnik, Ph.D., has received research funding from Janssen Scientific Affairs, LLC, and Genentech, Inc., through grants to Drs. Nuechterlein and Ventura. He is a consultant to Otsuka America Pharmaceutical, Inc.
Keith H. Nuechterlein, Ph.D., has received funding from Janssen Scientific Affairs, LLC, Posit Science, Inc., and Genentech, Inc. He has served as a consultant to Genentech, Inc. and Otsuka America Pharmaceutical, Inc.
Denise Gretchen Doorly, Ph.D., Gerhard S. Hellemann, Ph.D., Laurie Casaus, M.D., and Michael Boucher, M.D., have no conflicts of interest to disclose.
References
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, Knegtering H, 2016. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophrenia research. [DOI] [PubMed] [Google Scholar]
- Austin SF, Mors O, Budtz-Jørgensen E, Secher RG, Hjorthøj CR, Bertelsen M, Jeppesen P, Petersen L, Thorup A, Nordentoft M, 2015. Long-term trajectories of positive and negative symptoms in first episode psychosis: a 10year follow-up study in the OPUS cohort. Schizophrenia research 168(1), 84–91. [DOI] [PubMed] [Google Scholar]
- Austin SF, Mors O, Secher RG, Hjorthøj CR, Albert N, Bertelsen M, Jensen H, Jeppesen P, Petersen L, Randers L, 2013. Predictors of recovery in first episode psychosis: the OPUS cohort at 10year follow-up. Schizophrenia research 150(1), 163–168. [DOI] [PubMed] [Google Scholar]
- Awad AG, Voruganti LN, 2013. The impact of newer atypical antipsychotics on patient-reported outcomes in schizophrenia. CNS drugs 27(8), 625–636. [DOI] [PubMed] [Google Scholar]
- Bell M, Bryson G, Greig T, Corcoran C, Wexler BE, 2001. Neurocognitive enhancement therapy with work therapy: effects on neuropsychological test performance. Archives of General Psychiatry 58(8), 763–768. [DOI] [PubMed] [Google Scholar]
- Bell M, Fiszdon JM, Greig T, Wexler BE, Bryson G, 2007. Neurocognitive enhancement therapy with work therapy in schizophrenia: 6-month follow-up of neuropsychological performance. Journal of Rehabilitation Research and Development 44(5), 761. [DOI] [PubMed] [Google Scholar]
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Øhlenschlæger J, Le Quach P, Christensen TØ, Krarup G, Jørgensen P, Nordentoft M, 2009. Course of illness in a sample of 265 patients with first-episode psychosis—five-year follow-up of the Danish OPUS trial. Schizophrenia research 107(2), 173–178. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS, 1998. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin 24(3), 413–424. [DOI] [PubMed] [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, Rejas J, 2010. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. Journal of Clinical Psychiatry 71(3), 280. [DOI] [PubMed] [Google Scholar]
- Bracy OL, 1994. Cognitive functioning and rehabilitation. Journal of Cognitive Rehabilitation 12(2), 12–16. [Google Scholar]
- Bryson G, Bell M, Lysaker P, 1997. Affect recognition in schizophrenia: A function of global impairment or a specific cognitive deficit. Psychiatry Research 71, 105–113. [DOI] [PubMed] [Google Scholar]
- Cella M, Preti A, Edwards C, Dow T, Wykes T, 2017. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clinical Psychology Review 52, 43–51. [DOI] [PubMed] [Google Scholar]
- Chang WC, Tang JYM, Hui CLM, Wong GHY, Chan SKW, Lee EHM, Chen EYH, 2013. The relationship of early premorbid adjustment with negative symptoms and cognitive functions in first-episode schizophrenia: a prospective three-year follow-up study. Psychiatry research 209(3), 353–360. [DOI] [PubMed] [Google Scholar]
- Ergül C, Üçok A, 2015. Negative symptom subgroups have different effects on the clinical course of schizophrenia after the first episode: a 24-month follow up study. European Psychiatry 30(1), 14–19. [DOI] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S, 2010. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophrenia bulletin 36(4), 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P, 2015. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophrenia bulletin 41(4), 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S, Mucci A, Bitter I, Libiger J, Bucci P, Fleischhacker WW, Kahn RS, Group ES, 2013. Persistent negative symptoms in first episode patients with schizophrenia: results from the European First Episode Schizophrenia Trial. European Neuropsychopharmacology 23(3), 196–204. [DOI] [PubMed] [Google Scholar]
- Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, Rucci P, Gibertoni D, Aguglia E, Amore M, 2016. Pathways to functional outcome in subjects with schizophrenia living in the community and their unaffected first-degree relatives. Schizophrenia research 175(1), 154–160. [DOI] [PubMed] [Google Scholar]
- Gretchen-Doorly D, Kite RE, Subotnik KL, Detore NR, Ventura J, Kurtz AS, Nuechterlein KH, 2012. Cardiorespiratory endurance, muscular flexibility and strength in first-episode schizophrenia patients: use of a standardized fitness assessment. Early Interv Psychiatry 6(2), 185–190. [DOI] [PubMed] [Google Scholar]
- Gretchen-Doorly D, Subotnik KL, Kite RE, Alarcon E, Nuechterlein KH, 2009. Development and evaluation of a health promotion group for individuals with severe psychiatric disabilities. Psychiatr Rehabil J 33(1), 56–59. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Reichenberg A, Bowie CR, 2006. Cognition and aging in psychopathology: focus on schizophrenia and depression. Annu Rev Clin Psychol 2, 389–409. [DOI] [PubMed] [Google Scholar]
- Hovington CL, Bodnar M, Joober R, Malla AK, Lepage M, 2012. Identifying persistent negative symptoms in first episode psychosis. BMC psychiatry 12(1), 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Aguglia E, Altamura AC, Ayuso Gutierrez JL, Brunello N, Fleischhacker WW, Gaebel W, Gerlach J, Guelfi JD, Kissling W, Lapierre YD, Lindstrom E, Mendlewicz J, Racagni G, Carulla LS, Schooler NR, 1998. Guidelines for depot antipsychotic treatment in schizophrenia. European Neuropsychopharmacology 8(1), 55–66. [DOI] [PubMed] [Google Scholar]
- Kane JM, Kishimoto T, Correll CU, 2013. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 12(3), 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Huang C-L, Chang Y-C, Chen P-W, Lin C-Y, Tsai GE, Lane H-Y, 2013. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophrenia research 146(1), 231–237. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J, McGurk S, Mueser K, Khan A, Wance D, Hoffman L, Wolfe R, Xie H, 2008. A randomized controlled trial of cognitive remediation among inpatients with persistent mental illness. Psychiatric Services 59(3), 241. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J, 1986. Manual for the Expanded Brief Psychiatric Rating Scale (BPRS). Schizophrenia Bulletin 12, 594–602. [Google Scholar]
- Marder SR, Conley R, Ereshefsky L, Kane JM, Turner MS, 2003. Dosing and switching strategies for long-acting risperidone. Journal of Clinical Psychiatry 64(suppl 16), 41–46. [PubMed] [Google Scholar]
- Marder SR, Galderisi S, 2017. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16(1), 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Schiano D, Mueser KT, Wolfe R, 2010. Implementation of the thinking skills for work program in a psychosocial clubhouse. Psychiatric rehabilitation journal 33(3), 190. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT, 2007. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry 164(12), 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Revheim N, 1999. Computer assisted learning in psychiatric rehabilitation. Psychiatric Rehabilitation Skills 3(1), 77–98. [Google Scholar]
- Medalia A, Revheim N, Herlands T, 2009. Cognitive remediation for psychological disorders. Oxford University Press, New York. [Google Scholar]
- Medalia A, Saperstein AM, 2013. Does cognitive remediation for schizophrenia improve functional outcomes? Current Opinion in Psychiatry 26(2), 151–157. [DOI] [PubMed] [Google Scholar]
- Mezquida G, Cabrera B, Bioque M, Amoretti S, Lobo A, González-Pinto A, Espliego A, Corripio I, Vieta E, Castro-Fornieles J, 2017. The course of negative symptoms in first-episode schizophrenia and its predictors: A prospective two-year follow-up study. Schizophrenia Research. [DOI] [PubMed] [Google Scholar]
- Mucci A, Merlotti E, Üçok A, Aleman A, Galderisi S, 2016. Primary and persistent negative symptoms: concepts, assessments and neurobiological bases. Schizophrenia research. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J, 1992. Developmental processes in schizophrenic disorders: Longitudinal studies of vulnerability and stress. Schizophrenia Bulletin 18(3), 387–425. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Turner LR, Ventura J, Becker DR, Drake RE, 2008. Individual placement and support for individuals with recent-onset schizophrenia: Integrating supported education and supported employment. Psychiatric Rehabilitation Journal 31(4), 340–349. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Ventura J, McEwen SC, Gretchen-Doorly D, Vinogradov S, Subotnik KL, 2016. Enhancing cognitive training through aerobic exercise after a first schizophrenia episode: theoretical conception and pilot study. Schizophrenia bulletin 42(suppl 1), S44–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Ventura J, Subotnik KL, Medalia A, Bell MD, Hayata JN, 2014. Developing a cognitive training strategy for first-episode schizophrenia: Integrating bottom-up and top-down approaches. American Journal of Psychiatric Rehabilitation in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, Drake RJ, 2015. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophrenia research 168(1), 213–222. [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2006. National consenus statement on mental health recovery. Substance Abuse and Mental Health Services Administration, National Mental Health Information Center, Rockville, MD. [Google Scholar]
- Subotnik KL, Casaus LR, Ventura J, Luo JS, Hellemann GS, Gretchen-Doorly D, Marder S, Nuechterlein KH, 2015. Long-Acting Injectable Risperidone for Relapse Prevention and Control of Breakthrough Symptoms After a Recent First Episode of Schizophrenia. A Randomized Clinical Trial. JAMA Psychiatry 72(8), 822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subotnik KL, Ventura J, Gretchen-Doorly D, Hellemann GS, Agee ER, Casaus LR, Luo JS, Villa KF, Nuechterlein KH, 2014. The impact of second-generation antipsychotic adherence on positive and negative symptoms in recent-onset schizophrenia. Schizophr Res 159(1), 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üçok A, Ergül C, 2014. Persistent negative symptoms after first episode schizophrenia: a 2-year follow-up study. Schizophrenia research 158(1), 241–246. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP, 1993. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters.”. International Journal of Methods in Psychiatric Research 3(4), 221–244. [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH, 2009. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res 113(2–3), 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ, Gretchen-Doorly D, Ered A, Villa KF, Hellemann GS, Nuechterlein KH, 2015. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophr Res 161(2–3), 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am J Psychiatry 168(5), 472–485. [DOI] [PubMed] [Google Scholar]