Abstract
l-Cysteine is a well-known sulfur-containing non-essential amino acid that can be oxidized to cysteine, which possesses a variety of pharmacological actions, including antioxidant and anti-inflammatory activities. Traumatic brain injury (TBI) is defined as a closed head injury that leads to temporary alterations in neural function and further leads to pathophysiological processes. In the present study, rats were categorized into sham, control, 100 mg/kg l-cysteine, and 200 mg/kg l-cysteine groups and then the levels of lipid peroxidation, reduced glutathione (GSH), catalase, superoxide dismutase (SOD), reactive oxygen species (ROS), and mRNA and protein expression of microtubule-associated protein 2 (MAP2) were determined. Following supplementation with l-cysteine, there were reductions in lipid peroxidation and ROS levels, whereas catalase, SOD, and GSH levels increased. Additionally, the mRNA expression of MAP2 in the control rats was drastically reduced by 67% compared to the sham rats. However, supplementation with 100 mg/kg of l-cysteine and 200 mg/kg of l-cysteine significantly increased MAP2 mRNA expression by 84.8% and 169.7%, respectively. Similarly, MAP2 protein expression was drastically reduced by 61% in control rats compared to sham rats, but supplementation with 100 mg/kg of l-cysteine and 200 mg/kg of l-cysteine significantly increased MAP2 protein expression by 41% and 94.9%, respectively. Taken together, these data suggest that supplementation with l-cysteine significantly reduced lipid peroxidation and ROS levels, but increased antioxidant levels and the mRNA and protein expression of MAP2 in rats following TBI.
Keywords: l-Cysteine, Traumatic brain injury, Reduced glutathione, Lipid peroxidation, Rats
Introduction
l-Cysteine is a well-known sulfur-containing non-essential amino acid that is oxidized to cysteine (Lee et al. 2013), which exerts antioxidant activities (Lee et al. 2013; Elias et al. 2005; Miura et al. 2014). l-Cysteine is also important for glutathione (GSH), which is a key antioxidant molecule in cellular systems (Fechter et al. 2008); accordingly, Jain et al. (2016) reported that oral supplementation with l-cysteine upregulates circulating levels of GSH. Thioredoxin is important dithiol-disulfide oxidoreductase present in living cells. Thioredoxin reductase, thioredoxin, and NADPH are major components of the thioredoxin system. Thioredoxin system plays key role in NADPH-dependent protein disulfide reductase and DNA synthesis and repair. Reduced thioredoxin is crucial in antioxidant defense and regulation of transcription factors (Arnér and Holmgren 2006). Furthermore, Li et al. (2016) reported that rats treated with N-acetyl-cysteine exhibited reductions in neuropathic pain via the inhibition of matrix metalloproteinases (MMPs), and Eakin et al. (2014) showed that supplementation with N-acetyl-cysteine improves behavioral parameters following brain injury. Additionally, N-acetyl-cysteine exerts antioxidant and anti-inflammatory activities in the cerebral cortex of aspartame-treated rats (Saleh 2015).
Traumatic brain injury (TBI) is defined as a closed head injury that leads to temporary alterations in neural function and further leads to pathophysiological processes (Kim and Han 2017). Although the major symptoms of TBI include depression, headaches, dizziness, vomiting, fatigue, and cognitive slowing (Ruff 2011; D’Hemecourt 2011), the molecular mechanisms underlying the neural dysfunction in TBI have yet to be fully elucidated. TBI is followed by excitotoxic cascade events and oxidative stress, which lead to further neural dysfunction (Bobyn et al. 2002). The accelerated rate of oxidative stress leads to reductions in the levels of GSH and other antioxidant enzymes (Karanth and Jeevaratnam 2005). Microtubule-associated protein 2 (MAP2) is a well-known cytoskeletal protein present in the neuronal perikarya and dendrites (Buddle et al. 2003); several studies have shown that this protein serves as a marker of neuronal damage (Miyazawa et al. 1993; Buddle et al. 2003; Fontaine-Lenoir et al. 2006). Therefore, the present study analyzed the effects of l-cysteine on the levels of MAP2 and antioxidant markers in a rat model of brain injury.
Materials and methods
Rats
For the present study, male albino rats (180–200 g) were purchased from the animal house of The Second Xiangya Hospital of Central South University in Changsha, Hunan, China. The rats were maintained in standard polypropylene cages under standard atmospheric conditions with a relative humidity of 60 ± 5%, a temperature of 25 ± 0.5 °C, and a 12-h light/dark cycle. All animal experiments were conducted under the guidelines of the Experimental Laboratory Committee of the Department of Neurosurgery. The Ethical Committee of the Second Xiangya Hospital of Central South University approved all animal care and experimental protocols (YRRWE2019112). All animal surgeries were conducted under anesthesia, and all efforts were made to reduce animal suffering.
Experimental TBI procedure
Experimental TBI in the rats was induced as described previously (Xiong et al. 2013). Briefly, rats were placed in isoflurane chamber, and rats were anesthetized until non-responsive to tail pinch and paw. Then, rat chest down on scored tin foil and head was positioned directly on the path of a falling weight. If the rats wake-up or move before placing in scored tin foil, repeat the above steps until non-responsive to tail pinch and paw. Then, weight is allowed to fall vertically on the rat head through the plastic guide tube. Then, rats land in the supine position and undergone 180° rotation. Then, rats were removed from the collection sponge, and lidocaine was applied on the head. Time to the right was determined using a digital timer, and then rats were returned to the home cage.
Experimental groups
The rats were categorized into Group I (sham), Group II (control), Group III (100 mg/kg of l-cysteine), and Group IV (200 mg/kg of l-cysteine); normal saline was used to dissolve the l-cysteine, and the control and sham rats were administered saline. In the l-cysteine groups, the drug was administered to the rats for 21 consecutive days via the oral route. Dose-volume was adjusted to 0.5 ml, and the dose was given each day. Each group contained six rats.
Determination of lipid peroxidation levels
Lipid peroxidation levels in the serum were determined by measuring the malondialdehyde (MDA) content via thiobarbituric acid reactive species (TBARS), which is a commonly used index of the lipid peroxidation rate. The final lipid peroxidation product was determined at 534 nm (Jordão et al. 2004).
Determinations of antioxidant markers
Reduced serum GSH levels were measured according to the methods of Ye et al. (2010); serum GSH contents were measured using Ellman’s reaction. The final products were determined from their absorbance at 412 nm. Serum GSH peroxidase (Gpx) levels were measured according to the methods of Baydas et al. (2002), and all Gpx activity was determined by measuring the absorbance at 340 nm. Serum catalase and SOD levels were determined according to the methods of Ye et al. (2010). Briefly, catalase activity was determined following the addition of serum (500 µl), phosphate buffer (500 µl), TiOSO4 (500 µl), and water (500 µl) to the sample tube; the final product of the reaction was determined by measuring the absorbance at 420 nm. The SOD activity was determined following the addition of serum (0.1 ml), phosphate buffer (1.2 ml), nitro blue tetrazolium (0.3 ml), and nicotinamide adenine dinucleotide (NADH; 0.2 ml); the final product of the reaction was measured at 560 nm.
Determination of reactive oxygen species (ROS) levels
Serum ROS levels were determined using nitric oxide assays (Roesslein et al. 2013).
Dihydrorhodamine was used as a substrate for measuring the free radicals, including hydroxyl radicals, nitric oxide, and peroxynitrite. ROS and free radical oxidized dihydrorhodamine to the corresponding fluorescent product, rhodamine. This is assay has some limitation such as dihydrorhodamine conversion is not very specific due to dihydrorhodamine reacts with superoxide, nitric oxide, and other oxidants. Further, the intermediate oxidative product of dihydrorhodamine can increase the fluorescent signal intensity.
Reverse transcription (RT)-polymerase chain reaction (PCR) analysis
First, RNA was extracted from the homogenates of hippocampal tissues. Next, chloroform (0.3 ml) was added to the homogenates, and the mixtures were shaken for 60 s and centrifuged at 10,000 rpm for 15 min at 4 °C. Then, an equal volume of isopropyl alcohol was added to the supernatants, and they were centrifuged at 10,000 rpm for 15 min at 4 °C. The obtained RNA pellets were washed in diethyl pyrocarbonate-treated water, gel electrophoresis was performed to check RNA integrity, and the purity was determined at 260 nm. Then, the RNA was converted into cDNA by adding oligo dT primer (0.5 ng) and total RNA (1 µg) in a thermal cycler for 10 min at 65 °C. Finally, 10 mM dNTPs (2 µl), 5 × RT buffer (4 µl), and reverse transcriptase (100 U) were added to the reaction tubes, and they were incubated in a thermal cycler for 60 min at 37 °C and then for 10 min at 90 °C. The specific primers for MAP2 were as follows: sense, 5′-TGGCATTGACCTCCCTAAAGAG-3′, and anti-sense, 5′-TTGCTTCCGTTGGCATTTCG-3′. PCR was performed by adding forward and reverse primers (1 µl each, 10 pM), PCR master mix (12.5 µl), and cDNA (1 µl) with the final volume adjusted to 25 µl using sterile water. The PCR took place within a thermal cycler (CFX96™; Bio-Rad, Hercules, CA) using GAPDH as the PCR control. The relative level of MAP2 mRNA was measured using the 2−∆∆CT method (Higashikata et al. 2004).
Immunohistochemistry
The dissected brain sections were fixed in formalin and then embedded in paraffin. Subsequently, the sections were deparaffinized, rehydrated with xylene and a graded alcohol series, and H2O2 (0.3%) was used to inhibit endogenous peroxidase activity. Then, bovine serum albumin (BSA, 2%) was used to block non-specific binding sites, and the sections were treated with anti-MAP2 antibodies (1:300 dilutions, ab5392; Abcam, Cambridge, UK) overnight. Next, the homogenates were treated with a goat anti-rat antibody (1:300 dilutions, ab6840; Abcam) for 1 h (Balic et al. 2011) and the samples were analyzed under a confocal microscope to examine MAP2 expression. Relative staining intensity was quantified by normalizing the backgrounds of images depicting similar regions in treated and control brain tissue.
Statistical analyses
All data are presented as a mean and a standard deviation (SD). An analysis of variance (ANOVA) and Tukey’s post hoc tests was applied to assess the data; P values < 0.05 were considered to indicate statistical significance.
Results
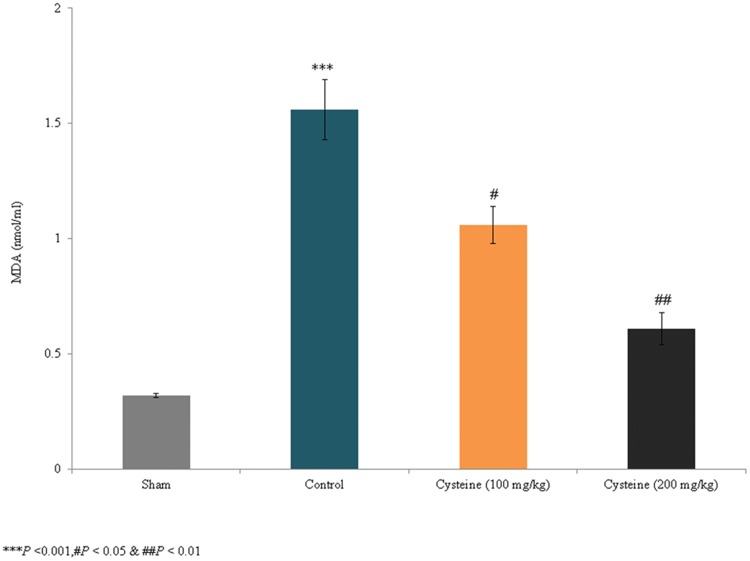
The present study assessed the effects of l-cysteine on the levels of MAP2 and antioxidant markers in a rat model of experimental brain injury. TBARS levels have been extensively used to determine lipid oxidation through the MDA product of membrane polyunsaturated fatty acids. In the present study, lipid peroxidation substantially increased by 387.5% in the control rats (P < 0.001; Fig. 1) but decreased by 32% and 60.1% in the 100 mg/kg and 200 mg/kg l-cysteine groups, respectively (P < 0.05; Fig. 1). Thus, the treatment of rats with 200 mg/kg of l-cysteine following brain injury reduced lipid peroxidation 60.9%.
Fig. 1.
Effects of l-cysteine on serum lipid peroxidation levels in a rat model of TBI. The MDA content is expressed as nmol/ml. All experimental data are presented as a mean and a standard deviation (n = 6); all statistical analyses were performed with an ANOVA. ***P < 0.001 compared to the sham rats; ##P < 0.01 and #P < 0.05 compared to the control rats (TBI)
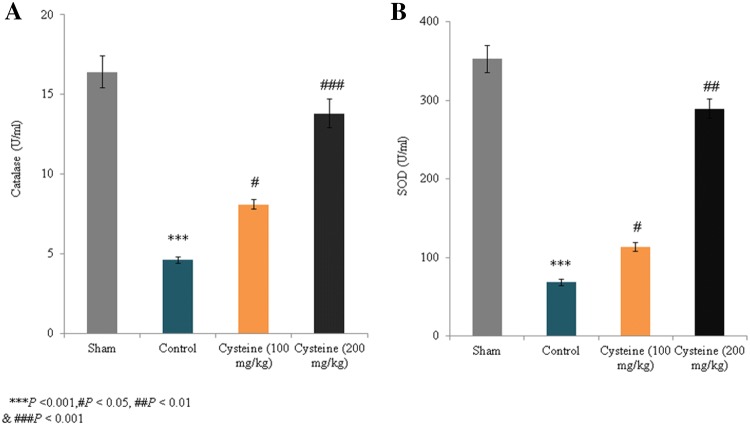
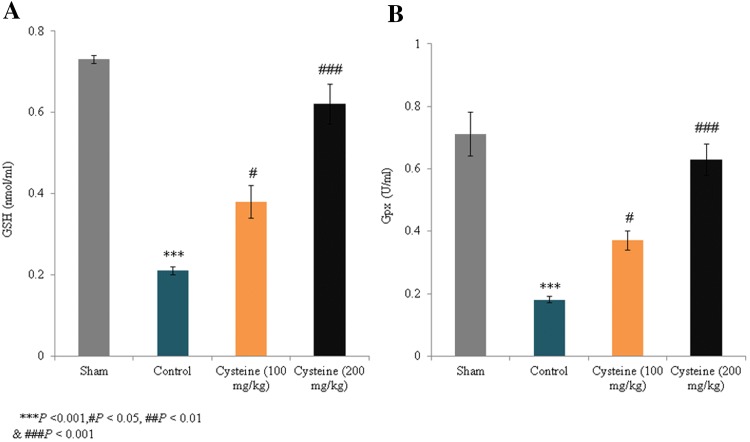
The activity levels of catalase and SOD in the control group decreased by 76.1% and 80.6%, respectively, compared to the sham rats (P < 0.001; Fig. 2). However, in the 100 mg/kg and 200 mg/kg l-cysteine groups, catalase activity increased by 76.1% and 200%, respectively (P < 0.05; Fig. 2a), while SOD activity increased by 65.9% and 323.6%, respectively (P < 0.05; Fig. 2b). Although the GSH and Gpx levels in the control group decreased by 71.2% and 74.6%, respectively, compared to the sham rats (P < 0.001; Fig. 3), the GSH levels in the 100 mg/kg and 200 mg/kg l-cysteine groups increased by 80.9% and 195.2%, respectively (P < 0.05; Fig. 3a), and Gpx activity increased by 47.9% and 250%, respectively (P < 0.05; Fig. 3b). Thus, the treatment of TBI rats with 200 mg/kg of l-cysteine maintained the catalase, SOD, GSH, and Gpx levels close to those observed in sham rats.
Fig. 2.
Effects of l-cysteine on serum catalase and SOD activity in a rat model of TBI. Catalase and SOD activity is expressed as U/ml. All experimental data are presented as a mean and a standard deviation (n = 6); all statistical analyses were performed with an ANOVA. ***P < 0.001 compared to the sham rats. ###P < 0.001, ##P < 0.01, and #P < 0.05 compared to the control rats (TBI)
Fig. 3.
Effects of l-cysteine on the serum GSH and Gpx levels in a rat model of TBI. GSH contents are expressed as nmol/ml and Gpx activities are expressed as U/ml. All experimental data are presented as a mean and a standard deviation (n = 6); all statistical analyses were performed with an ANOVA. ***P < 0.001 compared to the sham rats; #P < 0.05 and ###P < 0.001 compared to the control rats (TBI)
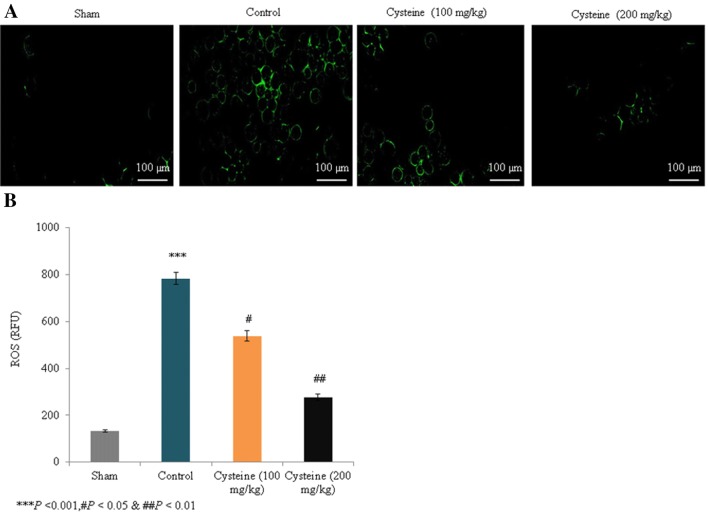
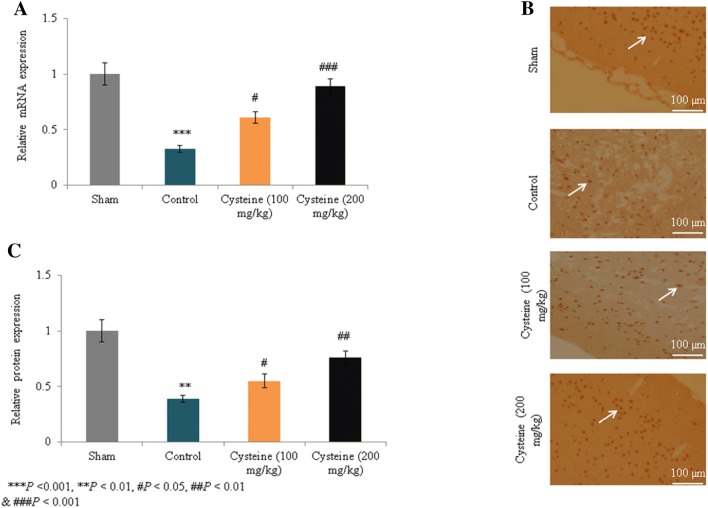
In the control rats, ROS levels increased substantially by 491% compared to the sham rats (P < 0.001; Fig. 4); Figure 4a shows fluorescence images of the ROS levels. However, supplementation with 100 mg/kg and 200 mg/kg of l-cysteine significantly reduced ROS levels by 31.3% and 64.6%, respectively (P < 0.05; Fig. 4b). The mRNA expression of MAP2 in the control rats was drastically reduced by 67% compared to the sham rats (P < 0.001; Fig. 5a), but supplementation with 100 mg/kg and 200 mg/kg of l-cysteine significantly increased the mRNA expression levels by 84.8% and 169.7%, respectively (P < 0.05; Fig. 5a). Figure 5b shows immunohistochemical images of the MAP2 expression levels. In the control rats, protein expression of MAP2 was drastically reduced by 61% compared to the sham rats (P < 0.001; Fig. 5c), but supplementation with 100 mg/kg and 200 mg/kg of l-cysteine significantly increased MAP2 protein expression by 41% and 94.9%, respectively (P < 0.05; Fig. 5c).
Fig. 4.
Effects of l-cysteine on serum ROS in a rat model of TBI. ROS levels are expressed as relative fluorescence units (RFU), all experimental data are presented as a mean and a standard deviation (n = 6). All statistical analyses were performed with an ANOVA. ***P < 0.001 compared to the sham rats; #P < 0.05 and ##P < 0.01 compared to the control rats (TBI). Scale bar: 100 µm. (Magnification, × 20)
Fig. 5.
Effects of l-cysteine on the mRNA and protein expression levels of (MAP2) in a rat model of TBI. a Relative mRNA expression of MAP2. b Immunohistochemical images of MAP2. c Relative protein expression of MAP2. All experimental data are presented as a mean and a standard deviation (n = 6); all statistical analyses were performed with an ANOVA. ***P < 0.001 compared to the sham rats; ###P < 0.001, ##P < 0.01, and #P < 0.05 compared to the control rats (TBI). Scale bar: 100 µm. (Magnification, × 20)
Discussion
The present study investigated the effects of l-cysteine on the levels of MAP2 and antioxidant markers in a rat model of experimental brain injury. TBI is a closed head injury that leads to temporary alterations in neural function and pathophysiological processes (Kim and Han 2017). Although the major symptoms of TBI include depression, headaches, dizziness, vomiting, fatigue, and cognitive slowing (Ruff 2011; D’Hemecourt 2011), the molecular mechanisms underlying the dysfunction in TBI have yet to be fully elucidated. Additionally, TBI is followed by excitotoxic cascade events and oxidative stress that can lead to additional neural dysfunction (Bobyn et al. 2002).
Saravanan et al. (1995) reported that l-cysteine attenuated lipid peroxidation in urolithiatic rats. Researchers have reported that the inhibition of protein disulfide isomerase through lipid peroxidation induces cysteine modification (Carbone et al. 2005). In the present study, supplementation with cysteine (200 mg/kg) significantly reduced lipid peroxidation levels to levels close to those observed under normal conditions. Higher levels of oxidative stress lead to reductions in cellular levels of GSH and other antioxidant enzymes (Karanth and Jeevaratnam 2005). Researchers have demonstrated the antioxidant activities of l-cysteine (Lee et al. 2013; Elias et al. 2005; Miura et al. 2014). l-Cysteine is an important component of GSH, which is a critical antioxidant molecule in cellular systems (Fechter et al. 2008). Jain et al. (2016) reported that oral supplementation with l-cysteine upregulated circulating levels of GSH. Furthermore, Li et al. (2016) reported that rats treated with N-acetyl-cysteine exhibited reductions in neuropathic pain due to the inhibition of MMPs, and Eakin et al. (2014) showed that N-acetyl-cysteine improved behavioral parameters following brain injury. Researchers have reported the excess of dietary l-cysteine is harmful than methionine to certain species (Dilger et al. 2007). However, we did not observe any side effects except mild weight loss during l-cysteine treatment in this present study.
Hoffer et al. (2017) have reported that the repositioning drugs for traumatic brain injury-N-acetyl cysteine and Phenserine. Bavarsad Shahripour et al. (2014) have reported that the therapeutic potential of N-acetylcysteine in neurological disorder recovery. Hasegawa et al. (2012) demonstrated the anti-inflammatory activities of cysteine in human endothelial cells, and other researchers have reported that N-acetyl-cysteine induced antioxidant and anti-inflammatory activities in the cerebral cortex of aspartame-treated rats (Saleh 2015). MAP2 is a well-known cytoskeletal protein that is present in neuronal perikarya and dendrites (Buddle et al. 2003) and serves as a marker for neuronal damage (Miyazawa et al. 1993; Buddle et al. 2003; Fontaine-Lenoir et al. 2006). Landino et al. (2004) reported that cysteine oxidates tau and MAP2 and found that changes in the redox statuses of several proteins could play crucial roles in the pathophysiology of various neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. In the present study, supplementation with cysteine increased the mRNA and protein expression of MAP2, indicating that l-cysteine was protective against brain injury.
Conclusion
Taken together, these data indicate that supplementation with l-cysteine exerted a protective effect against TBI through the increased antioxidant levels, and the increased mRNA and protein expression of MAP2.
Limitation of the study
Limitation of the present study lack of knock-down/overexpression of MAP2 and western-blotting assays.
Acknowledgement
This study was supported by National Natural Sciences Foundation of China (Grant No.81801721).
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Arnér ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16(6):420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Balic M, Rapp N, Stanzer S, Lin H, Strutz J, Szkandera J, Daidone MG, Samonigg H, Cote RJ, Dandachi N. Novel immunofluorescence protocol for multimarker assessment of putative disseminating breast cancer stem cells. Appl Immunohistochem Mol Morphol. 2011;19:33–40. doi: 10.1097/PAI.0b013e3181ebf4e8. [DOI] [PubMed] [Google Scholar]
- Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4(2):108–122. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydas G, Gursu MF, Yilmaz S, Canpolat S, Yasar A, Cikim G, Canatan H. Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci Lett. 2002;323:195–198. doi: 10.1016/S0304-3940(02)00144-1. [DOI] [PubMed] [Google Scholar]
- Bobyn PJ, Franklin JL, Wall CM, Thornhill JA, Juurlink BH, Paterson PG. The effects of dietary sulfur amino acid deficiency on rat brain glutathione concentration and neural damage in global, hemispheric hypoxia-ischemia. Nutr Neurosci. 2002;5(6):407–416. doi: 10.1080/1028415021000055952. [DOI] [PubMed] [Google Scholar]
- Buddle M, Eberhardt E, Ciminello LH, Levin T, Wing R, DiPasquale K, Raley-Susman KM. Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res. 2003;978(1–2):38–50. doi: 10.1016/S0006-8993(03)02758-6. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol. 2005;18(8):1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- d’Hemecourt P. Subacute symptoms of a sports-related concussion: outpatient management and return to play. Clin Sports Med. 2011;30:63–72. doi: 10.1016/j.csm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Toue S, Kimura T, Sakai R, Baker DH. Excess dietary l-cysteine, but not l-cystine, is lethal for chicks but not for rats or pigs. J Nutr. 2007;137(2):331–338. doi: 10.1093/jn/137.2.331. [DOI] [PubMed] [Google Scholar]
- Eakin K, Baratz-Goldstein R, Pick CG, Zindel O, Balaban CD, Hoffer ME, Lockwood M, Miller J, Hoffer BJ. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617. doi: 10.1371/journal.pone.0090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias RJ, McClements DJ, Decker EA. Antioxidant activity of cysteine, tryptophan, and methionine residues in continuous phase beta-lactoglobulin in oil-in-water emulsions. J Agric Food Chem. 2005;53(26):10248–10253. doi: 10.1021/jf0521698. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Nelson-Miller A, Gearhart C. Depletion of liver glutathione levels in rats: a potential confound of nose-only inhalation. Inhal Toxicol. 2008;20(9):885–890. doi: 10.1080/08958370801975329. [DOI] [PubMed] [Google Scholar]
- Fontaine-Lenoir V, Chambraud B, Fellous A, David S, Duchossoy Y, Baulieu EE, Robel P. Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc Natl Acad Sci USA. 2006;103(12):4711–4716. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Ichiyama T, Sonaka I, Ohsaki A, Okada S, Wakiguchi H, Kudo K, Kittaka S, Hara M, Furukawa S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin Exp Immunol. 2012;167(2):269–274. doi: 10.1111/j.1365-2249.2011.04519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashikata T, Yamagishi M, Sasaki H, Minatoya K, Ogino H, Ishibashi-Ueda H, Hao H, Nagaya N, Tomoike H, Sakamoto A. Application of real-time RT-PCR to quantifying gene expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human abdominal aortic aneurysm. Atherosclerosis. 2004;177:353–360. doi: 10.1016/j.atherosclerosis.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Pick CG, Hoffer ME, Becker RE, Chiang YH, Greig NH. Repositioning drugs for traumatic brain injury-N-acetyl cysteine and Phenserine. J Biomed Sci 9. 2017;24(1):71. doi: 10.1186/s12929-017-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Kanikarla-Marie P, Warden C, Micinski D. l-cysteine supplementation upregulates glutathione (GSH) and vitamin D binding protein (VDBP) in hepatocytes cultured in high glucose and in vivo in the liver, and increases blood levels of GSH, VDBP, and 25-hydroxy-vitamin D in Zucker diabetic fatty rats. Mol Nutr Food Res. 2016;60(5):1090–1098. doi: 10.1002/mnfr.201500667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordão AA, Chiarello PG, Arantes MR, Meirelles MS, Vannucchi H. Effect of an acute dose of ethanol on lipid peroxidation in rats: the action of vitamin E. Food Chem Toxicol. 2004;42:459–464. doi: 10.1016/j.fct.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Karanth J, Jeevaratnam K. Oxidative stress and antioxidant status in rat blood, liver and muscle: effect of dietary lipid, carnitine, and exercise. Int J Vitam Nutr Res. 2005;75(5):333–339. doi: 10.1024/0300-9831.75.5.333. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Han SJ. A simple rat model of mild traumatic brain injury: a device for reproducing anatomical and neurological changes of mild traumatic brain injury. PeerJ. 2017;5:e2818. doi: 10.7717/peerj.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landino LM, Skreslet TE, Alston JA. Cysteine oxidation of tau and microtubule-associated protein-2 by peroxynitrite: modulation of microtubule assembly kinetics by the thioredoxin reductase system. J Biol Chem. 2004;279(33):35101–35105. doi: 10.1074/jbc.M405471200. [DOI] [PubMed] [Google Scholar]
- Lee S, Han KH, Nakamura Y, Kawakami S, Shimada K, Hayakawa T, Onoue H, Fukushima M. Dietary l-cysteine improves the antioxidative potential and lipid metabolism in rats fed a normal diet. Biosci Biotechnol Biochem. 2013;77(7):1430–1434. doi: 10.1271/bbb.130083. [DOI] [PubMed] [Google Scholar]
- Li J, Xu L, Deng X, Jiang C, Pan C, Chen L, Han Y, Dai W, Hu L, Zhang G, Cheng Z, Liu W. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain. 2016;157(8):1711–1723. doi: 10.1097/j.pain.0000000000000575. [DOI] [PubMed] [Google Scholar]
- Miura Y, Honda S, Masuda A, Masuda T. Antioxidant activities of cysteine derivatives against lipid oxidation in anhydrous media. Biosci Biotechnol Biochem. 2014;78(8):1452–1455. doi: 10.1080/09168451.2014.918496. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Bonnekoh P, Hossman KA. Temperature effect on immunostaining of microtubule-associated protein 2 and synaptophysin after 30 minutes of forebrain ischemia in rat, Acta. Neuropathol. 1993;85:526–532. doi: 10.1007/BF00230493. [DOI] [PubMed] [Google Scholar]
- Roesslein M, Hirsch C, Kaiser JP, Krug HF, Wick P. Comparability of in vitro tests for bioactive nanoparticles: a common assay to detect reactive oxygen species as an example. Int J Mol Sci. 2013;14(12):24320–24337. doi: 10.3390/ijms141224320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM. Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation. 2011;28:167–180. doi: 10.3233/NRE-2011-0646. [DOI] [PubMed] [Google Scholar]
- Saleh AAS. Anti-neuroinflammatory and antioxidant effects of N-acetyl cysteine in long-term consumption of artificial sweetener aspartame in the rat cerebral cortex. J Basic Appl Zool. 2015;72:73–80. doi: 10.1016/j.jobaz.2015.05.001. [DOI] [Google Scholar]
- Saravanan N, Senthil D, Varalakshmi P. Effect of L-cysteine on lipid peroxidation in experimental urolithiatic rats. Pharmacol Res. 1995;32(3):165–169. doi: 10.1016/S1043-6618(05)80010-6. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Dong J, Han B. Protective effect of reduced glutathione and venous systemic oxygen persufflation on rat steatotic graft following liver transplantation. J Surg Res. 2010;158:138–146. doi: 10.1016/j.jss.2009.01.002. [DOI] [PubMed] [Google Scholar]