Abstract
Objective:
To test alterations in placental cellular aging as one pathway by which maternal early adversity influences physiologic development in her offspring.
Methods:
Maternal report of her adverse childhood experiences (ACE) was obtained prenatally along with measures of prenatal stress and demographic information. Placentas (N=67) were collected at birth and telomere length (TL) was measured in four separate fetally-derived placental tissues: amnion, chorion, villus, and umbilical cord. At four months of age, infants completed the still-face paradigm (SFP) during which respiratory sinus arrhythmia (RSA) data were collected; RSA reactivity and RSA recovery was available from 44 and 41 infants respectively. Multi-level mixed effects models examined the impact of maternal ACE score on placental TL. Generalized linear models tested the relation between composite placental TL and infant RSA, as well as the moderation of maternal ACE score and infant RSA by composite placental TL.
Results:
Higher maternal ACE score significantly predicted shorter placental TL across tissues (β=−0.015; p=0.036) and infant RSA across the SFP. No direct relation was found between placental TL and RSA, however placental TL moderated the relation between ACE score and both infant RSA reactivity (β=0.025; P=0.005) and RSA recovery (β=−0.028; P=0.032). In infants with shorter placental TL, higher ACE score predicted greater RSA suppression during the still-face epoch relative to play period 1 and greater RSA augmentation during play period 2 relative to the still-face epoch.
Conclusions:
These data are the first, to our knowledge, to report that changes in placental TL influence the transgenerational impact of maternal early life adversity on the development of her offspring’s autonomic nervous system.
Keywords: Adverse Childhood Experiences, Prenatal Stress, Telomere Length, Placenta, Respiratory Sinus Arrhythmia, Developmental Origins of Health and Disease
1. Introduction
Although the impact of maternal prenatal stress on her offspring is established, recent data suggests that the influence of maternal adversity on her offspring is better conceptualized from a life-course perspective that includes a mother’s own childhood experiences (Gray et al., 2017; McDonnell and Valentino, 2016; Moog et al., 2016). Within an individual, adverse childhood experiences (ACEs) are hypothesized to contribute to the earlier onset of both mental and physical illness through accelerated aging (Danese and McEwen, 2012; Drury et al., 2012; Felitti et al., 1998; Ridout et al., 2017). Both elevated cellular stress, indexed by measurements of oxidative stress and telomere length (TL), and reduced parasympathetic regulation of cardiac function indexed by respiratory sinus arrhythmia (RSA), are pathways predicted to contribute to the health risks associated with early life adversity (Danese and McEwen, 2012; Fitzpatrick et al., 2006; Masi et al., 2007; Ridout et al., 2017). TL, RSA, and the cardiovascular responses to stressors are also hallmarks of the aging process (Masi et al., 2007; Stratton et al., 2003). Given their mechanistic role in linking adversity to health outcomes within an individual, determining if these biological outcomes contribute to transgenerational effects is warranted.
The Developmental Origins of Health and Disease (DoHaD) model proposes that the origins of health and disease are found in the perinatal period (Barker, 1990; Barker et al., 2002). Alterations in developing stress response systems, such as the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal axis (HPA), are hypothesized pathways by which the earliest of experiences drive future health trajectories. Respiratory sinus arrhythmia (RSA) is one index of the ANS stress response system that is sensitive to prenatal stress exposure and preconception adversity (i.e., ACE exposure) in the next generation (DiPietro et al., 2006; Gray et al., 2017). RSA is the natural beat-to-beat variation in heart rate, in synchrony with respiration, which indexes parasympathetic regulation of cardiac function (Grossman and Taylor, 2007). Dynamic changes in RSA across a stress paradigm reflect an individual’s ability both to mobilize biological resources in response to a challenge and to return to a regulated state after the stressor is addressed. Development of RSA stress responsivity profiles, including both reactivity and recovery, begins in utero (Monk et al., 2000). Therefore, changes to the intrauterine environment, as a consequence of maternal life-course experiences, have the potential to impact the developmental trajectory of RSA profiles. Previously, we reported that maternal ACE exposure influences infant RSA independent of prenatal stress (Gray et al., 2017). However, to date, what aspects of the fetal environment contribute to these changes to the next generation is unclear.
Transgenerational transmission of maternal preconception adversity is hypothesized to occur via alterations in multiple factors across development including epigenetic changes in the germline, alterations to the intrauterine environment, and differences in postnatal caregiving, or, more likely some combination of these factors (Bale, 2015). When contemplating transgenerational effects, it is notable that maternal ACE exposure is associated with both pregnancy complications and preterm birth, highlighting the influence of the intrauterine environment as a target for ACE-related impacts (Cammack et al., 2011; Christiaens et al., 2015; Smith et al., 2016). A key organ at the maternal-fetal interface linked to pregnancy complications and preterm birth with the potential to transmit transgenerational effects is the placenta (Biron-Shental et al., 2010a; Biron-Shental et al., 2010b; Moog et al., 2016; Waldorf and McAdams, 2013). Biological indicators of compromised placental function include hypoxia and elevated oxidative stress, two factors indexed by telomere length (TL) (Biron-Shental et al., 2010b; Jauniaux et al., 2006; Polenttini et al., 2015a). Further, placental TL shortening is etiologically related to the onset of labor and parturition, as well as certain pregnancy complications, suggesting broader implications for placental TL (Biron-Shental et al., 2010a; Biron-Shental et al., 2010b; Menon et al., 2016; Phillippe, 2015). To date, however, no studies have examined placental TL in relation to maternal ACE exposure.
To explore the role of the placenta in the transmission of maternal adversity across generations, this study examined changes in placental TL as a moderator of the relation between maternal ACE and infant RSA. Identification of the earliest developmental time-points when the transgenerational effects of maternal life-course adversity are recognizable provides unique opportunities for mitigation strategies designed to combat the recalcitrant risk associated with maternal adversity.
2. Methods and Materials
2.1. Population
Recruitment of pregnant mothers took place at prenatal clinics, WIC (Women, infants, and children) clinics, and other ongoing studies at any point during their pregnancy as part of a prospective study of the impact of maternal life course stress on infant development. Mothers were at least 18 years of age, carrying a singleton pregnancy, and English speaking. Sixty-seven placentas were collected as placental collection began later in the course of the parent grant due to the receipt of additional foundation funding. Placentas were excluded if exposed to formalin or there was grand multiparty. The majority of mothers were recruited during their third trimester of pregnancy (61.2%; n=41) with 34.3% (n=23) recruited in their second trimester and 4.5% (n=3) in their first trimester. Gestational age ranged from 34–41 weeks. This study was approved by the University Institutional Review Board.
2.2. Demographic and pregnancy-related data collection
Mothers provided information about multiple levels of their and their infant’s social ecology during the prenatal visit by using an interview-assisted computer survey administered face-to-face at the research site or at prenatal clinics (Questionnaire Development System; Nova Research, Bethesda, Maryland). Oral responses were recorded onto a computer by trained interviewers. Demographic data were collected via maternal report during the pregnancy and pregnancy-related data were abstracted from medical records. Data collected via maternal report included: race, maternal age at conception, prenatal stress indices, ACE survey, maternal prenatal smoking, and socioeconomic status (SES) indicators. Race was categorized into Black, White, and other based on statistical distribution. Pregnancy-related data abstracted from medical records included: gestational age, delivery mode, parity, and pregnancy complications. A composite pregnancy risk variable was generated that included gestational hypertension, gestational diabetes, fetal growth restriction, and eclampsia, as previously used (Jones et al., 2017).
2.3. Placental tissue sample collection
Placental collection occurred as previously described (Jones et al., 2017). Placental collection and dissection occurred within approximately one hour of delivery (mean = 0.75 ± 0.52 h); the duration between parturition and sample collection was missing for five deliveries and the mean duration by delivery type was imputed. Four tissue types were separately collected from each placenta. The amnion and chorion were sampled >4 cm from fusion to the placental disk after manual separation. Villus tissue was sampled below the chorionic plate within ~4 cm of the cord insertion site and visible vasculature was excised. Umbilical cord samples were collected within ~4 cm of the placental disk insertion and fused reflected membranes were removed. Placental tissues were washed thoroughly in 1 mol/L phosphate-buffered saline solution. Samples were flash frozen in liquid nitrogen and stored at −80°C.
2.4. DNA extraction and quality assessment
Genomic DNA was isolated from placental tissues using the QIAamp DNA Mini Kit according to manufacturer protocols (Qiagen, Valencia, CA; Invitrogen, Carlsbad, CA). All DNA Samples were evaluated for double-stranded DNA integrity and concentration by Qubit dsDNA BR assay kit (Invitrogen, Carlsbad, CA) and purity by NanoDrop-2000 (Thermo Fisher Scientific, Waltham, MA). DNA was stored at −20°C and underwent no more than three freeze thaw cycles.
2.5. Telomere Length (TL)
The average relative TL was determined separately for each placental tissue type from the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio by using an adapted monochrome multiplex quantitative real-time polymerase chain reaction (PCR) and a BioRad CFX96 with standard methods from our laboratory (Cawthon, 2009; Jones et al., 2017). Placental TL was assayed with commercially available genomic placental DNA from a single donor (BioChain) as a control on all plates. Each plate was run in duplicate with all samples in a different well position. Thus, six replicates, of both the single copy gene and the telomere repeat, were available for each placenta for each tissue type. All four tissues from the same placenta were run on the same plate. PCR efficiency criteria for telomere and albumin reactions were 90% to 110%. Coefficients of variation (CVs) were calculated within each triplicate (CV criteria <10%) and between plates (CV criteria <6%). Samples with unacceptably high CVs (>10% intra- or >6% interassay CV) were repeated (N=5). The grand CV for all placental tissue TL samples was 3.16% determined by the average of the triplicates from both plates.
2.6. Maternal Adverse Childhood Experiences (ACE)
Mothers reported on their Adverse Childhood Experiences at their prenatal study visit (Felitti et al., 1998). The cumulative ACE exposure, which was the sum of the number of events reported (range = 0–9), score was used.
2.7. Prenatal maternal stress index (PNMS)
A composite PNMS risk metric consisting of five indicators was derived through factor analysis as previously reported (Gray et al., 2017). Indicators included the 10-item Pregnancy Related Anxiety Scale (Rini et al., 1999), Chronic Strain Questionnaire (Dunkel Schetter et al., 2013), Prenatal Life Events Scale – Revised (Lobel et al., 2008), the four-item version of the Perceived Stress Scale (Cohen et al., 1983), the 10-item Edinburgh Depression Scale (Cox et al., 1987). A composite prenatal risk variable was created by a summation score (range = 0–5) of positive endorsements of each the five PNMS indices. The cumulative PNMS score was used.
2.8. Socioeconomic status (SES) index
A summary score of SES was indexed from education, employment, home ownership, income, savings and government assistance status (range: 0–6) as previously reported (Jones et al., 2018). A score of six represents the highest SES score.
2.9. The Still-Face Paradigm (SFP)
At four months of age, 53 mother-infant dyads completed laboratory visits; fourteen dyads did not have an SFP due to difficulty in follow-up. During the visit, dyads completed the SFP, a validated social stressor for this age range shown to elicit ANS reactivity. The SFP included three epochs, each of which were 2.5 minutes in duration: free play (PP1), maternal neutral face and no interaction (SF; Still-face epoch), and resumption of free play (PP2). Infants were seated in a car seat facing their mother at eye level approximately three feet away. The mother was instructed not to engage in physical contact with the infant. If an infant displayed twenty seconds of continuous distress during any epoch, the epoch was ended. Of the 53 dyads, RSA reactivity data were not available due to the following reasons: one infant had excessive movement artifact, one due to human error, and seven did not complete a minimum of 64 seconds in both the PP1 and SF epochs of the SFP for RSA measurement. For RSA recovery values, an additional three infants did not complete a minimum of 64 seconds during PP2 due to infant distress. Thus, the resulting analytic sample for RSA reactivity was 44 and for RSA recovery was 41. Mothers of infants without RSA data for any reason were younger at the age of conception (t=2.02; P=0.048) and placentas were collected closer to parturition (t=2.04; P=0.045); analytic samples did not differ on any other demographic.
2.10. Respiratory sinus arrhythmia (RSA) stress responsivity
Heart period data were collected from continuous electrocardiography (ECG) recording during the SFP with previously established methods from our laboratory using James Long Company equipment (Caroga Lake, NY) (Jones et al., 2018). Following data collection, ECG signal processing and analysis was conducted offline. R-waves were algorithmically identified and misidentified or missing R-waves were corrected manually. IBIs between R-waves were prorated to equal time intervals of 125 ms and HR was assessed in beats per minute (bpm).
RSA was estimated with power spectral analysis using a discrete Fourier transform with a 64-second Hanning window and 50% overlap between consecutive windows. Spectral power was quantified in the high frequency band (0.30–0.75 Hz), a valid frequency range for assessing RSA in four month old infants (Bar-Haim et al., 2000).
2.11. Statistical analyses
2.11.1. Sample characteristics
Descriptive statistics characterized the sample overall and relations between relevant covariates using spearman correlation coefficients and t-tests where appropriate. Placental TL was normally distributed within tissue type and highly correlated between tissue types (ρ=0.40–0.70; Table 2). Infant RSA data were not normally distributed and were winsorized to three standard deviations from the mean and natural log transformed.
Table 2.
Correlation matrix of study variables
| Telomere length (T/S ratio) |
RSA PP1 |
RSA SF |
RSA PP2 |
ACE score |
PNMS score |
Race | Infant sex |
SES | Prenatal smoking |
Pregnancy complications |
Gestational age, wk |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cord | Amnion | Chorion | Villus | ||||||||||||
| Telomere length (T/S ratio) | |||||||||||||||
| Cord | 1 | ||||||||||||||
| Amnion | 0.70**** | 1 | |||||||||||||
| Chorion | 0.69**** | 0.59**** | 1 | ||||||||||||
| Villus | 0.57**** | 0.48**** | 0.40*** | 1 | |||||||||||
| RSA PP1 | −0.07 | −0.13 | −0.11 | −0.02 | 1 | ||||||||||
| RSA SF | −0.20 | −0.12 | −0.09 | 0.07 | 0.68**** | 1 | |||||||||
| RSA PP2 | −0.34* | −0.11 | −0.20 | −0.09 | 0.60**** | 0.56*** | 1 | ||||||||
| ACE score | −0.23 | −0.18 | −0.12 | −0.27* | 0.10 | 0.10 | −0.00 | 1 | |||||||
| PNMS score | 0.03 | −0.01 | 0.05 | −0.08 | 0.09 | −0.09 | −0.17 | 0.33** | 1 | ||||||
| Race | 0.11 | 0.07 | 0.25* | 0.17 | −0.21 | −0.08 | −0.21 | −0.12 | −0.03 | 1 | |||||
| Infant sex | −0.02 | 0.06 | 0.12 | 0.05 | 0.18 | 0.19 | 0.27 | 0.12 | −0.04 | −0.03 | 1 | ||||
| SES | 0.12 | −0.05 | 0.08 | 0.17 | 0.07 | 0.04 | −0.09 | −0.22 | −0.19 | 0.47**** | −0.05 | 1 | |||
| Prenatal smoking | −0.03 | −0.04 | 0.09 | −0.15 | 0.16 | −0.11 | −0.13 | 0.28* | 0.27* | 0.00 | 0.14 | −0.28* | 1 | ||
| Pregnancy complications | −0.25* | −0.29* | −0.38** | −0.06 | −0.15 | −0.16 | −0.06 | 0.12 | 0.06 | −0.03 | −0.11 | −0.08 | −0.02 | 1 | |
| Gestational age, wk | 0.13 | 0.02 | 0.19 | −0.01 | 0.01 | −0.05 | −0.28 | 0.02 | 0.08 | 0.07 | −0.13 | 0.17 | 0.03 | −0.33** | 1 |
| Maternal age at conception, yr | 0.08 | 0.01 | 0.01 | 0.16 | 0.01 | −0.07 | 0.02 | −0.23 | −0.05 | 0.33** | 0.08 | 0.45*** | −0.13 | −0.2 | −0.05 |
P<0.0001
P<0.001
P<0.01
P<0.05
Race: 0 = Black; 1 = White; 2 = Other; Infant sex: 1 = Male; 2 = Female.
2.11.2. ACE exposure and placental TL
To assess the impact of maternal ACE exposure and placental TL, multilevel analysis using mixed-effects linear regression models (MLM) with a random intercept were conducted. To first assess the impact of cumulative ACE score on placental TL, a MLM including only a dummy code for placental tissue type and cumulative ACE score was conducted (Model 1). Next, an adjusted model examined the impact of cumulative ACE score on placental TL accounting for the following covariates: placental tissue type, PNMS score, race, infant sex, SES, pregnancy complications, maternal prenatal smoking, and gestational age (Model 2). Covariates were chosen based on empirical relevance with TL or significant bivariable associations.
2.11.3. ACE exposure on RSA stress responsivity
To confirm our previous results linking maternal ACE exposure to infant RSA in this subset of infants, multilevel analysis using mixed-effects linear regression models (MLM) and a random intercept were conducted. To examine the impact of ACE exposure on RSA stress responsivity, a MLM including linear time, and quadratic time, cumulative ACE score, and the interaction of ACE score and quadratic time was conducted.
2.11.4. Moderation analysis of ACE exposure and RSA stress responsivity by composite placental TL
A generalized linear model failed to find an association between composite placental TL and RSA reactivity or RSA recovery, as such moderation analyses were used to examine how composite placental TL impacted the relation between maternal ACE exposure and RSA stress responsivity. This was tested by generalized linear models using maximum likelihood estimates with an interaction of cumulative ACE score and composite placental TL to predict RSA reactivity and RSA recovery, independently. The composite placental TL variable was generated for each individual by computing z-scores for each TL estimate by tissue type and then creating a sum of all four scores for each individual, subsequently referred to as composite placental TL. To assess RSA stress responsivity, change scores were generated for RSA reactivity by subtracting RSA during the SF epoch from PP1 (i.e., Δ = SF – PP1) and RSA recovery by subtracting RSA during PP2 from the SF epoch (i.e., Δ = PP2 – SF). A negative RSA value reflects parasympathetic withdrawal (i.e., RSA suppression) while a positive value represents parasympathetic engagement (i.e., RSA augmentation). The covariate structure was limited to infant sex, race, and maternal prenatal smoking given their empirical relevance to maternal-child health studies. To preserve statistical power, other covariates that did not independently contribute were not included.
3. Results
3.1. Demographics
Sixty-seven placentas yielded a total of 268 samples for TL measurement across placental tissue types. Descriptive outcomes of the sample demographics are listed in Table 1. A majority of mothers were Black (Table 1; 56.7%) and were approximately 28 years of age at conception. Mothers reported a mean ACE score of 2.31 ± 2.32 and mean PNMS score of 1.33 ± 1.45. The gestational period averaged 39.02 ± 1.41 weeks and infants weighed on average 3.35 ± 0.60 kg at birth. There were more male infants (56.7%) than female infants.
Table 1.
Demographic outcomes
| Demographic outcome, mean (SD) | Total (N=67) | Range | |
|---|---|---|---|
| ACE score | 2.31 (2.32) | 0–9 | |
| PNMS score | 1.33 (1.45) | 0–5 | |
| Maternal conception age (yr) | 27.96 (5.96) | 17–40 | |
| Gestational age (wk) | 39.02 (1.41) | 33.60–41.30 | |
| SES | 2.75 (2.10) | 0–6 | |
| Duration to sample collection (h) | 0.75 (0.52) | 0.10–2.13 | |
| Parity | 1.30 (1.44) | 0–5 | |
| % (n) | |||
| Maternal race | |||
| Black | 56.7 (38) | ||
| White | 29.9 (20) | ||
| Other | 13.4 (9) | ||
| Infant sex | |||
| Male | 56.7 (38) | ||
| Female | 43.3 (29) | ||
| Delivery mode | |||
| Vaginal | 52.2 (35) | ||
| C-section | 47.8 (32) | ||
| Maternal prenatal smoking | |||
| No | 85.1 (57) | ||
| Yes | 14.9 (10) | ||
| Pregnancy complications | |||
| No | 77.6 (52) | ||
| Yes | 22.4 (15) | ||
The correlations between covariates are listed in Table 2. SES and maternal age at conception were associated with race, such that Black mothers reported lower SES and conceived infants at a younger age. Maternal ACE score and PNMS score did not differ by race and were not correlated with SES, gestational age, pregnancy complications, or maternal age at conception. Lower SES (Odds ratio (OR)=0.63; P=0.040, CI=0.41–0.98), higher ACE (OR=1.36; P=0.027, CI=1.04–1.8) and higher PNMS (OR=1.84; P=0.011; CI=1.15–2.9) were all associated with prenatal smoking. Prenatal smoking was not associated with race, any placental tissue TL, or individual RSA measurements. TL was correlated across all placental tissue types. Shorter villus TL was associated with higher ACE score (ρ=−0.27; P=0.027), however other tissue types did not independently reach statistical significance. PNMS score was not associated with TL of any placental tissue type. RSA was significantly correlated across all three epochs but was not associated with any other covariate.
3.2. ACE exposure across placental TL tissue types
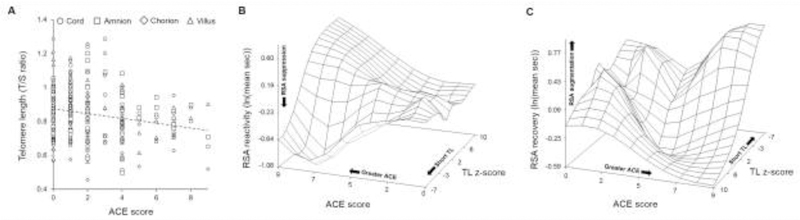
In the base model, utilizing 268 TL observations across fetally-derived placental tissues, higher ACE score predicted shorter placental TL (Figure 1A; Table 3; Model 1; β=−0.014; P=0.030). Maternal age at conception, parity, duration between parturition and sample collection, or delivery mode did not account for a significant portion of variance and were excluded from subsequent models. The adjusted model accounted for the following covariate structure: placental tissue type, PNMS score, race, infant sex, SES, pregnancy complications, maternal prenatal smoking, and gestational age. In the adjusted model, the impact of ACE score remained significant (Table 3; Model 2; β=−0.015; P=0.036) and pregnancy complications were marginally associated with shorter placental TL (β=−0.071; P=0.057).
Figure 1. The transgenerational impact of maternal ACE exposure.
In panel A, greater ACE score was predictive of shorter fetal placental TL. All placental tissue TL measurements are plotted by tissue type against ACE score with a linear trend line. In panels B and C, surface plots of the moderation of ACE score and RSA stress responsivity by composite placental TL are presented with RSA reactivity in panel B and RSA recovery in panel C. A spline interpolation was used to smooth the surface plot. RSA reactivity is defined as the difference in RSA during the SF epoch from PP1 (i.e., Δ = SF – PP1) and RSA recovery is defined as the difference in RSA during PP2 relative to the SF epoch (i.e., Δ = PP2 – SF). In order to visualize the surface plot in panel C, the axis was rotated 250° around the Y-axis.
Table 3.
The impact of ACE exposure across placental TL tissue types
| Model 1: Main effects | Model 2: Cumulative ACE | |||
|---|---|---|---|---|
| β | P-value | β | P-value | |
| Tissue type | −0.003 | 0.66 | −0.003 | 0.66 |
| ACE score | −0.014 | 0.030 | −0.015 | 0.036 |
| PNMS score | 0.009 | 0.45 | ||
| Infant sex | 0.026 | 0.40 | ||
| Race | 0.026 | 0.28 | ||
| SES | −0.002 | 0.81 | ||
| Pregnancy complications | −0.071 | 0.057 | ||
| Maternal prenatal smoking | −0.010 | 0.84 | ||
| Gestational age | 0.002 | 0.87 | ||
Bolded P-value signifies P < 0.05
3.3. ACE exposure and RSA stress responsivity
In the confirmatory analysis of our previous study utilizing 44 infants (N=129 RSA observations), ACE score significantly interacted with quadratic time to predict RSA across the SFP (β=−0.009; P=0.049).
3.4. The moderation of maternal ACE exposure and RSA by composite placental TL
Composite placental TL was not associated with RSA reactivity (β=0.002; P=0.93) or RSA recovery (β =−0.019; P=0.35). In the adjusted model, controlling for sex, race, and maternal prenatal smoking, composite placental TL and maternal ACE score interacted to predict both RSA reactivity (Figure 1B; Table 4; β=0.025; P=0.005) and RSA recovery (Figure 1C; Table 4; β=−0.028; P=0.032). In infants with shorter composite placental TL, higher ACE score was associated with greater RSA suppression during the SF epoch relative to PP1 and greater RSA augmentation during PP2 relative to the SF epoch (Figure 1B & 1C). However, among infants of lower ACE mothers, composite placental TL was not associated with RSA reactivity or RSA recovery. Maternal prenatal smoking was significantly associated with greater RSA suppression during the SF epoch relative to PP1 (β=−0.584; P=0.0003).
Table 4.
The moderation of ACE exposure and RSA stress responsivity by composite placental TL
| RSA reactivity | RSA recovery | |||
|---|---|---|---|---|
| Sample size | 44 | 41 | ||
| β | P-value | β | P-value | |
| ACE score | 0.037 | 0.16 | −0.065 | 0.12 |
| Placental TL | −0.059 | 0.018 | 0.037 | 0.29 |
| ACE x placental TL | 0.025 | 0.005 | −0.028 | 0.032 |
| Infant sex | 0.11 | 0.30 | 0.078 | 0.58 |
| Race | 0.096 | 0.20 | −0.058 | 0.58 |
| Maternal prenatal smoking | −0.584 | 0.0003 | −0.059 | 0.80 |
Bolded P-value signifies P < 0.05
4. Discussion
This is the first study to demonstrate the impact of maternal ACE exposure on TL, a marker of cellular stress, in the placenta, an effect that was robust to the inclusion of covariates. Beyond this we demonstrate that composite placental TL moderated the link between maternal ACE and later infant autonomic development, providing the first evidence of a physiologic consequence of accelerated placental aging in infants.
In our study of mostly term infants, we demonstrate that both maternal ACE exposure and pregnancy complications are associated with placental TL. These findings are consistent with the existing literature linking maternal ACE exposure to both preterm birth and various pregnancy complications, many of which have independent associations with placental physiology and function (Christiaens et al., 2015; Menon et al., 2012; Menon et al., 2014 Smith et al., 2016). Oxidative stress and inflammation, both of which are associated with shorter TL, represent two likely pathways linking these findings as shown in our conceptual model and supported by a broader literature (Figure 2) (Entringer et al., 2018; Godfrey et al., 2002; Schoots, et al., 2018). For example, telomere fragments from the chorioamniotic membrane are shed into the amniotic fluid and trigger an inflammatory response thereby contributing to the onset of parturition (Polettini et al., 2015a). In both preterm and term births, as well as pregnancies complicated by preterm premature rupture of membranes, cellular senescence, oxidative stress, inflammation and shorter TL have all been variously associated with active labor and/or parturition (Behnia et al., 2015; Menon et al., 2012 Menon et al., 2014; Menon et al., 2016; Polettini et al., 2015b). Thus, the earlier presentation of these molecular profiles associated with labor and/or parturition and linked to TL dynamics, suggests that, even in low risk pregnancies, maternal life-course adversity may still lead to elevated risk in the next generation. To further examine this, we tested whether the interaction of either ACE score and pregnancy complications, or composite placental TL and pregnancy complications, influenced infant RSA; neither interaction was significant (data not shown).
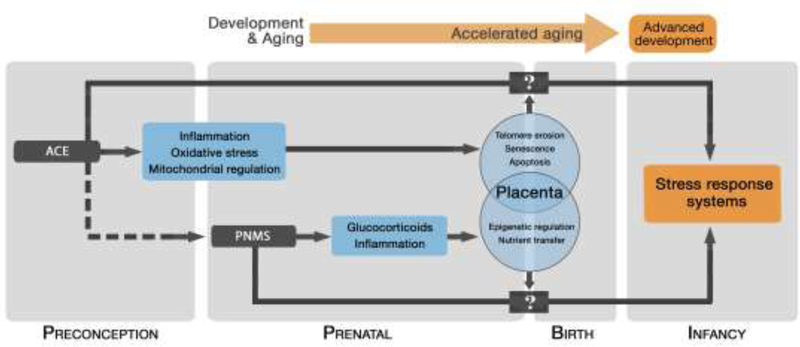
Figure 2. A model for transgenerational transmission of maternal life-course adversity.
The proposed conceptual model for the transgenerational transmission of maternal life-course adversity through accelerated aging. The dotted line represents the independent correlation between PNMS and ACE. Our model includes the preconception period as an independent period through which maternal experiences biologically influence the next generation, partially through placental factors. We propose, based on our data, a direct relation between maternal ACE exposures and accelerated cellular aging in the placenta. Further, the advanced ANS development associated with maternal ACE exposure is moderated by placental cellular aging. We leave open the direct intermediary factors (represented by boxes with “?”). Given that PNMS did not predict placental TL, we propose an alternative pathway, with some potential overlap (e.g. inflammation), by which maternal preconception and maternal prenatal adversity influence the next generation.
Our conceptual model proposes that placental cellular aging influences the relation between maternal preconception adversity and infant physiologic stress reactivity (Figure 2). In infants with shorter composite placental TL, higher maternal ACE score was associated with enhanced RSA reactivity and RSA recovery. Interestingly, this model coincides with data that links altered placental vascular flow, potentially as a function of elevated oxidative stress, to accelerated cardiac development (Burton and Jauniaux, 2018; Rodriguez-Rodriguez et al., 2018; Thornburg et al., 2010). These accentuated patterns of RSA stress responsivity can be considered an advanced developmental pattern, findings consistent, to some extent, with previous reports of the association between TL and RSA reactivity in adolescents (Kroenke et al., 2011; Woody et al., 2017). The lack of a direct relation between composite placental TL and infant RSA indicate that multiple, likely synergistic pathways, contribute to the transmission of maternal adversity across generations. Together these results suggest that accelerated cellular aging in the placenta, as a function of maternal life-course adversity, advances infant ANS development, potentially in expectation of an adverse postnatal environment.
There are limitations to the current study. The total number of placental samples was small. The use of multi-level mixed effects regression models partially mitigates the concern of sample size by nesting repeated placental TL measurements within an individual and allowing for examination of all 268 placental TL samples together. Post-hoc analysis of maternal ACE score on placental TL by tissue type revealed a similar pattern of high ACE score and shorter TL, but this only reached statistical significance in villus tissue. In a larger sample, examination of differences in TL measured in specific tissues with more putative direct links to preterm birth (i.e., amnion and chorion) and IUGR (i.e., villus) may provide additional insight (Arias-Ortega et al., 2016; Rakow et al., 2013). Given the low prevalence of individual pregnancy complications, a composite variable was generated and thus the testing of individual pregnancy compilations on placental TL was not possible, although gestational diabetes was independently associated with placental TL (data not shown). PNMS exhibited no impact on placental TL differing from previous studies testing TL in cord blood (Entringer et al., 2013; Marchetto et al., 2016). However, given differences in the cellular composition between the placenta and cord blood, this discrepancy is not unexpected. We also failed to find sex differences in placental TL, although females have been shown to exhibit longer placental TL (Martens et al., 2017). Larger studies are needed to examine the moderation of maternal life-course adversity on placental TL by infant sex in light of sex differences in both prenatal programming and TL (Barrett and Richardson, 2011). Although race differences were not found in this study, the persistence of racial disparities in pregnancy complications and birth outcomes highlight the need for future studies that are sufficiently powered to examine race differences (Bryant et al., 2010; Geronimus et al., 2010). There was variation in the trimester of maternal recruitment and assessment of PNMS, however trimester of pregnancy when recruited did not influence the results. Lastly, maternal ACE exposure was assessed through retrospective self-report and, while this is consistent with previous studies, it is subject to bias.
5. Conclusion
Recent literature continues to bolster the observed negative impacts of ACE exposure across health outcomes and across generations. Our data suggests that shorter placental TL is one molecular pathway by which ACEs may influence health across generations. Even after considering perinatal outcomes, infants born to mothers with high ACE scores remain at risk, likely carrying undetected advancements in their stress response systems, similar to the accelerated amygdala-prefrontal cortex coupling observed in post-institutionalized children (Gee et al., 2013). Given the established plasticity of the ANS, and its responsiveness to early caregiving, our findings provide heightened support for interventions that increase sensitive, contingent caregiving for their infants, particularly in high ACE mothers, as an innovative approach to buffering the transgenerational mental and physical health risks associated with ACE exposure (Feldman, 2007; Gee et al., 2014).
Highlights.
High maternal ACE exposure predicted shorter placental TL
Placental TL moderated the relation between maternal ACE and infant RSA
Infant health may be influenced by maternal preconception adversity
Maternal ACE-related infant ANS differences are influenced by placental aging
Acknowledgements
The authors would like to thank the mothers and their infants who participated in the study and all of the staff and students who assisted in the recruitment of participants, conducting laboratory visits, collection of psychophysiological data, DNA extractions, and many other aspects of the study.
Source of Funding
This study was funded by the National Institutes of Health [1R01MH101533-01, 3R01MH101533-02S3 {SD}], the Tulane University Oliver Fund (SD), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development [K12HD043451 {SD & SG} & L30HD085275 {SG}]. The funding sources had no direct involvement in the study or manuscript preparation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflict of interest.
Contributor Information
Mr. Christopher W. JONES, New Orleans, LA; Tulane Brain Institute, Department of Neuroscience, Tulane University
Mr. Kyle C. ESTEVES, New Orleans, LA; Department of Psychiatry and Behavioral Sciences, Tulane University School of Medicine.
Dr. Sarah A.O. GRAY, New Orleans, LA; Department of Psychology, Tulane University, School of Science and Engineering
Ms. Tegan N. Clarke, New Orleans, LA; Department of Psychiatry and Behavioral Sciences, Tulane University School of Medicine
Mr. Keegan CALLERAME, New Orleans, LA; Department of Psychiatry and Behavioral Sciences, Tulane University School of Medicine
Dr. Katherine P. THEALL, New Orleans, LA; Department of Global Community Health and Behavioral Sciences, Tulane University School of Public Health and Tropical Medicine
Dr. Stacy S. DRURY, New Orleans, LA; Tulane Brain Institute, Department of Neuroscience, Tulane University; Department of Psychiatry and Behavioral Sciences, Tulane University School of Medicine
References
- Arias-Ortega R, Echeverria J, Guzmán-Huerta M, Camargo-Marín L, Gaitán-González M, Borboa-Olivares H, Portilla-Islas E, Camal-Ugarte S, Vargas-García C, Ortiz M, 2016. Respiratory sinus arrhythmia in growth restricted fetuses with normal Doppler hemodynamic indices. Early Hum Dev 93, 17–23. [DOI] [PubMed] [Google Scholar]
- Bale TL, 2015. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 16, 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA, 2000. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Barker D, Thornburg K, 2013. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta 34, 841–845. [DOI] [PubMed] [Google Scholar]
- Barker DJ, 1990. The fetal and infant origins of adult disease. BMJ 301, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EL, Richardson DS, 2011. Sex differences in telomeres and lifespan. Aging cell 10, 913–921. [DOI] [PubMed] [Google Scholar]
- Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, Saade GR, Menon R 2015. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol 213, e1–16. [DOI] [PubMed] [Google Scholar]
- Biron-Shental T, Halevy RS, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A, 2010a. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR). Early Hum Dev 86, 451–456. [DOI] [PubMed] [Google Scholar]
- Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A, 2010b. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol 202, 381.e1–7. [DOI] [PubMed] [Google Scholar]
- Bryant AS, Worjoloh A, Caughey AB, Washington AE, 2010. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol 202, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, 2018. Development of the human placenta and fetal heart: synergic or independent? Front Physiol 9, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD, 2011. The association between early life adversity and bacterial vaginosis during pregnancy. Am J Obstet Gynecol 204, 431.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, 2009. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, Hegadoren K, Olson DM, 2015. Adverse childhood experiences are associated with spontaneous preterm birth: a case–control study. BMC Med 13, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS, 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav 106, 29–39. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, 2006. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging cell 5, 325–330. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP, 2006. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev 77, 573–587. [DOI] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong J, Fox NA, Zeanah CH, Nelson CA, 2012. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry 17, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C, Schafer P, Lanzi RG, Clark-Kauffman E, Raju TN, Hillemeier MM, Network CCH, 2013. Shedding light on the mechanisms underlying health disparities through community participatory methods: The stress pathway. Perspect Psychol Sci 8, 613–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD, 2018. The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci 373, 20170151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, Simhan HN, Wadhwa PD, 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol 208, 134.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, 2007. Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry 48, 329–354. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A, 2006. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165, 14–21. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N, 2013. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 110, 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N, 2014. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci 25, 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD, 2010. Do US black women experience stress-related accelerated biological aging? Hum Nat 21, 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, 2002. The role of the placenta in fetal programming-a review. Placenta 23, S20–27. [DOI] [PubMed] [Google Scholar]
- Gray SA, Jones CW, Theall KP, Glackin E, Drury SS, 2017. Thinking across generations: unique contributions of maternal early life and prenatal stress to infant physiology. J Am Acad Child Adolesc Psychiatry 56, 922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Taylor EW, 2007. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol 74, 263–285. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P, 2014. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton J, 2006. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update 12, 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CW, Gambala C, Esteves KC, Wallace M, Schlesinger R, O’Quinn M, Kidd L, Theall KP, Drury SS, 2017. Differences in placental telomere length suggest a link between racial disparities in birth outcomes and cellular aging. Am Journal Obstet Gynecol 216, 294.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CW, Gray SA, Theall KP, Drury SS, 2018. Polymorphic variation in the SLC5A7 gene influences infant autonomic reactivity and self-regulation: A neurobiological model for ANS stress responsivity and infant temperament. Psychoneuroendocrinology 97, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradović J, Lin J, Blackburn E, Stamperdahl JL, Boyce WT, 2011. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med 73, 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA, 2008. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol 27, 604–615. [DOI] [PubMed] [Google Scholar]
- Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R, Haussmann MF, 2016. Prenatal stress and newborn telomere length. Am Journal Obstet Gynecol 215, 94.e1–8. [DOI] [PubMed] [Google Scholar]
- Martens DS, Cox B, Janssen BG, Clemente DB, Gasparrini A, Vanpoucke C, Lefebvre W, Roels HA, Plusquin M, Nawrot TS, 2017. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging. JAMA pediatrics 171, 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT, 2007. Respiratory sinus arrhythmia and diseases of aging: obesity, diabetes mellitus, and hypertension. Biol Psychol 74, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell CG, Valentino K, 2016. Intergenerational effects of childhood trauma: evaluating pathways among maternal ACEs, perinatal depressive symptoms, and infant outcomes. Child Maltreatment 21, 317–326. [DOI] [PubMed] [Google Scholar]
- Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M, 2016. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 8, 216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Boldogh I, Hawkins HK, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN, 2014. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 184, 1740–1751. [DOI] [PubMed] [Google Scholar]
- Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN, 2013. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One 8, e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN, 2012. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One 7, e31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, Hurtado A, 2000. Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate. Dev Psychobiol 36, 67–77. [PubMed] [Google Scholar]
- Moog NK, Buss C, Entringer S, Shahbaba B, Gillen DL, Hobel CJ, Wadhwa PD, 2016. Maternal Exposure to Childhood Trauma Is Associated During Pregnancy With Placental-Fetal Stress Physiology. Biol Psychiatry 79, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippe M, 2014. Cell-free fetal DNA—a trigger for parturition. N Engl J Med 370, 2534–2536. [DOI] [PubMed] [Google Scholar]
- Phillippe M, 2015. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod Sci 22, 1186–1201. [DOI] [PubMed] [Google Scholar]
- Polenttini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R, 2015a. Telomere fragment induced amnion cell senescence: A contributor to partrutition. PLoS One 10, e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenttini J, Dutta EH, Behnia F, Saade GR, Torloni MR, Menon R, 2015b. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: A systematic review of the literature. Placenta 36, 969–973. [DOI] [PubMed] [Google Scholar]
- Rakow A, Katz-Salamon M, Ericson M, Edner A, Vanpée M, 2013. Decreased heart rate variability in children born with low birth weight. Pediatr Res 74, 339–343. [DOI] [PubMed] [Google Scholar]
- Ridout K, Levandowski M, Ridout S, Gantz L, Goonan K, Palermo D, Price L, Tyrka A, 2017. Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 23, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA, 1999. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol 18, 333–345. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez P, Ramiro-Cortijo D, Reyes-Hernández CG, de Pablo ALL, González MC, Arribas SM, 2018. Implication of oxidative stress in fetal programming of cardiovascular disease. Front Physiol 9, 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL, 2018. Oxidative stress in placental pathology. Placenta 69, 153–161. [DOI] [PubMed] [Google Scholar]
- Smith MV, Gotman N, Yonkers KA, 2016. Early childhood adversity and pregnancy outcomes. Matern Child Health J 20, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton JR, Levy WC, Caldwell JH, Jacobson A, May J, Matsuoka D, Madden K, 2003. Effects of aging on cardiovascular repsonses to parasympathetic withdrawal. J Am Coll Cardiol 41, 2077–2083. [DOI] [PubMed] [Google Scholar]
- Thornburg K, O’tierney P, Louey S, 2010. The placenta is a programming agent for cardiovascular disease. Placenta 31, S54–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf KMA, McAdams RM, 2013. Influence of infection during pregnancy on fetal development. Reproduction 146, R151–R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody A, Hamilton K, Livitz IE, Figueroa WS, Zoccola PM, 2017. Buccal telomere length and its associations with cortisol, heart rate variability, heart rate, and blood pressure responses to an acute social evaluative stressor in college students. Stress 20, 249–257. [DOI] [PubMed] [Google Scholar]