SUMMARY
Background
Adults with HIV have an increased burden of non-AIDS-defining cancers (NADC), myocardial infarction (MI), end-stage liver disease (ESLD), and end-stage renal disease (ESRD). The objective of this study was to estimate the population attributable fractions (PAFs) of preventable or modifiable HIV-related and traditional risk factors separately for NADC, MI, ESLD, and ESRD outcomes, interpreted as the proportion of cases of each outcome that could be avoided if adults with HIV were “unexposed” to these risk factors.
Methods
Participants receiving care in academic and community-based outpatient HIV clinical cohorts in the US and Canada that contributed validated NADC, MI, ESLD, and ESRD outcomes and traditional and HIV-related risk factors to the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) from 1 Jan 2000 to 31 Dec 2015 were included. Traditional risk factors included tobacco smoking, hypertension, elevated total cholesterol, type 2 diabetes, renal impairment (stage 4 chronic kidney disease [CKD]), and hepatitis C virus (HCV) and hepatitis B virus (HBV) infection. HIV-related risk factors included low CD4 count (<200 cells per μL), detectable plasma HIV RNA (>400 copies per mL), and history of a clinical AIDS diagnosis. Population attributable fractions and 95% confidence intervals ([,]) were estimated to quantify the proportion of outcomes that could be avoided if the risk factor was prevented.
Findings
In each of the study populations for the four outcomes (N=61,500 contributing n=1,405 NADCs, N=29,515 contributing n=247 Mis, N=35,044 contributing n=397 ESLD events, and N=35,620 contributing n=255 ESRD events), ~17% were age ≥50 years at study entry, ~50% were non-White, and ~80% were men. Preventing smoking would avoid 24% [13%, 35%] of NADCs and 37% [7%, 66%] of MIs. Preventing elevated total cholesterol and hypertension would avoid the greatest proportion of MIs (44% [30%, 58%] and 42% [28%, 56%], respectively). For ESLD, the PAF was greatest for at-risk alcohol use (35% [9%, 60%]) and HCV infection (33% [17%, 48%]). For ESRD, the PAF was greatest for hypertension (39% [26%, 51%]) followed by elevated total cholesterol (22% [13%, 31%]), detectable HIV RNA (19% [9%, 31%]), and low CD4 count (13% [4%, 21%]).
Interpretation
The substantial proportion of NADC, MI, ESLD, and ESRD outcomes that could be prevented with interventions on traditional risk factors elevates the importance of screening for these risk factors, improving the effectiveness of prevention (or modification) of these risk factors, and creating sustainable care models to implement such interventions during the decades of life adults living with HIV are receiving care.
Funding
National Institutes of Health, Centers for Disease Control and Prevention, the US Agency for Healthcare Research and Quality, the US Health Resources and Services Administration, the Canadian Institutes of Health Research, the Ontario Ministry of Health and Long Term Care, and the Government of Alberta.
Keywords: HIV, cancer, myocardial infarction, end stage renal disease, end stage liver disease, population attributable fraction
INTRODUCTION
Adults receiving antiretroviral therapy (ART) and aging with HIV have a greater burden of chronic non-communicable diseases (NCDs) compared to adults without HIV.1 This increased risk is hypothesized to be the result of a combination of factors, including: 1) increased prevalence of traditional risk factors for NCDs in adults with HIV;2,3 2) the possible synergistic effect of viral co-infection, particularly with hepatitis C virus (HCV) co-infection;4 3) HIV-associated immunosuppression, immune activation, inflammation, and hypercoaguability5 (which is blunted but not normalized with ART6); and 4) ART drugs, including previous exposure to first-generation nucleoside analogue reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs).7 Although some specific antiretroviral agents have been associated with an increased risk of some NCDs, initiation of ART at higher CD4 counts results in a decreased risk of cancer in adults aging with HIV, demonstrating the potential importance of early ART initiation for NCD prevention.8 Despite the known roles of these traditional and HIV-related risk factors in NCD development among adults with HIV, it is unknown how much of the NCD burden can be attributed to each of these factors.
Over the last two decades, cancer, cardiovascular disease (CVD), and liver disease have become the top causes of non-AIDS-related death.9,10 End-stage liver disease (ESLD) also results in a large proportion of deaths and is responsible for substantial morbidity among adults with HIV due to the high prevalence of chronic hepatitis B virus (HBV) and HCV infection in adults with HIV.11–13 Although rare, end-stage renal disease (ESRD) is elevated compared to the general population14 and has been hypothesized to increase due to the combined effect of nephrotoxic ART regimens and age-associated declines in renal function.15
Our goal was to estimate the proportion of non-AIDS-defining cancers (NADC), myocardial infarction (MI), ESLD, and ESRD events that can be attributed to traditional and HIV-related risk factors among adults with HIV who have been successfully linked into care. To this end, we analyzed traditional and HIV-related risk factors for these four validated NCD outcomes among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Our population attributable fraction (PAF) approach quantifies the proportion of NCDs that could be eliminated if particular risk factors were not present, and can be used to inform prioritization of such interventions in order to preserve the health of adults with HIV.
METHODS
Study population
The NA-ACCORD is the largest consortium of HIV cohorts in the US and Canada and is the North American region of the International Epidemiologic Databases to Evaluate AIDS (leDEA) project, supported by the National Institutes of Health. Details on this collaboration have been published previously.16 Briefly, the NAACCORD consists of single- and multi-site clinical and interval cohorts in various settings ranging from community health centers to academic institutions. The cohorts have prospectively collected data on >180,000 adults with HIV (≥18 years old) from more than 200 sites in the US and Canada (for a map of sites: www.naaccord.org). Each cohort submits comprehensive data on enrolled participants to the Data Management Core (University of Washington, Seattle, WA), where the data undergo quality control, are harmonized across cohorts, and are transmitted to the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, MD), which conducted the analyses presented here.
For these analyses, US clinical cohorts within the NA-ACCORD with complete access to both inpatient and outpatient electronic medical records (EMR) validated the occurrences of NADC, ESRD, ESLD, and MI among individuals. The human subjects research activities of the NA-ACCORD and each of the participating cohort studies have been reviewed and approved by their respective local institutional review boards and the Johns Hopkins School of Medicine. The Johns Hopkins Bloomberg School of Public Health internal review board approved the present study.
Data sources
The protocol for ascertainment and validation of cancer, type 1 MI, ESLD, and ESRD (outcomes of interest) within the NA-ACCORD has been previously published and are elaborated upon in the Appendix (pages 1-3).17–19 First NADC (which excludes cervical cancer, non-Hodgkin lymphoma, and6 Kaposi’s sarcoma) was our cancer outcome of interest; non-melanoma skin cancers and pre-cancers were also excluded. Only type 1 Mis that were the result of athero-thrombotic coronary events from plaque rupture were included; type 2 MIs have different causes (e.g. hypotension, hypoxia, and stimulant-induced spasm resulting in increased oxygen demand or decreased supply).
Traditional risk factors were chosen based on prior literature identifying risk factors for the outcomes of interest and data availability and included: cigarette smoking (ever/never), elevated total cholesterol (≥240 mg/dL, and statin prescription was accounted for separately in analyses), hypertension (clinical diagnosis and an antihypertensive medication prescription), type 2 diabetes (diabetes-specific medication, or a diagnosis with a diabetes-related medication, or a glycosylated hemoglobin ≥6.5%), stage 4 chronic kidney disease (CKD, eGFR <30 vs. ≥30 mL/min/1.73m2 using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation). Elevated total cholesterol, hypertension, diabetes, and stage 4 CKD were time-varying with the restriction that once an individual met the definition, the classification was not reversible.
At-risk alcohol use (ever reporting ≥3 drinks/day or ≥7 drinks/week for women, or ≥4 drinks/day or ≥14 drinks/week for men) and body mass index (BMI) measurements were available for a subgroup of participants enrolled in contributing cohorts that measured these risk factors.
We classified ever (vs. never) HCV infection (having a positive HCV antibody test, a detectable HCV RNA, or the presence of an HCV genotype test) and HBV infection (having a positive HBV surface antigen test, a positive HBV e antigen test, or a detectable HBV DNA test result) as preventable and modifiable traditional risk factors due to their causal relationships with liver cancer and ESLD. Additional details on the definitions and timing of the measurements for the outcome of interests, the traditional and HIV-related risk factors, and the non-modifiable covariates can be found in the Appendix (pages 1-3).
Traditional HIV-related risk factors included: CD4 T-lymphocyte cell (CD4) count (categorized using the clinically meaningful cut-off for severe immune deficiency threshold of <200 vs. ≥200 cells per μL, HIV virologic suppression (HIV-1 RNA ≤400 copies per mL), a history of a clinical AIDS-defining illness excluding the criteria of a CD4 count <200 cells per μL.20 CD4 count and HIV virologic suppression were time-varying. As is true in clinical care, a history of AIDS was also time-varying with the restriction that once an individual met the definition, the classification was not reversible. CD4 counts <200 cells per μL and detectable viral loads are modifiable with effective ART, and AIDS is preventable with early HIV diagnosis and ART initiation. ART prescription was not considered as a risk factor because of the associations of ART with CD4 count, HIV RNA, and clinical AIDS diagnosis. Investigations of specific antiretroviral agents, such as abacavir or tenofovir, require additional adjustment for confounding by indication are outside the scope of our study.
Non-modifiable covariates included age, sex (male and female), race and ethnicity (White, Black, Hispanic, and other), and HIV acquisition risk group (men who have sex with men [MSM], those reporting current or prior injection drug use [IDU], heterosexual, and other) were self-reported at enrollment into the NA-ACCORD. Although current IDU is a modifiable risk factor, IDU as the suspected mode of HIV transmission is not modifiable.
Statistical analysis
All analyses were conducted separately for each outcome (NADC, MI, ESLD, and ESRD), and an individual could contribute data to each of the outcome-specific analyses. Calendar periods in which validated outcomes were actively ascertained (“observation window”) were defined for each outcome and for each cohort. Participants entered our nested studies at enrollment into the NA-ACCORD, the start of the outcome observation window, or 1 January 2000, whichever came later. Participants were followed until the latest of the following: event of interest, death, one year after the date of the last CD4 or HIV RNA measurement, the end of the outcome observation window, or 31 December 2009 for ESRD and ESLD outcomes, 31 December 2013 for the MI outcome, or 31 December 2014 for the cancer outcome. We excluded prevalent cases of our events of interest.
As PAFs incorporate the prevalence of risk factors among persons with the outcome (estimated as a simple proportion) and the risk of the outcome, we estimated adjusted (aHR) hazard ratios and 95% confidence intervals using Cox proportional hazard models with piecewise constant baseline hazard functions. The population attributable fractions (PAFs) approach for disease incidence described by Laaksonen was used because it accommodated time-varying risk factors available from a cohort study design.21 Using this approach, risk factors that varied over time had a PAF estimate that was different than the PAF estimated using a standard formula (PAF = prevalence in the cases * [ (adjusted risk estimate − 1) / adjusted risk estimate]); however, risk factors that varied little over time had similar estimates across approaches. Death would preclude the outcome of interest. The Laaksonen approach accounted for the mortality rate as well as the associations between death and the risk factors of interest in the estimation of the PAF.21 Participant follow-up time was divided into 2-year intervals. Age, sex, race/ethnicity, and IDU status were accounted for in adjusted models that produced PAF estimates and 95% confidence intervals. Schoenfeld residual global tests and Kolmogorov-type supremum tests were used to test the assumption of proportional hazards for all risk factors of interest.22
A sensitivity analysis was performed after removing lung cancer from the NADC outcome as it was the most common cancer and has a very strong relationship with smoking. A sensitivity analysis was performed after removing elevated total cholesterol from the ESRD outcome due to the relationship of renal impairment and dyslipidemia.23
Sub-group analyses were performed for the MI and ESLD outcomes among persons with BMI and at-risk alcohol data, respectively; cohorts that did not contribute data on these risk factors were excluded from the sub-group analyses.
All analyses were performed using SAS version 9.3 (SAS Institute); statistical interpretation was guided by a p-value <0.05 and PAF 95% confidence intervals that do not cross 0%.
Role of the Funding Source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. YJ had full access to all the data in the study and KNA had final responsibility for the decision to submit for publication.
RESULTS
Flow charts depicting selection of the NA-ACCORD participants for each outcome can be found in the Appendix (page 4). Median follow-up time was similar across the four outcomes, ranging from 3.1 years for ESLD to 3.7 years for NADC (Appendix page 5). Type-specific NADC outcomes can be found in the Appendix page 6. Of the 1,405 NADCs observed, 16% (n=230) were lung, 16% (n=225) were anal, 12% (n=167) were prostate, (n=96) 7% were Hodgkin’s lymphoma, 6% (n=90) were liver, 6% (n=83) were oral cavity and pharynx, 6% (n=82) were breast, 5% (n=69) were melanoma, and 5% (n=65) were colon and rectum cancers. Each of the other type-specific cancers accounted for <5% of the 1,405 cancer diagnoses.
Prevalence of preventable or modifiable risk factors
The prevalence of the preventable or modifiable risk factors at study entry among persons with and without the outcomes of interest are shown in Table 1. For all four incident outcomes, there were greater proportions of participants with a smoking history, HCV infection, HBV infection, low (<200 cells per μL) CD4 count, and a history of a clinical AIDS diagnosis among persons with an incident outcome compared to those who did not develop the outcomes. Persons with an incident MI were more likely to have elevated total cholesterol, hypertension, diabetes, and stage 4 CKD compared to those who did not have an incident MI. Persons with an ESRD diagnosis were more likely to have a detectable viral load as compared to those without an incident diagnosis, but the proportion with detectable HIV RNA was similar by incident diagnoses status for all of the other outcomes. Those with an incident ESRD diagnosis also had greater proportions with elevated total cholesterol, hypertension, and diabetes compared to those who did not develop ESRD.
Table 1:
Characteristics at study entry, by non-AIDS-defining cancers, myocardial infarction (MI), end-stage liver disease (ESLD), and end-stage renal disease (ESRD) diagnoses, NA-ACCORD
| NADC | MI | ESLD | ESRD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No diagnosis | Diagnosis | No diagnosis | Diagnosis | No diagnosis | Diagnosis | No diagnosis | Diagnosis | |||||||||
| n= 60,095 | n= 1,405 | n= 29,168 | n= 347 | n= 34,657 | n= 387 | n= 35,365 | n= 255 | |||||||||
| Demographics | ||||||||||||||||
| Age | ||||||||||||||||
| <40 years | 29,429 | 49% | 317 | 23% | 13,749 | 47% | 63 | 18% | 16,641 | 48% | 124 | 32% | 16,344 | 46% | 94 | 37% |
| 40-49 years | 20,584 | 34% | 550 | 39% | 10,217 | 35% | 144 | 41% | 12,474 | 36% | 160 | 41% | 13,042 | 37% | 101 | 40% |
| 50-59 years | 8,239 | 14% | 390 | 28% | 4,220 | 14% | 106 | 31% | 4,528 | 13% | 80 | 21% | 4,835 | 14% | 41 | 16% |
| ≥60 | 1,843 | 3% | 148 | 11% | 982 | 3% | 34 | 10% | 1,014 | 3% | 23 | 6% | 1,144 | 3% | 19 | 7% |
| Male | 46,330 | 77% | 1,093 | 78% | 23,475 | 80% | 298 | 86% | 27,354 | 79% | 334 | 86% | 27,974 | 79% | 178 | 70% |
| Race and ethnicity | ||||||||||||||||
| White | 25,075 | 42% | 692 | 49% | 13,429 | 46% | 193 | 56% | 14,560 | 42% | 207 | 53% | 15,022 | 42% | 30 | 12% |
| Black | 21,658 | 36% | 534 | 38% | 10,831 | 37% | 123 | 35% | 11,693 | 34% | 108 | 28% | 11,452 | 32% | 205 | 80% |
| Hispanic | 7,683 | 13% | 111 | 8% | 3,106 | 11% | 20 | 6% | 4,379 | 13% | 43 | 11% | 4,756 | 13% | 12 | 5% |
| Other | 3,033 | 5% | 44 | 3% | 1,308 | 4% | 10 | 3% | 1,269 | 4% | 7 | 2% | 1,333 | 4% | 1 | 0% |
| Unknown/Missing | 2,646 | 4% | 24 | 2% | 494 | 2% | 1 | 0% | 2,756 | 8% | 22 | 6% | 2,802 | 8% | 7 | 3% |
| HIV transmission risk | ||||||||||||||||
| MSM | 31,370 | 52% | 742 | 53% | 16,103 | 55% | 193 | 56% | 17,514 | 51% | 180 | 47% | 17,114 | 48% | 67 | 26% |
| IDU | 6,885 | 11% | 204 | 15% | 2,971 | 10% | 44 | 13% | 4,231 | 12% | 97 | 25% | 3,912 | 11% | 52 | 20% |
| Heterosexual contact | 15,397 | 26% | 343 | 24% | 7,559 | 26% | 84 | 24% | 9,224 | 27% | 73 | 19% | 9,065 | 26% | 108 | 42% |
| Other/Unknown/Missing | 6,443 | 11% | 116 | 8% | 2,535 | 9% | 26 | 7% | 3,688 | 11% | 37 | 10% | 5,274 | 15% | 28 | 11% |
| Traditional Risk Factors | ||||||||||||||||
| Smoking | ||||||||||||||||
| Never | 13,604 | 23% | 236 | 17% | 6,891 | 24% | 55 | 16% | 6,916 | 20% | 57 | 15% | 7,645 | 22% | 41 | 16% |
| Ever | 37,013 | 62% | 1,106 | 79% | 21,884 | 75% | 292 | 84% | 20,588 | 59% | 289 | 75% | 20,711 | 59% | 182 | 71% |
| Missing | 9,478 | 16% | 63 | 4% | 393 | 1% | 0 | 0% | 7,153 | 21% | 41 | 11% | 7,009 | 20% | 32 | 13% |
| Elevated TC | 10,037 | 17% | 325 | 23% | 7,287 | 25% | 211 | 61% | 7,032 | 20% | 66 | 17% | 6,779 | 19% | 108 | 42% |
| Hypertension | 5,230 | 9% | 237 | 17% | 2,809 | 10% | 89 | 26% | 2,234 | 6% | 53 | 14% | 2,365 | 7% | 68 | 27% |
| Diabetes | 1,660 | 3% | 68 | 5% | 766 | 3% | 27 | 8% | 855 | 2% | 20 | 5% | 928 | 3% | 29 | 11% |
| Stage 4 CKD | 889 | 1% | 35 | 2% | 610 | 2% | 20 | 6% | 634 | 2% | 26 | 7% | 479 | 1% | 83 | 33% |
| Statin Prescription | 2,260 | 4% | 95 | 7% | 1,449 | 5% | 50 | 14% | 812 | 2% | 11 | 3% | 1,090 | 3% | 16 | 6% |
| Hepatitis C infection | 10,379 | 17% | 356 | 25% | 5,392 | 18% | 92 | 27% | 6,700 | 19% | 197 | 51% | 6,189 | 18% | 88 | 35% |
| Hepatitis B infection | 4,814 | 8% | 156 | 11% | 1,766 | 6% | 30 | 9% | 3,535 | 10% | 108 | 28% | 2,225 | 6% | 31 | 12% |
| HIV-related risk factors | ||||||||||||||||
| Low CD4 count | 13,337 | 22% | 396 | 28% | 7,034 | 24% | 112 | 32% | 8,875 | 26% | 139 | 36% | 8,805 | 25% | 93 | 36% |
| Detectable HIV RNA | 34,408 | 57% | 809 | 58% | 16,523 | 57% | 184 | 53% | 19,862 | 57% | 217 | 56% | 19,422 | 55% | 176 | 69% |
| Clinical AIDS diagnosis | 10,158 | 17% | 353 | 25% | 6,284 | 22% | 109 | 31% | 7,392 | 21% | 102 | 26% | 7,695 | 22% | 94 | 37% |
| ART regimen | ||||||||||||||||
| Treatment naïve | 25,216 | 42% | 452 | 32% | 11,478 | 39% | 90 | 26% | 14,170 | 41% | 96 | 25% | 13,065 | 37% | 75 | 29% |
| PI-based ART | 12,394 | 21% | 406 | 29% | 5,842 | 20% | 114 | 33% | 7,707 | 22% | 117 | 30% | 7,606 | 16% | 51 | 20% |
| NNRTI-based ART | 10,289 | 17% | 217 | 15% | 5,121 | 18% | 44 | 13% | 5,465 | 16% | 50 | 13% | 6,756 | 15% | 35 | 14% |
| Other ART | 5,591 | 9% | 155 | 11% | 2,402 | 8% | 43 | 12% | 2,858 | 8% | 56 | 14% | 3,278 | 7% | 35 | 14% |
| Treatment experienced but not prescribed ART | 6,558 | 11% | 174 | 12% | 4,325 | 15% | 56 | 16% | 4,457 | 13% | 68 | 18% | 4,660 | 13% | 59 | 23% |
Abbreviations:
MSM=men who have sex with men
IDU=history of injection drug use
Heterosexual=heterosexual contact
TC=total cholesterol
CKD=chronic kidney disease
PI=protease inhibitor
NNRTI=non-nucleoside reverse transcriptase inhibitor
All p-values were <0.05 except: sex (p=0.61) and HIV transmission risk group (p=0.24) for non-AIDS-defining cancers; statin prescription (p=0.57) and detectable HIV RNA (p=0.053) for ESLD; and sex (p=0.77) and detectable HIV RNA (p=0.26) for MI.
Associations between preventable or modifiable risk factors and outcomes
Other factors influencing the PAFs are the aHRs for each preventable or modifiable risk factor and outcome, which were calculated allowing some risk factors to vary with time (i.e., elevated total cholesterol, hypertension, type 2 diabetes, stage 4 CKD, low CD4 count, detectable HIV RNA, history of AIDS-defining illness) and which were adjusted for age (time-varying), sex, race/ethnicity, and a history of IDU.
For NADC, the preventable or modifiable risk factors with significant associations were smoking, low CD4 count, detectable HIV RNA, a history of clinical AIDS diagnosis, and HBV infection (Figure 1; see aHRs). In a sensitivity analysis excluding persons with lung cancer diagnoses, the effect of smoking attenuated, but there was still a strong, statistically significant relationship between smoking and NADC.
Figure 1:
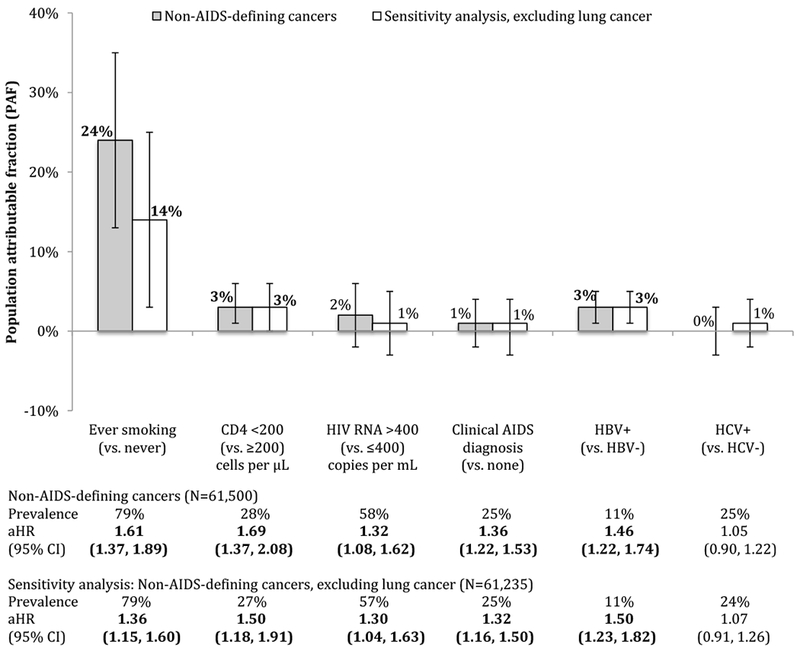
Population attributable fractions (and 95% confidence intervals) for traditional- and HIV-related risk factors for non-AIDS-defining cancer, overall (N=61,500) and excluding lung cancer (N=61,235), NA-ACCORD
Abbreviations:
95% CI: 95% confidence interval
aHR: adjusted hazard ratio
HBV: hepatitis B infection
HCV: hepatitis C infection
Prevalence is the prevalence of the risk factor among those with the outcome at study entry; the calculation of the population attributable fraction (PAF) allows for time-varying risk factors
aHRs were adjusted for age, sex, race and ethnicity, history of injection drug use, and all the risk factors shown in the figure.
Bold indicates statistically significant estimates.
A total of 265 participants with lung cancer were excluded in the sensitivity analysis; 230 with lung cancer as a first cancer diagnosis, 9 with lung cancer a cancer diagnosis after another cancer diagnosis, and 26 participants who had a lung cancer diagnosis reported after the close of the observation window. For the sensitivity analysis excluding lung cancer, there were 61,235 participants, of whom 1,1166 had a cancer diagnosis while under observation and 60,069 who did not.
For MI, smoking, elevated total cholesterol, hypertension, stage 4 CKD, a low CD4 count, detectable HIV RNA, and HCV infection were statistically significantly associated (Figure 2; see aHRs). In a sub-group analysis restricting to persons with available BMI measurements (N=16,687 or 57%), obesity did not have a statistically significant relationship with MI in the adjusted model, and the associations of elevated total cholesterol, diabetes, stage 4 CKD, low CD4 count, and HCV infection with MI strengthened; all other associations attenuated.
Figure 2:
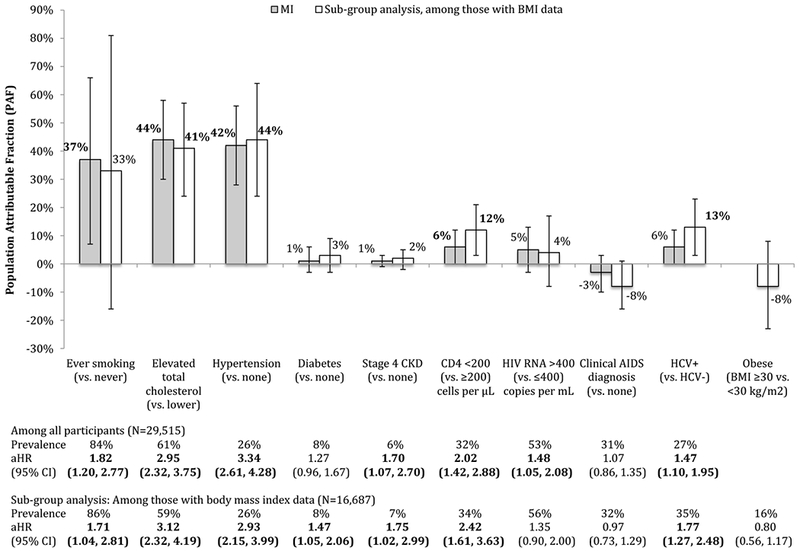
Population attributable fractions (and 95% confidence intervals) for traditional- and HIV-related risk factors for myocardial infarction (MI), overall (N=29,515) and among those with body mass index (BMI) data (N=16,687), NA-ACCORD
Abbreviations:
95% CI: 95% confidence interval
aHR: adjusted hazard ratio
CKD: chronic kidney disease
HBV: hepatitis B infection
HCV: hepatitis C infection
Prevalence is the prevalence of the risk factor among those with the outcome at study entry; the calculation of the population attributable fraction (PAF) allows for time-varying risk factors.
aHRs were adjusted for age, sex, race and ethnicity, history of injection drug use, and all the risk factors shown in the figure.
Bold indicates statistically significant estimates.
For the sub-group analysis restricted to the 16,687 (57%) participants with body mass index (BMI) data, 227 had a type 1 MI diagnosis while under observation and 16,460 did not.
For ESLD, each of low CD4 count, detectable HIV RNA, a history of a clinical AIDS diagnosis, HBV infection, and HCV infection showed statistically significant associations (Figure 3; see aHRs). In a sub-group analysis restricted to those with known at-risk alcohol status (N=12,158 or 35%), at-risk alcohol use showed a statistically significant association with ESLD. The inclusion of at-risk alcohol use in the model diminished the effects of smoking and a history of a clinical AIDS diagnosis, strengthened the association with low CD4 count, and attenuated the effects of the remaining risk factors.
Figure 3:
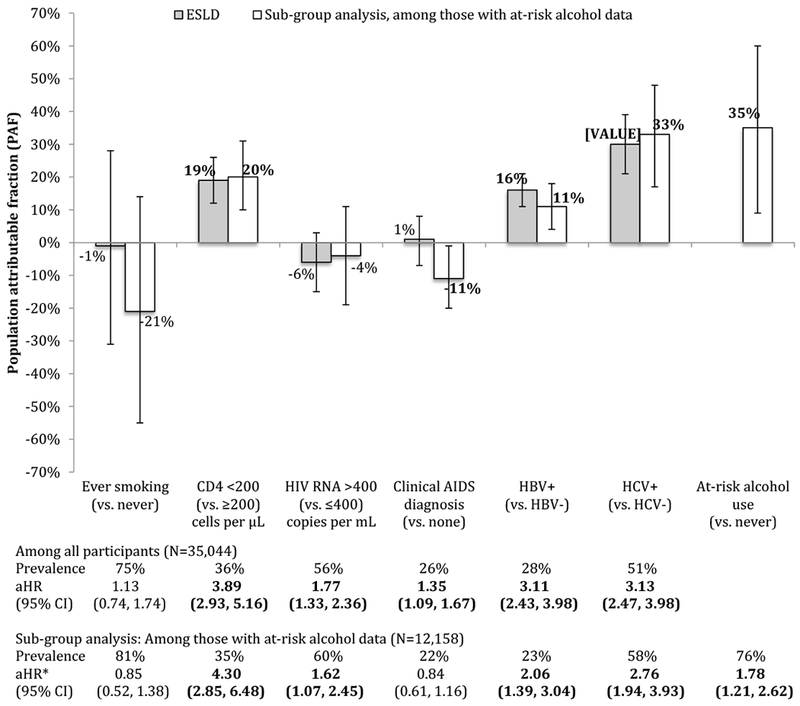
Population attributable fractions (and 95% confidence intervals) for traditional- and HIV-related risk factors for end-stage liver disease (ESLD), overall (N=35,044) and among those with at-risk alcohol use data (N=12,158), NA-ACCORD
Abbreviations:
95% CI: 95% confidence interval
aHR: adjusted hazard ratio
HBV: hepatitis B infection
HCV: hepatitis C infection
Prevalence is the prevalence of the risk factor among those with the outcome at study entry; the calculation of the population attributable fraction (PAF) allows for time-varying risk factors.
aHRs were adjusted for age, sex, race and ethnicity, history of injection drug use, and all the risk factors shown in the figure.
Bold indicates statistically significant estimates.
For the sub-group analysis restricted to the 12,158 (35%) participants with body mass index (BMI) data, 176 had an ESLD diagnosis while under observation and 11,982 did not.
For ESRD, each of elevated total cholesterol, hypertension, diabetes, low CD4 count, detectable HIV RNA, and a history of clinical AIDS diagnosis showed statistically significant associations (Figure 4; see aHRs).
Figure 4.
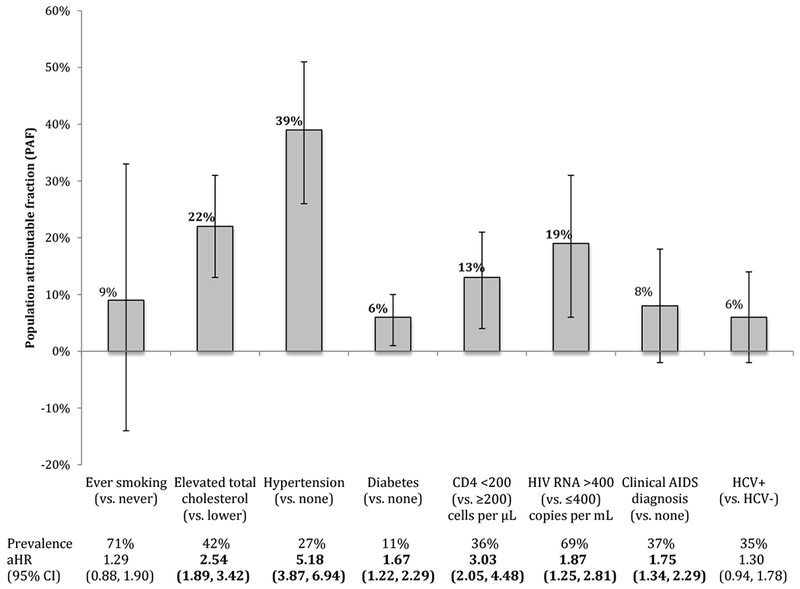
Population attributable fractions (and 95% confidence intervals) for traditional- and HIV-related risk factors for end-stage renal disease (N=35,620), NA-ACCORD
Abbreviations:
95% CI: 95% confidence interval
aHR: adjusted hazard ratio
HCV: hepatitis C infection
Prevalence is the prevalence of the risk factor among those with the outcome at study entry; the calculation of the population attributable fraction (PAF) allows for time-varying risk factors.
aHRs were adjusted for age, sex, race and ethnicity, history of injection drug use, and all the risk factors shown in the figure. This analysis of PAFs for factors associate with ESRD did not include accounting for stages of chronic kidney disease.
Bold indicates statistically significant estimate
Schoenfeld residual global test approach and the Kolmogorov-type supremum test of the proportional hazards assumption demonstrated proportionality for all risk factors presented.
Population attributable fractions
The PAFs for the preventable or modifiable risk factors stratified by outcome can be seen in Figures 1–4. The PAF for smoking was substantial for NADC and MI, but was far less and not significant for ESLD or ESRD. For NADC, the PAF for smoking was far greater than the PAFs for HIV-related risk factors and persisted after excluding lung cancer cases from the NADC outcome.
For MI, elevated total cholesterol and hypertension had higher PAFs than smoking; these PAFs were much larger than those for HIV-related risk factors. In the sub-group analysis among persons with BMI measurements, after adjustment for BMI, the magnitude of the PAFs for traditional risk factors did not meaningfully change (however, the PAF for smoking was no longer statistically significant), and the magnitude of the low CD4 count and HCV infection PAFs increased slightly.
HCV infection had the highest PAF for ESLD, followed by low CD4 count and HBV infection. In the subgroup analysis among persons with at-risk alcohol use measurements, the PAF for at-risk alcohol use was greater than HCV.
Hypertension had the highest PAF for ESRD. There were similar PAFs for elevated total cholesterol, detectable HIV RNA, followed by low CD4 count, and a history of a clinical AIDS diagnosis. Smoking had a non-statistically significant PAF of 9%. In the sensitivity analysis removing total elevated cholesterol, the PAFs for the remaining risk factors were consistent (data not shown).
See Appendix (pages 7-8) to view the PAFs grouped by risk factors (as opposed to outcomes).
DISCUSSION
Our study sought to answer the question “How much NCD in adults with HIV could be avoided if the effects of certain risk factors were prevented or eliminated from the population?” In our population of adults with HIV who were successfully linked to care, we found that traditional risk factors, including smoking, elevated total cholesterol, hypertension, and chronic HCV infection accounted for a larger share of the NADC, MI, ESLD, and ESRD incident outcomes than HIV-related risk factors. The PAF approach accounts for not only the risk of the outcome associated with the risk factor, but also the prevalence of the risk factor among persons with the outcome. A high PAF can result from a risk factor with a weak or moderately strong association with the outcome but a high prevalence; conversely, a low PAF can result from a strong risk factor with a low prevalence. Using both pieces of information, the evidence from our study is clear: screening for traditional risk factors, effective interventions reducing the burden of traditional risk factors, and a sustainable model of care with the capacity to provide traditional risk factor interventions over the decades of life experienced with HIV must be balanced with the continued focus on maintaining HIV viral suppression after ART initiation in order to avoid sizeable proportions of NADC, MI, ESLD, and ESRD.
Many interventions used to reduce traditional risk factor burden in adults with HIV were developed in the general population and may need modification to improve effectiveness in adults with HIV. For example, smoking cessation programs with reduced financial barriers to nicotine replacement therapy and increased social support may improve the low success rates of smoking cessation programs; a challenge that plagues both adults with and without HIV, but has a greater impact on adults with HIV due to the 2-fold higher prevalence of smoking compared to the general pouplation.24–27 Similarly, interventions for alcohol use reduction, and screening and treating dyslipidemia, hypertension, diabetes, may need tailoring to better meet the needs of adults with HIV to have a substantial impact. Beyond the individual-level interventions, structural and policy interventions on traditional risk factors may have large impacts among adults with HIV carrying a heavier burden of traditional risk factors. For example, a recent simulation study suggested lowering nicotine levels in cigarettes could result in a 15.6% decrease in smoking prevalence (from 12.8% to 10.8% in the overall US population) in the first year of the policy.28 The benefit of this structural intervention would not be limited to reducing smoking prevalence among adults with HIV but would also benefit the general population. Evaluating the effectiveness of modified interventions, and cost analyses for effective interventions, are needed.
Individual-level traditional risk factor interventions must be accessible to adults with HIV; herein lie substantial challenges. Driven by the increased life expectancy of adults with HIV initiating ART at early stages of disease, the size of the population of adults with HIV will continue to increase within the context of current and projected shortages of HIV care providers in some settings.29 There is a need for sustainable models that have the capacity for risk factor interventions (many of which may need to be offered numerous times over decades of life with HIV) and management of complex healthcare needs and multidisciplinary teams when NCDs are not avoided. General practitioners, nurse practitioners, and care coordinator models that are common in other medically complex settings (such as oncology and transplantation) should be evaluated for implementation as the medical home for adults with HIV. More formal adoption of geriatric medicine principles and patient-centered care approaches in HIV care models may be particularly beneficial.30–32
We caution against common misinterpretation of PAFs. PAFs should be interpreted as the “proportion of disease cases over a specified time that would be prevented following elimination of the exposure, assuming the exposure is causal.”33 The relationships between the risk factors included in this investigation and each outcome range from meeting Bradford Hill’s causal criteria (e.g., smoking and NADC) to being incompletely verified as causal (e.g., HIV RNA, which is a time-dependent surrogate for inflammation from uncontrolled viremia). The interpretation of the disease association of PAFs in our study must be that of prevention (e.g., the proportion of the outcome that could be avoided if all members of the study population never started smoking). Perhaps it is more realistic to attempt to modify these risk factors (as opposed to prevent). Although we did not directly assess the impact of modification, it is commonly assumed that the proportions of outcomes avoided by modifying the risk factor (e.g., smoking cessation) will likely be similar to that of preventing the risk factor (i.e., smoking prevention), albeit less because the risks of the outcomes do not immediately drop to that of the unexposed group (i.e., non-smokers) at the time of modification (i.e., cessation).34 We also caution against inappropriately summing the single risk factor PAFs in an attempt to derive the total fraction of disease risk attributable to all of the factors as the limited conditions under which this approach is valid do not apply to this study.35
It is possible that the PAFs for many of the traditional risk factors are underestimated. For example, the proportion of outcomes avoided if smoking was prevented is likely underestimated because current and former smokers are combined in the “ever” category, thus increasing the prevalence of this exposure but diluting the strength of the relationships between smoking and the outcomes. Because hypertension is categorized as diagnosed and treated hypertension, the prevalence is likely underestimated and the relationships between hypertension and the outcomes may be diluted (systolic and diastolic blood pressure measurements were not available for this study). This may also be the case for diabetes, as those with undiagnosed diabetes and no HbA1c test do not meet the criteria to classify the individual as having diabetes, thus reducing the prevalence of diabetes and potentially diluting the relationship between diabetes and the outcomes. The PAF for HCV infection may also be impacted by the definition of have a positive HCV antibody, which includes those who resolve their HCV infection, thus overestimating the prevalence of HCV but likely diluting the relationships with the outcomes. It should be noted that direct acting antivirals for HCV were not licensed until the last year of our study.
Although we examined consistently measured risk factors across four validated NCD events, a limitation specific to our study includes the potential that additional preventable or modifiable causal risk factors were not considered, including specific antiretroviral drugs and regimens (and associated metabolic effects), diet, physical activity, and proteinuria. To estimate the PAF for specific antiretroviral drugs and regimens, methods are needed to account for confounding by indication (perhaps by incorporating weights into the PAF approach) so that specific antiretroviral drugs and regimens do not show spuriously protective PAFs. Further, investigations into causal interactions of specific antiretroviral drugs or regimens and risk factors are needed to incorporate the complexities of HIV treatments when estimating the burden of these outcomes that could be avoided. It is also worth noting that risk factors that are not preventable or modifiable (such as the estimated 66% of cancer-driving mutations that are due to random DNA replication errors in healthy, dividing cells) may also be at play but unaccounted for.36 Although we cannot weigh the benefit of intervention upon these omitted risk factors, we believe we have included the most relevant known risk factors for each outcome. Additionally, current smoking was not included as a possible category of smoking, which is an important limitation. Given the high prevalence of smoking and the low cessation rates among adults with HIV, we suspect the PAF for ever smoking presented here is between the unknown PAFs for current and former smoking.26,27 Finally, our findings cannot be interpreted as the effect of HIV itself on these outcomes because all patients in this study were infected with HIV; the goal was to assess the population-level impact of traditional and HIV-related risk factors on these outcomes among adults aging with HIV.
In summary, preventing the high burden of NCDs among adults aging with HIV will require prioritization of interventions. Our findings show individual and structural or policy-level interventions on traditional risk factors in the context of antiretroviral-induced chronic viral suppression could prevent a substantial proportion of NCDs. Interventions to prevent and address smoking, elevated total cholesterol, and hypertension are particularly important for reducing NADC, MI and ESRD outcomes. HCV infection and at-risk alcohol use prevention and treatment are critical to reducing the burden of ESLD. Modifications to individual-level interventions and models of HIV care, and implementing structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid NCDs and preserve health among successfully antiretroviral-treated adults aging with HIV.
Supplementary Material
PANEL.
Evidence before this study
We searched PubMed with the terms “HIV and cancer,” “HIV and myocardial infarction,” “HIV and end stage liver disease,” and “HIV and end stage renal disease” for articles published in English up to January, 2018. Studies have shown there is an increased burden of non-AIDS defining cancers, myocardial infarction, end-stage renal, and end-stage liver disease, among adults aging with HIV compared to those without HIV. Although both traditional HIV-related risk factors have been associated with these outcomes, the proportion of outcomes attributed to traditional and HIV-related risk factors can help guide programs and policies for interventions.
Added value of this study
To our knowledge, this is the first study to estimate population attributable fractions for four age-related conditions in a large study population of adults with HIV. Risk factors were grouped as traditional or HIV-related to provide direction to programs and policies for preserving health among adults aging with HIV.
Implications of all available evidence
ART initiation is essential to preserving health of adults with HIV; however, traditional risk factors contribute greatly to the burden of age-related diseases. Focusing on reducing traditional risk factors after ART initiation and viral suppression, improving the effectiveness of risk factor interventions in adults with HIV, and implementing care models that can sustain a focus on traditional risk factor reduction is essential to preserving health among adults with HIV.
ACKNOWLEDGEMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Centers for Disease Control and Prevention. KN Althoff was supported by K01AI093197, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Additionally, this work was supported by National Institutes of Health grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K23EY013707, K24AI065298, K24AI118591, K24DA000432, K24DA035684, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R01AG053100, R01DA026770, R24AI067039, R24AG044325, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794,U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214 and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Cancer Institute, National Institute for Mental Health and National Institute on Drug Abuse, the Johns Hopkins Center for AIDS Research (P30 AI094189) and the Sidney Kimmel Comprehensive Cancer Center research program grant (P30 CA006973).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Althoff K, Smit M, Reiss P, Justice AC. HIV and ageing: Improving quantity and quality of life. Curr Opin HIV AIDS. 2016. September; 11 (5):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: A meta-analysis. AIDS. 2016. January;30(1):273–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients – association with antiretroviral therapy. Results from the DAD study. AIDS. 2003. May 23;17(8):1179–1193. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002. July 10;288(2): 199–206. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011. ;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nl Wada, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015. February 20;29(8):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friis-Møller N, Smieja M, Klein D. Antiretroviral therapy as a cardiovascular disease risk factor: fact or fiction? A review of clinical and surrogate outcome studies. Curr Opin HIV AIDS. 2008. May;3(3):220–225. [DOI] [PubMed] [Google Scholar]
- 8.ÁH Borges, Neuhaus J, Babiker AG, et al. Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis. 2016. December 15;63(12):1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet. 2014. July 19;384(9939):241–248. [DOI] [PubMed] [Google Scholar]
- 10.Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013. April; 14(4): 195–207. [DOI] [PubMed] [Google Scholar]
- 11.Klein MB, Rollet-Kurhajec KC, Moodie EEM, et al. Mortality in HIV-hepatitis C co-infected patients in Canada compared to the general Canadian population (2003-2013). AIDS. 2014. August 24;28(13): 1957–1965. [DOI] [PubMed] [Google Scholar]
- 12.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect Dis 2016. July; 16(7):797–808. [DOI] [PubMed] [Google Scholar]
- 13.Sun H-Y, Sheng W-H, Tsai M-S, et al. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: A review. World J Gastroenterol 2014. October 28;20(40):14598–14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015. March 15;60(6):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocroft A, Lundgren JD, Ross M, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: A prospective international cohort study. Lancet HIV. 2016. January;3(1):e23–32. [DOI] [PubMed] [Google Scholar]
- 16.Gange SJ, Kitahata MM, Saag MS, et al. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007. April;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: A cohort study. Ann Intern Med. 2015. October 6;163(7):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane HM, Heckbert SR, Drozd DR, et al. Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. Am J Epidemiol. 2014. April 15;179(8): 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitahata MM, Drozd DR, Crane HM, et al. Ascertainment and verification of end-stage renal disease and end-stage liver disease in the North American AIDS Cohort Collaboration on Research and Design. AIDS Res Treat. 2015;2015:923194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro KG, Ward JW, Slutsker L, et al. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992. December 18; 41(RR17):1–19. [PubMed] [Google Scholar]
- 21.Laaksonen MA, Virtala E, Knekt P, Oja H, Harkanen T. SAS Macros for Calculation of Population Attributable Fraction in a Cohort Study Design. J Stat Softw. 2011. July;43(7):1–25. [Google Scholar]
- 22.Hiller L, Marshall A, Dunn J. Assessing violations of the proportional hazards assumption in Cox regression: does the chosen method matter? Trials. 2015;16(Suppl2):P134.25873248 [Google Scholar]
- 23.Tsimihodimos V, Mitrogianni Z, Elisaf M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc Med J. 2011. ;5:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Althoff KN. The shifting paradigm of care for adults living with HIV: Smoking cessation for longer life. J Infect Dis. 2016. December 1;214(11): 1618–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercié P, Arsandaux J, Katlama C, et al. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): A randomised controlled phase 3 clinical trial. Lancet HIV 2018. March;5(3):e126–35. [DOI] [PubMed] [Google Scholar]
- 26.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann Intern Med 2015. March 3;162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 27.Hessol NA, Weber KM, D’Souza G, et al. Smoking cessation and recidivism in the Women’s Interagency Human Immunodeficiency Virus Study. Am J Prev Med 2014. July;47(1):53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apelberg BJ, Feirman SP, Salazar E, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med. 2018. May;378(18): 1725–1733. [DOI] [PubMed] [Google Scholar]
- 29.Walensky RP, Del Rio C, Armstrong WS. Charting the future of infectious disease: Anticipating and addressing the supply and demand mismatch. Clin Infect Dis. 2017. May;64(10): 1299–1301. [DOI] [PubMed] [Google Scholar]
- 30.Boyd CM, Lucas GM. Patient-centered care for people living with multimorbidity. Curr Opin HIV AIDS. 2014. July 9(4):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh HK, Del Carmen T, Freeman R, Glesby MJ, Siegler EL. From one syndrome to many: Incorporating geriatric consultation into HIV care. Clin Infect Dis. 2017. August 1;65(3):501–506. [DOI] [PubMed] [Google Scholar]
- 32.Guaraldi G, Rockwood K. Geriatric-HIV medicine Is born. Clin Infect Dis. 2017. August 1;65(3):507–509. [DOI] [PubMed] [Google Scholar]
- 33.Rockhill B, Newman B, Weinberg C. Use and Misuse of Population Attributable Fractions. Am J Pub Health 1998. January;88(1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000. August 5; 321(7257): 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter SD. The estimation and interpretation of attributable risk in health research. Biometrics 1976. December; 32(4): 829–849. [PubMed] [Google Scholar]
- 36.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017. March 24;355(6331): 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


