Abstract
Intrinsic biological mechanisms transduce psychological stress into physiological adaptation that requires energy, but the role of mitochondria and mitochondrial DNA (mtDNA) in this process has not been defined in humans. Here, we show that similar to physical injury, exposure to psychological stress increases serum circulating cell-free mtDNA (ccf-mtDNA) levels. Healthy midlife adults exposed on two separate occasions to a brief psychological challenge exhibit a 2–3-fold increase in ccf-mtDNA, with no change in ccf-nuclear DNA levels, establishing response magnitude and specificity for ccf-mtDNA. In cell-based studies, we show that glucocorticoid signaling – a consequence of psychological stress in humans – is sufficient to induce mtDNA extrusion in a time frame consistent with stress-induced ccf-mtDNA increase. Collectively, these findings provide evidence that acute psychological stress induces ccf-mtDNA and implicate neuroendocrine signaling as a potential trigger for ccf-mtDNA release. Further controlled work is needed to confirm that observed increases in ccf-mtDNA result from stress exposure and to determine the functional significance of this effect.
Keywords: psychosocial stress, mitochondria, neuroendocrine, mitokine, psychobiology
1. Introduction
In response to perceived threat, humans and other mammals generate an integrated physiological response (the “fight-or-flight response”) involving the activation of multiple physiological systems. Every aspect of the stress response entails increased energy demand and thus necessarily engages mitochondrial energy production and signaling (Picard et al., 2018). The stress response is believed to have evolved to promote adaptation and increase the probability of survival (Weiner, 1992); however, chronic activation of stress reactivity systems is associated with increased disease risk (Cohen et al., 2018; McEwen, 1998). Even brief exposure to a psychological stressor (i.e., an imagined threat) is sufficient to alter gene expression and elevate systemic markers of inflammation (Marsland et al., 2017; Schwaiger et al., 2016), reflecting the existence of intrinsic brain-body processes that transduce psychological stress into biological changes. But little is known about the cellular events that occur acutely in response to psychological stress.
Recent animal studies suggest that chronic stress adversely influences multiple aspects of mitochondrial function and structural integrity (Cai et al., 2015; Liu and Zhou, 2012; Magariños et al., 1997) (reviewed in (Picard and McEwen, 2018)). Outside of the nucleus, mitochondria are the only organelle to contain their own genome – the mitochondrial DNA (mtDNA). Although the circular mtDNA is normally sequestered inside mitochondria, after physical stressors, such as trauma, infection, or strenuous exercise in humans, mtDNA molecules are found in the circulation as circulating cell-free mitochondrial DNA (ccf-mtDNA) (Boyapati et al., 2017; Hummel et al., 2018; Nakahira et al., 2013; Stawski et al., 2017; Zhang et al., 2010). Owing to its bacterial origin, ccf-mtDNA is immunogenic and triggers inflammation (Pinti et al., 2014; Zhang et al., 2010). During cell death, mtDNA is also actively released into the cytosol by selective molecular permeabilization of the mitochondrial membranes (McArthur et al., 2018). Furthermore, ccf-mtDNA is also actively released by human lymphocytes and triggers immune activation (Ingelsson et al., 2018), demonstrating that specific mechanisms exist to regulate mitochondrial genome release. Thus, given the ability of mitochondria-derived ccf-mtDNA to trigger inflammation, and evidence that mitochondria are a target of physical stress, it is possible that mitochondria play a signaling role in response to psychological threat in humans.
In addition to its elevation in injury and severe health conditions, higher ccf-mtDNA levels have been found in suicide attempters (Lindqvist et al., 2016) and patients with major depressive disorder (Lindqvist et al., 2018), representing cross-sectional evidence for a possible link between psychological states and ccf-mtDNA. Recently, psychological stress was shown to induce a rapid 1.7 fold increase in plasma ccf-mtDNA in a small study of 20 men (Hummel et al., 2018). Activation of the hypothalamic-pituitary-adrenal (HPA)-axis and the peripheral release of glucocorticoids is a primary neuroendocrine mediator of physiological responses to psychological stress (Denson et al., 2009). Interestingly, alterations of the HPA axis may also be implicated in the regulation of ccf-mtDNA levels in humans (Lindqvist et al., 2016) and mtDNA gene expression in animals (Hunter et al., 2016).
Here we examined whether an acute psychosocial stress known to elicit the coordinated physiological stress response (Carroll et al., 2011; Dickerson and Kemeny, 2004; Marsland et al., 2017) is sufficient to affect serum ccf-mtDNA levels in humans. We sampled blood at three time points to examine dynamic changes in ccf-mtDNA levels in response to a social-evaluative stressor, with a repeated challenge on a second visit one month later. To ascertain whether changes in ccf-mtDNA were due to non-specific release of bulk cellular material, we also assessed levels of circulating DNA from the nucleus (nDNA). In secondary analyses, we also explored whether the magnitude of stress-related changes in circulating ccf-mtDNA differed by sex. Finally, we used time lapse imaging in living human cells in vitro to test if neuroendocrine signaling influences mtDNA localization. Our results show that exposure to a brief psychological stressor is associated with a rapid, robust, and specific increase in ccf-mtDNA, stronger in men than women, without a parallel increase in nDNA. Overall, these findings implicate mitochondria and mtDNA signaling in the acute physiological response to psychological stress in humans.
2. Materials and Methods
2.1. Study cohort
Samples and data for the present study were obtained from the Vaccination and Immunity Project, a longitudinal study investigating the association of psychosocial, physiologic, and behavioral factors with antibody response to hepatitis B vaccination in a middle age adult population (Carroll et al., 2011; Prather et al., 2009). A total of 50 participants (30 men, 20 women, 88% Caucasian) aged between 41–58 years were included in the present study and 32 participants (64%) completed both visits.
Participants were non-smokers, in good general health (no history of myocardial infarction, asthma, cancer treatment in the past year, psychotic illness, or other systemic diseases affecting the immune system) free from medication interfering with nervous, endocrine, and immune system (excepted for oral contraceptives) for the 3 months prior to the beginning of the study. Pregnant and lactating women were ineligible. Participants were free of symptoms of infection, antibiotic use, vaccination and did not had a tattoo for the 2 weeks prior to the laboratory visits. Informed consent was obtained in compliance with guidelines of the University of Pittsburgh Institutional Review Board.
2.2. Experimental stress procedure
The detailed study design is illustrated in Supplemental Information (SI) (Fig. S1A) and has been previously reported (Carroll et al., 2011; Prather et al., 2009). Prior to receiving the hepatitis B vaccination series, participants attended two laboratory sessions scheduled 1 month apart. On each occasion, participants abstained from caffeine, food, and beverage (excepted water) for 12 h, non-prescription medications and physical activity for 24 h and alcohol beverages for 48 h before coming into the laboratory between 7:00 and 9:00 AM. The stress protocol was conducted in the morning and in the fasting state to avoid potential confounding from daily stress-related and diet-related factors prior to testing. On arrival, an intravenous catheter was inserted into the antecubital fossa of one arm for collection of blood samples. Participants then rested quietly for a 30-minute adaptation period and a pre-task sample of blood was drawn and mood states were assessed using the Profile of Mood States questionnaire (POMS) (McNair, 1971; Usala and Hertzog, 1989). Participants then performed a simulated public speaking task, consisting of 2 minutes of preparation for a speech defending themselves against an alleged transgression (shoplifting or traffic violation) followed by 3 minutes of videotaped speech delivery (see (Carroll et al., 2011; Prather et al., 2009)). We have shown previously that this task elicits reproducible cardiovascular and immune stress responses (Carroll et al., 2011; Marsland et al., 2002; Marsland et al., 1995; Prather et al., 2009). Mood states were collected and post-task blood samples were drawn immediately following completion of the task and again after the subject had rested quietly for 30 minutes (+30 min). The procedure was the same at the second laboratory visit except subjects were told that their “performance on the first speech task was slightly below average when compared with other participants’ speeches,” and participants were asked to “try to be more persuasive when delivering this speech.”
2.3. Serum DNA extraction and quantification
Blood samples were allowed to clot, centrifuged at 1,000 × g for 10 minutes, and the serum was frozen at −80°C until further processing (Fig. S1B). Each sample was thawed, visually inspected, and subjected to an additional centrifugation at 2,000 × g for 5 minutes in 1.5ml microfuge tubes to prevent potential cellular contamination prior to DNA extraction.
There was no relationship between sample color (yellow, pink due to erythrocyte lysis, or opacity) and the amount of DNA detected. Similar to the previously reported fraction of mtDNA molecules that are freely circulating and those enclosed in small vesicles (~19%) (Ye et al., 2017), removal of membrane-bound fractions by centrifugation of plasma at 10,000 × g and 18,000 × g reduced circulating mtDNA levels by ~31% in our samples. All analyses shown are therefore representative of both free DNA and small membrane-bound pools of circulating mtDNA.
Total nucleic acids were extracted from serum by proteinase K and ethanol precipitation as previously described (Kolesar et al., 2012), and circulating levels of mtDNA measured by duplex quantitative real-time PCR (qPCR) with Taqman chemistry (Belmonte et al., 2016). Linearity of assays was confirmed using titration of mixed total DNA from 30 human placentas, which was used to calculate relative abundance of individual samples, and read counts validated by digital PCR. Two different reactions were run in parallel for each sample, with each measure performed in triplicates, including a standard curve for each reaction plate. Reactions consisted in both a mtDNA and nDNA amplicons: mt-ND1 and B2m for mtDNA1 and nDNA1, respectively; and mt-CYTB and Gusb for mtDNA2 and nDNA2, respectively (see SI Table 1 for details of primers/probes). Two different amplicons for both the mitochondrial and nuclear genomes were used to ensure that mtDNA and nDNA quantifications are invariant of potential inter-individual sequence variation. Both mtDNA1 and mtDNA2 yielded highly correlated results (Fig. S1D).
2.4. Dexamethasone stimulation of primary human fibroblast
Primary human male fibroblasts (hFB1m) obtained from a healthy donor (passages 9–13) were seeded on glass cover slips and grown in DMEM supplemented with non-essential amino acids and physiological glucose concentration (5.5mM). Cells were acutely exposed to the synthetic glucocorticoid receptor agonist dexamethasone (DEX) at a widely used concentration of 100 nM (Alm et al., 2012; Gerö and Szabo, 2016; Kim et al., 2013) across a series of exposures (12hr, 24hr, 48hr, 3d, 4d, 6d, 7d). At each time point, respiratory capacity was determined using the Seahorse XFe96 Extracellular Flux Analyzer (Agilent) (Mookerjee et al., 2017; Tan et al., 2015) 24 hours after seeding 20,000 cells per well. A concentration of 4uM FCCP was previously established through a titration experiment to elicit maximal uncoupling in this cell type. The working concentration of DEX used was further confirmed based on a dose-response DEX titration experiment (0, 0.01, 0.05, 0.1, 0.5, 1, 5, 10uM DEX) at 12hr, the time point that elicited maximal induction of spare respiratory capacity (see Results and supporting information for details). These experiments confirmed that 100nM DEX elicited maximal increase in mitochondrial respiratory capacity in these cells. Thus, 100nM DEX was used in triple immunofluorescence microscopy experiments.
2.4.1. Immunofluorescence and image analysis
We used immunofluorescence microscopy to simultaneously visualize the glucocorticoid receptor (GR), mitochondria, and the mtDNA. Following DEX stimulation, cells were fixed with 4% paraformaldehyde (PFA) for 15 minutes. Cells were washed 3 times with PBS and then permeabilized with 90% Methanol (diluted in PBS) for 20 minutes at −30°C. Cells were washed with PBS three times and blocked with 10% Normal Goat Serum (NGS) for 60 minutes at room temperature (RT). Primary antibody incubation was as follows: Tom20 (Santa Cruz (F10); sc-17764), mouse monoclonal IgG2a, 1:100 in 2% NGS; Glucocorticoid receptor (GR) (Santa Cruz (G-5); sc-393232, which recognizes aa. 121–420), mouse monoclonal IgG2b, 1:100 in 2% NGS; Anti-DNA antibody (American Research Product (AC-30–10); #03–61014), mouse monoclonal IgM, 1:100 in 2% NGS. Coverslips were incubated at 4°C, overnight. For secondary antibody incubation cells were first washed 3 times with PBS and then incubated in darkness for 60 minutes at RT, as follows: Mouse IgG2a-594, 1:500 in 2% NGS; Mouse IgG2b-488, 1:500 in 2% NGS; Mouse IgM-AMCA, 1:500 in 2% NGS. Cells were washed 3 times with PBS and mounted with ProLong Gold (Thermo Fisher Scientific #P10144). Images were taken on an Olympus IX81 inverted fluorescence microscope equipped with a digitized stage (ProScan; Prior Scientific), a 63x/1.35 oil objective (Olympus, MA), a corresponding fluorescence filter set and a 2.0 neutral density filter using a CoolSNAP HQ camera (Roper Scientific/Photometrics, AZ) and MetaMorph software (Molecular Devices, CA).
For GR translocation analyses, at least five images were analyzed per time point, and experiments repeated three times. For each cell analyzed, regions of interest (ROIs) were averaged for the cytoplasm (six ROIs), nucleus (three), and background (three). For analyses of extruded mtDNA located outside mitochondria, a mask using the mitochondrial channel (Tom20) was used to remove mtDNA nucleoids inside mitochondria, and nucleoid number and size were automatically determined. Each extruded mtDNA particle was analyzed manually and classified as being in immediate contact with a mitochondrion (attached) or without any overlapping pixel with mitochondria (free floating). Nucleoid size was also measured for all nucleoids at each time point and expressed as μm2. A line scan was performed to assess co-localization of mitochondria and mtDNA nucleoids (see Fig. 5). All images were analyzed for densitometry or colocalization using Image J (Rasband, 2011), and all data is the average of three separate experiments.
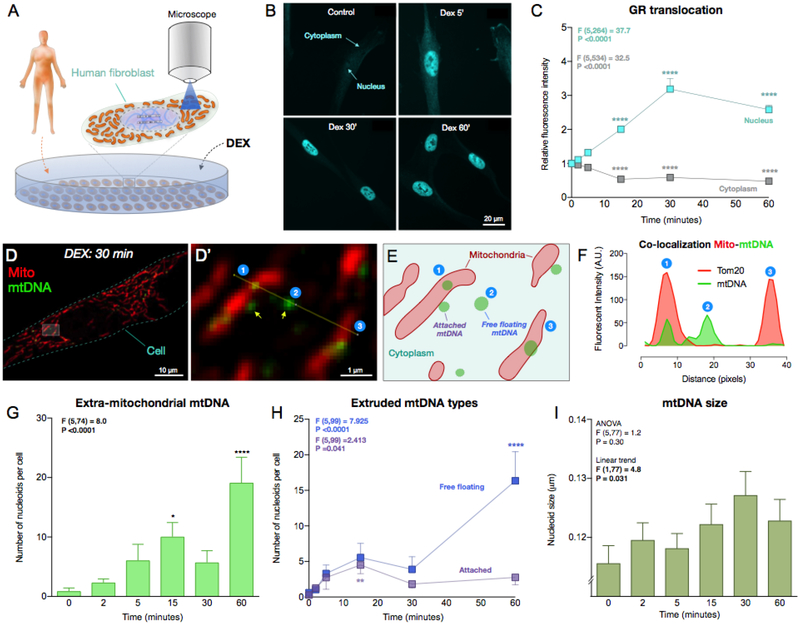
Fig 5. Glucocorticoid stimulation causes mtDNA extrusion in human cells.
(A) Schematic of the experimental design. Primary male human fibroblasts were acutely exposed to 100nM dexamethasone (DEX) for 0, 2, 5, 15, 30 and 60 minutes, and mitochondria and mtDNA visualized by immunofluorescence. (B) Representative pictures of human fibroblasts after DEX treatment analyzed for GR subcellular localization. (C) Glucocorticoid receptor (GR) fluorescence was quantified in both the nucleus and the cytoplasm following DEX stimulation, which induced a marked translocation of GR to the nucleus. One Way ANOVA and Least Significant Difference (LSD) pairwise comparisons. (D) A human fibroblast exposed to DEX stimulation for 30 minutes, and dual-labeled by immunofluorescence for a ubiquitous marker of mitochondria (Tom20) and mtDNA. (D’) Higher magnification of boxed area in (D). (E) Cartoon of the mitochondria and nucleoids observed in (D’). Note the two different forms of extruded mtDNA: in contact to the surface of mitochondria (attached), or free floating in the cytoplasm (free floating). (F) Line profile intensity quantification for Tom20 and mtDNA along the yellow line in D’ showing: ➀ A nucleoid inside the mitochondrion; ➁ A nucleoid extruded into the cytoplasm (no mito-Tom20 staining); and ➂ A mitochondrion without mtDNA. (G) Time-course of the number of extruded mtDNA nucleoids after acute DEX stimulation. One Way ANOVA and Least Significant Difference (LSD) pairwise comparisons, n = 82 cells. (H) Time-course of sub-types of mtDNA nucleoids: the number of attached and free floating mtDNA. One Way ANOVA and LSD pairwise comparisons, n = 105 cells. (I) Size of extruded mtDNA (averaged per cell). One Way ANOVA and test for linear trend, n = 105 cells. Data are means ± SEM. Pairwise comparisons presented are relative to DEX 0 minute. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
2.5. Data analysis
Statistical analyses were performed using SAS 9.3, SPSS 23, and Prism 7.0 (Graphpad). mtDNA and nDNA data obtained from the human study were natural log transformed before analyses or analyzed using non-parametric tests. mtDNA/nDNA ratio was computed to estimate the relative abundance of mitochondrial and nuclear DNA levels. Paired two-tailed student T-tests were used to assess pre- to post-task within-person change in positive affect (calm, well-being) and negative affect (anxiety and anger) at session 1 and session 2 (n=49). Pearson correlation was used to examine the association between change in affect and change in mtDNA (from post to +30). Linear regression was used to compare baseline nDNA and mtDNA levels between sessions (n=24 for paired data at session 1 and 2) and to investigate the association between mtDNA1 and mtDNA2 amplicons at pre, post and +30 min (n=69 paired data at session 1 and 2). There was one data point detected as low mtDNA1 amplicon and high mtDNA2 amplicon (SI, Fig. S1,D), excluding this value in a sensitivity analysis did not change the findings. To evaluate main effects of the stressor on mtDNA and nDNA concentration, repeated-measures analyses of variance (ANOVAs) were conducted (performed only on complete case set of data (pre, post, +30) n=31 at session 1 and n=31 at session 2), followed by Least Significant Difference (LSD) pairwise comparisons when indicated. Measures of effect size were calculated using partial eta-squared (ηp2). For analyses where the assumption of sphericity (tested using Mauchly’s test) was not met, the Greenhouse-Geisser correction was applied. In a model that included both sessions (performed only on participants without missing data (pre, post, +30), n=62), we tested for a sex × time interaction using repeated-measures analyses of covariance (ANCOVAs). Sex differences were further explored by stratifying analysis. For cell culture experiments, difference in mtDNA nucleoids and GR characteristics between the DEX conditions (0, 2, 5, 15, 30, 60 minutes) were assessed using One Way ANOVA, test for linear trend (when non-significant) (Altman, 1991), and LSD pairwise comparisons. Two-tailed statistical significance was accepted as P<0.05.
3. Results
Serum levels of two mtDNA (mt-ND1: mtDNA1, and mt-CYTB: mtDNA2) and two nDNA (B2m: nDNA1, and Gusb: nDNA2) amplicons were measured by qPCR (Fig. 1A,B). This dual-amplicon approach insures that results are invariant to sequence differences that may exist between individuals, and differentiates between mitochondrial and nuclear genome release. A total of 50 healthy, midlife individuals (20 women, 30 men; mean age = 50 years, range: 41–58, 88% Caucasian) were studied. Serum was collected at three time points: i) pre: before the social-evaluative stress test; ii) post: immediately after the stress test; and iii) +30 min: 30 minutes after the end of the stressor (Fig. 1C). A validation visit took place one month later when all measures were repeated on the same participants. The detailed study design is illustrated in Supplemental Information (SI) (Fig. S1).
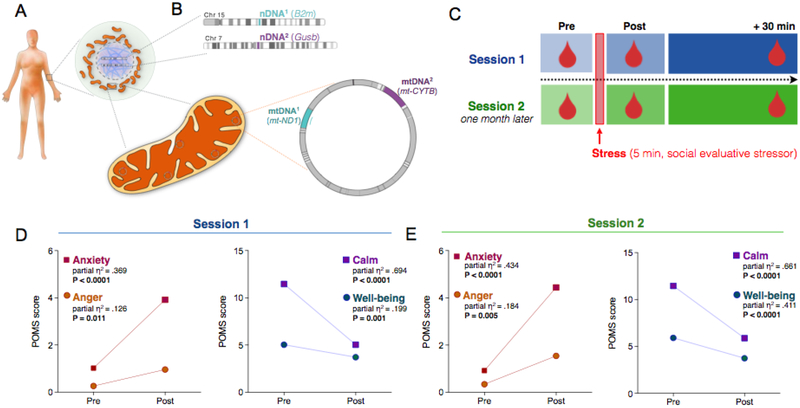
Fig. 1. Study design to assess levels of circulating cell-free mitochondrial (ccf-mtDNA) and nuclear DNA (ccf-nDNA) in response to induced psychological stress.
(A) Human cells contain nuclear DNA in the nucleus (purple) and cytoplasmic mitochondria (orange) with multiple copies of their own genome, the mtDNA. (B) Two different mtDNA amplicons, mtDNA1 (mt-ND1) and mtDNA2 (mt-CYTB), and two different nDNA amplicons nDNA1 (B2m) and nDNA2 (Gusb), were amplified from circulating cell-free serum using quantitative PCR. (C) Schematic of the experimental study design with three serial blood draws (pre, post, +30 min). A subsequent validation session 2 which was conducted one month later. (D) Stress-induced elevation of negative (left) and decrease in positive (right) items pre- and post-stress at session 1 and (E) at session 2, confirming the successful experimental manipulation of psychological states. Paired two-tailed student T-tests, n = 49 per session.
3.1. ccf-mtDNA shows substantial inter-individual and intra-individual variability over one month
There was substantial inter-individual variability in baseline (pre-task) ccf-mtDNA1 (mt-ND1) and ccf-mtDNA2 (mt-CYTB) levels as well as intra-individual differences in baseline ccf-mtDNA1 and ccf-mtDNA2 between sessions 1 and 2 (one month apart) (SI, Fig. S2A,B). The between-person coefficient of variation (C.V.) was 98% when averaged across both mtDNA amplicons measured at baseline at both sessions (SI, Fig. S2E). In comparison, the average within-subject C.V. between baseline measure across the two sessions was 33%. Moreover, for each participant baseline mtDNA levels were only moderately correlated between sessions 1 and 2 (SI, Fig. S2G,H), indicating a moderate degree of stability in baseline ccf-mtDNA and suggesting that ccf-mtDNA may represent a variable “state” characteristic. For the nuclear genome, the average between-person C.V. across both baseline nDNA amplicons and both sessions was 69% and the within-person variability across sessions was on average 36%; session 1 and 2 baseline ccf-nDNA values were not correlated (SI, Fig. S2G,H).
3.2. Acute psychological stress increases ccf-mtDNA levels
Participants were exposed to a 5-minute psychological stress task involving the preparation (2 min) and delivery (3 min) of a speech to defend themselves against a false accusation (SI, Fig. S1). To validate the effectiveness of this task as an experimental stressor, we examined indices of individual psychological states (i.e., mood) from pre- to post-task. Consistent with our previous report (Carroll et al., 2011), at both sessions, the stressor caused a significant increase in negative mood (anxiety, anger) and decrease in positive mood (calm, well-being) (Fig. 1D–E), demonstrating successful manipulation of the psychological state.
In response to this brief psychological challenge, ccf-mtDNA levels increased from pre to +30 min post-stress, exhibiting a large effect size (mtDNA1, ηp2 = .569, P<0.0001, Fig. 2A, mtDNA2, ηp2 = 0.600, P<0.0001, SI, Fig. S3A). The majority of participants (93%) showed an increase in both ccf-mtDNA1 and ccf-mtDNA2 after 30 minutes. Interestingly, ccf-mtDNA levels did not change significantly immediately after the stressor, between pre and post samples, indicating some delay in this response.
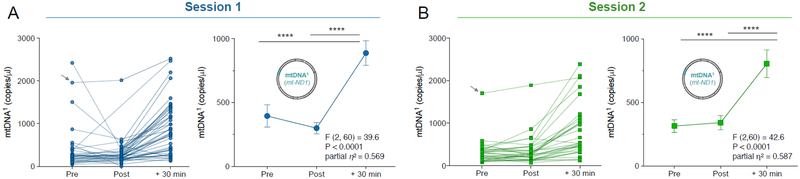
Fig. 2. Psychological stress selectively increases serum circulating cell-free mitochondrial DNA.
(A) Individual (left) and group mean (right) ccf-mtDNA1 (mt-ND1) responses to acute psychological stress at session 1. (B) Validation of the results in (A) in a repeat session 2 one month later. Arrow: a participant with unusually high mtDNA1 baseline values at both sessions. Data are means and SEM. Repeated measure ANOVA on log-transformed data and Least Significant Difference (LSD) pairwise comparisons, n = 31 per session, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
The significant release of mtDNA at +30 min post stress was replicated at a second visit, one month later. Again, individuals showed a large increase in the levels of both ccf-mtDNA amplicons, with 94% of participants showing an elevation in both ccf-mtDNA1 and ccf-mtDNA2. The effect size for session 2 was comparable to session 1 (mtDNA1, ηp2 = .587, P<0.0001, Fig. 2B mtDNA2, ηp2 = .556, P<0.0001, SI, Fig. S3B). The replication of this stress-induced mtDNA increase confirmed that similar to physical injury and sepsis (Boyapati et al., 2017), psychosocial stress is associated with robust elevations in serum ccf-mtDNA levels in human subjects within 30 minutes.
3.3. Stress selectively increases ccf-mtDNA
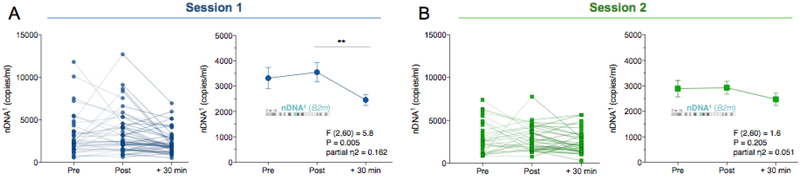
We next examined whether the increased ccf-mtDNA was specific to mitochondria or reflected a general increase in circulating total cellular genomic material. The same serum samples were analyzed for nuclear genome content. In contrast to ccf-mtDNA, circulating levels of nDNA1 (B2m) or nDNA2 (Gusb) did not increase in response to stress. In fact, both nuclear sequences decreased at +30 min (nDNA1: ηp2 = .162, P<0.01, Fig. 3A; nDNA2: ηp2 = .174, P<0.05, SI, Fig. S4A). Because the mitochondrial and nuclear genomes were quantified in duplex reactions (i.e. in the same reaction), this result cannot be due to a sampling error. In the validation session one month later, ccf-nDNA1 and ccf-nDNA2 were not significantly different over time (nDNA1, ηp2 = 0.051, P = 0.20, Fig. 3B; nDNA2, ηp2 = 0.068, P = 0.132, SI, Fig. S4B).
Fig. 3. Psychological stress does not increase serum circulating cell-free nuclear DNA.
(A) Responses to acute psychological stress at session 1 for the nuclear DNA (ccf-nDNA1, B2m) and (B) at the validation session 2. Data are means and SEM. Repeated measure ANOVA on log transformed data and Least Significant Difference (LSD) pairwise comparisons, n = 31 per session, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
Furthermore, the ratio of the number of circulating mitochondrial to nuclear genomes (mtDNA/nDNA) also illustrates the selective increase of mtDNA over nDNA after stress. The baseline mtDNA/nDNA ratio was ~140 for DNA1 and ~63 for DNA2 (SI, Fig. S5A–D). Relative to pre ratio, the mtDNA1/nDNA1 ratio at +30 min rose by > 2-fold during both first and second visits (P<0.0001, SI, Fig. S5A,B). The direction and magnitude of effect was confirmed with the second set of amplicons mtDNA2/nDNA2 (SI, Fig. S5C,D). Together, these data show that psychological stress selectively increases circulating cell-free mitochondrial but not nuclear DNA, and that their circulating levels are likely modulated by different biological mechanisms.
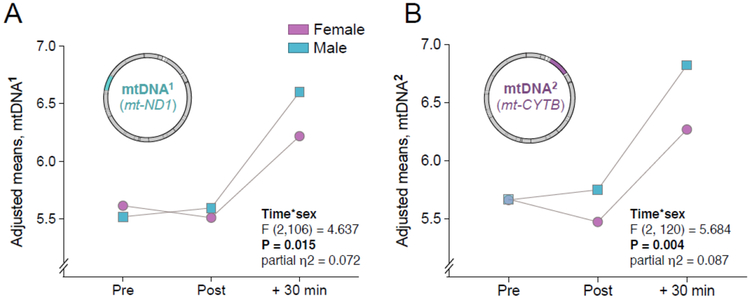
3.4. Stress-induced ccf-mtDNA elevation differs by sex
Next, we conducted exploratory analyses to investigate whether sex moderated the magnitude of stress-induced ccf-mtDNA responses. The complete results of sex-stratified analyses of the levels of ccf-mtDNA in response to stress are presented in SI Fig. S6. In a model that included both sessions, a significant sex by time interaction was found for mtDNA1 (F (2,105) = 4.60, P < 0.05, ηp2 = 0.195) and mtDNA2 (F (2,120) = 5.68, P < 0.01, ηp2 = 0.087) (Fig. 4A,B), with higher + 30 min ccf-mtDNA levels observed in males than females, suggesting that the magnitude of psychological stress-induced ccf-mtDNA release may differ by sex. The effect sizes also reveal consistently larger effects of stress on ccf-mtDNA for males than females (SI, Fig. S7), particularly at session 2. In comparison, there was no evidence that sex moderated nuclear genome levels and the effects sizes for nDNA were substantially smaller than for mtDNA (SI, Fig. S7B). This finding further reinforces the notion that circulating levels of mitochondrial and nuclear genome levels are differentially regulated.
Fig. 4. Stress by sex interaction on the levels of circulating cell-free mtDNA.
(A) Adjusted means for ccf-mtDNA1 responses to acute psychological stress. (B) Same as in (A) for ccf-mtDNA2. Repeated measure ANOVA on log transformed data, n = 62 for both sessions combined together.
3.5. Stress-induced mood change and ccf-mtDNA elevation
We conducted exploratory analyses to investigate the association between stress-induced changes in mood states and ccf-mtDNA (SI, Fig. S8). At session 1, no significant associations were observed. At session 2, there was a significant positive association between stress-related ccf-mtDNA increases and anxiety (r2 = 0.17, p < 0.05), and an inverse association with calmness (r2 = 0.17, p < 0.05) (SI, Fig. S8B,D). These data are consistent with the notion that subjective psychological states (more negative, less positive) account for a fraction of psychological stress-induced ccf-mtDNA levels in serum.
3.5. Neuroendocrine signaling triggers mtDNA extrusion in living cells
Finally, we sought to explore whether mtDNA localization could be influenced by neuroendocrine stress signaling, such as glucocorticoids, in human cells. We stimulated primary human fibroblasts in vitro for up to an hour with the glucocorticoid hormone mimetic dexamethasone (DEX, 100nM) (Fig. 5A) and concurrently quantified the intracellular distribution of the glucocorticoid receptor (GR). The DEX concentrations used was confirmed to elicit maximal changes in mitochondrial respiratory capacity by monitoring cellular oxygen consumption (SI, Fig. S9). As expected, DEX stimulation caused a rapid redistribution of GR from the cytoplasm to the nucleus peaking at 30 min, demonstrating in this system the activation of glucocorticoid signaling (Fig. 5B–C).
During DEX stimulation, we simultaneously tracked mitochondria and mtDNA. Under normal conditions, >99.5% of mtDNA appear as punctate structures, termed nucleoids, inside mitochondria. Strikingly, acute glucocorticoid stimulation increased the number of mtDNA nucleoids located outside mitochondria as early as 15 min (Fig. 5D–G). Extruded mtDNA were categorized as “mitochondrion-attached” or “free-floating,” presumably representing the early and late stages of mtDNA extrusion, respectively. Accordingly, the number of attached nucleoids showed a gradual increase peaking at 15 minutes, and free-floating mtDNA increased and peaked at 60 minutes (Fig. 5H). This process was associated with increased mtDNA nucleoid size (+10.4% at 30 min) (test for linear trend F (1,77) = 4.8, P < 0.05), consistent with the release and “decondensation” of mtDNA known to occur during other forms of cellular stress (Fig. 5I) (Ashley and Poulton, 2009; West et al., 2015).
4. Discussion
The organism’s ability to adapt to stress depends on the concerted action of molecular factors secreted within minutes to hours, enabling the transfer of information across physiological system, which ultimately promotes survival of the organism. The present findings show that a brief psychological stressor is sufficient to cause a robust and rapid increase in serum ccf-mtDNA, implicating the mitochondrial genome as a stress-inducible cytokine, or “mitokine”. In addition, our findings contribute new knowledge to the existing correlational studies on psychiatric populations and raise the possibility that mitochondria dynamically respond, within minutes, to the organism’s psychological state. On both occasions of testing, stress was associated with a selective elevation in ccf-mtDNA levels, but not ccf-nDNA, within 30 minutes. Consistent with preclinical studies showing deleterious effects of acute psychological stress on mitochondrial structure and function (Picard and McEwen, 2018) and previous work showing elevated plasma ccf-mtDNA in response to induced psychological stress in men (Hummel et al., 2018), the current findings identify ccf-mtDNA as a possible biological marker of mitochondrial stress secondary to acute psychological challenge in humans, and provide proof-of-concept evidence that neuroendocrine factors may contribute to this psychobiological effect.
Clinically, increases in ccf-mtDNA have been ascribed to tissue damage, with higher levels predicting mortality (Boyapati et al., 2017). In a prospective study of 443 critically ill patients in the intensive care unit, elevated ccf-mtDNA levels were associated with a 7-fold increased risk of death within the next month (Nakahira et al., 2013). Levels of ccf-mtDNA also appear to increase with age. In a study of 831 healthy individuals, compared to children and young adults aged 1–41 years, older adults aged 50–89 showed 3.8 fold higher ccf-mtDNA levels, and those >90 years showed a 7.0-fold elevation (Pinti et al., 2014). Together, these findings raise the possibility that levels of ccf-mtDNA increase with age and are functionally significant, predicting increased disease and mortality risk (Boyapati et al., 2017). Thus, further understanding of mechanisms that regulate ccf-mtDNA levels is warranted.
In relation to psychological stress, our findings add to initial cross-sectional evidence in two psychiatric populations – non-violent suicide attempters and patients with major depressive disorder – that show elevated levels of plasma ccf-mtDNA when compared with matched controls (Lindqvist et al., 2016; Lindqvist et al., 2018). In our study, the elevation pre/post- to +30 min across two sessions yielded Cohen’s d values ranging from 0.85–1.23, demonstrating a robust and rapid effect of psychological stress on serum ccf-mtDNA. Other than mtDNA, mitochondrial proteins such as heat shock protein 60 (Hsp60), cytochrome c, and prohibitins have also been detected in the circulation of healthy individuals (Mengwasser et al., 2004; Pullerits et al., 2004; Shamaei-Tousi et al., 2007). In one study, psychological distress, high levels of job demand, and low levels of emotional support were found to be associated with circulating plasma mitochondrial Hsp60 levels (Shamaei-Tousi et al., 2007). In another study of 20 healthy young men, plasma ccf-mtDNA rapidly increased by 0.7-fold after a social-evaluative stress (Hummel et al., 2018). Extending these findings, we show that serum ccf-mtDNA is acutely and robustly responsive to psychological state, and more so in men than women, and thus should be considered as a dynamic stress-inducible marker.
In line with some, but not all, previous reports suggesting sex differences in the magnitude of neuroendocrine responses to social-evaluative stress (Chan et al., 2017; Goel et al., 2014; Juster et al., 2016), we observed greater stress-related increase in ccf-mtDNA in men compared to women. While further work is needed to replicate and explain these findings, it should be noted that several aspects of mitochondria biology, including respiratory capacity, sensitivity to permeability transition, and reactive oxygen species production have been shown to differ by sex, with increased vulnerability generally observed in males (Ventura-Clapier et al., 2017). There are also significant metabolic differences between women and men (Mittelstrass et al., 2011) and some mitochondrial disorders show sexual dimorphism even at a young age before differences in sex hormones appear, with boys being more susceptible than girls (Rahman et al., 1996; Van Erven et al., 1987).
The current study also broadens prior assumptions that ccf-mtDNA is a consequence of physical injury to raise the possibility that ccf-mtDNA may also be responsive to psychological states. However, the full downstream physiological effects of acute increases in ccf-mtDNA remain to be determined. One possibility is that ccf-mtDNA contributes to the delayed effects of psychological stress on inflammation and metabolism (Boyapati et al., 2017; Kiecolt-Glaser et al., 2015). Mitochondria are the only organelle with their own DNA, which is recognized as bacterial-like and activates pattern-recognition receptors both intracellularly and extracellularly (West and Shadel, 2017). In animals, injection of mtDNA in the blood triggers systemic inflammation in a toll-like receptor 9-dependent manner (Zhang et al., 2010). Furthermore, in humans, inflammatory diseases have been associated with elevated ccf-mtDNA (Caielli et al., 2016), and the anti-inflammatory mediator acetylcholine may prevent mtDNA release from mitochondria (Lu et al., 2014). Consistent with this idea, older individuals with elevated circulating levels of ccf-mtDNA tend to have higher levels of proinflammatory cytokines IL-6 and TNF-α, and mtDNA functions as an adjuvant that potentiates the production of TNF-α in isolated monocytes (Pinti et al., 2014). Having established the rapid inducibility of ccf-mtDNA, further research is thus warranted to determine if the increase in ccf-mtDNA secondary to acute psychological stress is necessary and sufficient to trigger stress-induced inflammation in humans.
It has been demonstrated that acute psychological stress evokes systemic inflammation (Marsland et al., 2017; Rohleder, 2014). In humans, stress-evoked inflammatory responses peak around 90–120 minutes following the onset of acute laboratory stress (Marsland et al., 2017). Thus, the present study shows that the elevation in serum ccf-mtDNA at 30 minutes precedes the established time frame for the inflammatory response, which would be consistent with the immunogenic nature of the mtDNA. We previously showed in this cohort that higher negative emotional responses to the stressor were positively associated with IL-6 responses from baseline to 30 minutes after stress (Carroll et al., 2011). But since we observed a ccf-mtDNA increase at the same time point, and could not assess later time points in this cohort, the current study does not allow us to test whether the ccf-mtDNA increase contributed to subsequent inflammation.
To determine the precise pathways at the basis of stress induced ccf-mtDNA increase, there is need for denser and longer kinetic analyses of ccf-mtDNA in relation to neuroendocrine and inflammatory markers, as well as controlled animal and cell-based studies. In addition, future studies should distinguish between plasma and serum levels since ccf-mtDNA concentration measured in serum may be substantially higher due to additional release of cellular DNA during coagulation in serum (Xia et al., 2009). Importantly, in addition to the within-subject repeated sampling design, future studies should also include a resting comparison group in addition to the stress condition to control for the time factor. Moreover, in light of our findings and of the multiple pathways known be involved in stress adaptation, future efforts to establish the contribution of specific neuroendocrine pathways to ccf-mtDNA regulation with stress should be designed with circadian variation of these parameters in mind, and include parallel assessments from multiple neuroendocrine, metabolic, and inflammatory mediators to assess their dynamic interactions.
Finally, it must be noted that the source of circulating DNA in humans, particularly in response to psychological stress, is not clear. The bulk release of cellular material as a result of cell death would result in an increase in both mitochondrial and nuclear genomes. In contrast, the current findings show a selective increase in ccf-mtDNA levels with no concomitant increase in nDNA. In fact, nDNA at session 1 was found to decrease +30min after stress. While the reasons for this phenomenon are unclear, this could reflect the endogenous activity (present during normal regulation of the organism) and potential induction of enzymes that degrade circulating DNA molecules – both mtDNA and nDNA (i.e., DNAse, which are induced with exercise (Velders et al., 2014)). Following acute stress, mtDNA may be selectively released at a greater rate than its degradation. The dissociable responses of nDNA and mtDNA to social-evaluative stress strongly argue against cellular death as the mechanism of stress-induced increases in ccf-mtDNA. Another possible mechanism involves the active release of mtDNA. Recent evidence describes molecular mechanisms of mtDNA release from the mitochondrial matrix to the cytoplasm (McArthur et al., 2018) and from the cell (i.e., leukocytes) to the circulation (Ingelsson et al., 2018), providing a biological basis for rapid and selective extrusion of mtDNA. Our data on primary human fibroblasts showing that glucocorticoid signaling induces mtDNA extrusion into the cytoplasm also suggests that canonical neuroendocrine stress mediators, including but not limited to glucocorticoids, may contribute to the observed association between stressful experiences and mtDNA release. Additional work is required to establish, in different cell types, the mechanisms for extracellular mtDNA release.
5. Conclusions
Overall, this study demonstrates an increase in serum ccf-mtDNA following acute psychological stress in humans. The current findings add to a growing literature on circulating DNAs (Boyapati et al., 2017), providing initial evidence that elevation of ccf-mtDNA occurs not only with physical injury, inflammatory diseases, critical illness, and aging, but also in response to acute psychological stress. Furthermore, this study provides evidence that neuroendocrine signaling induces mtDNA extrusion in human cells, suggesting potential pathways linking subjective psychological states to mitochondrial signaling. Overall, these findings raise the possibility that mitochondrial adaptations and mitokine signaling plays a role in the fight-or-flight response, and possibly in human stress pathophysiology.
Supplementary Material
Highlights.
Circulating cell-free mitochondrial DNA (ccf-mtDNA) is released from mitochondria
Ccf-mtDNA is a pro-inflammatory molecule elevated with aging and inflammatory diseases
Psychological stress rapidly and selectively increases serum ccf-mtDNA
The effect size for stress-induced elevation in serum ccf-mtDNA is stronger in men
Neuroendocrine signaling triggers mtDNA extrusion in primary human fibroblasts
Role of the funding source
This work was supported by NIH grant NR08237 to ALM, NIH grant GM110424 to BAK, the Wharton Fund, NIH grants GM119793, MH113011 to MP, and FONDECYT grant 3150623 to CBA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
The authors declare no conflict of interest.
References
- Alm JJ, Heino TJ, Hentunen TA, Väänänen HK, Aro HT, 2012. Transient 100 nM dexamethasone treatment reduces inter-and intraindividual variations in osteoblastic differentiation of bone marrow-derived human mesenchymal stem cells. Tissue Engineering Part C: Methods 18, 658–666. [DOI] [PubMed] [Google Scholar]
- Altman D, 1991. Practical Statistics for Medical Research Chapman & Hall London Google Scholar. Haung, et al [16] USA (Black). [Google Scholar]
- Ashley N, Poulton J, 2009. Anticancer DNA intercalators cause p53-dependent mitochondrial DNA nucleoid re-modelling. Oncogene 28, 3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte FR, Martin JL, Frescura K, Damas J, Pereira F, Tarnopolsky MA, Kaufman BA, 2016. Digital PCR methods improve detection sensitivity and measurement precision of low abundance mtDNA deletions. Scientific reports 6, 25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyapati RK, Tamborska A, Dorward DA, Ho G-T, 2017. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Research 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, Song L, Kretzschmar W, Gan X, Nicod J, 2015. Molecular signatures of major depression. Current Biology 25, 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, Baisch J, Phelps K, Clayton S, Gong M, 2016. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. Journal of Experimental Medicine, jem. 20151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL, 2011. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, behavior, and immunity 25, 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Houghton AB, Bale TL, 2017. Strained in Planning Your Mouse Background? Using the HPA Stress Axis as a Biological Readout for Backcrossing Strategies. Neuropsychopharmacology 42, 1749–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Murphy ML, Prather AA, 2018. Ten Surprising Facts About Stressful Life Events and Disease Risk. Annual review of psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Miller N, 2009. Cognitive appraisals and emotions predict cortisol and immune responses: a meta-analysis of acute laboratory social stressors and emotion inductions. Psychological bulletin 135, 823. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin 130, 355. [DOI] [PubMed] [Google Scholar]
- Gerö D, Szabo C, 2016. Glucocorticoids suppress mitochondrial oxidant production via upregulation of uncoupling protein 2 in hyperglycemic endothelial cells. PloS one 11, e0154813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V, 2014. Sex differences in the HPA axis. Comprehensive Physiology 4, 1121–1155. [DOI] [PubMed] [Google Scholar]
- Hummel E, Hessas E, Müller S, Beiter T, Fisch M, Eibl A, Wolf O, Giebel B, Platen P, Kumsta R, 2018. Cell-free DNA release under psychosocial and physical stress conditions. Translational Psychiatry 8, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Seligsohn M, Rubin TG, Griffiths BB, Ozdemir Y, Pfaff DW, Datson NA, McEwen BS, 2016. Stress and corticosteroids regulate rat hippocampal mitochondrial DNA gene expression via the glucocorticoid receptor. Proc Natl Acad Sci U S A 113, 9099–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson B, Söderberg D, Strid T, Söderberg A, Bergh A-C, Loitto V, Lotfi K, Segelmark M, Spyrou G, Rosén A, 2018. Lymphocytes eject interferogenic mitochondrial DNA webs in response to CpG and non-CpG oligodeoxynucleotides of class C. Proceedings of the National Academy of Sciences 115, E478–E487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, Raymond C, Desrochers AB, Bourdon O, Durand N, Wan N, Pruessner JC, Lupien SJ, 2016. Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology 63, 282–290. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Habash DL, Fagundes CP, Andridge R, Peng J, Malarkey WB, Belury MA, 2015. Daily stressors, past depression, and metabolic responses to high-fat meals: A novel path to obesity. Biological psychiatry 77, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-M, Kim Y-G, Park J-W, Lee J-M, Suh J-Y, 2013. The effects of dexamethasone on the apoptosis and osteogenic differentiation of human periodontal ligament cells. Journal of periodontal & implant science 43, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesar JE, Wang CY, Taguchi YV, Chou S-H, Kaufman BA, 2012. Two-dimensional intact mitochondrial DNA agarose electrophoresis reveals the structural complexity of the mammalian mitochondrial genome. Nucleic acids research 41, e58–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Fernström J, Grudet C, Ljunggren L, Träskman-Bendz L, Ohlsson L, Westrin Å, 2016. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Translational psychiatry 6, e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernström J, Westrin Å, Hough CM, Lin J, Reus VI, 2018. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhou C, 2012. Corticosterone reduces brain mitochondrial function and expression of mitofusin, BDNF in depression-like rodents regardless of exercise preconditioning. Psychoneuroendocrinology 37, 1057–1070. [DOI] [PubMed] [Google Scholar]
- Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, Joshi S, Wang H, Andersson U, Chavan SS, 2014. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Molecular Medicine 20, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Verdugo JMG, McEwen BS, 1997. Chronic stress alters synaptic terminal structure in hippocampus. Proceedings of the National Academy of Sciences 94, 14002–14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Henderson BN, Chambers WH, Baum A, 2002. Stability of individual differences in cellular immune responses to two different laboratory tasks. Psychophysiology 39, 865–868. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS, 1995. Stability of individual differences in cellular immune responses to acute psychological stress. Psychosomatic Medicine 57, 295–298. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain, behavior, and immunity 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H, 2018. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McNair DM, 1971. Profile of mood states instrument. Manual for the Profile of Mood States, 3–29. [Google Scholar]
- Mengwasser J, Piau A, Schlag P, Sleeman JP, 2004. Differential immunization identifies PHB1/PHB2 as blood-borne tumor antigens. Oncogene 23, 7430. [DOI] [PubMed] [Google Scholar]
- Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, 2011. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS genetics 7, e1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee SA, Gerencser AA, Nicholls DG, Brand, M.D.J.J.o.B.C., 2017. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. 292, 7189–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Kyung S-Y, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, 2013. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS medicine 10, e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, 2018. Psychological stress and mitochondria: A systematic review. Psychosom Med In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, Epel E, Sandi C, 2018. An energetic view of stress: Focus on mitochondria. Frontiers in neuroendocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, 2014. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm‐aging”. European journal of immunology 44, 1552–1562. [DOI] [PubMed] [Google Scholar]
- Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL, 2009. Gender differences in stimulated cytokine production following acute psychological stress. Brain, behavior, and immunity 23, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits R, Bokarewa M, Jonsson I-M, Verdrengh M, Tarkowski A, 2004. Extracellular cytochrome c, a mitochondrial apoptosis-related protein, induces arthritis. Rheumatology 44, 32–39. [DOI] [PubMed] [Google Scholar]
- Rahman S, Blok R, Dahl HH, Danks D, Kirby D, Chow C, Christodoulou J, Thorburn D, 1996. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Annals of neurology 39, 343–351. [DOI] [PubMed] [Google Scholar]
- Rasband WS, 2011. Imagej, us national institutes of health, bethesda, maryland, usa. http://imagej.nih.gov/ij/.
- Rohleder N, 2014. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic medicine 76, 181–189. [DOI] [PubMed] [Google Scholar]
- Schwaiger M, Grinberg M, Moser D, Zang JC, Heinrichs M, Hengstler JG, Rahnenführer J, Cole S, Kumsta R, 2016. Altered Stress-Induced Regulation of Genes in Monocytes in Adults with a History of Childhood Adversity. Neuropsychopharmacology 41, 2530–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamaei-Tousi A, Steptoe A, O’Donnell K, Palmen J, Stephens JW, Hurel SJ, Marmot M, Homer K, D’Aiuto F, Coates AR, 2007. Plasma heat shock protein 60 and cardiovascular disease risk: the role of psychosocial, genetic, and biological factors. Cell stress & chaperones 12, 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski R, Walczak K, Kosielski P, Meissner P, Budlewski T, Padula G, Nowak D, 2017. Repeated bouts of exhaustive exercise increase circulating cell free nuclear and mitochondrial DNA without development of tolerance in healthy men. PloS one 12, e0178216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Xiao H, Li F, Zeng L, Yin YJAN, 2015. The profiles of mitochondrial respiration and glycolysis using extracellular flux analysis in porcine enterocyte IPEC-J2. 1, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala PD, Hertzog C, 1989. Measurement of affective states in adults: Evaluation of an adjective rating scale instrument. Research on Aging 11, 403–426. [DOI] [PubMed] [Google Scholar]
- Van Erven P, Cillessen J, Eekhoff E, Gabreëls F, Doesburg W, Lemmens W, Slooff J, Renier W, Ruitenbeek W, 1987. Leigh syndrome, a mitochondrial encephalo (myo) pathy: a review of the literature. Clinical neurology and neurosurgery 89, 217–230. [DOI] [PubMed] [Google Scholar]
- Velders M, Treff G, Machus K, Bosnyák E, Steinacker J, Schumann U.J.C.b., 2014. Exercise is a potent stimulus for enhancing circulating DNase activity. 47, 471–474. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A, 2017. Mitochondria: a central target for sex differences in pathologies. Clinical Science 131, 803–822. [DOI] [PubMed] [Google Scholar]
- Weiner H, 1992. Perturbing the organism: The biology of stressful experience. University of Chicago press, Chicago. [Google Scholar]
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS, 2015. Mitochondrial DNA Stress Primes the Antiviral Innate Immune Response. Nature 520, 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Shadel GS, 2017. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nature Reviews Immunology 17, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Radpour R, Zachariah R, Fan AXC, Kohler C, Hahn S, Holzgreve W, Zhong XY, 2009. Simultaneous quantitative assessment of circulating cell-free mitochondrial and nuclear DNA by multiplex real-time PCR. Genetics and molecular biology 32, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Tang X, Liu C, Wen C, Li W, Lyu J, 2017. Accurate quantitation of circulating cell-free mitochondrial DNA in plasma by droplet digital PCR. Analytical and bioanalytical chemistry 409, 2727–2735. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ, 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.