Abstract
Purpose of Review
Standard clinical protocols for treating cerebral edema and intracranial hypertension after severe TBI have remained remarkably similar over decades. Cerebral edema and intracranial hypertension are treated interchangeably when in fact intracranial pressure (ICP) is a proxy for cerebral edema but also other processes such as extent of mass lesions, hydrocephalus, or cerebral blood volume. A complex interplay of multiple molecular mechanisms results in cerebral edema after severe TBI, and these are not measured or targeted by current clinically available tools. Addressing these underpinnings may be key to preventing or treating cerebral edema and improving outcome after severe TBI.
Recent Findings
This review begins by outlining basic principles underlying the relationship between edema and ICP including the Monro-Kellie doctrine and concepts of intracranial compliance/elastance. There is a subsequent brief discussion of current guidelines for ICP monitoring/management. We then focus most of the review on an evolving precision medicine approach towards cerebral edema and intracranial hypertension after TBI. Personalization of invasive neuromonitoring parameters including ICP waveform analysis, pulse amplitude, pressure reactivity, and longitudinal trajectories are presented. This is followed by a discussion of cerebral edema subtypes (continuum of ionic/cytotoxic/vasogenic edema and progressive secondary hemorrhage). Mechanisms of potential molecular contributors to cerebral edema after TBI are reviewed. For each target, we present findings from preclinical models, and evaluate their clinical utility as biomarkers and therapeutic targets for cerebral edema reduction. This selection represents promising candidates with evidence from different research groups, overlap/inter-relatedness with other pathways, and clinical/translational potential.
Summary
We outline an evolving precision medicine and translational approach towards cerebral edema and intracranial hypertension after severe TBI.
Keywords: Severe traumatic brain injury, Cerebral edema, Intracranial hypertension, Biomarkers, Therapeutic target
Introduction
After management of primary injury such as contusions, focal hematomas, or diffuse injury, cerebral edema is an important focus of neurocritical care in traumatic brain-injured patients [1•, 2,3]. Mechanisms underlying cerebral edema include a complex and expanding network of secondary injury molecular pathways that go beyond brain swelling caused by acute osmolar forces of central necrotic tissue from primary injury as described in the 1990s [4-6]. Cerebral edema often manifests as intracranial hypertension, which can be monitored clinically and is a key prognosticator of unfavorable outcome in traumatic brain injury (TBI) potentially resulting in irreversible brain injury, herniation, and death [7-19].
For decades, management of cerebral edema and resultant intracranial hypertension has relied on nonspecific therapies ranging from hyperosmolar agents to decompressive craniectomy. These therapies, while potentially life-saving, have questionable impact on functional outcome and often fail to target or prevent underlying mechanisms of cerebral edema [20-22]. Due to insufficient evidence, current guidelines from the Brain Trauma Foundation (BTF) provide standard recommendations on intracranial pressure (ICP) or cerebral perfusion pressure (CPP) targets regardless of underlying patient and injury characteristics [23]. With the advent of precision medicine, there is an increasing recognition of the likely inadequacy of a “one-size -fits-all” approach, particularly in a heterogenous disease like TBI [1•, 24•, 25]. Advances in research increasingly suggest the potential benefits of incorporating mechanistic endophenotypes in the care of TBI patients, including biomarkers, genetic information, radiographic nuances, and advanced neuromonitoring [14, 25-30]. Although this review discusses current monitoring and management of cerebral edema in TBI, the primary focus is on how translational strategies are evolving towards targeting molecular processes and individualizing patient-care.
Cerebral Edema and Intracranial Pressure
Principles
Cerebral edema is related to ICP through the Monro-Kellie doctrine, autoregulation, and pressure volume relationships including intracranial compliance and elastance (Box 1). Individual differences in these processes are a fundamental reason why a standard ICP threshold does not reflect the same degree of pathophysiology or edema generation among patients; this provides an impetus for personalizing these targets. It is important to note that cerebral edema is not the only cause of intracranial hypertension. A variety of other processes including primary injury, obstruction of cerebrospinal fluid (CSF) outflow, and others can cause intracranial hypertension. Nonetheless, cerebral edema resulting from secondary injury is a central cause of elevated ICP [3]. Continuous ICP monitoring is a standard proxy for cerebral edema and complements other tools such as neurological examinations and radiographic scans. Since its introduction into clinical practice more than five decades ago, ICP has been a cornerstone of guiding severe TBI (severe TBI) care in the USA and most European countries [31].
Box 1: Important Physiologic Principles of Cerebral Edema & Intracranial Pressure.
| •Monro-Kellie Hypothesis volume equilibrium between intracranial compartments of blood, CSF, brain parenchyma • CPP = MAP - ICP •Ohm’s Law: CBF =MAP - ICP /Resistance •Poiseuille’s Law: Resistance α1/radius4 Q = πr4 ΔP/ 8 ηL |
• Autoregulation maintaining CBF over a range of CPP • Compliance = ΔV/ΔP • Elastance = ΔP/ΔV • PVI (ml) =V/1og(Pp/Po) •Starling’s Forces driving water movement Jv=KO (πc - πi)+KH(Pc -Pi) |
CBF= cerebral blood flow; CPP= cerebral perfusion pressure; ICP= intracranial pressure; KO= osmotic conductivity filtration coefficient; KH= hydraulic conductivity filtration coefficient; L= vessel length; MAP = mean arterial pressure, η = viscosity, π = 3.14 in Poiseuille’s law; Pc= capillary hydrostatic pressure; Pi= interstitial hydrostatic pressure; πc= capillary oncotic pressure; πi= interstitial oncotic pressure; ΔP = pressure difference; Q= blood flow; Pp = peak ICP; Po= ICP before volume change; ΔV = change in volume
Box 1 Important physiologic principles of cerebral edema and intracranial pressure
Monro-Kellie, Compliance, and Elastance
Initially described in 1783, the Monro-Kellie doctrine states that with an intact skull, the intracranial volume is fixed and dependent on the individual components: brain parenchyma (essentially non-compressible), CSF, and blood [32]. Volume added to one compartment (e.g., contusion, cerebral edema) necessitates a decrease in one or all of the remaining components. Thus, within limits, and depending on the rate of volume expansion, increases in one compartment are buffered by compensatory changes in other compartments such as reduction in CSF, or vasoconstriction from hyperventilation resulting in decreased CBF per Poiseuille’s law (Box 1). Of note, autoregulation (the maintenance of CBF across a range of CPP values) may be regionally or globally disrupted after TBI.
An increasing recognition of the importance of this doctrine’s relationship with ICP developed in the 1960s, with the seminal mathematical volume-pressure description by Marmarou in 1973 [33-35]. Although initial increases in intracranial volumes are compensated for by readjustment in other compartments, once the compensatory mechanisms have been exhausted, ICP rises. The resultant increase in ICP may reflect both the degree of edema (i.e., volume added to the intracranial vault), as well the intracranial elastance/compliance which varies between individuals. Intracranial elastance is the change in pressure per unit change in volume (ΔP/ΔV); intracranial compliance (a more colloquial/familiar term) is the inverse of elastance, i.e., ΔV/ΔP (Box 1) [36]. Beyond a threshold that may also vary between individuals, this pressure volume relationship becomes exponential (Fig. 1) and requires therapeutic intervention to prevent ischemia and/or herniation. The pressure volume index (PVI) described by Marmarou (verified experimentally in a cat model) is the theoretical volume required to raise ICP tenfold, and is typically 20–25 ml (Box 1) [37]. For obvious reasons including potential concerns for provoking ICP crises, PVI is not measured clinically. Attention has shifted towards other tools such as ICP waveform evaluation or pressure reactivity indices (PRx) as discussed below (“Current management guidelines for intracranial hypertension” section).
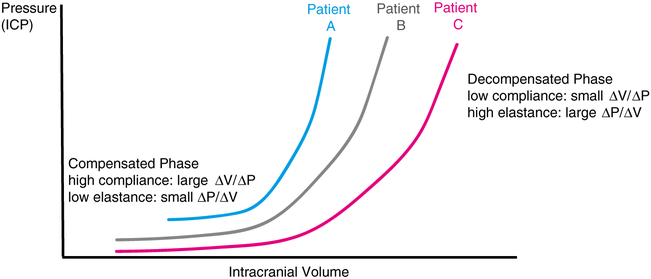
Fig. 1.
Intracranial pressure volume relationships. Classic curve demonstrating how intracranial pressure (ICP) is related to intracranial volume. In the compensated phase with low elastance, increase in intracranial volume does not significantly increase ICP. However, in the decompensated phase with high elastance, a small increase in intracranial volume exponentially increases ICP. Each patient has an individual pressure volume curve based on their respective baseline tissue properties and disease pathophysiology—schematic examples are provided in red (patient C), gray (patient B), and blue (patient A). At any given point, a patient may be located anywhere along the curve and these points may vary based on time as well as different brain regions
Current ICP Monitoring and Target Guidelines
Intracranial hypertension, particularly the pattern of ICP increase, “dose” of ICP, and ICP refractory to treatment is associated with unfavorable outcome and mortality after TBI [7-19, 38]. However, the benefit of ICP monitoring and management on clinical outcome lacks high-quality evidence. An exhaustive discussion of the most recent BTF guidelines [23] is beyond the scope of this review. There is no level 1 evidence to support either ICP monitoring or ICP-CPP targets in TBI. The 2017 BTF guidelines provide a level II-b recommendation for ICP monitoring to reduce in-hospital and acute (2 week) mortality, with a revised threshold of 22 mmHg. This threshold has increased from 20 mmHg compared with the 2007 version. Similarly, there is a level II-b recommendation to target CPP 60–70 mmHg to decrease 2-week mortality.
There exists significant controversy regarding the risks versus benefits of ICP monitoring as well as ICP- and CPP-directed treatment [31,38, 39]. A recent randomized controlled trial (RCT) performed in Bolivia and Ecuador by Chesnut et al. did not demonstrate superiority of ICP-guided therapy over imaging and clinical-examination-based care [39]. This was rapidly countered by a succession of large observational studies totaling > 14,000 patients, suggesting that ICP-guided treatment improves severe TBI mortality [39-43]. Some limitations of the Latin American RCT include a low treatment threshold (20 mmHg), external validity concerns due to increased mortality en route to a hospital, only 45% of patients transported by trained ambulance services, use of a composite endpoint and limited power to detect an effect on extended Glasgow Outcome Scale (GOS-E) score, and limited experience of some of the caregivers in managing severe TBI using ICP monitoring. Nonetheless, the data highlight how clinical and radiographic information may potentially complement ICP-directed therapy. While a commendable study, given its limitations and large body of contradictory observational data in the context of challenging decades of cemented practice, it has not changed standard of care in the USA.
Current Management Guidelines for Intracranial Hypertension
The 2017 BTF guidelines outline a systematic approach to ICP management [23]. There are no therapies with class 1 evidence available. Many institutional protocols follow a stepwise approach (Fig. 2).
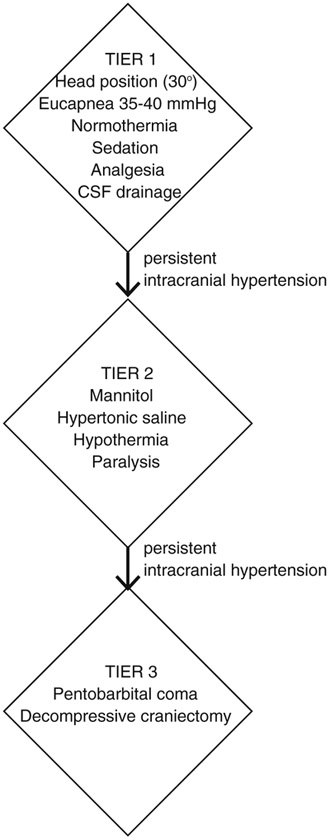
Fig. 2.
Example of tiered approach to intracranial hypertension. This schematic is one example of a potential graded approach to ICP management. The exact details may differ based on institutional/ individual practice. In general, tier 1 therapy involves less aggressive measures such as head positioning, eucapnia, normothermia, adequate sedation/analgesia, and CSF drainage. If intracranial hypertension persists (currently defined by the Brain Trauma Foundation as > 22 mmHg) despite these therapies, tier 2 reflect an escalation in care such as hyperosmolar therapy, hypothermia, or paralysis. Tier 3 strategies are utilized when there is refractory intracranial hypertension to tier 2 strategies. This typically indicates barbiturate coma or decompressive craniectomy
Tier 1
Within the first tier, adequate sedation and analgesia is necessary to minimize ICP spikes in patients with severe TBI; however, comparative data are lacking between agents and is often selected based on clinician discretion with regard to side-effect profile. While not specifically in TBI, there are emerging data from pediatric critical illness suggesting a potential role of pharmacogenomic responses and polymorphisms in selection of the ideal agents—in the referenced study, ABC1 genotypes were associated with response to fentanyl/dose requirements [44]. Also, a recent direct comparison of fentanyl to hypertonic saline therapy for ICP spikes in children favored hypertonic saline [45].
There are small retrospective studies demonstrating ICP reduction with continuous CSF drainage; however, the effect on mortality is unknown [46, 47]. The BTF level III recommendation is that continuous CSF drainage at the level of the midbrain may lower ICP more effectively than intermittent use [23].
Tier 2
Hyperosmolar therapy in the form of mannitol or varying concentrations of hypertonic saline (HTS) are commonly used to treat cerebral edema and intracranial hypertension after TBI. There are no randomized studies demonstrating clinical/ functional outcome improvement. Both agents effectively lower ICP in TBI and there are few prospective trials comparing their efficacy. Direct comparisons are challenging due to wide ranges of concentrations and formulations of HTS (1.9–23%, dextran containing vs. sodium chloride vs. sodium acetate). An equimolar comparison between mannitol and 15% HTS determined no difference in efficacy [48]. Recent meta-analyses suggest a marginal benefit of HTS, although the absolute difference of ~ 2 mmHg is of unclear clinical significance and the included trials had small sample sizes and significant methodological heterogeneity [22, 48, 49]. Of note, a prolonged hyperosmolar state from continuous HTS infusion results in cerebral adaptation with increasing CNS intracellular osmolyte production within 9–24 h, reaching a new steady state between 2 and 7 days. This theoretically reduces the oncotic-pressure gradient (driven by Starling forces, Box 1) and results in potential tolerance/reduced efficacy of continuous HTS infusions [50-53]. However, treatment with continuous HTS results in fewer episodes of intracranial hypertension in retrospective studies compared with hypotonic fluids, [54-57]. There are no trials comparing continuous infusions versus bolus therapy of HTS in TBI.
Hypothermia is another tier 2 strategy to reduce ICP. Although earlier studies of hypothermia as a treatment for intracranial hypertension were promising [58-61], a recent RCT (Eurotherm3235) evaluating the effect of hypothermia (32–35 °C) as a stage 2 treatment for ICP > 20 mmHg did not result in improved outcomes versus standard care [62•]. In this study, hypothermia effectively controlled ICP but there was a trend towards worse outcome. Of note, barbiturate treatment, a potential confounder and independent neuroprotectant, was used more frequently in the standard care group. Similar to cardiac arrest, questions remain regarding the appropriate degree of hypothermia versus targeted temperature management, versus “ultra-mild” hypothermia that may provide neuroprotection beyond fever prevention [63, 64]. There are no BTF recommendations regarding hypothermia for ICP control in adults, but there is a level 2 recommendation in children [65].
Tier 3
Decompressive craniectomy is considered a definitive treatment for intracranial hypertension. Nonetheless, its role, timing, and effect on functional outcome is debated. Results from an earlier RCT performed in Australia (DEcompressive CRAniectomy, DECRA) indicated that although early bifrontotemporoparietal decompressive craniectomy decreased ICP and length of ICU stay, it increased risk of unfavorable outcomes [20]. This did not change clinical practice likely due to several trial limitations including morbidity related to the extensive nature of surgical decompression potentially contributing to unfavorable outcomes/complications, biased differences in injury severity between groups (more severe patients in the craniectomy group), early timing of surgery, and the fact that it is not frequently practiced clinically in the USA. A subsequent RCT (RESCUEicp) evaluated the impact of craniectomy for refractory ICP and demonstrated a lower mortality and higher rate of upper severe disability (independent at home, requires assistance outside) compared with the medical group [21•]. However, there were also higher rates of vegetative state and lower severe disability, resulting in controversy regarding the overall benefit of surgery on functional outcome and highlighting the importance of preoperative discussions regarding expectations and potential quality of life.
None of the strategies discussed above represents targeted therapy against underlying mechanisms of cerebral edema; they are reactionary and non-specific. This approach is changing as research advances in molecular pathways contributing to cerebral edema are revealing promising potential targets (“Molecular Pathophysiology, Biomarkers, and Targeted Treatment of Cerebral Edema” section).
Future Personalization of Invasive Neuromonitoring
One concern with the current ICP guidelines is that they do not account for individual differences in injury patterns, secondary injury responses, or elastance/compliance. This may partially contribute to the observed outcome reported by Chesnut et al. where ICP-guided therapy did not improve outcomes. There has been an encouraging interest in developing precision medicine approaches to ICP- and CPP-based management including ICP waveform analysis, reactivity indices that evaluate autoregulation, and longitudinal trajectory analyses.
ICP Waveform Analysis
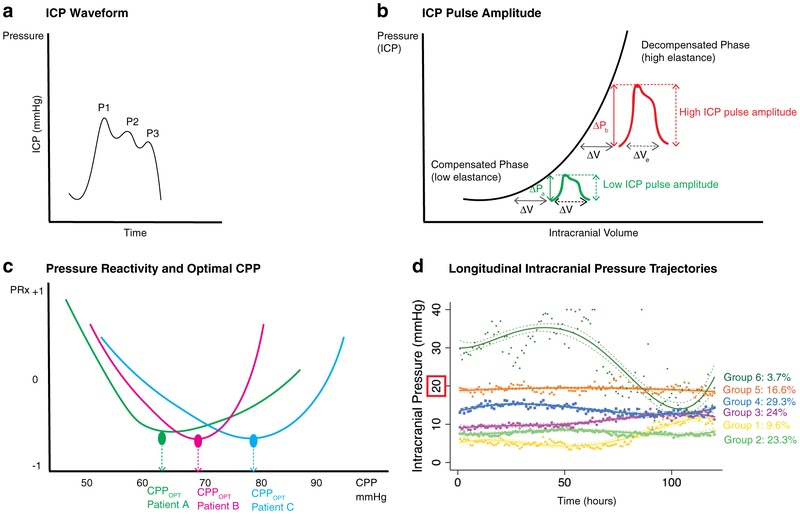
For each ICP measurement, the waveform has three consistent peaks: P1 (percussion wave), P2 (tidal wave), and P3 (dicrotic wave) as shown in Fig. 3A. ICP waveform pulse amplitude (ICPplse) is the magnitude of the pressure change (ΔP) that results from the pulsatile increase in cerebral blood volume (ΔV) occurring with each cardiac cycle. This amplitude, logically, is related to the intracranial elastance, i.e., with high elastance (ΔP/ΔV), a small increase in volume causes a large increase in pressure resulting in a large ICPplse (Fig. 3B). As with many basic physiologic principles, this relationship has been long recognized—it was first noted in 1866 by Leyden, and is now being exploited for potentially clinical utility [36]. Depending on compliance/elastance differences, ICPplse could vary within each patient, and between patients, thus providing an opportunity for individualized evaluation and potential intervention. In a recent work, Howell et al. describe an inverse correlation between ICPplse and Glasgow Motor Score (GMS) and a positive correlation with age. There was also a response noted with treatment where ICPplse significantly decreased after thiopental and craniectomy. Subsequent work evaluating the pulse amplitude index (PAx, derived from the correlation between mean arterial pressure and ICPplse) and correlation coefficient between ICPplse and CPP (RAC; R for correlation, A for amplitude, and C for CPP) that account for individual compliance and cerebral vasoreactivity have shown promise in prognosticating functional outcome even at low ICP values [28, 66, 67]. Limited data suggest their utility in determining optimal CPP (CPPOPT) similar to PRx [66]. Translation of these parameters into easily accessible bedside care may prove challenging. Nonetheless, although not ready for clinical practice, if validated, these continuous physiologic measures may provide an important opportunity for precision medicine.
Fig. 3.
Intracranial pressure waveforms, reactivity, and trajectories. 3A Basic ICP waveform comprising three peaks: P1 = percussion wave, P2 = tidal wave, P3 = dicrotic wave. 3B ICP pulse amplitude (ICPPLSE) demonstrated on a standard ICP-volume curve. In the compensated phase (low elastance), a small change in volume (e.g., with every cardiac cycle, ΔV in green), there is a small increase in ICP (ΔP in green), i.e., a low ICPPLSE. However, in the uncompensated phase/with high elastance, a small change in volume (ΔV in red) causes a large increase in ICP (ΔP in red), i.e., a large ICPPLSE. Modified from Jha et al., Meyers: Encyclopedia of Molecular Cell Biology and Molecular Medicine, In Press. 3C Graph of pressure reactivity index (PRx) on the Y axis with cerebral perfusion pressure (CPP) on the X axis. Curves are shown for three different patients: Patient A (green), patient B (red), and patient C (blue). The lowest (most negative) PRx is indicative of maximal pressure reactivity, thus identifying optimal CPP (CPPOPT). As evident from the three different patient curves, this value of CPPOPT is different for each individual. Modified from Jha et al., Meyers: Encyclopedia of Molecular Cell Biology and Molecular Medicine, In Press. 3D Graph of longitudinal intracranial pressure (ICP) trajectories over time in a cohort of severe TBI patients demonstrating six distinct trajectory groups. Trajectory groups are indicated in different colors with solid lines, and 95% confidence intervals in dashed lines. Individual measurements for each groups are shown in scatter points, color coordinate. Groups 1 (yellow) and 2 (green) have consistently low ICP with no/minimal spikes > 20 mmHg. Groups 3 (purple) and 4 (blue) have more frequent ICP spikes but no persistent intracranial hypertension. Group 5 (orange) has nearly continuous intracranial hypertension around 20 mmHg. Group 6 (dark green) has severe and rapid intracranial hypertension well above 20 mmHg. Modified from Jha et al., Crit Care Med, In Press
Pressure Reactivity Index
First described in 1997, PRx is another tool to evaluate cerebrovascular reactivity. This preceded PAx and RAC and consequently has the most data in support of its utility [68-72]. PRx is a moving correlation coefficient (range −1 to +1) between arterial pressure and ICP (40 consecutive samples averaged over 5 s). With intact autoregulation, increasing blood pressure reduces ICP (-PRx) versus with absent pressure reactivity, where ICP would passively rise (+PRx). Positive values, particularly > 0.25 have been associated with mortality, and those < 0.05 with favorable outcome [68, 69]. Persistently positive PRx for > 6 h demonstrating a long period of absent reactivity is similarly associated with unfavorable outcome [70, 71]. It follows that the lowest patient value of PRx reflects maximal pressure reactivity and allows identification of CPPOPT (Fig. 3C). Although some studies suggest that patients with mean CPP approximating CPPOPT have increased odds of favorable outcome, other work has indicated that PRx informing ICP-targeted management is more successful when PRx > 0.13 (i.e., impaired) while CPP-targeted management is favorable with intact pressure reactivity (PRx < 0.13) [69, 72, 73]. Unlike the waveform analysis parameters above, PRx does not incorporate measures of compliance/ elastance and its predictive value has been outperformed by PAx and RAC in a recent study evaluating multiple continuous cerebrovascular reactivity indices [28]. Importantly, like ICP, all of these measures of cerebrovascular reactivity and compliance are global measures that are not reflective of a process that is often heterogeneous [74]. Autoregulation, for example, may be intact in one brain region but impaired or even absent in another after TBI and thus the use of a single global metric to direct therapy could either appropriately or inappropriately impact different brain regions. This is an important limitation and underscores the need for eventual use in combination with other metrics including imaging, other advanced monitoring, molecular and/or biochemical markers, and biomarkers.
Longitudinal Trajectories
Point values of ICP (or any continuous variable/index) do not include information regarding potentially important temporal trends. Recent work has suggested that there are multiple different patterns of longitudinal ICP trajectories after severe TBI (Fig. 3D), and that these categories may prognosticate outcome [75]. Interestingly, although groups with persistent hypertension in this study predicted unfavorable outcome, so did groups that never/rarely experienced ICP values > 20 mmHg even in the absence of craniectomy. All groups prognosticating unfavorable outcome were found to have extremely low ICP variability, potentially reflective of cerebrovascular uncoupling. The presence of persistently low ICP may not reflect less cerebral edema/secondary injury—indeed in these patients, ICP may just not be an accurate reflection of this process that may still benefit from targeted treatment even though they do not reach the traditional “threshold” of 20–22 mmHg; rather, ICP may be low due to other factors such as age-related atrophy or compliance/elastance differences, or cerebrovascular uncoupling. There are many limitations to this exploratory work—the findings need to be validated, and, in their current form, these methods cannot categorize patients a priori into different groups, thus limiting the ability for immediate bedside translation. Nonetheless, the findings raise provocative questions about the utility of ICP monitoring in different groups, the potential need for different thresholds/ other biomarkers for cerebral edema, as well as patient selection for clinical trials.
Molecular Pathophysiology, Biomarkers, and Targeted Treatment of Cerebral Edema
ICP is an imperfect but practical measurement proxy for cerebral edema. It is a focal measure of a heterogeneous process, and does not reflect the complex multifaceted molecular pathways that culminate in cerebral edema. Acute brain swelling in contusions is thought to be secondary to the high osmolality of central necrotic tissue as characterized by Katayama et al. [4, 5, 76]. However, primary injury initiates a cascade of events that influence ion channels, cellular swelling, inflammation, and BBB integrity. Understanding these processes at a molecular level will facilitate biomarker and targeted therapy development.
Continuum of Ionic, Cytotoxic, and Vasogenic Edema
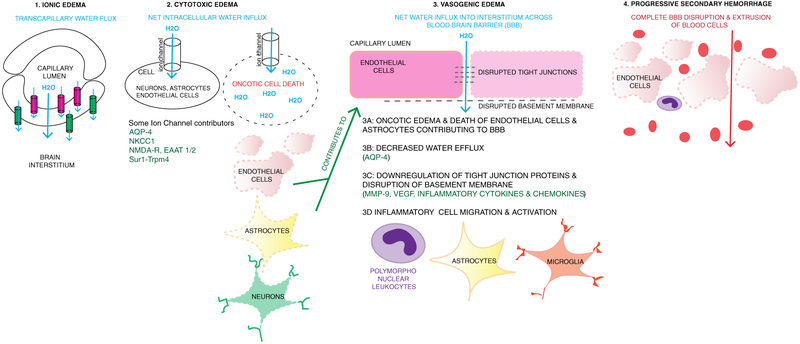
Historically, cerebral edema has been categorized as “cytotoxic” (cellular) edema versus “vasogenic” edema (from a leaky BBB); however, these distinctions are somewhat artificial since there is increasing evidence of mechanistic overlap between these forms of edema [3, 6, 77, 78]. As early as 1 h after TBI, ion pump failure/activation of select ion channels results in loss of homeostatic ionic gradients and water movement into the intracellular space [3, 14, 76]. This cellular swelling, a.k.a. cytotoxic edema, has been best characterized in astrocytes, however can occur in all cell types including endothelial cells and neurons [79]. When cytotoxic edema in endothelial cells results in oncotic cell death, it contributes to loss of integrity of the BBB and thus promotes vasogenic edema (which also occurs through a variety of additional processes including mechanical disruption, neuroinflammation, angiogenic factors, tight junction degradation, and cytoskeletal rearrangements) [3, 79]. In its extreme form, where there is complete dissolution of the BBB, progressive secondary hemorrhage occurs. Thus, cellular/cytotoxic edema, vasogenic edema, and secondary hemorrhage are inter-related on a continuum (Fig. 4) [80]. Ionic edema also occurs in endothelial cells and is analogous to cytotoxic edema; however, there is a polarity to transcapillary ion/water fluxes where water moves across the endothelial cell into the interstitium. Of note, isolated cellular swelling is merely redistribution of water from the interstitial to the intracellular space, thus does not increase absolute brain water content—this increase requires perfusion and is driven by external fluid entry into the central nervous system (CNS) via other sources such as the vasculature and/or glymphatic system [79-82]. In addition, it should be recognized that in some settings, the term “cellular swelling” may be preferable to the term cytotoxic edema, given that cellular swelling occurs predominantly in astrocyte foot processes and may often be less representative of a “toxic” than a homeostatic or mediator driven process such as glutamate uptake, potassium homeostasis, arachidonic acid release, and/or pH regulation [83, 84]. Thus, in its extreme form, for example, with endothelial cell rupture, cellular swelling may be cytotoxic, but at milder levels, it is homeostatic.
Fig. 4.
Continuum of ionic, cytotoxic and vasogenic edema, and progressive secondary hemorrhage. 1 Ionic edema involves transcapillary flux of ions and water across the capillary membrane. One hypothesis is that this is driven by osmotic forces. Ion channels expressed luminally (pink, such as NKCC1, Sur1-Trpm4) result in water movement into the endothelial cell, and then abluminal channels and transporters (green, e.g., Na+/K+ ATPase, Sur1-Trpm4) continue water movement across the cell into the interstitial space. The net movement of water (blue arrow) is from the capillary, across the endothelial cell, into the interstitial space. 2 Cytotoxic edema involves movement of water into cells including neurons, astrocytes, and endothelial cells. This occurs via various channels as listed including Sur1-Trpm4, NKCC1, EAAT1/2 and NMDA-R (glutamate channels), and AQP4. Intracellular influx of Na+ and water results in oncotic edema and cell death. When this occurs in endothelial cells and astrocytes containing podocytes that contribute to blood-brain barrier (BBB) integrity, it contributes to disruption of the BBB and vasogenic edema. 3 Vasogenic edema involves water and proteinaceous fluid movement across a disrupted BBB. Multiple mechanisms contribute to vasogenic edema including oncotic edema of endothelial cells and astrocytes (3A, 2), decreased water efflux via AQP4 (3B), downregulation and disruption of tight junction proteins and the basement membrane (3C) and recruitment and activation of inflammatory cells such as peripheral leukocytes, astrocytes, and microglia (3D.) Disruption and downregulation of tight junctions and basement membrane proteins involves many pathways including MMP- 9, VEGF, and inflammatory cytokines and chemokines. These are also involved in recruitment and activation of inflammatory cells. 4 Progressive secondary hemorrhage when mechanisms of cellular and vasogenic edema result in complete disruption of the BBB and extravasation of blood cells and products into the interstitium
Both cytotoxic and vasogenic components have been found to be significant contributors to cerebral edema after TBI, potentially occurring concurrently [3, 14, 85, 86]. The predominant edema type may vary based on injury phenotype, genetics, secondary insults, treatments, and timing after injury. Cytotoxic edema in humans has been reported within the first hour after TBI [76]. Vasogenic edema can also form early since BBB permeability is immediately influenced by mechanical shearing forces. Significant BBB permeability has been described within the first few hours post-injury in different TBI models, persisting for 3–4 days with a possible second phase after 5–7 days resulting from microglial activation [3, 87-91].
Key Molecular Contributors to Cerebral Edema: Mechanisms, Biomarkers, and Therapeutic Targets
A comprehensive review of all the pathways reported to contribute to cerebral edema is beyond the scope of this review; indeed, many of these pathways are likely yet to be discovered. The roles of some key players and their reported molecular mechanisms are highlighted. These were selected based on a growing body of evidence suggesting their importance including repeated observations by multiple laboratories and promise in terms of translation as biomarkers and/or clinical therapeutic targets. No new biomarkers or therapies are currently available for clinical use to aid in the diagnosis, monitoring, or treatment of cerebral edema, but they represent important avenues for future research. As discussed below, many of these pathways interact in a cybernetic fashion, suggesting potential synergistic effects of novel therapies targeting cerebral edema.
Aquaporin-4
Mechanism
Although both AQP1 and aquaporin-4 (AQP4) are prevalent in the CNS, the role of AQP4 in both vasogenic and cytotoxic edema is better characterized [92]. Supramolecular assemblies of the M23 AQP4 isoform tetramers have been reported to aggregate into orthogonal arrays and facilitate water movement [80, 92]. This pathway has reported connections with other molecular contributors to cerebral edema, including a tripartite-complex association between Sur1-Trpm4-AQP4, a glutamate uptake channel EAAT1/2, and inflammatory cytokines [79, 93, 94]. Increased intracellular water movement mediated by AQP4 channels on perivascular astrocytic foot processes contributes to cytotoxic edema. Decreased water elimination via AQP4 through perivascular astrocytic foot processes, subpial processes (into the subarachnoid CSF), subependymal processes (into the ventricle), and glymphatic processes contributes to vasogenic edema [81, 95, 96]. Given these opposing forces, the spatio-temporal expression of AQP4 may be important to determining (and treating) AQP4 contributions to edema formation versus elimination [80]. These challenges are likely one reason for conflicting reports in TBI where some studies report downregulation of AQP4 within the first 48 h [97-101], and others report upregulation [102-104]. AQP4 downregulation may specifically occur in regions of BBB disruption [97-101]. A study in murine closed head injury demonstrated a global increase in cortical and striatal AQP4 (peak day 7), with perivascular AQP4 downregulation by day 3, suggesting that changes in AQP4 localization and polarization while potentially worsening vasogenic edema may be a compensatory mechanism to counteract cytotoxic edema [105]. In AQP4 −/−mice subjected to mild-TBI, decreased astrocytic foot-process edema was observed; however, these effects were smaller than seen with AQP4 deletion in pure cytotoxic edema models, potentially a consequence of a concomitant decrease in AQP4-dependent clearance of vasogenic edema [106].
Biomarker
Study of AQP4 in human TBI is limited but promising. In a study containing 9 TBI patients, AQP4 expression was seen around the site of injury at 15 h, persisting at 8 days [107]. In 20 patients with TBI, CSF levels of AQP4 by Western blot were significantly higher vs. control [108]. Unexpectedly, AQP levels continued to increase in patients without intracranial hypertension, however remained unchanged in patients with multiple measures > 20 mmHg; the authors speculated a negative feedback loop whereby persistent intracranial hypertension impeded AQP4 expression. Although AQP4 polymorphisms have been associated with outcome after TBI, their ability to prognosticate development of cerebral edema or intracranial hypertension remains to be defined [109]. Findings from these exploratory human studies are consistent with underlying pathophysiology but require validation.
Therapeutic Target
There are no human studies on AQP4 inhibition after severe TBI. In vivo TBI studies have shown conflicting results—many with improved edema, however a few with no-effect or even increased edema [99, 105, 106, 110-112]. In non-TBI models limited primarily to cytotoxic edema (such as water intoxication), AQP4−/− mice have reduced edema; however, in models with predominantly vasogenic edema (tumors, subarachnoid hemorrhage, abscess), there is worsening of edema [92, 113, 114] potentially consistent with the aforementioned importance of AQP4 in water elimination [81, 95, 96]. There are isolated reports of pharmacologic AQP4 inhibition showing benefit on edema reduction in TBI with multiple compounds via different mechanisms including AER-271, astaxanthin, ghrelin, and propofol that warrant further exploration in corroboratory preclinical work prior to translation to humans [115-119].
Glutamate
Mechanism
Glutamate excitotoxicity has been a subject of research across a spectrum of neuroinflammatory, neurode-generative and neuropsychiatric disorders including TBI for over 30 years [120-123]. Post-TBI glutamate excitotoxicity may have harmful contributions to many secondary injury cascades including cerebral edema. However, there may also be a delayed benefit to neuronal survival [124]. Increased extracellular glutamate after injury is related to processes such as synaptic release, cellular/neuronal lysis, and spreading depolarizations; levels may reach >200 μM [79]. This value far exceeds concentrations of 5–50 μM that result in astrocytic edema via EAAT1/2 channels that mediate glutamate influx by co-transporting Na+ and water [79, 80]. The impact of glutamate on cellular edema may also occur via other pathways including the ionotropic NMDA-receptor (causing intracellular influx of Na + and Ca2+), metabotropic receptors that may increase BBB permeability, or increasing endothelial cell apoptosis via nitric oxide and reactive oxygen species pathways [3].
Biomarker
Preclinical studies have reported elevated extracellular glutamate concentrations after TBI [125, 126]. Research in human patients has confirmed a significant increase in local microdialysate glutamate concentration post-TBI [127-131]. Earlier studies from different groups have demonstrated a consistent/reproducible association between elevated microdialysate glutamate concentration and intracranial hypertension, most pronounced in contusions and patients with secondary ischemia [127-129, 131]. Concentrations > 20 μmol/L were associated with sustained ICP and 6-month outcome [127, 129]. Longitudinal patterns of glutamate levels have been associated with mortality and outcome—declining levels have favorable prognoses versus patients with rising or sustained elevations in glutamate [127]. The concept of longitudinal patterns appears to be a consistent theme, observed for ICP trajectories and other biomarkers (Sur1) [26]. A recent human TBI study (n = 20) correlated CSF glutamate and lactate levels with 3-day mortality; however, prediction on ICP or cerebral edema measures was not performed [130].
Therapeutic Target
Glutamate inhibition has been studied across many neurological disorders using multiple approaches including pre-/postsynaptic receptor antagonists, competitive antagonists, and non-competitive antagonists such as MK-801, selfotel, magnesium, memantine, ketamine, amantadine, and NA-1 [127, 132-136]. Some of these compounds have been tested in human TBI trials however failed to show clinical benefit (ICP/cerebral edema were not evaluated as outcome measures) [136-138]. NA-1, an eicosapeptide that inhibits the interactions of postsynaptic scaffolding protein PSD-95 to NMDA glutamate receptors, reduced the number of ischemic microemboli in patients undergoing endovascular repair of intracranial aneurysms and is emerging as a potential neuroprotectant. It is being evaluated in a multicenter phase 3 randomized placebo-controlled clinical trial of acute stroke (ClinicalTrials.gov NCT02930018). Reducing ischemic effects may also decrease cytotoxic edema after TBI, given the well-recognized relationship between excitotoxicity and intracranial hypertension [139].
Inflammatory Chemokines and Cytokines
Mechanism
The role of neuroinflammation in TBI is complex and detailed in other reviews [140, 141]. It plays a complex role in both injury and repair. Release of damage-associated molecular patterns with both primary and secondary injury initiates an immune response including release of pro-inflammatory cytokines (tumor necrosis factor (TNF); interleukins IL-6, IL-8, IL-1β; tumor growth factor (TGF-β)); chemokines (CXCL1, CXCL2, CCL2); inflammatory cell adhesion molecules (intracellular adhesion molecule (ICAM); vascular adhesion molecule (VCAM)); recruitment of systemic inflammatory cells (first neutrophils, and subsequently monocytes and lymphocytes); activation of resident cells (microglia, astrocytes, neurons, endothelial cells); activation of pathways involving AQP4, MMP, Sur1-Trpm4, and bradykinin; and decreased expression of tight junction proteins (claudin, occludin) [3, 87, 124, 140, 142-158].These processes are interconnected (e.g., release of chemokines further recruits and activates inflammatory cells), and contribute to BBB disruption and vasogenic edema after TBI [80].
Biomarker
TGF-β, a pro-inflammatory cytokine associated with BBB disruption, has demonstrated a dose-dependent effect on paracellular permeability of the CNS endothelium in vitro [159]. In a small human study of TBI (n = 22), elevated CSF TGF-β was similarly associated with increased BBB permeability as measured by CSF/serum albumin quotient [151]. Increased CSF levels of IL-8, another upregulated pro-inflammatory cytokine after TBI known to increase BBB permeability, were associated with mortality in children, and BBB permeability in adults [152-155]. CSF complement levels (C3 and the soluble complement-derived membrane attack complex) have also been associated with post-TBI BBB permeability [160, 161]. A recent study in 114 patients used principal component analysis and identified CSF inflammatory biomarker profiles (containing high levels of soluble-ICAM-1, sFAS, IL-10, IL-6, sVCAM-1, IL-5, and IL8) highly predictive of unfavorable 6-month outcome [162]. Effect on ICP/other measures of edema were not evaluated.
Therapeutic Target
High dose corticosteroids, while antiinflammatory with effects on some of the aforementioned targets and reported benefit against cerebral edema, are not recommended in TBI [23]. Their utility, initially discovered against tumoral edema, was expanded to other neurological diseases including TBI in the 1960s. Subsequent RCTs were conflicting; however, after the large randomized placebo-controlled trial (Corticosteroid Randomization After Significant Head injury, CRASH, n = 10,008) demonstrating increased 2-week and 6-month mortality resulted in a level-I recommendation against their use in severe TBI [163, 164]. It remains unclear whether this finding resulted from a failure of corticosteroid treatment to mitigate key detrimental effects of neuroinflammation in TBI, the possibility that important beneficial effects of neuroinflammation were blunted, and/or that unwanted side effects of steroid therapy negated any potential benefit.
Another potential treatment class with anti-inflammatory effects that may decrease cerebral edema is the peroxisome proliferator-activated receptor agonists such as pioglitazone and rosiglitazone. In vitro and in vivo, these have been shown to inhibit pathways implicated in vasogenic edema including cytokines (TNF, IL-1β), NF-κB, ICAM-1, and matrix metalloproteinase-9 (MMP-9) with resultant BBB stabilization [3, 165-168]. There are also purported benefits with reduction in lesion volume and neuronal survival [169, 170]. There are clinical studies of pioglitazone and rosiglitazone in stroke, multiple sclerosis, intracerebral hemorrhage, and brain tumors—while some of these trials have demonstrated promising anti-inflammatory effects, none directly assess the impact on BBB/vasogenic edema [171-175]. The trial in multiple sclerosis demonstrated decreased IL-6 and TNF-α in patients treated with pioglitazone [174]. At the time of this review, there are no human studies in TBI, although preclinical studies suggest that this may be a valuable target for exploration.
Finally, contemporary studies have suggested an important role for the danger signal high mobility group Box 1 (HMGB1) in initiating a molecular cascade that involves toll-like receptor signaling and result cytokine-mediated up-regulation of AQP4 with resultant cerebral edema and BBB permeability [94]. Several reports support that possibility including studies showing increased CSF levels of HMGB1 in clinical TBI [176]. However, recent targeted studies of TBI in conditional HMGB1 knockout mice failed to reveal any effect on either cerebral edema or BBB permeability vs. wild-type control mice (Aneja et al., Crit Care Med, ePub ahead of print). The role of this pathway in the development of cerebral edema remains to be more fully defined.
Matrix Metalloproteinase-9
Mechanism
MMP-9 is a zinc-dependent endopeptidase zymogen implicated in BBB disruption and vasogenic edema from extracellular matrix degradation (including basal lamina, tight junction proteins such as occludin, zona-occludins-1, c-Jun-N-Terminal-kinase signaling) and activation of cytokines and chemokines [3, 6, 124, 177]. It is predominantly expressed in neutrophils but is present in endothelial cells, other leukocytes, and weakly in astrocytes and neurons and released into the extracellular space after injury [3, 80, 124]. Preclinical studies in TBI suggest a connection between MMP-9 and the Na+-K+-2CL− cotransporter NKCC1 [178].
Biomarker
High microdialysate MMP-9 levels have been detected early in a small study of severe TBI (12–18 h, n = 8), with declining levels over time [179], and there was a trend towards higher MMP-9 levels in patients with severe brain tissue hypoxia. Interestingly, elevated MMP-8 but not MMP-9 levels in this study were associated with mortality. Another TBI study (n = 12) reported elevated peri-contusional microdialysate MMP-9 concentrations (versus contralateral) [144]. In large-hemispheric stroke studies, serum MMP-9 levels consistently correlate with malignant cerebral edema and were responsive to Sur1/Trpm4 inhibition with glibenclamide as discussed in the “Sulfonylurea Receptor 1- Transient Receptor Potential Cation Channel Subfamily M Member 4 (SUR1- TRPM4)” section [180-184]. While the data are limited, based on available research, MMP-9 appears to be a promising potential biomarker of cerebral edema and BBB breakdown that warrants further exploration in human TBI.
Therapeutic Target
Preclinical TBI models with MMP-9 inhibition (including SB-3CT, a highly selective MMP-2 and MMP-9 inhibitor) have shown improvement in BBB integrity (from reduced tight junction protein degradation and decreased vascular permeability) attenuated microglial activation, and reduced lesion volume; the effect on outcome has been controversial [145, 185-190]. In part, this may be due to the involvement of MMP-9 in neurovascular modeling and repair [124, 191]; timing of MMP-9 inhibition may thus be critical in terms of impact on edema and outcome. Glibenclamide, an indirect MMP inhibitor, has shown benefit against edema reduction in clinical stroke trials (“Sulfonylurea Receptor 1- Transient Receptor Potential Cation Channel Subfamily M Member 4 (SUR1- TRPM4)” section) [180, 184]; however, MMP-9 inhibition has not been studied in human TBI. Future studies evaluating the effect of MMP-9 inhibition in TBI are warranted.
NKCC1
Mechanism
NKCC1 is a Na+-K+2Cl− cotransporter that mediates ion and water transport across the plasma membrane to regulate volume/ion homeostasis [178]. In the presence of ATP, luminal NKCC1 transports Na+ into endothelial cells, which is then expelled into the interstitium by abluminal Na+- K+-ATPase. NKCC1 is rapidly upregulated after preclinical TBI (within 1 h), resulting in cellular swelling due to increased ion and water influx and reduced efflux. In animal models, the upregulation and contribution of NKCC1 to both cytotoxic and vasogenic edema are connected with other pathways including glutamate, IL-1β,MMP-9, and AQP4[115,178,192-196].
Biomarker
There are no reported studies of NKCC1 as a biomarker in humans.
Therapeutic Target
Bumetanide treatment (inhibiting NKCC1) in vitro reduced astrocyte swelling after FPI [192, 197]. Both cellular and vasogenic edema/BBB disruption were reduced in in vivo TBI models [115,178, 193-196]. Although potential synergy between bumetanide and glibenclimide has been proposed (the “Sulfonylurea Receptor 1- Transient Receptor Potential Cation Channel Subfamily M Member 4 (SUR1- TRPM4)” section) due to the opposite dependencies on intracellular ATP [194], this has not been experimentally evaluated. Several preclinical studies in in vitro and animal models of TBI support benefit of bumetanide or NKCC1 knockout mice on cerebral edema and neuronal death (Lu et al., Eur J Pharmacol 2006; 548:99–105; Lu et al., Neurol Res 29:404–9; Jayakumar et al., J Neurochem 2011; 117:437–438; Hui et al., Neurochem Int 2016; 94:23–31).
Sulfonylurea Receptor 1- Transient Receptor Potential Cation Channel Subfamily M Member 4 (SUR1- TRPM4)
Mechanism
First reported in ischemic stroke, Sur1 is upregulated after various forms of CNS injury (including TBI) and associates with a non-selective cation channel (Trpm4) causing channel opening, sodium (and water) influx, oncotic edema, and ultimately cell death [198, 199]. The Sur1-Trpm4 channel (an octamer comprising four Sur1 subunits and four Trpm4 subunits) is not present in the CNS under normal conditions, thus rendering it a unique potential target [199]. After TBI, it is upregulated in neurons, astrocytes, and endothelial cells, contributing to cytotoxic edema. With oncotic cell death of endothelial cells disrupting the BBB, this pathway also contributes to vasogenic edema and progressive secondary hemorrhage [198].
Biomarker
In a small prospective study of severe TBI (n = 28), CSF Sur1 levels were elevated in patients and undetectable in controls (patients with normal pressure hydrocephalus) [26]. Mean and peak Sur1 levels were higher in patients with radiographic cerebral edema. Similar to other biomarkers where longitudinal trajectories appear relevant (glutamate, Vascular endothelial growth factor (VEGF), MMP-9), no patients with declining Sur1 levels between 48 and 72 h had any episodes of intracranial hypertension. Spatially clustered ABCC8 (encoding Sur1) polymorphisms contained within a region of DNA encoding the Sur1-receptor site and Trpm4-pore interface have been reported to predict measures of cerebral edema (radiographic, mean/peak ICP, ICP trajectories) and outcome after severe TBI [30, 200, 201]. In the same cohort, clustered TRPM4 polymorphisms (contained within a region of DNA encoding the channel pore) also predicted intracranial hypertension after TBI and had important interactions with ABCC8 polymorphisms [202]. These studies all require validation but are consistent with known pathophysiology and exciting avenues for future research.
Therapeutic Target
In preclinical models, Sur1-Trpm4 inhibition with glibenclamide (aka glyburide) has shown promising edema-reducing effects (measured by brain water and ICP), as well as decreased progressive secondary hemorrhage [198, 203, 204]. There are also reported improvement in functional outcome and contusion volume [205, 206]. In human trials of ischemic stroke, this therapy has also demonstrated impact on edema reduction [180, 184]. Treatment with oral glibenclamide in two small RCTs showed decreased contusion expansion rate and improved short-term outcome [207, 208]. Results from a third placebo-controlled clinical trial in TBI ( NCT01454154) are pending, and a large phase II study is planned. Of all the targets mentioned, this currently appears the closest to clinical translation.
Vascular Endothelial Growth Factor-A
Mechanism
Vascular endothelial growth factor-A (VEGF-A) is a secreted glycoprotein upregulated in astrocytes, CNS endothelium, and macrophages after TBI that increases micro-vascular permeability and angiogenesis both in vitro and in vivo [209, 210]. Increased microvascular permeability is thought to occur via downregulation and/or degradation/ubiquination of tight junction proteins [211-213], and angiogenesis via recruitment of endothelial progenitor cells [214]. VEGF-A is activated by MMP-9, and similarly is also involved in neurogenesis and repair [215], thus making the timing of modulation important to any potential benefit on edema/outcome [80].
Biomarker
In rat microdialysate, VEGF was one of four growth factors with significantly elevated levels after fluid percussion injury versus sham; however, no association was demonstrated with vasogenic edema, BBB integrity, or outcome [216]. Increased levels of VEGF were seen in CSF peaking early after severe TBI in children [217]. In adults (n = 70) evaluating longitudinal VEGF trajectories, plasma VEGF levels initially decreased in the first 24 h after TBI, subsequently rose (day 4), peaked at day 14, and normalized by day 21 [218]. In this study, VEGF alone did not predict mortality or clinical course. However, its relationship to cerebral edema and/or ICP was not evaluated and merits further study in TBI.
Therapeutic Target
VEGF inhibition has been reported using vascular endothelial growth inhibitor (VEGI) or a VEGF-antibody, bevacizumab. Modulation of this pathway in fluid percussion injury using exogenous VEGI reduced tissue loss and upregulated tight junction proteins to protect the BBB [219]. Bevacizumab has demonstrated beneficial effects on the BBB and vasogenic edema in glioblatoma multiforme [220]. However, in controlled-cortical impact, bevacizumab treatment resulted in worsened contusion volume and neurologic deficits without decreasing vasogenic edema or BBB permeability, thus highlighting the potential importance of VEGF in angiogenesis/repair [221].
Conclusions
Our understanding the pathophysiology of cerebral edema after TBI is incomplete. The degree to which the processes discussed in this review are harmful versus protective remains to be defined and is crucial to informing future therapeutic modulation. Severe intracranial hypertension, while associated with mortality, is not generally associated with functional outcome in severe TBI survivors [38, 201, 222-227]. Conversely, cerebral edema that does not result in intracranial hypertension due to other factors such as age, atrophy, or elastance may impact outcome unfavorably. The current generic standard of care does not phenotype patients with severe TBI using anatomic, physiologic, genetic, and/or molecular approaches which represents a roadblock to understanding the pathobiology of cerebral edema and developing optimal targeted therapies to prevent or mitigate its development and/or its deleterious effects. However, many such efforts are underway. There are innumerable pathways contributing to cerebral edema, some of which may remain to be discovered; however, identification of key interconnected participants such as those described in this review provides an important foundation. Advances in technologies including biomarkers, genetics, “omics,” imaging, and neuromonitoring may eventually enable rapid bedside stratification of severe TBI patients based on individual pathophysiology—optimizing edema targeting strategies. However, integrating these data into a clinically usable format will likely be challenging and require significant collaborative and financial research support to facilitate translation. Multicenter preclinical and clinical research initiatives will be imperative such as Operation Brain Trauma Therapy (OBTT), Transforming Research and Clinical Knowledge in TBI (TRACK-TBI), and Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) [228-230], among others. Analogous to cancer chemotherapies, eventual treatment of cerebral edema may be multi-drug regimens targeting specific molecular and monitoring signatures, coupled to optimized physiology-based interventions and/or surgery. Quo vadis? Into the future.
Abbreviations
- AQP4
Aquaporin 4
- BBB
Blood-brain barrier
- BTF
Brain Trauma Foundation
- CBF
Cerebral blood flow
- CNS
Central nervous system
- CPP
Cerebral perfusion pressure
- CPPOPT
Optimal CPP
- CSF
Cerebrospinal fluid
- CXCL
Chemokine (C-X-C motif) ligand
- EAAT
Excitatory amino acid transporter
- GOS-E
Glasgow Outcome Scale-Extended
- GMS
Glasgow Motor Score
- HTS
Hypertonic saline
- ICAM
Intracellular adhesion molecule
- ICP
Intracranial pressure
- ICPplse
ICP pulse amplitude
- ICU
Intensive care unit
- IL
Interleukin
- MAP
Mean arterial pressure
- MMP-9
Matrix metalloproteinase-9
- NKCC1
Na+-K+2Cl− cotransporter
- NMDA
N-methyl-D-aspartate
- OBTT
Operation brain trauma therapy
- PAx
Pulse amplitude index
- PPAR
Peroxisome proliferator-activated receptor
- PVI
Pressure volume index
- RAC
Correlation coefficient between ICPplse and CPP; R for correlation, A for amplitude, and C for CPP
- RCT
Randomized controlled trial
- Sur1-Trpm4
Sulfonylurea receptor 1-transient receptor potential cation channel subfamily M member 4
- TBI
Traumatic brain injury
- TGF
Tumor growth factor
- TNF
Tumor necrosis factor
- VCAM
Vascular cell adhesion molecule
- VEGF
Vascular endothelial growth factor
- VEGI
Vascular endothelial growth inhibitor
Footnotes
Conflict of Interest Ruchira M. Jha reports grants from NIH/NINDS 1K23NS101036, grants from Dean’s Faculty Advancement Award, personal fees from Biogen, during the conduct of the study. Dr. Kochanek is supported by W81XWH-17-C-0064 from the U.S. Department of Defense
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.•.Stocchetti N, Carbonara M, Citerio G, Ercole A, Skrifvars MB, Smielewski P, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16:452–64Current review on management of severe TBI in the intensive care unit.
- 2.Stocchetti N, Maas AIR. Traumatic intracranial hypertension. N Engl J Med. 2014;370:2121–30. [DOI] [PubMed] [Google Scholar]
- 3.Winkler EA, Minter D, Yue JK, Manley GT. Cerebral edema in traumatic brain injury: pathophysiology and prospective therapeutic targets. Neurosurg Clin N Am. 2016;27:473–88. [DOI] [PubMed] [Google Scholar]
- 4.Katayama Y, Mori T, Maeda T, Kawamata T. Pathogenesis of the mass effect of cerebral contusions: rapid increase in osmolality within the contusion necrosis. Acta Neurochir Suppl. 1998;71: 289–92. [DOI] [PubMed] [Google Scholar]
- 5.Katayama Y, Kawamata T. Edema fluid accumulation within necrotic brain tissue as a cause of the mass effect of cerebral contusion in head trauma patients. Acta Neurochir Suppl (Wien). 2003;86:323–7. [DOI] [PubMed] [Google Scholar]
- 6.Jha RM, Kochanek PM, Simard JM. Central nervous system trauma: pharmacological and therapeutic approaches. Encycl Mol Cell Biol Mol Pharmacol. [Google Scholar]
- 7.Feldmann H, Klages G, Gärtner F, Scharfenberg J. The prognostic value of intracranial pressure monitoring after severe head injuries. Acta Neurochir Suppl (Wien). 1979;28:74–7. [DOI] [PubMed] [Google Scholar]
- 8.Feickert HJ, Drommer S, Heyer R. Severe head injury in children: impact of risk factors on outcome. J Trauma. 1999;47:33–8. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–16. [DOI] [PubMed] [Google Scholar]
- 10.Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56:498–503. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg HM, Gary HE, Aldrich EF, Saydjari C, Turner B, Foulkes MA, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–98. [DOI] [PubMed] [Google Scholar]
- 12.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. [DOI] [PubMed] [Google Scholar]
- 13.Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part I: the significance of intracranial pressure monitoring. J Neurosurg. 1979;50:20–5. [DOI] [PubMed] [Google Scholar]
- 14.Hudak AM, Peng L, Marquez de la Plata C, Thottakara J, Moore C, Harper C, et al. Cytotoxic and vasogenic cerebral oedema in traumatic brain injury: assessment with FLAIR and DWI imaging. Brain Inj. 2014;28:1602–9. [DOI] [PubMed] [Google Scholar]
- 15.Iaccarino C, Schiavi P, Picetti E, Goldoni M, Cerasti D, Caspani M, et al. Patients with brain contusions: predictors of outcome and relationship between radiological and clinical evolution. J Neurosurg. 2014;120:908–18. [DOI] [PubMed] [Google Scholar]
- 16.Tucker B, Aston J, Dines M, Caraman E, Yacyshyn M, McCarthy M, et al. Early brain edema is a predictor of in-hospital mortality in traumatic brain injury. J Emerg Med. 2017;53:18–29. [DOI] [PubMed] [Google Scholar]
- 17.Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008;109:678–84. [DOI] [PubMed] [Google Scholar]
- 18.Marmarou A, Anderson RL, Ward JD, Choi SC, Young HF, Eisenberg HM, et al. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg. 1991;75:S59–66. [Google Scholar]
- 19.Stocchetti N, Zanaboni C, Colombo A, Citerio G, Beretta L, Ghisoni L, et al. Refractory intracranial hypertension and “second-tier” therapies in traumatic brain injury. Intensive Care Med. 2008;34:461–7. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–502. [DOI] [PubMed] [Google Scholar]
- 21.•.Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl JMed. 2016;375:1119–30Recent landmark randomized controlled trial evaluating decomrpessive craniectomy for intracranial hypertension after TBI.
- 22.Kamel H, Navi BB, Nakagawa K, Hemphill JC, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011;39:554–9. [DOI] [PubMed] [Google Scholar]
- 23.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80:6–15. [DOI] [PubMed] [Google Scholar]
- 24.•.Nielson JL, Cooper SR, Yue JK, Sorani MD, Inoue T, Yuh EL,et al. Uncovering precision phenotype-biomarker associations in traumatic brain injury using topological data analysis. PLoS One. 2017;12:e0169490.Although not focused on severe TBI, this article provides an approach towards harnessing available technologies, big data (including genetics, biomarker information), and complex phenotyping to advance precision medicine in the field.
- 25.Jha RM, Kochanek PM. Adding insight to injury: a new era in neurotrauma. Lancet Neurol. 2017;16:578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha RM, Puccio AM, Chou SH-Y, Chang C-CH, Wallisch JS, Molyneaux BJ, et al. Sulfonylurea receptor-1: a novel biomarker for cerebral edema in severe traumatic brain injury. Crit Care Med. 2017;45:e255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias C, Silva MJ, Pereira E, Monteiro E, Maia I, Barbosa S, et al. Optimal cerebral perfusion pressure management at bedside: a single-center pilot study. Neurocrit Care. 2015;23:92–102. [DOI] [PubMed] [Google Scholar]
- 28.Zeiler FA, Donnelly J, Smielewski P, Menon DK, Hutchinson PJ, Czosnyka M. Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J Neurotrauma. 2018;35:1107–15. [DOI] [PubMed] [Google Scholar]
- 29.Jha RM, Puccio AM, Okonkwo DO, Zusman BE, Wallisch JS, Shutter LA, et al. ABCC8 tag single nucleotide polymorphisms correlate with edema and outcome in traumatic brain injury. Crit Care Med. 2016;44:260. [Google Scholar]
- 30.Jha RM, Koleck TA, Puccio AM, Okonkwo DO, Park S-Y, Zusman BE, et al. Regionally clustered ABCC8 polymorphisms in a prospective cohort predict cerebral oedema and outcome in severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchinson PJ, Kolias AG, Czosnyka M, Kirkpatrick PJ, Pickard JD, Menon DK. Intracranial pressure monitoring in severe traumatic brain injury. BMJ. 2013;346:f1000. [DOI] [PubMed] [Google Scholar]
- 32.Macintyre I A hotbed of medical innovation: George Kellie (1770-1829), his colleagues at Leith and the Monro-Kellie doctrine. J Med Biogr. 2014;22:93–100. [DOI] [PubMed] [Google Scholar]
- 33.Stern WE. Intracranial fluid dynamics: the relationship of intracranial pressure to the Monro-Kellie doctrine and the reliability of pressure assessment. J R Coll Surg Edinb. 1963;9:18–36. [PubMed] [Google Scholar]
- 34.Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978;48:332–44. [DOI] [PubMed] [Google Scholar]
- 35.Marmarou A A theoretical and experimental evaluation of the cerebrospinal fluid system (PhD Thesis). Philadelphia: Drexel University; 1973. [Google Scholar]
- 36.Hawthorne C, Piper I. Monitoring of intracranial pressure in patients with traumatic brain injury. Front Neurol. 2014;5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg. 1975;43:523–34. [DOI] [PubMed] [Google Scholar]
- 38.Chesnut R, Videtta W, Vespa P, Le Roux P. Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Intracranial pressure monitoring: fundamental considerations and rationale for monitoring. Neurocrit Care. 2014;21(Suppl 2):S64–84. [DOI] [PubMed] [Google Scholar]
- 39.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farahvar A, Gerber LM, Chiu Y-L, Carney N, Härtl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012;117:729–34. [DOI] [PubMed] [Google Scholar]
- 41.Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013;30:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talving P, Karamanos E, Teixeira PG, Skiada D, Lam L, Belzberg H, et al. Intracranial pressure monitoring in severe head injury: compliance with Brain Trauma Foundation guidelines and effect on outcomes: a prospective study. J Neurosurg. 2013;119:1248–54. [DOI] [PubMed] [Google Scholar]
- 43.Gerber LM, Chiu Y-L, Carney N, Härtl R, Ghajar J. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2013;119:1583–90. [DOI] [PubMed] [Google Scholar]
- 44.Horvat CM, Au AK, Conley YL, Kochanek PM, Li L, Poloyac SL, et al. ABCB1 genotype is associated with fentanyl requirements in critically ill children. Pediatr Res. 2017;82:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shein SL, Ferguson NM, Kochanek PM, Bayir H, Clark RSB, Fink EL, et al. Effectiveness of pharmacological therapies for intracranial hypertension in children with severe traumatic brain injury—results from an automated data collection system time-synched to drug administration. Pediatr Crit Care Med. 2016;17:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nwachuku EL, Puccio AM, Fetzick A, Scruggs B, Chang Y-F, Shutter LA, et al. Intermittent versus continuous cerebrospinal fluid drainage management in adult severe traumatic brain injury: assessment of intracranial pressure burden. Neurocrit Care. 2014;20:49–53. [DOI] [PubMed] [Google Scholar]
- 47.Griesdale DEG, McEwen J, Kurth T, Chittock DR. External ventricular drains and mortality in patients with severe traumatic brain injury. Can J Neurol Sci. 2010;37:43–8. [DOI] [PubMed] [Google Scholar]
- 48.Sakellaridis N, Pavlou E, Karatzas S, Chroni D, Vlachos K, Chatzopoulos K, et al. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J Neurosurg. 2011;114:545–8. [DOI] [PubMed] [Google Scholar]
- 49.Mortazavi MM, Romeo AK, Deep A, Griessenauer CJ, Shoja MM, Tubbs RS, et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg. 2012;116:210–21. [DOI] [PubMed] [Google Scholar]
- 50.Gullans SR, Verbalis JG. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289–301. [DOI] [PubMed] [Google Scholar]
- 51.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–9. [DOI] [PubMed] [Google Scholar]
- 52.McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–6. [DOI] [PubMed] [Google Scholar]
- 53.Doyle JA, Davis DP, Hoyt DB. The use of hypertonic saline in the treatment of traumatic brain injury. J Trauma. 2001;50:367–83. [DOI] [PubMed] [Google Scholar]
- 54.Hauer E-M, Stark D, Staykov D, Steigleder T, Schwab S, Bardutzky J. Early continuous hypertonic saline infusion in patients with severe cerebrovascular disease. Crit Care Med. 2011;39:1766–72. [DOI] [PubMed] [Google Scholar]
- 55.Wagner I, Hauer E-M, Staykov D, Volbers B, Dörfler A, Schwab S, et al. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke. 2011;42:1540–5. [DOI] [PubMed] [Google Scholar]
- 56.Froelich M, Ni Q, Wess C, Ougorets I, Härtl R. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37:1433–41. [DOI] [PubMed] [Google Scholar]
- 57.Diringer MN. New trends in hyperosmolar therapy? Curr Opin Crit Care. 2013;19:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu W, Zhang Y, Sheng H, Zhang J, Wang W, Liu W, et al. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22:229–35. [DOI] [PubMed] [Google Scholar]
- 59.Jiang J-Y, Xu W, Li W-P, Gao G-Y, Bao Y-H, Liang Y-M, et al. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:771–6. [DOI] [PubMed] [Google Scholar]
- 60.Yan Y, Tang W, Deng Z, Zhong D, Yang G. Cerebral oxygen metabolism and neuroelectrophysiology in a clinical study of severe brain injury and mild hypothermia. J Clin Neurosci. 2010;17:196–200. [DOI] [PubMed] [Google Scholar]
- 61.Jiang J, Yu M, Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93:546–9. [DOI] [PubMed] [Google Scholar]
- 62.•.Andrews PJD, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JKJ, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373:2403–12Recent landmark randomized controlled trial evaluating hypothermia for intracranial hypertension after TBI.
- 63.Jackson TC, Manole MD, Kotermanski SE, Jackson EK, Clark RSB, Kochanek PM. Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience. 2015;305:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kochanek PM, Jackson TC. The brain and hypothermia—from Aristotle to targeted temperature management. Crit Care Med. 2017;45:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. [DOI] [PubMed] [Google Scholar]
- 66.Zeiler FA, Donnelly J, Menon DK, Smielewski P, Hutchinson PJA, Czosnyka M. A description of a new continuous physiological index in traumatic brain injury using the correlation between pulse amplitude of intracranial pressure and cerebral perfusion pressure. J Neurotrauma. 2018. [DOI] [PubMed] [Google Scholar]
- 67.Aries MJH, Czosnyka M, Budohoski KP, Kolias AG, Radolovich DK, Lavinio A, et al. Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care. 2012;17:67–76. [DOI] [PubMed] [Google Scholar]
- 68.Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C,Smielewski P, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care. 2012;16: 258–66. [DOI] [PubMed] [Google Scholar]
- 69.Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus. 2008;25:E2. [DOI] [PubMed] [Google Scholar]
- 70.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–7 discussion 17. [DOI] [PubMed] [Google Scholar]
- 71.Czosnyka M, Smielewski P, Kirkpatrick P, Piechnik S, Laing R, Pickard JD. Continuous monitoring of cerebrovascular pressure-reactivity in head injury. Acta Neurochir Suppl. 1998;71:74–7. [DOI] [PubMed] [Google Scholar]
- 72.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D,Menon DK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–8. [DOI] [PubMed] [Google Scholar]
- 73.Howells T, Elf K, Jones PA, Ronne-Engström E, Piper I, Nilsson P, et al. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J Neurosurg. 2005;102:311–7. [DOI] [PubMed] [Google Scholar]
- 74.Darby JM, Yonas H, Marion DW, Latchaw RE. Local “inverse steal” induced by hyperventilation in head injury. Neurosurgery. 1988;23:84–8. [DOI] [PubMed] [Google Scholar]
- 75.Jha RM, Elmer J, Zusman BE, Desai S, Puccio AM, Okonkwo DO, et al. Intracranial pressure trajectories: a novel approach to informing severe traumatic brain injury phenotypes. Crit Care Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito J, Marmarou A, Barzó P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg. 1996;84:97–103. [DOI] [PubMed] [Google Scholar]
- 77.Marmarou A A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22:E1. [DOI] [PubMed] [Google Scholar]
- 78.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016;36:513–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34: 16180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014;37:620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mackert BM, Staub F, Peters J, Baethmann A, Kempski O. Anoxia in vitro does not induce neuronal swelling or death. J Neurol Sci. 1996;139:39–47. [PubMed] [Google Scholar]
- 84.Kochanek PM, Clark RS, Ruppel RA, Dixon CE. Cerebral resuscitation after traumatic brain injury and cardiopulmonary arrest in infants and children in the new millennium. Pediatr Clin North Am. 2001;48:661–81. [DOI] [PubMed] [Google Scholar]
- 85.Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–7. [DOI] [PubMed] [Google Scholar]
- 86.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg. 2006;104:720–30. [DOI] [PubMed] [Google Scholar]
- 87.Whalen MJ, Carlos TM, Wisniewski SR, Clark RS, Mellick JA, Marion DW, et al. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit Care Med. 2000;28:3710–7. [DOI] [PubMed] [Google Scholar]
- 88.Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: distribution and time course of protein extravasation. J. Neurotrauma. 1992;9:21–32. [DOI] [PubMed] [Google Scholar]
- 89.Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, et al. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res. 2010;88:3530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shetty AK, Mishra V, Kodali M, Hattiangady B. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front Cell Neurosci. 2014;8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeoh S, Bell ED, Monson KL. Distribution of blood-brain barrier disruption in primary blast injury. Ann Biomed Eng. 2013;41: 2206–14. [DOI] [PubMed] [Google Scholar]
- 92.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stokum JA, Kwon MS, Woo SK, Tsymbalyuk O, Vennekens R, Gerzanich V, et al. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia. 2018;66:108–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, et al. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filippidis AS, Carozza RB, Rekate HL. Aquaporins in brain edema and neuropathological conditions. Int J Mol Sci. 2016;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hubbard JA, Szu JI, Binder DK. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull. 2018;136:118–29. [DOI] [PubMed] [Google Scholar]
- 97.Cartagena CM, Phillips KL, Tortella FC, Dave JR, Schmid KE. Temporal alterations in aquaporin and transcription factor HIF1 α expression following penetrating ballistic-like brain injury (PBBI). Mol Cell Neurosci. 2014;60:81–7. [DOI] [PubMed] [Google Scholar]
- 98.Ke C, Poon WS, Ng HK, Pang JC, Chan Y. Heterogeneous responses of aquaporin-4 in oedema formation in a replicated severe traumatic brain injury model in rats. Neurosci Lett. 2001;301:21–4. [DOI] [PubMed] [Google Scholar]
- 99.Kiening KL, van Landeghem FKH, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, et al. Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci Lett. 2002;324:105–8. [DOI] [PubMed] [Google Scholar]
- 100.Liu H, Qiu G p, Zhuo F, Yu W h, Sun S q, Li F h, et al. Lost polarization of aquaporin4 and dystroglycan in the core lesion after traumatic brain injury suggests functional divergence in evolution. Biomed Res Int. 2015;2015:471631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C, Chen J, Lu H. Expression of aquaporin-4 and pathological characteristics of brain injury in a rat model of traumatic brain injury. Mol Med Rep. 2015;12:7351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taya K, Marmarou CR, Okuno K, Prieto R, Marmarou A. Effect of secondary insults upon aquaporin-4 water channels following experimental cortical contusion in rats. J Neurotrauma. 2010;27:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros M-P, Garcia-Segura LM. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10:e0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu H, Lei X-Y, Hu H, He Z-P. Relationship between AQP4 expression and structural damage to the blood-brain barrier at early stages of traumatic brain injury in rats. Chin Med J. 2013;126:4316–21. [PubMed] [Google Scholar]
- 105.Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, et al. “Hit & Run” model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yao X, Uchida K, Papadopoulos MC, Zador Z, Manley GT, Verkman AS. Mildly reduced brain swelling and improved neurological outcome in aquaporin-4 knockout mice following controlled cortical impact brain injury. J Neurotrauma. 2015;32:1458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu H, Yao H, Zhang W, Zhang L, Ding W, Zhang S, et al. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J Zhejiang Univ Sci B. 2005;6:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo Pizzo M, Schiera G, Di Liegro I, Di Liegro CM, Pál J, Czeiter E, et al. Aquaporin-4 distribution in control and stressed astrocytes in culture and in the cerebrospinal fluid of patients with traumatic brain injuries. Neurol Sci. 2013;34:1309–14. [DOI] [PubMed] [Google Scholar]
- 109.Dardiotis E, Paterakis K, Tsivgoulis G, Tsintou M, Hadjigeorgiou GF, Dardioti M, et al. AQP4 tag single nucleotide polymorphisms in patients with traumatic brain injury. J Neurotrauma. 2014;31: 1920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J-Q, Zhang C-C, Jiang S-N, Lu H, Wang W. Effects of aquaporin 4 knockdown on brain edema of the uninjured side after traumatic brain injury in rats. Med Sci Monit. 2016;22:4809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fukuda AM, Adami A, Pop V, Bellone JA, Coats JS, Hartman RE, et al. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J Cereb Blood Flow Metab. 2013;33:1621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, et al. The role of hypoxia-inducible factor-1 α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114: 92–101. [DOI] [PubMed] [Google Scholar]
- 113.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–63. [DOI] [PubMed] [Google Scholar]
- 114.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–3. [DOI] [PubMed] [Google Scholar]
- 115.Zhang M, Cui Z, Cui H, Cao Y, Zhong C, Wang Y. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 2016;17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ding Z, Zhang J, Xu J, Sheng G, Huang G. Propofol administration modulates AQP-4 expression and brain edema after traumatic brain injury. Cell Biochem Biophys. 2013;67:615–22. [DOI] [PubMed] [Google Scholar]
- 117.Wang X, Zhang J, Yang Y, Dong W, Wang F, Wang L, et al. Progesterone attenuates cerebral edema in neonatal rats with hypoxic-ischemic brain damage by inhibiting the expression of matrix metalloproteinase-9 and aquaporin-4. Exp Ther Med. 2013;6:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wallisch J, Jha R, Vagni V, Feldman K, Dixon C, Farr G, et al. Effect of the novel aquaporin-4 antagonist AER-271 in combined TBI plus hemorrhagic shock in mice. Crit Care Med. 2015;43:6–7. [Google Scholar]
- 119.Lopez NE, Krzyzaniak MJ, Blow C, Putnam J, Ortiz-Pomales Y, Hageny A-M, et al. Ghrelin prevents disruption of the blood-brain barrier after traumatic brain injury. J Neurotrauma. 2012;29:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ballarin B, Tymianski M. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol Sin. 2018;39:661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pál B Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell Mol Life Sci. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kostic M, Zivkovic N, Stojanovic I. Multiple sclerosis and glutamate excitotoxicity. Rev Neurosci. 2013;24:71–88. [DOI] [PubMed] [Google Scholar]
- 123.Plitman E, Nakajima S, de la Fuente-Sandoval C, Gerretsen P, Chakravarty MM, Kobylianskii J, et al. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur. Neuropsychopharmacol. 2014;24:1591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nilsson P, Hillered L, Pontén U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10:631–7. [DOI] [PubMed] [Google Scholar]
- 126.Hillered L, Persson L, Carlson H, Ungerstedt U, Ronne-Engström E, Nilsson P. Studies on excitatory amino acid receptor-linked brain disorders in rat and man using in vivo microdialysis. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):695A–6A. [DOI] [PubMed] [Google Scholar]
- 127.Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010;113:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koura SS, Doppenberg EM, Marmarou A, Choi S, Young HF, Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir Suppl. 1998;71:244–6. [DOI] [PubMed] [Google Scholar]
- 129.Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg. 1998;89:507–18. [DOI] [PubMed] [Google Scholar]
- 130.Stefani MA, Modkovski R, Hansel G, Zimmer ER, Kopczynski A, Muller AP, et al. Elevated glutamate and lactate predict brain death after severe head trauma. Ann Clin Transl Neurol. 2017;4:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zauner A, Bullock R, Kuta AJ, Woodward J, Young HF. Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir Suppl. 1996;67:40–4. [DOI] [PubMed] [Google Scholar]
- 132.Sönmez A, Sayin O, Gürgen SG, Çalişir M. Neuroprotective effects of MK-801 against traumatic brain injury in immature rats. Neurosci Lett. 2015;597:137–42. [DOI] [PubMed] [Google Scholar]
- 133.Imer M, Omay B, Uzunkol A, Erdem T, Sabanci PA, Karasu A, et al. Effect of magnesium, MK-801 and combination of magnesium and MK-801 on blood-brain barrier permeability and brain edema after experimental traumatic diffuse brain injury. Neurol Res. 2009;31:977–81. [DOI] [PubMed] [Google Scholar]
- 134.Görgülü A, Kiriş T, Unal F, Türkoğlu U, Küçük M, Cobanoğlu S. Protective effect of the N-methyl-D-aspartate receptor antagonists, MK-801 and CPP on cold-induced brain oedema. Acta Neurochir (Wien). 1999;141:93–8. [DOI] [PubMed] [Google Scholar]
- 135.Day NL, Carle MS, Floyd CL. Post-injury administration of a combination of memantine and 17β-estradiol is protective in a rat model of traumatic brain injury. Neurochem Int. 2017;111:57–68. [DOI] [PubMed] [Google Scholar]
- 136.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. [DOI] [PubMed] [Google Scholar]
- 137.Willis C, Lybrand S, Bellamy N. Excitatory amino acid inhibitors for traumatic brain injury. Cochrane Database Syst Rev. 2004;CD003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gultekin R, Huang S, Clavisi O, Pattuwage L, König TC, Gruen R. Pharmacological interventions in traumatic brain injury: can we rely on systematic reviews for evidence? Injury. 2016;47:516–24. [DOI] [PubMed] [Google Scholar]
- 139.Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6. [PMC free article] [PubMed] [Google Scholar]
- 140.Simon DW, McGeachy MJ, Bayir H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Corrigan F, Mander KA, Leonard AV, Vink R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation. 2016;13:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alluri H, Wilson RL, Anasooya Shaji C, Wiggins-Dohlvik K, Patel S, Liu Y, et al. Melatonin preserves blood-brain barrier integrity and permeability via matrix metalloproteinase-9 inhibition. PLoS One. 2016;11:e0154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guilfoyle MR, Carpenter KLH, Helmy A, Pickard JD, Menon DK, Hutchinson PJA. Matrix metalloproteinase expression in contusional traumatic brain injury: a paired microdialysis study. J Neurotrauma. 2015;32:1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, et al. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One. 2013;8:e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. [DOI] [PubMed] [Google Scholar]
- 147.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57. [DOI] [PubMed] [Google Scholar]
- 148.Walker K, Perkins M, Dray A. Kinins and kinin receptors in the nervous system. Neurochem Int. 1995;26:1–16 discussion 17. [DOI] [PubMed] [Google Scholar]
- 149.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, et al. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113(Pt 11):2085–90. [DOI] [PubMed] [Google Scholar]
- 150.Wójciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–65. [DOI] [PubMed] [Google Scholar]
- 151.Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, et al. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J Neurotrauma. 1999;16:617–28. [DOI] [PubMed] [Google Scholar]
- 152.Buttram SDW, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, et al. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–17. [DOI] [PubMed] [Google Scholar]
- 153.Helmy A, Carpenter KLH, Menon DK, Pickard JD, Hutchinson PJA. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maier B, Schwerdtfeger K, Mautes A, Holanda M, Müller M, Steudel WI, et al. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock. 2001;15:421–6. [DOI] [PubMed] [Google Scholar]
- 155.Whalen MJ, Carlos TM, Kochanek PM, Wisniewski SR, Bell MJ, Clark RS, et al. Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Crit Care Med. 2000;28:929–34. [DOI] [PubMed] [Google Scholar]
- 156.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction “opening”: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–28. [DOI] [PubMed] [Google Scholar]
- 157.Woodcock TM, Frugier T, Nguyen TT, Semple BD, Bye N, Massara M, et al. The scavenging chemokine receptor ACKR2 has a significant impact on acute mortality rate and early lesion development after traumatic brain injury. PLoS One. 2017;12: e0188305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.da Fonseca ACC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, et al. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90:323–32. [DOI] [PubMed] [Google Scholar]
- 160.Stahel PF, Morganti-Kossmann MC, Perez D, Redaelli C, Gloor B, Trentz O, et al. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J Neurotrauma. 2001;18:773–81. [DOI] [PubMed] [Google Scholar]
- 161.Kossmann T, Stahel PF, Morganti-Kossmann MC, Jones JL, Barnum SR. Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J Neuroimmunol. 1997;73:63–9. [DOI] [PubMed] [Google Scholar]
- 162.Kumar RG, Rubin JE, Berger RP, Kochanek PM, Wagner AK. Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain Behav Immun. 2016;53:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–9. [DOI] [PubMed] [Google Scholar]
- 164.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRCCRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–8. [DOI] [PubMed] [Google Scholar]
- 165.Thal SC, Heinemann M, Luh C, Pieter D, Werner C, Engelhard K. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-γ-independent mechanisms. J Neurotrauma. 2011;28:983–93. [DOI] [PubMed] [Google Scholar]
- 166.Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma. 2007;24:1119–31. [DOI] [PubMed] [Google Scholar]
- 167.Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett. 2005;388:7–12. [DOI] [PubMed] [Google Scholar]
- 168.Thal SC, Neuhaus W. The blood-brain barrier as a target in traumatic brain injury treatment. Arch Med Res. 2014;45:698–710. [DOI] [PubMed] [Google Scholar]
- 169.Yi J-H, Park S-W, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, et al. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol. 2011;227:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gonzales NR, Shah J, Sangha N, Sosa L, Martinez R, Shen L, et al. Design of a prospective, dose-escalation study evaluating the safety of pioglitazone for hematoma resolution in intracerebral hemorrhage (SHRINC). Int J Stroke. 2013;8:388–96. [DOI] [PubMed] [Google Scholar]
- 172.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mandrekar-Colucci S, Sauerbeck A, Popovich PG, McTigue DM. PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro. 2013;5:e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 2016;73:520–8. [DOI] [PubMed] [Google Scholar]
- 175.Hau P, Kunz-Schughart L, Bogdahn U, Baumgart U, Hirschmann B, Weimann E, et al. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas—a phase II study. Oncology. 2007;73:21–5. [DOI] [PubMed] [Google Scholar]
- 176.Au AK, Aneja RK, Bell MJ, Bayir H, Feldman K, Adelson PD, et al. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J Neurotrauma. 2012;29:2013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lu L, Wang M, Yuan F, Wei X, Li W. Roles of elevated 20-HETE in the breakdown of blood brain barrier and the severity of brain edema in experimental traumatic brain injury. Mol Med Rep. 2018;17:7339–45. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J, Pu H, Zhang H, Wei Z, Jiang X, Xu M, et al. Inhibition of Na+-K+-2Cl-cotransporter attenuates blood-brain-barrier disruption in a mouse model of traumatic brain injury. Neurochem Int. 2017;111:23–31. [DOI] [PubMed] [Google Scholar]
- 179.Roberts DJ, Jenne CN, Léger C, Kramer AH, Gallagher CN, Todd S, et al. A prospective evaluation of the temporal matrix metalloproteinase response after severe traumatic brain injury in humans. J Neurotrauma. 2013;30:1717–26. [DOI] [PubMed] [Google Scholar]
- 180.Kimberly WT, Battey TWK, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Serena J, Blanco M, Castellanos M, Silva Y, Vivancos J, Moro MA, et al. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005;36:1921–6. [DOI] [PubMed] [Google Scholar]
- 182.Jha R, Battey TWK, Pham L, Lorenzano S, Furie KL, Sheth KN, et al. Fluid-attenuated inversion recovery hyperintensity correlates with matrix metalloproteinase-9 level and hemorrhagic transformation in acute ischemic stroke. Stroke. 2014;45:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, et al. Does inhibiting Sur1 complement rt-PA in cerebral is chemia? Ann N Y Acad Sci. 2012;1268:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15:1160–9. [DOI] [PubMed] [Google Scholar]
- 185.Muradashvili N, Benton RL, Saatman KE, Tyagi SC, Lominadze D. Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metab Brain Dis. 2015;30:411–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Semple BD, Noble-Haeusslein LJ, Gooyit M, Tercovich KG, Peng Z, Nguyen TT, et al. Early gelatinase activity is not a determinant of long-term recovery after traumatic brain injury in the immature mouse. PLoS One. 2015;10:e0143386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener. 2012;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jia F, Yin YH, Gao GY, Wang Y, Cen L, Jiang J-Y. MMP-9 inhibitor SB-3CT attenuates behavioral impairments and hippocampal loss after traumatic brain injury in rat. J Neurotrauma. 2014;31:1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao B-Q, Wang S, Kim H-Y, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–5. [DOI] [PubMed] [Google Scholar]
- 192.Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD. Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J Neurochem. 2011;117:437–48. [DOI] [PubMed] [Google Scholar]
- 193.Lu K-T, Huang T-C, Tsai Y-H, Yang Y-L. Transient receptor potential vanilloid type 4 channels mediate Na-K-Cl-co-transporter-induced brain edema after traumatic brain injury. J Neurochem. 2017;140:718–27. [DOI] [PubMed] [Google Scholar]
- 194.Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury—synergistic roles of NKCC1 and SUR1/TRPM4. J Neurosurg. 2010;113: 622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu K-T, Cheng N-C, Wu C-Y, Yang Y-L. NKCC1-mediated traumatic brain injury-induced brain edema and neuron death via Raf/ MEK/MAPK cascade. Crit Care Med. 2008;36:917–22. [DOI] [PubMed] [Google Scholar]
- 196.Lu K-T, Wu C-Y, Cheng N-C, Wo Y-YP, Yang J-T, Yen H-H, et al. Inhibition of the Na+ -K+ -2Cl- -cotransporter in choroid plexus attenuates traumatic brain injury-induced brain edema and neuronal damage. Eur J Pharmacol. 2006;548:99–105. [DOI] [PubMed] [Google Scholar]
- 197.Jayakumar AR, Taherian M, Panickar KS, Shamaladevi N, Rodriguez ME, Price BG, et al. Differential response of neural cells to trauma-induced swelling in vitro. Neurochem Res. 2018;43:397–406. [DOI] [PubMed] [Google Scholar]
- 198.Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, et al. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J Neurotrauma. 2009;26:2257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32:1699–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jha RM, Puccio AM, Okonkwo DO, Zusman BE, Park S-Y, Wallisch J, et al. ABCC8 single nucleotide polymorphisms are associated with cerebral edema in severe TBI. Neurocrit Care. 2017;26:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jha RM, Elmer J, Zusman B, Puccio A, Okonkwo D, Shutter L, et al. Intracranial pressure trajectories: a novel tool to inform severe TBI phenotypes. Crit Care Med. 2018;46:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jha R, Puccio A, Okonkwo D, Zusman B, Koleck T, Wallisch J, et al. 785 TRPM-4 single nucleotide polymorphisms correlate with cerebral edema in traumatic brain injury. Crit Care Med. 2018;46:378. [Google Scholar]
- 203.Jha RM, Molyneaux BJ, Jackson TC, Wallisch JS, Park S-Y, Poloyac S, et al. Glibenclamide produces region-dependent effects on cerebral edema in a combined injury model of traumatic brain injury and hemorrhagic shock in mice. J Neurotrauma. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zweckberger K, Hackenberg K, Jung CS, Hertle DN, Kiening KL, Unterberg AW, et al. Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience. 2014;272:199–206. [DOI] [PubMed] [Google Scholar]
- 205.Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 2010;69:1177–90. [DOI] [PubMed] [Google Scholar]
- 206.Jha R, Yan H, Dixon CE, Poloyac S, Jackson T, Hoshitsuki K, et al. Evaluation of glibenclamide in the pittsburgh controlled cortical impact model of traumatic brain injury: an OBTT Consortium Study. J Neurotrauma. 2015;32:119. [Google Scholar]
- 207.Khalili H, Derakhshan N, Niakan A, Ghaffarpasand F, Salehi M, Eshraghian H, et al. Effects of oral glibenclamide on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injuries: a randomized double-blind placebo-controlled clinical trial. World Neurosurg. 2017;101:130–6. [DOI] [PubMed] [Google Scholar]
- 208.Zafardoost P, Ghasemi AA, Salehpour F, Piroti C, Ziaeii E. Evaluation of the effect of glibenclamide in patients with diffuse axonal injury due to moderate to severe head trauma. Trauma Mon. 2016;21:e25113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Suzuki R, Fukai N, Nagashijma G, Asai JI, Itokawa H, Nagai M, et al. Very early expression of vascular endothelial growth factor in brain oedema tissue associated with brain contusion. Acta Neurochir Suppl. 2003;86:277–9. [DOI] [PubMed] [Google Scholar]
- 210.Chodobski A, Chung I, Koźniewska E, Ivanenko T, Chang W, Harrington JF, et al. Early neutrophilic expression of vascular endothelial growth factor after traumatic brain injury. Neuroscience. 2003;122:853–67. [DOI] [PubMed] [Google Scholar]
- 211.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284:21036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280: H434–40. [DOI] [PubMed] [Google Scholar]
- 214.Salehi A, Zhang JH, Obenaus A. Response of the cerebral vasculature following traumatic brain injury. J Cereb Blood Flow Metab. 2017;37:2320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Clausen F, Marklund N, Hillered L. Acute inflammatory biomarker responses to diffuse traumatic brain injury in the rat monitored by a novel microdialysis technique. J Neurotrauma. 2018. [DOI] [PubMed] [Google Scholar]
- 217.Shore PM, Jackson EK, Wisniewski SR, Clark RSB, Adelson PD, Kochanek PM. Vascular endothelial growth factor is increased in cerebrospinal fluid after traumatic brain injury in infants and children. Neurosurgery. 2004;54:605–11 discussion 611. [DOI] [PubMed] [Google Scholar]
- 218.Li M, Jia Q, Chen T, Zhao Z, Chen J, Zhang J. The role of vascular endothelial growth factor and vascular endothelial growth inhibitor in clinical outcome of traumatic brain injury. Clin Neurol Neurosurg. 2016;144:7–13. [DOI] [PubMed] [Google Scholar]
- 219.Gao W, Zhao Z, Yu G, Zhou Z, Zhou Y, Hu T, et al. VEGI attenuates the inflammatory injury and disruption of blood-brain barrier partly by suppressing the TLR4/NF-κB signaling pathway in experimental traumatic brain injury. Brain Res. 2015;1622:230–9. [DOI] [PubMed] [Google Scholar]
- 220.Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2014;CD008218. [DOI] [PubMed] [Google Scholar]
- 221.Tado M, Mori T, Fukushima M, Oshima H, Maeda T, Yoshino A, et al. Increased expression of vascular endothelial growth factor attenuates contusion necrosis without influencing contusion edema after traumatic brain injury in rats. J Neurotrauma. 2014;31:691–8. [DOI] [PubMed] [Google Scholar]
- 222.Badri S, Chen J, Barber J, Temkin NR, Dikmen SS, Chesnut RM, et al. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38:1800–9. [DOI] [PubMed] [Google Scholar]
- 223.Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, et al. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care. 2006;4:8–13. [DOI] [PubMed] [Google Scholar]
- 224.Lannoo E, Van Rietvelde F, Colardyn F, Lemmerling M, Vandekerckhove T, Jannes C, et al. Early predictors of mortality and morbidity after severe closed head injury. J Neurotrauma. 2000;17:403–14. [DOI] [PubMed] [Google Scholar]
- 225.Czosnyka M, Hutchinson PJ, Balestreri M, Hiler M, Smielewski P, Pickard JD. Monitoring and interpretation of intracranial pressure after head injury. Acta Neurochir Suppl. 2006;96:114–8. [DOI] [PubMed] [Google Scholar]
- 226.Struchen MA, Hannay HJ, Contant CF, Robertson CS. The relation between acute physiological variables and outcome on the Glasgow Outcome Scale and Disability Rating Scale following severe traumatic brain injury. J Neurotrauma. 2001;18:115–25. [DOI] [PubMed] [Google Scholar]
- 227.Levin HS, Eisenberg HM, Gary HE, Marmarou A, Foulkes MA, Jane JA, et al. Intracranial hypertension in relation to memory functioning during the first year after severe head injury. Neurosurgery. 1991;28:196–9 discussion 200. [DOI] [PubMed] [Google Scholar]
- 228.Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30:1831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kochanek PM, Bramlett HM, Dixon CE, Shear DA, Dietrich WD, Schmid KE, et al. Approach to modeling, therapy evaluation, drug selection, and biomarker assessments for a multicenter pre-clinical drug screening consortium for acute therapies in severe traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33:513–22. [DOI] [PubMed] [Google Scholar]
- 230.Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76:67–80. [DOI] [PubMed] [Google Scholar]