Abstract
Introduction:
Non-white minorities are at higher risk for chronic kidney disease than non-Hispanic whites. Better cardiorespiratory fitness is associated with slower declines in estimated glomerular filtration rate and a lower incidence of chronic kidney disease. Little is known regarding associations of fitness to racial disparities in chronic kidney disease.
Methods:
A prospective cohort of 3,842 young adults without chronic kidney disease completed a maximal treadmill test at baseline in 1985–1986. Chronic kidney disease status was defined as estimated glomerular filtration rate of <60 mL/min/1.73 m2 during 10-, 15-, 20-, 25-, and 30-year follow-up assessments (through 2006). Analyses were completed in 2019. Multivariable Cox models were used to determine hazard ratios and 95% CI for incidence of chronic kidney disease. Multivariable models included race, gender, age, field center, education, baseline estimated glomerular filtration rate, and time-varying covariates of healthy diet index, smoking status, alcohol intake, BMI, systolic blood pressure, and fasting glucose. Percent attenuation quantified the association of fitness to racial disparities in chronic kidney disease.
Results:
Chronic kidney disease incidence was higher among blacks (n=83/1,941, 1.61 per 1,000 person years) than whites (43/1,901, 0.82 per 1,000 person years). Every 1-minute shorter treadmill duration was associated with 1.14 (95% CI=1.04, 1.25) times higher risk of chronic kidney disease. Blacks were 1.72 (95% CI=1.13, 2.63) times more likely to develop chronic kidney disease compared with whites. The risk was reduced to 1.54 (95% CI=1.01, 2.39) with fitness added. This suggests that fitness is associated with 20.4% (95% CI=5.8, 43.0%) of the excess risk of chronic kidney disease attributable to race.
Conclusions:
Low fitness is a modifiable factor that may contribute to the racial disparity in chronic kidney disease.
INTRODUCTION
Chronic kidney disease (CKD) is an independent contributor to end-stage renal disease, cardiovascular disease morbidity, and mortality.1–3 With a prevalence estimate of 13% in the U.S. population,4 preventing CKD and treating in early stages to stop progression to end-stage renal disease is particularly important. Racial and ethnic minorities have a higher risk for CKD.5,6 Although a proportion of the elevated risk of CKD may be attributable to genetic differences (i.e., apolipoprotein L1 [APOL1]),7 a large proportion may be explained by the higher burden of hypertension and diabetes.8,9 Adults who maintain higher levels of physical activity and fitness are less likely to develop hypertension and diabetes compared with less active and less fit individuals.10–13 By extension, epidemiologic and exercise trial research demonstrate that higher fitness is associated with reduced risk of rapid decline in estimated glomerular filtration rate (eGFR), proteinuria, and CKD incidence.14–17
Fitness is an independent predictor for all-cause and disease-specific mortality across race/ethnic groups.18,19 Prior investigations described lower levels of physical activity and poorer fitness among blacks compared with whites.20–22 This present study determines the associations of low fitness with racial disparities in CKD incidence. The hypothesis is that lower fitness among blacks partially explains the higher rates of CKD in black versus white adults.
METHODS
Study Sample
The Coronary Artery Risk Development in Young Adults (CARDIA) cohort study recruited 5,115 black and white young adults in 1985–1986 from four areas of the U.S. (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; Oakland, California). CARDIA recruited a balanced cohort across race, age, gender, and educational attainment.23 Participants have undergone in-person examinations at baseline and years 2, 5, 7, 10, 15, 20, 25, and 30. Retention rates among surviving participants at exams were 91%, 86%, 81%, 79%, 74%, 72%, 72%, and 71%, respectively. Appendix Figure 1 provides a flow chart of sample selection. After applying exclusions for having CKD at baseline; missing fitness, CKD, or covariate data at baseline; and no follow-up visit documenting CKD status there were 3,842 participants. All participants provided written informed consent. IRB approvals were received from each field center institution.
Measures
The primary exposure was cardiorespiratory fitness measured at baseline with a maximal graded exercise treadmill test. The test used a modified Balke protocol consisting of 2-minute stages (maximum 18 minutes) of progressively increasing speed and grade. Stage 1 was 3.0 miles per hour at 2% grade (4.1 METs), progressing to Stage 9 at 5.6 miles per hour at 25% grade (19.0 METs). A previous publication further details the conversion formula from treadmill duration to METs.24 The test was terminated for the following reasons: volitional fatigue (84.1%), shortness of breath (7.4%), refusal to continue (1.4%), abnormal electrocardiogram, blood pressure, or chest pain (1.5%), medical reason (4.7%), or completion of protocol (0.3%).
The primary outcome was incident CKD, defined as an eGFR of <60 mL/minute/1.73 m2 during any follow-up assessment at year 10, 15, 20, 25, and 30. The CKD–Epidemiology Collaboration equation estimated GFR25 from fasting serum creatinine measurements available at years 0, 10, 15, 20, 25, and 30, measured by nephelometry according to National Institute of Standards and Technology standards.26
Years 10 through 30 examinations included a collection of urine albumin and creatinine using a single, untimed spot urine sample. The nephelometric procedure assessed albumin and the Jaffe method measured creatinine. Urine albumin creatinine ratio (UACR) was calculated following previous calculations used in CARDIA accounting for racial and gender differences in creatinine excretion.27
Age, race, gender, and education were queried during clinic visits. An interview administered diet history questionnaire collected alcohol intake as average mL/day. Diet was quantified based on the Healthy Eating Index using data collected from CARDIA’s comprehensive food frequency questionnaire.28 Smoking history classified participants as current, former, or never smokers. Body weight and height measured with light clothing to the nearest 0.09 kg and 0.5 cm, respectively, was used to calculate BMI (kg/m2). Fasting glucose was measured using standard laboratory techniques. Three blood pressure measurements were taken by trained staff and the mean of the last two measurements was calculated.
Statistical Analyses
All analyses were conducted in 2018–2019 using SAS statistical software, version 9.4. Chi-square tests and t-tests compared baseline categorical and continuous variables, respectively. Fitness, quantified as treadmill test duration, by race and gender was compared as a continuous variable of and as a categorical variable. Within this sample, groups were created as low fitness (lowest gender-specific quintile), moderate fitness (Quintiles 2–3), high fitness (upper Quintiles 4–5), and as has been done previously to emphasize the group at highest risk (<20th percentile).29,30
Extended Cox regression models using PROC PHREG examined the association of baseline fitness with incident CKD. Extended Cox models allow for a more robust model by accounting for covariates at each timepoint rather than using static baseline variables.31 The primary exposure, fitness, was assessed both as a continuous variable (per 1 minute lower treadmill duration) and a categorical variable comparing low versus high (reference), low versus moderate (reference), and moderate versus high (reference). Participants who did not develop CKD by their last available exam were censored. Incidence of CKD was confirmed at 5-year intervals. Therefore, knowledge of event occurrence is between two successive visits rather than the exact date. The discrete model option handled time to event ties. The discrete-time method better accounts for interval censored data at non-continuous treat times.32
Model 1 included baseline fitness only; Model 2 added demographics (race, gender, age, maximum education, field center); Model 3 added time-varying lifestyle factors (diet, smoking, alcohol); Model 4 added time-varying cardiometabolic risk factors (BMI, systolic blood pressure, fasting glucose). Model 5 added baseline eGFR. The proportional hazards assumption was not violated in a model restricted to baseline values for all exposures. Multiple imputation using SAS PROC MIanalyze handled missing covariate data.33 All variables were included in the imputation methods. A range of 0%–2.4% of covariate data were missing for variables during each follow-up exam except diet, with 11% missing at the year 20 exam. Ten replication data sets imputed the values for the 6.0% total missing data points. Sensitivity analyses comparing before and after incorporating multiple imputation into the extended Cox models showed stable estimates. Given no significant interactions by gender or race X fitness using multiplicative interaction terms, results were pooled for the entire sample.
Testing the hypothesis that baseline fitness was associated with racial disparities in CKD incorporated two different strategies, percentage attenuation and population attributable fraction (PAF). Percentage attenuation quantified the reduction in the effect estimate associated with race in a multivariable model including all established confounders (Model 1) when adding fitness (Model 2). The percentage excess risk explained used the following formula: 100 × (βModel 1 − βModel 2)/(βModel 1), where β = log(hazard ratio [HR])34 and 95% CIs using bootstrap methods with 1,000 re-samplings.
PAF quantifies the proportion of cases of incident CKD that could be prevented if all participants moved from low fitness to moderate or high fitness. The formula for PAF follows: Pd (HRadj - 1)/HRadj × 100%, where Pd is the proportion of low fitness individuals among cases, and HRadj is the RR of incident CKD in the fully adjusted model.35,36 Race-specific extended Cox regression analysis determined the HR for the association between fitness and incident CKD.
The definition of CKD as eGFR <60 mL/min/1.73 m2 was selected as the primary outcome because it allowed for the longest follow-up and largest sample size possible in the CARDIA study. To test the robustness of the association, secondary analysis added albuminuria to the CKD definition using extended Cox regression models with incident CKD defined as low eGFR <60 mL/minute/1.73 m2 or UACR>30 mg/g at years 15, 20, 25, and 30 follow-up. This analysis included 2,714 participants with measured UACR and did not have albuminuria at year 10, the first visit with UACR available.
Moderate to vigorous physical activity (MVPA) was added (with and without fitness included) to the Cox regression models. MVPA was assessed with a questionnaire asking about frequency of participation in sports and exercise during the past 12 months.37 No associations were found between self-reported MVPA and incident CKD and therefore not included in the final results. Models were also tested quantifying cardiometabolic covariates using categorical definitions for obesity,38 diabetes,39 and Stage 1 and Stage 2 hypertension,40 and did not impact the results. Variants in the gene encoding APOL1 were also considered. Renal risk variants in APOL1 are associated with a more rapid decline in eGFR in blacks.7 A sensitivity analysis included a Cox regression model, adding APOL1 as a dichotomous variable (two copies of the gene variant versus zero or one copy) in the fully adjusted model.41
RESULTS
Table 1 provides baseline characteristics for the total sample and across fitness categories. Participants’ mean age was 24.8 (SD=3.6) years, 50.5% (n=1,941) black, and 53.5% (n=2,055) women. Participants in the low fitness group had lower educational attainment and higher measures of BMI, blood pressure, and fasting glucose. Mean eGFR for the total sample was 97 (SD=17) mL/min/1.73 m2 at baseline and the median UACR was 3.9 mg/g (IQR, 2.7–5.9) at year 10.
Table 1.
Baseline Characteristics for Total Sample and by Fitness Groups
| Characteristics | Total | Low fitness |
Moderate fitness |
High fitness |
|---|---|---|---|---|
| n | 3,842 | 736 | 1,564 | 1,542 |
| Age, years | 24.8 ± 3.6 | 25.4 ± 3.6 | 24.6 ± 3.7 | 24.9 ± 3.5 |
| Black race, n (%) | 1,941 (50.5) | 553 (75.1) | 893 (57.1) | 495 (32.1) |
| Women, n (%) | 2,055 (53.5) | 414 (56.3) | 814 (52.1) | 827 (53.6) |
| Education attainment, years Smoking | 15.5 ± 3.0 | 14.6 ± 4.0 | 15.2 ± 2.6 | 16.2 ± 2.6 |
| Never, n (%) | 2,252 (58.6) | 380 (51.6) | 888 (56.8) | 984 (63.8) |
| Former, n (%) | 497 (12.9) | 80 (10.9) | 184 (11.8) | 233 (15.1) |
| Current, n (%) | 1,093 (28.5) | 276 (37.5) | 492 (31.5) | 325 (21.1) |
| Alcohol intake, ml/day | 11.7 ± 21.1 | 11.6 ± 24.0 | 12.0 ± 22.1 | 11.4 ± 18.5 |
| Healthy Diet Index score | 44.7 ± 12.7 | 40.6 ± 11.1 | 42.3 ± 11.7 | 48.5 ± 13.2 |
| BMI (kg/m2) | 24.4 ± 4.8 | 29.1 ± 6.7 | 24.3 ± 3.9 | 22.3 ± 2.6 |
| SBP (mmHg) | 110 ± 11 | 113 ± 11 | 110 ± 10 | 109 ± 11 |
| DBP (mmHg) | 69 ± 9 | 70 ± 10 | 68 ± 9 | 68 ± 9 |
| Fasting glucose (mg/dL) | 82.3 ± 13.0 | 83.4 ± 13.4 | 82.7 ± 16.1 | 81.4 ± 8.4 |
| Maximal treadmill duration, minutes | ||||
| Women | 8.3 ± 2.3 | 5.2 ± 1.0 | 7.6 ± 0.6 | 10.5 ± 1.4 |
| Men | 11.8 ± 2.3 | 8.3 ± 1.4 | 11.2 ± 0.7 | 13.9 ± 1.1 |
| Maximal treadmill METs | ||||
| Women | 11.0 ± 2.1 | 8.2 ± 1.0 | 10.4 ± 0.7 | 13.1 ± 1.3 |
| Men | 14.2 ± 2.1 | 11.0 ± 1.3 | 13.7 ± 0.8 | 16.2 ± 0.9 |
| eGFR, CKD-EPI equation (mL/minute/1.73 m2) | 97.0 ± 16.8 | 99.4 ± 17.5 | 98.2 ± 17.3 | 94.6 ± 15.7 |
| UACR (mg/g) at year 10 (n=2,714) | 3.9 (2.7, 5.9) | 4.3 (2.9, 7.0) (n=577) | 3.8 (2.7, 5.8) (n=1,026) | 3.7 (2.6, 5.5) (n=1,111) |
| APOL1 (n=3,433), High riska n (%) | 217 (6.3) | 66 (10.0) | 106 (7.6) | 45 (3.3) |
Notes: Values for categorical variables are given as n and percentages; values for continuous variables are given as mean ± SD or median (IQR). Low fitness: bottom lowest gender-specific quintile (bottom 20%); Moderate fitness: quintiles 2–3 (middle 20%–60%); High fitness: quintiles 4–5 (top >60%).
High risk category for APOL1 are individuals with two copies of the gene variant.
APOL1, apolipoprotein L1; DBP, diastolic blood pressure; eGFR, estimated Glomerular Filtration Rate; SBP, systolic blood pressure; UACR, Urine Albumin Creatinine Ratio
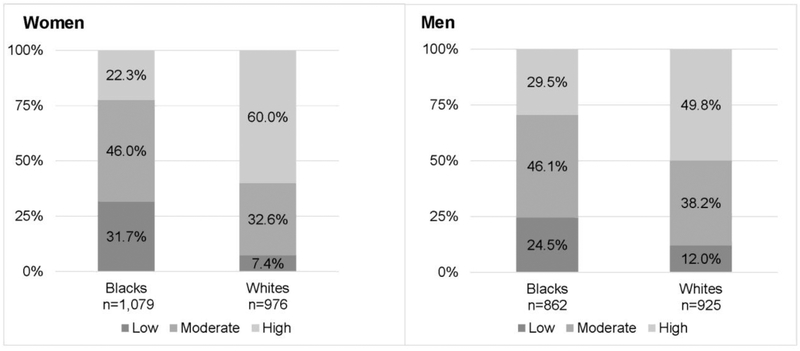
The prevalence of being in the low fitness group was 31.7% for black women vs 7.4% for white women (p<0.001) and 24.5% for black men vs 12.0% for white men (p<0.001; Figure 1). Appendix Figure 1 displays the unadjusted and adjusted treadmill durations, of which blacks had significantly shorter durations than whites (p<0.001).
Figure 1.
Distribution of low, moderate, and high fitness by race in women and men.
Note: Low fitness: bottom lowest gender-specific quintile (bottom 20%); Moderate fitness: quintiles 2–3 (middle 20%–60%); High fitness: quintiles 4–5 (upper >60%). p<0.01 for difference in fitness group proportions by race for both women and men.
Average time from baseline to end of surveillance was 27.9 years. During 102,685 person years of follow-up, 4.5% of blacks and 2.3% of whites developed CKD, compared with whites (blacks: n=83, 1.61 [95% CI=0.82, 3.13] cases per 1,000 person years; whites: n=43, 0.81 [95% CI=0.61, 1.10] cases per 1,000 person years). During follow-up, those who developed CKD had declines in eGFR by an average of 51.4 (SD=27.3) mL/min/1.73 m2 between year 10 and year 30 (99.1 [SD=22.2] at year 10 to 48.4 [SD=18.1] mL/min/1.73 m2 at year 30). The mean eGFR decreased by an average of 17.6 (SD=14.4) mL/min/1.73 m2 for those without CKD (111.0 [SD=15.7] at year 10 to 93.0 [SD=14.5] mL/min/1.73 m2 at year 30).
Table 2 shows the extended Cox regression models for incident CKD. Each 1-minute lower treadmill duration was associated with a 1.14 times higher incidence of CKD (HR=1.14, 95% CI=1.04, 1.25) in the fully adjusted model. Participants in the low fitness group were 2.44 (95% CI=1.42, 4.20) times more likely compared with the high fitness group and 1.83 (95% CI=1.19, 2.85) times more likely compared with the moderate fitness group to have incident CKD after adjusting for all covariates. Appendix Table 1 provides a summary of Cox models in the subset (n=2,714) with measured UACR available. When adding albuminuria to the incident CKD definition, the associations remained similar.
Table 2.
Associations of Baseline Fitness With Incident CKDa in Full Sample and Fitness Groups
| Association of fitness | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
|
| Duration with CKD | |||||
| Fitness per 1 minute lower duration | 1.13 (1.06,1.21) | 1.17 (1.07, 1.27) | 1.16 (1.06,1.26) | 1.14 (1.04,1.25) | 1.14 (1.04,1.25) |
| Groups with CKD | |||||
| Low vs high fitness (ref) | 3.76 (2.38, 5.94) | 2.70 (1.65, 4.40) | 2.57 (1.56, 4.24) | 2.42 (1.40, 4.10) | 2.44 (1.42, 4.20) |
| Low vs moderate fitness (ref) | 2.35 (1.56, 3.53) | 1.98 (1.31, 3.00) | 1.93 (1.27, 2.93) | 1.83 (1.18, 2.83) | 1.83 (1.19, 2.85) |
| Moderate vs high fitness (ref) | 1.60 (1.02, 2.52) | 1.37 (0.86, 2.18) | 1.33 (0.83, 2.13) | 1.32 (0.82,2.10) | 1.34 (0.83,2.13) |
Notes: Boldface indicates statistical significance (p<0.05). Model 1: Unadjusted. Model 2: Model 1 + gender, race, age, maximal education throughout study, and field center. Model 3: Model 2 + time varying healthy eating index score, smoking status, alcohol intake. Model 4: Model 3 + time varying BMI, systolic BP, and fasting glucose. Model 5: Model 4+ baseline eGFR. Low fitness: bottom lowest gender-specific quintile (bottom 20%); Moderate fitness: quintiles 2–3 (middle 20%–60%); High fitness: quintiles 4–5 (top >60%).
Chronic Kidney Disease (CKD) defined as eGFR <60 mL/min/1.73m2.
HR, hazard ratio.
In supplementary analysis (n=3,433) including APOL1 as a covariate, blacks who carried two gene variants, had 2.14 (95% CI=1.23, 3.74) times higher risk of incident CKD versus individuals with zero or one gene variant. Fitness test duration remained significantly associated with incident CKD when APOL1 was added to the final model (HR=1.14, 95% CI=1.04, 1.25).
Black race was significantly associated with incident CKD (HR=1.72, 95% CI=1.13, 2.63). Adding fitness duration to the model attenuated the HR to 1.54 (95% CI=1.01, 2.39), resulting in fitness associated with 20.4% (95% CI=5.8%, 43.0%) of the excess risk of incident CKD attributable to race. In race-specific extended Cox regression analysis, 22.5% and 10.8% of incident CKD was attributable to low fitness in blacks and whites, respectively (Table 3).
Table 3.
Attributable Fraction of Fitness to CKDa by Race and Total Sample
| Variable | Prevalence of low fitness in
full population (%) |
Prevalence of low
fitness in people eventually developing CKD (Pd) |
Adjusted HR for low vs mod/high fit |
Population attributable fraction (PAF)c |
||
|---|---|---|---|---|---|---|
| Low fitd / Total | % | Low fit events / Total events |
% | HRb (95% CI) | % | |
| Blacks | 553/1,941 | 28.5 | 38/82 | 46.3 | 1.94 (1.20,3.16) | 22.5 |
| Whites | 183/1,901 | 9.6 | 9/43 | 20.9 | 2.07 (0.89, 4.83) | 10.8 |
| Overall | 736/3,842 | 19.2 | 47/125 | 37.6 | 2.00 (1.31,3.04) | 18.9 |
Chronic kidney disease (CKD) defined as eGFR <60 mL/min/1.73m2.
HR adjusted for baseline gender, age, field center, maximal education throughout study, time varying healthy diet index, smoking status, alcohol intake, BMI, systolic BP, and fasting glucose, and baseline eGFR.
PAF=(Pd (HRadj −1))/HRadj × 100%, Where Pd is the proportion of inactive people among cases, and HRadj is the HR of CKD, comparing low to mod/high fitness, adjusted for confounding factors.
Low fit: bottom lowest gender-specific quintile (bottom 20%).
eGFR, estimated Glomerular Filtration Rate; HR, hazard ratio.
DISCUSSION
In this population-based sample of young adults followed for up to 30 years, low fitness may be associated with the higher rates of incident CKD in blacks versus whites. The relationship of low fitness with the development of risk factors for CKD, such as hypertension and diabetes, is well established. This study adds to the literature by demonstrating that even after accounting for these intermediate factors, low fitness itself is associated with CKD. The findings also expand upon prior research, finding that the proportion of CKD associated with low fitness was twofold greater in blacks versus whites.
The results are consistent with two epidemiologic studies on fitness and incident CKD. Among 5,812 male Veterans, every 1 MET higher maximal treadmill test resulted a lower risk of CKD (HR=0.72, 95% CI=0.75, 0.82).14 The Cooper Center Longitudinal Study of 17,979 men and women fitness per 1 MET increment was associated with an HR of 0.94 (95% CI=0.89, 0.99) for incident CKD.17 In these studies, as with the current, the low fitness group had a significantly higher risk for CKD compared with both the moderate and high fitness groups. This provides evidence for the robustness of the negative impact of low fitness on CKD. Even modest improvements in fitness or avoidance of very low fitness may have a meaningful impact on incident CKD.42 MVPA and fitness can help prevent the occurrence or progression of CKD through counteracting metabolic and cardiovascular risk factors and maintaining muscle mass and function.10–13 The Kidney Disease Improving Global Outcomes clinical practice guidelines recommends regular MVPA in patients with CKD to improve exercise capacity, reduce cardiovascular risk, and improve health-related quality of life.47
Blacks were nearly two times more likely to develop CKD compared with whites, consistent with previous studies from CARDIA and other cohorts.5,6,48 Fitness explained 20% of the excess risk in blacks versus whites. Additionally, the proportion of CKD attributable to low fitness was estimated as twofold greater in blacks versus whites (PAF: 22.5% versus 10.8%). A National Health and Nutrition Examination Survey study looked at collective lifestyle factors, finding that smoking, BMI, alcohol, and MVPA explained 23.7% of the excess prevalence of CKD among blacks.49 Another study among the National Health and Nutrition Examination Survey cohort found that 20% of the excess prevalence of CKD among low SES adults was explained by a combination of smoking, alcohol intake, sedentary time, diet, and MVPA.50 This study also analyzed MVPA separately and found that MVPA contributed to a greater degree (8%) among the blacks-only analysis versus the entire pooled sample (4% of the effect).
These studies included the behavior of MVPA, whereas cardiorespiratory fitness is a physiological measure, partially explained by MVPA as well as genetics, environmental, and physiologic factors.51 MVPA can have measurement error resulting in to smaller effect estimates compared with its physiologic trait of fitness. This has been shown in prior studies of which fitness has a stronger magnitude of association with cardiovascular morbidity and mortality compared with self-reported MVPA.52–54 This may also explain the absence of significant associations of MVPA with incident CKD in the sensitivity analyses.
Additional contributors to the racial disparity in CKD include clinical factors, such as higher prevalence and severity of diabetes and hypertension, and social determinants like education, healthcare access, and neighborhood conditions.48,55 The present study found an independent association of fitness with CKD incidence after adjustment for other factors. The relationship of fitness with race may also have indirect associations through these contributors. Social determinants of health including lack of safe places to exercise, limited time, and psychosocial stressors can interfere with the ability to maintain fitness-enhancing activity.55 Recent research has demonstrated that blacks may have a lower magnitude of improvement in fitness responses from formal exercise interventions.56,57 Therefore, it may be more difficult to improve fitness levels in blacks versus whites, which could play a role in fitness’ contributions to race disparities in CKD.
Fitness can indirectly impact CKD by way of established associations with obesity, hypertension, and diabetes.44,58–60 The extended Cox models accounted for time-updated risk factor development. The effect remained statistically significant suggesting that fitness may act on multiple pathways, independent of obesity, diabetes, and hypertension, to influence CKD. Other mechanisms through which fitness has benefits on CKD may be anti-inflammatory effects, maintenance of muscle mass and function, and endothelial health.45,58,61,62 As a pleiotropic factor, modifying fitness in young adults could be effective for reducing the later burden of CKD.
Strengths of this study include 30 years of follow-up, rigorously measured outcomes, utilizing an objective physiological measure of fitness, a wealth of covariate data and a diverse sample in race, gender, and SES. Furthermore, time-varying covariates allowed for more robust adjustment for factors that substantially affect the risk of CKD.47 Additionally, supplemental analyses included the APOL1 gene variant. Fitness remained significantly associated with incident CKD when taking into account this indicator of genetic predisposition for CKD.
Limitations
The PAF should be interpreted with care as it assumes a causal relationship between the risk factor and outcome. The observational design limits the ability to make conclusions regarding the causal pathway of fitness’ association with race differences in CKD. The PAF provides the maximum impact fitness could have on incident CKD, assuming an improvement from low to moderate or high fitness occurs among all individuals. However, lifestyle interventions impact individuals differentially and health behaviors are difficult to maintain. There were few cases of CKD during follow-up, which is expected given the baseline assessment began with healthy, young adults. There is potential of selection bias resulting from participant dropout from sickness. There may be residual confounding for factors not measured that may have associations with CKD and therefore, are not adjusted for in analyses. This study could not confirm the stability of eGFR over 3 months as recommended,47 because this measure occurred once every 5 years. However, eGFR is more stable at younger ages, therefore a more reliable measure of kidney function during young and middle adulthood versus a sample of older adults. Furthermore, the sensitivity analyses including albuminuria into the CKD definition found similar associations.
CONCLUSIONS
The findings suggest that low fitness during young adulthood is a significant risk factor for the development of CKD. Given that blacks have poorer fitness compared with whites in this study and others,22,63,64 physical activity interventions are an important strategy18 to improve fitness and lower the likelihood of developing CKD. If such strategies are successful in blacks, it may be possible to reduce a portion of the racial disparity in CKD.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the participants, staff, and investigators of the Coronary Artery Risk Development in Young Adults (CARDIA) study.
The CARDIA Study is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute.
This research was supported, in part, by a grant from the American Heart Association (15SFDRN25080331).
The sponsors had no role in the study design; collection, analysis, and interpretation of the data; writing the report; or the decision to submit the report for publication. The research reported here does not represent the official views of the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AEP and MRC worked on study conception and interpreting data. AEP and LRP analyzed the data. AEP drafted the manuscript. AEP, LRP, MW, TI, CEL, RM, PJS, SS, and MRC worked on critical revisions of the manuscript and approved the final version.
Preliminary analyses of article contents were presented at the American Heart Association Epidemiology and Prevention Lifestyle Scientific Sessions in March 2018.
RM has interest in Abbot Laboratories, AbbVie, Inc., and Teva Pharmaceuticals Industries Ltd. All other authors report no financial disclosures.
REFERENCES
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–1270. 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, Newsome B, Kramer H, et al. Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):101–107. 10.2215/CJN.06450611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33(5):409–415. 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsa A, Kao WHL, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryson CL, Ross HJ, Boyko EJ, Young BA. Racial and ethnic variations in albuminuria in the U.S. Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48(5):720–726. 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis. 2015;22(2):88–95. 10.1053/j.ackd.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Zhang D, Liu Y, et al. Dose-response association between physical activity and incident hypertension: a systematic review and meta-analysis of cohort studies. Hypertension. 2017;69(5):813–820. 10.1161/HYPERTENSIONAHA.116.08994. [DOI] [PubMed] [Google Scholar]
- 11.Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527–2545. 10.1007/s00125-016-4079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaccardi F, O’Donovan G, Webb DR, et al. Cardiorespiratory fitness and risk of type 2 diabetes mellitus: a 23-year cohort study and a meta-analysis of prospective studies. Atherosclerosis. 2015;243(1):131–137. 10.1016/j.atherosclerosis.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 14.Kokkinos P, Faselis C, Myers J, et al. Exercise capacity and risk of chronic kidney disease in U.S. Veterans: a cohort study. Mayo Clin Proc. 2015;90(4):461–468. 10.1016/j.mayocp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Nylen ES, Gandhi SM, Kheirbek R, Kokkinos P. Enhanced fitness and renal function in type 2 diabetes. Diabet Med. 2015;32(10):1342–1345. 10.1111/dme.12789. [DOI] [PubMed] [Google Scholar]
- 16.Park M, Ko Y, Song SH, Kim S, Yoon H-J. Association of low aerobic fitness with hyperfiltration and albuminuria in men. Med Sci Sports Exerc. 2013;45(2):217–223. 10.1249/MSS.0b013e318271b39f. [DOI] [PubMed] [Google Scholar]
- 17.DeFina LF, Barlow CE, Radford NB, Leonard D, Willis BL. The association between midlife cardiorespiratory fitness and later life chronic kidney disease: the Cooper Center Longitudinal Study. Prev Med. 2016;89:178–183. 10.1016/j.ypmed.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(14):1622–1639. 10.1016/j.jacc.2018.08.2141. [DOI] [PubMed] [Google Scholar]
- 19.Harber MP, Kaminsky LA, Arena R, et al. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis. 2017;60(1):11–20. 10.1016/j.pcad.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Sidney S, Sternfeld B, Haskell WL, Quesenberry CP, Crow RS, Thomas RJ. Seven-year change in graded exercise treadmill test performance in young adults in the CARDIA study. Med Sci Sports Exerc. 1998;30(3):427–433. 10.1097/00005768-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Ceaser TG, Fitzhugh EC, Thompson DL, Bassett DR. Association of physical activity, fitness, and race: NHANES 1999–2004. Med Sci Sports Exerc. 2013;45(2):286–293. 10.1249/MSS.0b013e318271689e. [DOI] [PubMed] [Google Scholar]
- 22.Swift DL, Staiano AE, Johannsen NM, et al. Low cardiorespiratory fitness in African Americans: a health disparity risk factor? Sports Med. 2013;43(12):1301–1313. 10.1007/s40279-013-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 24.Sidney S, Haskell WL, Crow R, et al. Symptom-limited graded treadmill exercise testing in young adults in the CARDIA study. Med Sci Sports Exerc. 1992;24(2):177–183. 10.1249/00005768-199202000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs DR, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155(12):1114–1119. 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 28.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91(9):1104–1112. [PubMed] [Google Scholar]
- 29.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092–3100. 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 30.Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. 10.1001/jama.1996.03540030039029. [DOI] [PubMed] [Google Scholar]
- 31.Powell T, Bagnell M. Your “Survival” Guide to Using Time‐Dependent Covariates. http://support.sas.com/resources/papers/proceedings12/168-2012.pdf. Published 2012. Accessed June 27, 2017.
- 32.Allison P Discrete time methods for the analysis of event histories In: Sociological Methodology. San Francisco: Jossey-Bass; 1982:61–98. 10.2307/270718. [DOI] [Google Scholar]
- 33.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1–406. [PubMed] [Google Scholar]
- 35.Al Tunaiji H, Davis JC, Mackey DC, Khan KM. Population attributable fraction of type 2 diabetes due to physical inactivity in adults: a systematic review. BMC Public Health. 2014;14:469 10.1186/1471-2458-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. 10.2105/AJPH.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: Cardia and the Minnesota Heart Health Program. J Cardiopul Rehabil. 1989;9(11):448–459. 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl 1):S8–S16. 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 40.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426–e483. 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 41.Peralta CA, Bibbins-Domingo K, Vittinghoff E, et al. APOL1 genotype and race differences in incident albuminuria and renal function decline. J Am Soc Nephrol. 2016;27(3):887–893. 10.1681/ASN.2015020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32:349–365. 10.1146/annurev-publhealth-031210-101151. [DOI] [PubMed] [Google Scholar]
- 43.Bowlby W, Zelnick LR, Henry C, et al. Physical activity and metabolic health in chronic kidney disease: a cross-sectional study. BMC Nephrol. 2016;17:187 10.1186/s12882-016-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–393. 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Craenenbroeck AHV, Craenenbroeck EMV, Kouidi E, Vrints CJ, Couttenye MM, Conraads VM. Vascular effects of exercise training in CKD: current evidence and pathophysiological mechanisms. Clin J Am Soc Nephrol. 2014;9(7):1305–1318. 10.2215/CJN.13031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015;8(6):753–765. 10.1093/ckj/sfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150. 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 48.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19(7):1261–1270. 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 49.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363–2370. 10.1097/01.ASN.0000026493.18542.6A. [DOI] [PubMed] [Google Scholar]
- 50.Vart P, Gansevoort RT, Crews DC, Reijneveld SA, Bültmann U. Mediators of the association between low socioeconomic status and chronic kidney disease in the United States. Am J Epidemiol. 2015;181(6):385–396. 10.1093/aje/kwu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeFina LF, Haskell WL, Willis BL, et al. Physical activity versus cardiorespiratory fitness: two (partly) distinct components of cardiovascular health? Prog Cardiovasc Dis. 2015;57(4):324–329. 10.1016/j.pcad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Carnethon MR, Evans NS, Church TS, et al. Joint associations of physical activity and aerobic fitness on the development of incident hypertension. Hypertension. 2010;56(1):49–55. 10.1161/HYPERTENSIONAHA.109.147603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33(5):754–761. 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers J, Kaykha A, George S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117(12):912–918. 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 55.Norton JM, Moxey-Mims MM, Eggers PW, et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27(9):2576–2595. 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swift DL, Johannsen NM, Lavie CJ, et al. Racial differences in the response of cardiorespiratory fitness to aerobic exercise training in Caucasian and African American postmenopausal women. J Appl Physiol. 2013;114(10):1375–1382. 10.1152/japplphysiol.01077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swift DL, Johannsen NM, Earnest CP, Newton RL, McGee JE, Church TS. Cardiorespiratory fitness and exercise training in African Americans. Prog Cardiovasc Dis. 2017;60(1):96–102. 10.1016/j.pcad.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Gould DW, Graham-Brown MPM, Watson EL, Viana JL, Smith AC. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology (Carlton). 2014;19(9):519–527. 10.1111/nep.12285. [DOI] [PubMed] [Google Scholar]
- 59.Naderi N, Kleine C-E, Park C, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61(2):168–181. 10.1016/j.pcad.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lakkis JI, Weir MR. Obesity and kidney disease. Prog Cardiovasc Dis. 2018;61(2):157– 167. 10.1016/j.pcad.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Wilkinson TJ, Shur NF, Smith AC. “Exercise as medicine” in chronic kidney disease. Scand J Med Sci Sports. 2016;26(8):985–988. 10.1111/sms.12714. [DOI] [PubMed] [Google Scholar]
- 62.Dungey M, Hull KL, Smith AC, Burton JO, Bishop NC. Inflammatory factors and exercise in chronic kidney disease. Int J Endocrinol. 2013;2013:569831 10.1155/2013/569831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59(1):126–134. 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zelle DM, Klaassen G, van Adrichem E, Bakker SJL, Corpeleijn E, Navis G. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol. 2017;13(3):152–168. 10.1038/nrneph.2016.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.