Abstract
This study investigates the association between neighborhood disadvantage from adolescence to young adulthood and metabolic syndrome using a life course epidemiology framework. Data from the United States-based National Longitudinal Study of Adolescent to Adult Health (n=9,500) and a structural equation modeling approach were used to test neighborhood disadvantage across adolescence, emerging adulthood, and young adulthood in relation to metabolic syndrome. Adolescent neighborhood disadvantage was directly associated with metabolic syndrome in young adulthood. Evidence supporting an indirect association between adolescent neighborhood disadvantage and adult metabolic syndrome was not support. Efforts to improve cardiometabolic health may benefit from strategies earlier in life.
Keywords: Neighborhood Disadvantage, Metabolic Syndrome, Sensitive Periods, Life course epidemiology, Population Health
INTRODUCTION
Metabolic syndrome—clinically defined by the presence of central adiposity, dyslipidemia hypertension, and insulin sensitivity (Grundy et al. 2005)—has been linked to increased risk of adverse cardiometabolic health, including cardiovascular disease and type 2 diabetes (Galassi, Reynolds, and He 2006; Laaksonen et al. 2002; Malik et al. 2004; McNeill et al. 2005). Cardiovascular disease is the leading cause of death in the United States (Heron 2018) and, while risk factors can manifest as early as childhood, diagnosis generally occurs much later in life (Morrison, Friedman, and Gray-McGuire 2007). Therefore, metabolic syndrome is a reasonable outcome in studies of cardiometabolic risk in young adults, before permanent biological damage occurs, to help identify at-risk populations prior to the onset of cardiovascular disease. The prevalence of metabolic syndrome is relatively low during early life (3% among children), but becomes increasingly prevalent in adulthood, with 23–44% of adults ≥19 years of age impacted (Alexander et al. 2003; Beltrán-Sánchez et al. 2013; Friend, Craig, and Turner 2013). Given metabolic syndrome’s rising prevalence with age, it is increasingly important to determine risk factors earlier in the life course that contribute to the development of metabolic syndrome for cardiovascular disease prevention.
Individual-level determinants of metabolic syndrome are well-established, including factors such as low socioeconomic status (Chichlowska et al. 2009; Gustafsson, Persson, and Hammarström 2011; Langenberg et al. 2006; Schooling et al. 2008; Yang et al. 2017), physical inactivity (He et al. 2014; Zhang et al. 2017), and poor diet quality (Lutsey, Steffen, and Stevens 2008; Rodriguez-Monforte et al. 2017). Within the last two decades, researchers have begun to acknowledge the importance of neighborhood-level conditions, beyond the previously mentioned individual-level risk factors, in shaping cardiovascular health (Barber et al. 2016; Diez-Roux et al. 1997; Diez Roux et al. 2001, 2016; Diez Roux, Jacobs, and Kiefe 2002; Gebreab et al. 2017; Glass, Rasmussen, and Schwartz 2006; Krishnan et al. 2010; Suglia et al. 2016). Individuals residing in disadvantaged neighborhoods experience limited food and physical activity environments, including limited availability and access to healthy food choices (Morland et al. 2002) and poor infrastructures for physical activity (Gordon-Larsen et al. 2006). Moreover, residing in disadvantaged neighborhoods is associated with increased exposure to chronic stressors, such as greater crime, discrimination, and despair (Ross and Mirowsky 2001; Sampson, Raudenbush, and Earls 1997). Taken together, determining the role of neighborhood-level disadvantage, independent of individual-level factors, in shaping the development of metabolic syndrome may be helpful in identifying targets for intervention to reduce the burden of cardiovascular disease.
Prior cross-sectional studies of neighborhood-level SES (i.e. disadvantage) and metabolic syndrome have consistently suggested that residing in more disadvantaged neighborhoods confer increased risk of metabolic syndrome (Chichlowska et al. 2008; Clark et al. 2013; Keita et al. 2014). However, such research designs preclude inference of the temporal effects of neighborhood disadvantage on metabolic syndrome and limits the ability to account for factors that select individuals into a given neighborhood (Diez Roux 2004; Oakes 2004). Therefore, it remains unclear whether contemporaneous associations between neighborhood-level disadvantage and metabolic syndrome are partially or entirely confounded by earlier life exposures.
Prospective studies of neighborhood disadvantage across the life course offer a unique opportunity to understand the mechanisms linking early life exposures to adult health. Life course epidemiology, which posits that social and biological factors may likely act independently, cumulatively, and interactively over the life course to impact adult health and disease (Kuh et al. 2003), provides a useful framework for conceptualizing these mechanisms. Residing in a disadvantaged neighborhood is correlated across life stages, such that individuals who experience neighborhood disadvantage in early life are more likely to experience adult neighborhood disadvantage (Gustafsson et al. 2013; Van Ham et al. 2014), with both likely influencing cardiometabolic health. Prior research suggests that residing in disadvantaged neighborhoods for longer time periods is associated with overweight and obesity (Do and Zheng 2017; Lippert 2016; Lippert et al. 2017; Sheehan et al. 2017), weight gain (Powell-Wiley et al. 2014), high blood pressure and hypertension (Lippert et al. 2017), and cardiovascular mortality (Xiao et al. 2018).
A focus of life course epidemiology has been identifying exposures during gestation, childhood, adolescence, and early adulthood that impact health outcomes later in life. Two theoretical models within the life course epidemiology framework may help to understand how neighborhood disadvantage in earlier life stages influence metabolic syndrome in adulthood: (1) the sensitive period model; and (2) chains of risk model. The sensitive period model describes a life stage when an exposure has a greater impact on disease risk than it would at other life stages (Kuh and Shlomo 2004). To test the sensitive period life course model, control for subsequent life course exposures to neighborhood disadvantage is necessary. Under this life course model, neighborhood disadvantage during adolescence may serve as a sensitive period directly altering an individual’s susceptibility to metabolic syndrome in adulthood by impacting their likelihood to develop unhealthy norms around diet and physical activity.
Second, a chains of risk model, a form of the accumulation of risk life course model, postulates that exposures are linked over the life course to influence later health (Kuh and Shlomo 2004). Two possible chains of risk models exist: one whereby earlier exposure increases risk of later exposures while also independently impacting disease risk, and another whereby earlier exposures set off a chain of exposures that has no direct (independent) effect on disease risk except through the final link in the chains of exposures. Applied to the present study, it is plausible that living in a highly disadvantaged neighborhood early in life is associated with an increased risk of living in a highly disadvantaged neighborhood in adulthood, which in turn is associated with an increased risk of metabolic syndrome in adulthood. Early life exposure to a disadvantaged neighborhood may operate entirely through this pathway (and thus retain only an indirect effect on adulthood risk of metabolic syndrome) or may operate partially through this pathway (and thus retain an indirect and direct effect on adulthood risk of metabolic syndrome).
Longitudinal measures of neighborhood attributes are necessary for testing life course models and to more accurately assess long-term, dynamic exposures. Building upon previous research, this study applies a life course framework and structural equation modeling (SEM) approach to examine the sensitive period and chains of risk models to determine the association between neighborhood disadvantage during the transition from adolescence to adulthood and metabolic syndrome during adulthood. Using data from a nationally representative, longitudinal study of adolescents followed into adulthood, we explicitly tested whether: (1) the association between neighborhood disadvantage in adolescence and metabolic syndrome in adulthood remains after controlling for neighborhood disadvantage in subsequent life stages (sensitive period model); or (2) the association between neighborhood disadvantage in adolescence and metabolic syndrome in adulthood operated through subsequent neighborhood disadvantage (i.e. chains of risk model).
METHODS
The National Longitudinal Study of Adolescent to Adult Health (Add Health) is an ongoing, nationally representative longitudinal study of adolescents in grades 7–12 during the 1994–1995 school year in the United States (Harris et al. 2013). Students were recruited from 132 middle and high schools. In 1994, in-school surveys were administered to 90,118 students selected from a stratified random sample of all high schools. A subsample of these students was randomly selected from the school rosters to participate in home-based interviews (n=20,745). A second wave of in-home interviews were conducted among those in grades 8–12 in 1996 followed by a third wave of data collection in 2001–2002 during emerging adulthood (ages 18–26) and a fourth wave in 2008–2009 during young adulthood (ages 24–32) among Wave I participants who participated in the in-home survey.
Individuals included in our study were those who participated in Waves I, III, and IV (n=13,034) without missing data on any component of the metabolic syndrome measure taken at Wave IV (n=11,422). We only included females who were not pregnant at the time of any of the three interviews (n=10,762), as anthropometric and physiological markers do not compare among pregnant and non-pregnant women. Further, only US-born participants were included (n=10,077) due to the established health differences by nativity status (Crosnoe 2006; Harris, Perreira, and Lee 2009; Hummer et al. 1999; Singh and Miller 2004). Finally, participants with available Wave IV sampling weights to produce nationally-representative estimates were included resulting in a final analytic sample size of 9,500. We compared sociodemographic characteristics of those excluded from our analytic sample (n=7,711) to those who responded across all three waves (n=13,034). Participants who responded across each of the three waves where more likely to be non-Hispanic white (p=0.01), have a parent with more than a high school education (p<0.001), and have lived in their current residence since birth (p<0.001), but no difference was observed by parent age at baseline (p=0.58). Further, among the individuals who participated across all three waves (n=13,034), we compared characteristics for those who were excluded due to missing metabolic syndrome components (n=1,612) and the participants who remained in the sample (n=11,422). Similarly, we observed that those with all metabolic syndrome measures were more likely to be non-Hispanic white (p=0.001) and had slightly higher mean body mass index (BMI) at baseline (p=<0.01), but did not differ on educational attainment (p=0.37).
Exposures
Neighborhood characteristics were measured at the census-tract level using contextual data appended to Add Health (Harris, 2013). Information from the 1990 U.S. Census and the 2005–2009 U.S. American Community Survey were used to measure neighborhood disadvantage during adolescence (Wave I), emerging adulthood (Wave III), and young adulthood (Wave IV). Five census-tract level indicators were chosen a priori based on availability of measures in the 1990 and 2005–2009 instruments to reflect aspects of neighborhood income/wealth, education, and household structure (Sampson et al. 1997). The five census-tract level indicators included: percent households with incomes below the federal poverty line; percent of households receiving public assistance; civilian unemployment rate; percent of persons 25 years or older with no high school diploma or equivalency; and percent of female-headed households. To determine the final neighborhood disadvantage construct, we used factor analysis with Varimax orthogonal factor rotation and indicators were included based on the factor loadings, standardized regression coefficients, and measurement model fit.
Outcome
During young adulthood (Wave IV, ages 24–32), metabolic syndrome was defined according to the third Adult Treatment Panel (ATP III) guidelines (Grundy et al. 2005) and included the following five components: waist circumference, blood pressure, high density lipoprotein-cholesterol (HDL-c), triglycerides, and insulin resistance. Participants were classified as having metabolic syndrome if they met at least 3 of the 5 criteria. The ATP III definition was modified slightly to align with the available Add Health data (Table 1). First, the lab that provided the assayed specimens for lipids used two different assays for samples. After extensive data cleaning and quality control efforts, Add Health only released the rank-ordering (by deciles) as a more reliable measure than the absolute measures from conversion strategies. Therefore, we departed from the clinical cutoffs (<50 mg/dL for women, <40 mg/dL in men, or lipid lowering drug treatment) to classify reduced HDL-C as membership in the lowest category for women and lowest two deciles for men. This was based on evidence showing 11.9% of women and 31.4% of men have reduced HDL-C (Carroll, Kit, and Lacher 2012). Similarly, the top three deciles of triglycerides for men and the top two deciles of triglycerides for women were used to define elevated triglycerides based on national estimates for men (29.6%) and women (17.8%) 20–39 years of age (Ervin 2009). Second, insulin sensitivity was defined using pre-diabetic value of glycosylated hemoglobin (HbA1c) instead of the ATP III definition of fasting glucose ≥100 mg/dL or antidiabetic medication as participants were not required to fast before their interview. As a result, data contain a combination of both fasting and non-fasting glucose measures. Instead of combining the two measures, we used HbA1c, a measure of blood glucose in a person’s body over 2–3 months prior to their interview. HbA1c provides a more stable measure of metabolic dysregulation than glucose, which varies widely by dietary intake prior to measurements. Other studies have also utilized the similar alternative definitions for measures of metabolic syndrome in Add Health (Gaydosh et al. 2018; Kane et al. 2017).
Table 1.
ATP III definition and modified ATP III definition (Add Health) for metabolic syndrome
| Component | ATP III Definition | Modified ATP III Definition |
|---|---|---|
| Central obesity | High Waist Circumference: Women: ≥88 cm Men: ≥102 cm |
ATP III Criteria |
| High blood pressure | Systolic/diastolic blood pressure: ≥130/85 mm Hg or antihypertensive drug treatment |
ATP III Criteria |
| Reduced HDL-cholesterol | HDL-cholesterol level: Men: <40 mg/dL Women: <50 mg/dL |
HDL-cholesterol level: Men: lowest two deciles Women: lowest decile |
| Elevated triglycerides | Triglyceride level: ≥150 mg/dL | Triglyceride levels: Men: highest three deciles Women: highest two deciles |
| Insulin sensitivity | Fasting glucose level: ≥110 mg/dL | HbAlc* level: >5.6% |
Covariates
Covariates included participants’ baseline age (Wave I), sex, race/ethnicity (Non-Hispanic white, Non-Hispanic black, Hispanic, other), parental educational attainment based on highest level achieved by mother and father (less than high school, high school or equivalent, some college, college degree or more), same residence since birth (yes/no), and participant’s baseline self-reported health using general question of health status (excellent, very good, good, fair, poor). Self-reported health was included as an important covariate to account for potential residential selection issues due to childhood health status. Values were grouped to create a three-level variable – excellent/very good, good, fair/poor. Variables were defined as shown in Table 1.
Statistical Analysis
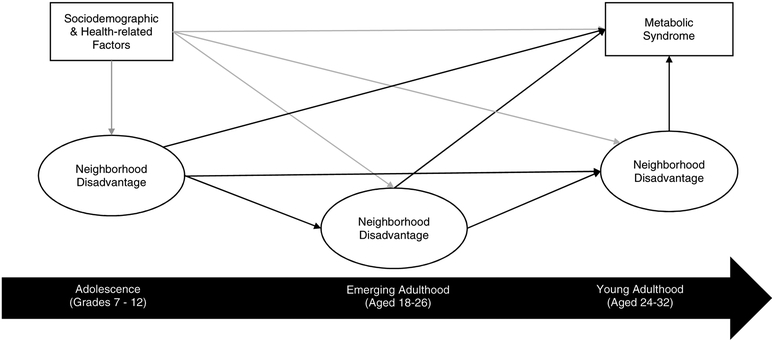
We employed a structural equation modeling approach (SEM) to examine the pathways linking neighborhood disadvantage across the transition from adolescence to young adulthood on metabolic syndrome in young adulthood. Figure 1 depicts a theoretical model of life course neighborhood disadvantage and metabolic syndrome, which was used to guide our analysis. SEM models include a measurement model (i.e. factor model) and structural model (i.e. regression) to estimate the direct and indirect effects. The findings from the factor analysis was tested in the measurement model (latent constructs for neighborhood disadvantage across each time period). The structural model estimated: (1) direct effect of adolescent neighborhood disadvantage on young adult metabolic syndrome, independent of all other pathways (sensitive periods model); and (2) indirect effect of adolescent neighborhood disadvantage on young adult metabolic syndrome, mediated by emerging adulthood neighborhood disadvantage and/or young adult neighborhood disadvantage (chains of risk model). We included sociodemographic and health-related factors as controls (age, sex, race/ethnicity, parental education, living in same residence since birth, and self-reported health) to adjust for potential confounding.
Figure 1.
Conceptual model of neighborhood disadvantage from adolescence to young adulthood and metabolic syndrome in young adulthood. Black lines represent paths estimated in the SEM. Gray lines reflect potential confounding and mediating pathways not directly estimated in the SEM.
All SEM analyses were conducted in MPlus version 8 (Muthén & Muthén, Los Angelos, CA). We used a probit link function, theta parameterization, and weighted least squares estimator (WLSMV) to appropriately model the binary outcome, metabolic syndrome (yes versus no). Missing covariate data (n=56 missing parent education, 21 missing race/ethnicity, 74 missing residence at birth, and 3 missing child self-reported health) was accounted for in MPlus using full information maximum likelihood estimation. Goodness-of-fit statistics for the final models were calculated using the root mean squared error of approximation (RMSEA) and comparative fit index (CFI). Models with a RMSEA <0.05, CFI>0.90, and SRMR <0.08 were considered to fit the data well (Bentler 1990; Browne and Cudeck 1993; Hu and Bentler 1999).
RESULTS
Weighted descriptive statistics, adjusted for clustering of the sample design, for selected adolescent and adult characteristics of Add Health participants included in this analysis are shown in Table 2. The study sample had a mean age of 15 years, 52% were female, and 72% were non-Hispanic white. Sixty-two percent of participants had at least one parent with some college education or higher. Twenty-five percent of the study sample was classified with metabolic syndrome. Of the measured risk factors of metabolic syndrome, 52% had central obesity, 43% with elevated blood pressure, 26% with elevated triglycerides, 17% with reduced LDL-cholesterol, and 32% with elevated HbA1c.
Table 2.
Descriptive statistics* of selected characteristics; National Longitudinal Study of Adolescent to Adult Health (Add Health) from 1994–2008 (n=9,500)
| N (%) | Mean (SE) | |
|---|---|---|
| Adolescent characteristics (Wave I) | ||
| Age in 1994 (years) | 15.4(0.1) | |
| Sex | ||
| Female | 5,092 (50.0) | |
| Male | 4,408 (50.0) | |
| Race/ethnicity | ||
| Non-Hispanic white | 5,687 (72.2) | |
| Non-Hispanic black | 2,007 (14.7) | |
| Hispanic | 1,238 (9.6) | |
| Other | 547 (3.5) | |
| Parental Education | ||
| <High school | 1,035 (10.4) | |
| High school or equivalent | 2,443 (27.4) | |
| More than high school | 5.966 (62.1) | |
| Lived at same residence since birth | ||
| Yes | 2,071 (21.6) | |
| No | 7,355 (78.4) | |
| Childhood self-reported health | ||
| Excellent/Very good | 6,355 (66.8) | |
| Good | 2,426 (25.9) | |
| Fair/Poor | 716 (7.3) | |
| Metabolic syndrome (Wave IV) | ||
| Central obesity (high waist circumference) | 5,041 (51.9) | |
| Elevated blood pressure | 3,985 (43.4) | |
| Elevated triglycerides | 2,342 (25.7) | |
| Reduced LDL-cholesterol | 1,519 (16.6) | |
| Elevated HbAlc | 3,254 (32.0) | |
| Metabolic Syndrome | 2,407 (24.8) | |
N’s are unweighted and means and percentages are weighted with probability sampling weights and standard errors adjusted for the cluster design.
Table 3 describes the prevalence of neighborhood disadvantage characteristics during adolescence, emerging adulthood, and young adulthood. Adolescents in our study sample resided in neighborhoods characterized by 12% of families below the federal poverty level, 9% of families receiving public assistance, 27% of households without an adult with a high school diploma, and 7% female-headed households. During emerging and young adulthood, neighborhoods in which participants resided had similar presence of families living below poverty with approximately 11% at both time points. Also, during emerging and young adulthood, the neighborhood presence of families receiving public assistance and households without an adult with a high school diploma declined, while the percent of female-headed increased.
Table 3.
Neighborhood characteristics* during the transition from adolescence to young adulthood; National Longitudinal Study of Adolescent to Adult Health from 1994–2008 (n=9,500)
| Neighborhood Variables | Adolescence (Wave I) % (SE) |
Emerging Adulthood (Wave III) % (SE) |
Young Adulthood (Wave IV) % (SE) |
|---|---|---|---|
| Families living below poverty level | 11.7 (0.8) | 10.8 (0.5) | 11.4 (0.5) |
| Families receiving public assistance | 8.6 (0.5) | 3.6 (0.2) | 2.6 (0.1) |
| Household with no adult having HS diploma | 26.8 (0.9) | 20.0 (0.7) | 15.6 (0.5) |
| Unemployed | 7.4 (0.3) | 6.7 (0.3) | 8.0 (0.2) |
| Female-headed households | 6.8 (0.3) | 23.8 (0.7) | 26.7 (0.7) |
Statistics are weighted and adjusted for clustering
Measurement models
Unstandardized and standardized factor loadings for neighborhood disadvantage during adolescence, emerging adulthood, and young adulthood are presented in Supplemental Table 1. Across each time point, indicators of the latent variables reflecting neighborhood disadvantage were statistically significant at p<0.001 and the measurement model fit was appropriate (RMSEA = 0.032, CFI = 0.938, SRMR = 0.044). A general SEM diagram depicting neighborhood disadvantage in adolescence in relation to metabolic syndrome in young adulthood is provided in Supplemental Figure 1. Neighborhood disadvantage in adolescence was positively associated with neighborhood disadvantage in both emerging and young adulthood and neighborhood disadvantage in emerging adulthood was also positively associated with neighborhood disadvantage in young adulthood, accounting for all other pathways (Supplemental Table 2).
Is adolescence a sensitive period when neighborhood disadvantage is associated with metabolic syndrome in adulthood (sensitive period model)?
The adjusted total, direct, and indirect effects of neighborhood disadvantage across the transition from adolescence to young adulthood on metabolic syndrome are summarized in Table 4. The SEM fit the data well (RMSEA = 0.020, CFI = 0.913, SRMR = 0.065). In our model, neighborhood disadvantage in adolescence was associated with metabolic syndrome. A positive total effect of neighborhood disadvantage in adolescence on metabolic syndrome in adulthood was observed. The direct effect of neighborhood disadvantage in adolescence to metabolic syndrome in young adulthood, independent of indirect neighborhood disadvantage pathways ub emerging and young adulthood and potential confounding factors—adolescent age, parent education, race/ethnicity, sex, childhood self-reported health, and resident of neighborhood at birth—was also observed (direct effect: standardized coefficient = 0.057, p-value = 0.024).
Table 4.
Standardized direct and indirect effects* of neighborhood disadvantage across the transition from adolescence to young adulthood and metabolic syndrome in young adulthood; Add Health from 1994–2008 (n=9,500)
| Metabolic syndrome | ||
|---|---|---|
| Standardized Estimate | p-value | |
| Adolescent Neighborhood Disadvantage | ||
| Total Effect | 0.076 | 0.001 |
| Direct Effect | 0.057 | 0.024 |
| Total Indirect Effect | 0.018 | 0.187 |
| Decomposition of Indirect Effects | ||
| via Emerging Adult Neighborhood Disadvantage | 0.020 | 0.126 |
| via Young Adult Neighborhood Disadvantage | −0.001 | 0.869 |
| via Emerging and Young Adult Neighborhood Disadvantage | −0.001 | 0.869 |
| Emerging Adult Neighborhood Disadvantage | ||
| Total Effect | 0.040 | 0.119 |
| Direct Effect | 0.041 | 0.126 |
| Total Indirect Effect | −0.001 | 0.869 |
| Decomposition of Indirect Effects | ||
| Via Young Adult Neighborhood Disadvantage | −0.001 | 0.869 |
| Young Adult Neighborhood Disadvantage | ||
| Direct Effect | −0.004 | 0.869 |
All models adjusted for adolescent age, parent education, race/ethnicity, sex, childhood self-reported health, resident of neighborhood at birth
Does neighborhood disadvantage in adolescence impact metabolic syndrome in young adulthood through exposure to neighborhood disadvantage in emerging and young adulthood (chains of risk model)?
We did not find evidence of an indirect effect from neighborhood disadvantage in adolescence to metabolic syndrome in adulthood through neighborhood disadvantage in emerging and young adulthood (total indirect effect: standardized coefficient = 0.018, p=0.19). Furthermore, there was no evidence of direct effects between neighborhood disadvantage during emerging (total direct effect: standardized coefficient = 0.041, p=0.13) or young adulthood (total direct effect: standardized coefficient = −0.004, p= 0.87) on metabolic syndrome, when adjusting for potential confounding factors and neighborhood disadvantage at earlier life stages.
DISCUSSION
We tested two life course models to assess how neighborhood disadvantage across the life course influences metabolic syndrome in adulthood. First, we tested whether adolescence served as a sensitive period of development during which exposure to neighborhood disadvantage independently impacted metabolic syndrome in young adulthood. Our findings were in support of the sensitive period model, where neighborhood disadvantage in adolescence was associated with an increased risk of young adulthood metabolic syndrome, independent of all other hypothesized mediating pathways, including neighborhood disadvantage in emerging and young adulthood. Second, we tested whether a chains of risk model was supported across the life course. We did not find evidence of this life course model. Despite the finding that neighborhood disadvantage tracked across the transition from adolescence to young adulthood, the final “link” in the chains of neighborhood disadvantage was not associated with metabolic syndrome. Moreover, unlike previous research, neighborhood disadvantage in emerging adulthood and young adulthood was not associated with metabolic syndrome in young adulthood, after accounting for adolescent neighborhood disadvantage.
No previous study has assessed exposure to neighborhood disadvantage during early life (e.g. adolescence) as a sensitive period for development of metabolic syndrome; however, previous studies of life course neighborhood disadvantage in relation to weight status are suggestive of an association between early life exposure to neighborhood disadvantage and risk of obesity in adulthood (Harris et al. 2009; Kravitz-Wirtz 2016). One study using data from the Panel Study on Income Dynamics to examine the timing of exposure to neighborhood disadvantage during childhood (e.g. early childhood [1–5 years], late childhood [6–11 years], and adolescence [12–17 years]) found that exposure to neighborhood disadvantage during adolescence was associated with greater odds of self-reported obesity at least once in early adulthood (18–30 years of age), independent of the childhood period (Kravitz-Wirtz 2016).
Adolescence is an important stage of development characterized by increased desire for autonomy and greater exploration of one’s neighborhood environment (Harris, 2010). During this time, adolescents begin making choices surrounding health-related behaviors (e.g. physical activity and dietary habits) that continue into adulthood (Gordon-Larsen et al.,2004; Biddle et al., 2010; Nelson et al., 2008; Kane et al., 2017). At the same time, a large proportion of outside-the-home time spent by adolescents is in their residential neighborhoods, which provide physical space for social interactions (Leventhal, Dupéré, and Brooks-Gunn 2009), as well as provide contexts for food choices and physical activity. Neighborhood disadvantage has been associated with lower access to healthy food choices and recreational facilities (Gordon-Larsen et al. 2006; Morland et al. 2002). Collectively, exposure to neighborhood disadvantage during adolescence may begin to shape norms and attitudes around health behaviors that impact risk of metabolic syndrome. In addition to exposure to unhealthy behavioral practices, residing in disadvantaged neighborhoods exposes adolescents to stressful conditions that may bolster risk of poor cardiometabolic health in adulthood through increases in allostatic load and cardiometabolic-related physiologic dysfunction (McEwen 2000). While beyond the scope of the current study, future research should explore such risk factors (e.g. health behaviors, mental health, socioeconomic position, stressors) that mediate the association between adolescent neighborhood disadvantage and young adulthood metabolic syndrome.
Interestingly, we did not find evidence of an indirect effect of neighborhood disadvantage in adolescence and metabolic syndrome in adulthood through neighborhood disadvantage in later life stages (i.e. young adulthood) or direct effects of neighborhood disadvantage in emerging and young adulthood on metabolic syndrome in adulthood when accounting for adolescent neighborhood disadvantage. The transition from adolescence to young adulthood introduces individuals to additional social environments (i.e. post-secondary education, work) that may diminish residential neighborhood effects in later life stages. The transitory nature of these early adult life stages may make residential locations less salient for health, given young adults likely view their current neighborhoods as non-permanent. Furthermore, our results offer a unique perspective in that neighborhood disadvantage in young adulthood may only serve as a proxy measure for earlier life disadvantage. Traditional methods that do not account for early life effects may result in bias due to missing historic confounding by antecedent early life environments. Indeed, when we examined the cross-sectional association between neighborhood disadvantage and metabolic syndrome during young adulthood without controlling for adolescent neighborhood disadvantage, a significant positive association was observed; however, this association disappears once we longitudinally account for earlier exposure to neighborhood disadvantage in adolescence as shown in our full model.
This study is not without limitations. First, self-reported weight and height was available at baseline to estimate BMI during adolescence, which might influence future neighborhood selection. We performed a sensitivity analysis to include adolescent BMI as a control measure influencing neighborhood disadvantage across the life course (i.e. adolescence, emerging adulthood, and young adulthood) and metabolic syndrome in adulthood and the findings of our SEM remained relatively unchanged. Second, although we controlled for important known factors that select individuals to reside in a given neighborhood (e.g., parent education, race/ethnicity, resident of neighborhood at birth), the potential for additional unmeasured confounding remains. Further, we did not include all possible pathways through which neighborhood disadvantage in adolescence might impact metabolic syndrome in adulthood as the focus of our paper was one specific pathway – neighborhood disadvantage in later life stages. However, future research will build on this work to further establish causality and explore additional pathways, such as health behaviors. Third, while adolescence is an important period of development during which neighborhood environment impacts metabolic syndrome development, earlier life stages, such as childhood or birth, may be most influential and adolescence serves only as a proxy. Unfortunately, we did not have data (i.e. census tract, zip code) to assess neighborhood environment earlier in life; however, we were able to adjust for whether the participant was a resident of the neighborhood at birth using self-reported years lived at residence at baseline. In addition, a sensitivity analysis was conducted to adjust for self-reported birth weight as an early life proxy of health and socioeconomic status and our results did not change. Fourth, neighborhood boundaries were defined at the census-tract level to compare our results to previous research. Census-tracts are subdivisions of counties; however, this may not capture the most salient residential environment, particularly among emerging and young adults as indicated in our findings. Lastly, the ATP III definition for metabolic syndrome was modified to align with our data. Specifically, decile ranks for cholesterol and triglycerides were used because absolute lipid levels were unavailable and hemoglobin A1C was used to represent insulin sensitivity as participants were not required to provide fasting blood samples. However, the decile ranks used in our analysis were comparable to the national prevalence of reduced HDL-cholesterol and elevated triglycerides during the time period of young adulthood and have been included in previous studies of metabolic syndrome in Add Health (Gaydosh et al. 2018; Lippert et al. 2017; Yang et al. 2017).
Despite these limitations, our study has important strengths. This is the first study to employ a life course framework to guide theoretical and statistical approaches for investigating neighborhood disadvantage during adolescence and metabolic syndrome in adulthood. This analysis was conducted in one of the largest, nationally representative, longitudinal studies in the United States with more than two decades of data collection. Because of Add Health’s rich multi-level data across the life course, we were able to account for several factors (e.g. residence at birth, parental education) that may select adolescents into neighborhoods and also predict later life metabolic health. The findings of this study underscore the need for future research using multiple measures of neighborhood disadvantage to test other possible mechanisms across the life course that might mediate the effect of neighborhood disadvantage during adolescence on cardiometabolic health in adulthood.
In conclusion, the findings of this study are consistent with a sensitive period life course model – whereby the deleterious effects of neighborhood disadvantage in adolescence are associated with the risk of metabolic syndrome in adulthood, independent of later life experiences of neighborhood disadvantage. While exposure to neighborhood disadvantage in adolescence was associated with metabolic syndrome in adulthood, no association between neighborhood disadvantage in emerging adulthood or young adulthood was observed when accounting for neighborhood disadvantage in adolescence. Furthermore, we did not find evidence that neighborhood disadvantage during adolescence was operating through experiences of neighborhood disadvantage in later life to impact metabolic syndrome (chains of risk model), further supporting our findings of a sensitive period model. This study provides the most stringent test to date of the association between neighborhood disadvantage and metabolic syndrome and pinpoints the life stage in which these exposures are likely to be more influential for an individual’s risk of developing metabolic syndrome in adulthood. Policy and practice decisions based on cross-sectional evidence provided to-date would have invested in concurrent neighborhoods and likely found little to no impact. Based on this new, longitudinal evidence, investments made earlier in the life course—during adolescence and maybe before—may be more effective in reducing the burden of adverse cardiometabolic health.
Supplementary Material
Supplemental Figure 1. Diagram of SEM for standardized (standard errors) direct and indirect effects of neighborhood disadvantage effects in adolescence, emerging adulthood, and young adulthood on adult metabolic syndrome, Add Health. Solid black lines represent direct and indirect effects; gray lines represent potential confounding pathways. *P<0.05; **P<0.001 (RMSEA = 0.020; CFI=0.913; SRMR = 0.065)
RESEARCH HIGHLIGHTS.
Neighborhood disadvantage in adolescence associated with metabolic syndrome in adulthood
Adult neighborhood disadvantage not associated with metabolic syndrome in adulthood
Efforts to reduce poor cardiometabolic health may be most effective in early life
Acknowledgments:
This research uses data from the National Longitudinal Study of Adolescent to Adult Health (Add Health), a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill. The project was funded by grant P01HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. The research was supported by the National Institute on Minority Health and Health Disparities (K99MD012808), Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32HD007168), and National Institute of Environmental Health Sciences (T32ES007018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None
REFERENCES
- Alexander CM, Landsman PB, Teutsch SM, and Haffner SM. 2003. “NCEP-Defined Metabolic Syndrome, Diabetes, and Prevalence of Coronary Heart Disease Among NHANES III Participants Age 50 Years and Older.” Diabetes. [DOI] [PubMed] [Google Scholar]
- Barber Sharrelle, Hickson Demarc A., Wang Xu, Sims Mario, Nelson Cheryl, and Diez-Roux Ana V.. 2016. “Neighborhood Disadvantage, Poor Social Conditions, and Cardiovascular Disease Incidence among African American Adults in the Jackson Heart Study.” American Journal of Public Health 106(12):2219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Sánchez Hiram, Harhay Michael O., Harhay Meera M., and McElligott Sean. 2013. “Prevalence and Trends of Metabolic Syndrome in the Adult U.S. Population, 1999–2010.” Journal of the American College of Cardiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM 1990. “Comparative Fit Indexes in Structural Models.” Psychological Bulletin 107:238–46. [DOI] [PubMed] [Google Scholar]
- Browne MW and Cudeck R. 1993. Alternative Ways of Assessing Model Fit. In Bollen KA & Long JS (Eds.). [Google Scholar]
- Carroll Margaret D., Kit Brian K., and Lacher David A.. 2012. “Total and High-Density Lipoprotein Cholesterol in Adults: National Health and Nutrition Examination Survey, 2009–2010.” NCHS Data Brief (92):1–8. [PubMed] [Google Scholar]
- Chichlowska KL, Rose KM, Diez-Roux Ana V, Golden Sherita H., Mcneill Annie M., and Heiss Gerardo. 2009. “Life Course Socioeconomic Conditions and Metabolic Syndrome in Adults: The Atherosclerosis Risk in Communities (ARIC) Study.” North 19(12):916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichlowska Kristal L., Rose Kathryn M., Diez-Roux Ana V., Golden Sherita H., McNeill Annie M., and Heiss Gerardo. 2008. “Individual and Neighborhood Socioeconomic Status Characteristics and Prevalence of Metabolic Syndrome: The Atherosclerosis Risk in Communities (ARIC) Study.” Psychosomatic Medicine 70(9):986–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark Cheryl R., Ommerborn Mark J., Hickson De Marc A., Grooms Kya N., Sims Mario, Taylor Herman A., and Albert Michelle A.. 2013. “Neighborhood Disadvantage, Neighborhood Safety and Cardiometabolic Risk Factors in African Americans: Biosocial Associations in the Jackson Heart Study.” PLoS ONE 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnoe Robert. 2006. “Health and the Education of Children from Racial/Ethnic Minority and Immigrant Families.” Journal of Health and Social Behavior 47(1):77–93. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, Cooper LS, Watson RL, and Szklo M. 1997. “Neighborhood Environments and Coronary Heart Disease: A Multilevel Analysis.” American Journal of Epidemiology 146(1):48–63. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, and Watson RL. 2001. “Neighborhood of Residence and Incidence of Coronary Heart Disease.” The New England Journal of Medicine 345(2):99–106. [DOI] [PubMed] [Google Scholar]
- Diez Roux Ana V. 2004. “Estimating Neighborhood Health Effects: The Challenges of Causal Inference in a Complex World.” Social Science and Medicine. [DOI] [PubMed] [Google Scholar]
- Diez Roux Ana V, Jacobs David R., and Kiefe Catarina I.. 2002. “Neighborhood Characteristics and Components of the Insulin Resistance Syndrome in Young Adults: The Coronary Artery Risk Development in Young Adults (CARDIA) Study.” Diabetes Care 25(11):1976–82. [DOI] [PubMed] [Google Scholar]
- Diez Roux Ana V, Mujahid Mahasin S., Hirsch Jana A., Moore Kari, and Moore Latetia V. 2016. “The Impact of Neighborhoods on CV Risk.” Global Heart 11(3):353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong Do, D. and Zheng Cheng. 2017. “A Marginal Structural Modeling Strategy Investigating Short and Long-Term Exposure to Neighborhood Poverty on BMI among U.S. Black and White Adults.” Health & Place 46(May):201–9. [DOI] [PubMed] [Google Scholar]
- Bethene Ervin, R.. 2009. “Prevalence of Metabolic Syndrome among Adults 20 Years of Age and over, by Sex, Age, Race and Ethnicity, and Body Mass Index: United States, 2003–2006.” National Health Statistics Reports (13):1–7. [PubMed] [Google Scholar]
- Friend Amanda, Craig Leone, and Turner Steve. 2013. “The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature.” Metabolic Syndrome and Related Disorders 11(2):71–80. [DOI] [PubMed] [Google Scholar]
- Galassi Andrea, Reynolds Kristi, and He Jiang. 2006. “Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis.” American Journal of Medicine 119(10):812–19. [DOI] [PubMed] [Google Scholar]
- Gaydosh Lauren, Schorpp Kristen M., Chen Edith, Miller Gregory E., and Harris Kathleen Mullan. 2018. “College Completion Predicts Lower Depression but Higher Metabolic Syndrome among Disadvantaged Minorities in Young Adulthood.” Proceedings of the National Academy of Sciences 115(1):109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreab Samson Y., Hickson DeMarc A., Sims Mario, Wyatt Sharon B., Davis Sharon K., Correa Adolfo, and Diez-Roux Ana V. 2017. “Neighborhood Social and Physical Environments and Type 2 Diabetes Mellitus in African Americans: The Jackson Heart Study.” Health & Place 43:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass Thomas A., Rasmussen Meghan D., and Schwartz Brian S.. 2006. “Neighborhoods and Obesity in Older Adults: The Baltimore Memory Study.” American Journal of Preventive Medicine 31(6):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Larsen Penny, Nelson Melissa C., Page Phil, and Popkin Barry M.. 2006. “Inequality in the Built Environment Underlies Key Health Disparities in Physical Activity and Obesity.” Pediatrics 117(2):417–24. [DOI] [PubMed] [Google Scholar]
- Grundy Scott M., Cleeman James I., Daniels Stephen R., Donato Karen A., Eckel Robert H., Franklin Barry A., Gordon David J., Krauss Ronald M., Savage Peter J., Smith Sidney C. Jr, Spertus John A., and Costa Fernando. 2005. “Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement.” Circulation 112(17):2735–52. [DOI] [PubMed] [Google Scholar]
- Gustafsson Per E., Persson Mats, and Hammarström Anne. 2011. “Life Course Origins of the Metabolic Syndrome in Middle-Aged Women and Men: The Role of Socioeconomic Status and Metabolic Risk Factors in Adolescence and Early Adulthood.” Annals of Epidemiology 21(2):103–10. [DOI] [PubMed] [Google Scholar]
- Gustafsson Per E., San Sebastian Miguel, Janlert Urban, Theorell T??res, Westerlund Hugo, and Hammarstr??m Anne. 2013. “Residential Selection across the Life Course: Adolescent Contextual and Individual Determinants of Neighborhood Disadvantage in Mid-Adulthood.” PLoS ONE 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham Van, Maarten Lina Hedman, Manley David, Coulter Rory, and Östh John. 2014. “Intergenerational Transmission of Neighbourhood Poverty: An Analysis of Neighbourhood Histories of Individuals.” Transactions of the Institute of British Geographers 39(3):402–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Kathleen Mullan. 2013. “The Add Health Study: Design and Accomplishments.” Carolina Population Center, University of North Carolina at Chapel Hill. [Google Scholar]
- Harris Kathleen Mullan, Carolyn Tucker Halpern Jon Hussey, Whitsel Eric A., Ley Killeya-Jones Joyce Tabor, Elder Glen, Hewitt John, Shanahan Michael, Williams Redford, Siegler Ilene, and Smolen Andrew. 2013. “Social, Behavioral, and Genetic Linkages from Adolescence Into Adulthood.” American Journal of Public Health 103(S1):S25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Kathleen Mullan, Perreira Krista M., and Lee Dohoon. 2009. “Obesity in the Transition to Adulthood: Predictions across Race/Ethnicity, Immigrant Generation, and Sex.” Archives of Pediatrics & Adolescent Medicine 163(11):1022–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Dan, Xi Bo, Xue Jian, Huai Pengcheng, Zhang Min, and Li Jun. 2014. “Association between Leisure Time Physical Activity and Metabolic Syndrome: A Meta-Analysis of Prospective Cohort Studies.” Endocrine 46(2):231–40. [DOI] [PubMed] [Google Scholar]
- Heron Melonie. 2018. “Deaths. Leading Causes for 2016.” National Vital Statistics Reports 67(6):1–76. [PubMed] [Google Scholar]
- Hu Li Tze and Bentler Peter M.. 1999. “Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives.” Structural Equation Modeling 6(1):1–55. [Google Scholar]
- Hummer Robert A., Rogers Richard G., Nam Charles B., and LeClere Felicia B.. 1999. “Race/Ethnicity, Nativity, and US Adult Mortality.” Social Science Quarterly 136–53. [Google Scholar]
- Kane Jennifer B., Harris Kathleen Mullan, Morgan S. Philip, and Guilkey David K.. 2017. “Pathways of Health and Human Capital from Adolescence into Young Adulthood.” Social Forces 96(3):949–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita Akilah Dulin, Judd Suzanne E., Howard Virginia J., Carson April P., Ard Jamy D., and Fernandez Jose R.. 2014. “Associations of Neighborhood Area Level Deprivation with the Metabolic Syndrome and Inflammation among Middle- and Older- Age Adults.” BMC Public Health 14(1):1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz-Wirtz Nicole. 2016. “Temporal Effects of Child and Adolescent Exposure to Neighborhood Disadvantage on Black/White Disparities in Young Adult Obesity.” Journal of Adolescent Health 58(5):551–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan Supriya, Cozier Yvette C., Rosenberg Lynn, and Palmer Julie R.. 2010. “Socioeconomic Status and Incidence of Type 2 Diabetes: Results from the Black Women’s Health Study.” American Journal of Epidemiology 171(5):564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, and Power C. 2003. “Life Course Epidemiology (Glossary).” J Epidemiol Community Health 57(10):778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh Diana and Shlomo Yoav Ben. 2004. A Life Course Approach to Chronic Disease Epidemiology. Oxford University Press. [Google Scholar]
- Laaksonen David E., Lakka Hanna-Maaria, Niskanen Leo K., Kaplan George A., Salonen Jukka T., and Lakka Timo A.. 2002. “Metabolic Syndrome and Development of Diabetes Mellitus: Application and Validation of Recently Suggested Definitions of the Metabolic Syndrome in a Prospective Cohort Study.” American Journal of Epidemiology 156(11):1070–77. [DOI] [PubMed] [Google Scholar]
- Langenberg Claudia, Kuh Diana, Wadsworth Michael E. J., Brunner Eric, and Hardy Rebecca. 2006. “Social Circumstances and Education: Life Course Origins of Social Inequalities in Metabolic Risk in a Prospective National Birth Cohort.” American Journal of Public Health 96(12):2216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal Tama, Dupéré Véronique, and Brooks-Gunn Jeanne. 2009. “Neighborhood Influences on Adolescent Development.” Handbook of Adolescent Psychology 2:411–43. [Google Scholar]
- Lippert Adam M. 2016. “Stuck in Unhealthy Places.” Journal of Health and Social Behavior 57(1):1–21. [DOI] [PubMed] [Google Scholar]
- Lippert Adam M., Evans Clare Rosenfeld, Razak Fahad, and Subramanian SV. 2017. “Associations of Continuity and Change in Early Neighborhood Poverty with Adult Cardiometabolic Biomarkers in the United States: Results from the National Longitudinal Study of Adolescent to Adult Health, 1995–2008.” American Journal of Epidemiology 185(9):765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsey Pamela L., Steffen Lyn M., and Stevens June. 2008. “Dietary Intake and the Development of the Metabolic Syndrome: The Atherosclerosis Risk in Communities Study.” Circulation 117(6):754–61. [DOI] [PubMed] [Google Scholar]
- Malik S, Wong ND, Franklin SS, V Kamath T, L’Italien GJ, Pio JR, and Williams GR. 2004. “Impact of the Metabolic Syndrome on Mortality from Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults 1.” Circulation 110(1524–4539 (Electronic)):1245–50. [DOI] [PubMed] [Google Scholar]
- McEwen Bruce S. 2000. “Allostasis and Allostatic Load: Implications for Neuropsychopharmacology.” Neuropsychopharmacology 22(2):108–24. [DOI] [PubMed] [Google Scholar]
- McNeill Ann Marie, Rosamond Wayne D., Girman Cynthia J., Sherita Hill Golden Maria I. Schmidt, East Honey E., Ballantyne Christie M., and Heiss Gerardo. 2005. “The Metabolic Syndrome and 11-Year Risk of Incident Cardiovascular Disease in the Atherosclerosis Risk in Communities Study.” Diabetes Care 28(2):385–90. [DOI] [PubMed] [Google Scholar]
- Morland Kimberly, Wing Steve, Diez Roux Ana, and Poole Charles. 2002. “Neighborhood Characteristics Associated with the Location of Food Stores and Food Service Places.” American Journal of Preventive Medicine 22(1):23–29. [DOI] [PubMed] [Google Scholar]
- Morrison JA, Friedman LA, and Gray-McGuire C. 2007. “Metabolic Syndrome in Childhood Predicts Adult Cardiovascular Disease 25 Years Later: The Princeton Lipid Research Clinics Follow-up Study.” Pediatrics 120(2):340–45. [DOI] [PubMed] [Google Scholar]
- Michael Oakes, J.. 2004. “The (Mis)Estimation of Neighbor- Hood Effects: Causal Inference for a Practicable so- Cial Epidemiology.” Social Science and Medicine 58(10):1929–1952. [DOI] [PubMed] [Google Scholar]
- Powell-Wiley Tiffany M., Ayers Colby, Agyemang Priscilla, Leonard Tammy, Berrigan David, Rachel Ballard-Barbash Min Lian, Das Sandeep R., and Hoehner Christine M.. 2014. “Neighborhood-Level Socioeconomic Deprivation Predicts Weight Gain in a Multi-Ethnic Population: Longitudinal Data from the Dallas Heart Study.” Preventive Medicine 66:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Monforte Miriam, Sanchez Emilia, Barrio Francisco, Costa Bernardo, and Flores-Mateo Gemma. 2017. “Metabolic Syndrome and Dietary Patterns: A Systematic Review and Meta-Analysis of Observational Studies.” European Journal of Nutrition 56(3):925–47. [DOI] [PubMed] [Google Scholar]
- Ross Catherine E. and Mirowsky John. 2001. “Neighborhood Disadvantage, Disorder, and Health.” Journal of Health and Social Behavior. [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, and Earls F. 1997. “Neighborhoods and Violent Crime: A Multilevel Study of Collective Efficacy.” Science (New York, N.Y.) 277(5328):918–24. [DOI] [PubMed] [Google Scholar]
- Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, and Leung GM. 2008. “Life-Course Origins of Social Inequalities in Metabolic Risk in the Population of a Developing Country.” American Journal of Epidemiology 167(4):419–28. [DOI] [PubMed] [Google Scholar]
- Sheehan Connor M., Cantu Phillip A., Powers Daniel A., Margerison-Zilko Claire E., and Cubbin Catherine. 2017. “Long-Term Neighborhood Poverty Trajectories and Obesity in a Sample of California Mothers.” Health and Place 46(March):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Gopal K. and Miller Barry A.. 2004. “Health, Life Expectancy, and Mortality Patterns among Immigrant Populations in the United States.” Can J Public Health 95(3):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia Shakira F., Shelton Rachel C., Hsiao Amber, Wang Y. Claire, Rundle Andrew, and Link Bruce G.. 2016. “Why the Neighborhood Social Environment Is Critical in Obesity Prevention.” Journal of Urban Health 93(1):206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Qian, Berrigan David, Powell-Wiley Tiffany M., and Matthews Charles E.. 2018. “Ten-Year Change in Neighborhood Socioeconomic Deprivation and Rates of Total, Cardiovascular Disease, and Cancer Mortality in Older US Adults.” American Journal of Epidemiology 187(12):2642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Yang Claire, Gerken Karen, Schorpp Kristen, Boen Courtney, and Harris Kathleen Mullan. 2017. “Early-Life Socioeconomic Status and Adult Physiological Functioning: A Life Course Examination of Biosocial Mechanisms.” Biodemography and Social Biology 63(2):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Dongdong, Liu Xuejiao, Liu Yu, Sun Xizhuo, Wang Bingyuan, Ren Yongcheng, Zhao Yang, Zhou Junmei, Han Chengyi, Yin Lei, Zhao Jingzhi, Shi Yuanyuan, Zhang Ming, and Hu Dongsheng. 2017. “Leisure-Time Physical Activity and Incident Metabolic Syndrome: A Systematic Review and Dose-Response Meta-Analysis of Cohort Studies.” Metabolism 75:36–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Diagram of SEM for standardized (standard errors) direct and indirect effects of neighborhood disadvantage effects in adolescence, emerging adulthood, and young adulthood on adult metabolic syndrome, Add Health. Solid black lines represent direct and indirect effects; gray lines represent potential confounding pathways. *P<0.05; **P<0.001 (RMSEA = 0.020; CFI=0.913; SRMR = 0.065)