Abstract
Purpose
The study examined the relationships among pain, pain coping and sleep and assessed factors (age, gender, frequency and intensity of pain) that affect pain, coping, and sleep in children with sickle cell disease (SCD).
Methods
Participants (66) were 39 children (M = 11.5 years) and 27 adolescents (M = 15.5 years) with SCD who completed an electronic visual analog scale (eVAS), Pain Coping Questionnaire, and Pittsburg Sleep Quality Index.
Results
About two thirds of the children reported pain the previous month. No significant differences were found between pain and age, gender, pain intensity or frequency. Most children coped with pain by seeking information, problem solving, seeking social support, and positive self-statements. There were significant negative correlations in males between worse pain severity and behavioral distraction and internalizing or catastrophizing. The majority (91.2%) had mild to severe sleep disturbances, with 18.2% requiring sleeping medication three or more times a week. There were no significant differences between sleep and age, gender, pain intensity or frequency.
Conclusion
Children with SCD experience pain that affects sleep patterns and the way they cope with pain. Nurses need to concurrently assess pain, coping and sleep and promote sleep hygiene and positive coping strategies during pain episodes.
Keywords: children, sickle cell disease, pain, coping, sleep
Introduction
One of the major characteristics of sickle cell disease (SCD), a genetic blood disorder which affects one out of five African American children born in the United States, are episodic exacerbations of pain (Benjamin, 2008, CDC, 2011). Children with SCD pain frequently experience sleep disturbances and daytime sleepiness, putting them at risk for a decreased quality of life (Long, Krishnamurthy & Palermo, 2008; Smaldone, Honig & Byrne, 2007). Adolescents also experience less desirable health outcomes, such as depression and physical impairment, if they use ineffective coping strategies during the pain experience and do not get satisfactory sleep (Lynch, Kashikar-Zuck, Goldschneider & Jones, 2007; Rogers, et al., 2011). Unfortunately, sleep disruptions are predictive of severe pain the next day and intense pain during the day is predictive of sleep disturbances during the night (Valrie, Redding-Lallinger & Daeschner, 2007). A bi-directional theory to explain this co-existing relationship between pain and sleep was proposed by Lewin and Dahl (1999). An explanation for this relationship between sleep and pain may be the result of shared neurotransmitter pathways (Pieh, Popp, Geisler, & Hajak, 2011). Pieh and colleagues further posited that sleep deprivation reduces the pain threshold, possibly by negatively influencing opioid protein synthesis and opioid receptors. However, it is not clear what factors have mediating effects on pain exacerbations, coping ability, and disruptive sleep.
The specific aims of this study were: 1) to examine relationships among pain, pain coping, and sleep, and 2) to assess factors such as age, gender, frequency and severity of pain episodes that may have significant effects on pain, pain coping, and sleep in children and adolescents with SCD. It was hypothesized that more pain is associated with more negative emotion-based coping skills and more problems with sleep.
Background and Significance
The pathogenesis of a painful sickle cell crisis is complex but some identified risk factors are the type and severity of SCD, sickle cell anemia, dehydration, infection or hypoxemia related to upper airway obstruction (Anie & Green, 2012; Hargrave, Wade, Evans, Hewes, & Kirkham, 2003). Some children and adolescents with SCD may experience over ten painful episodes a year, although not all require hospitalization. However, in two studies, 30% of all children with SCD pain required at least one hospitalization a year (Jacob, et al., 2012; Unal, Toros, Kutuk, & Uyaniker, 2011). A common complication of SCD is acute chest syndrome, which leads to hypoxia, which is a lowering of oxygen in the blood. Hypoxia causes excruciating pain and can lead to the cellular death of bones, tissues and organs, thus presenting a life-threatening sickle cell crisis. Unfortunately, children and adolescents frequently wait until pain is severe and intolerable before seeking medical treatment (Sanders, Labott, Molokie, Shelby & Desimone, 2010). This delay frequently necessitates emergency department visits or preventable hospitalizations, resulting in a painful personal and financial burden (Boulet, Yanni, Creary, & Olney, 2010).
Hargrave, Wade, Evans, Hewes & Kirham (2003) found that during sleep, there was a highly significant association between hypoxemia and a high rate of painful sickle cell related episodes in children. Kirkham and Datta (2001) focused on sleep disordered breathing, such as obstructive sleep apnea, because it has been linked to increased pain. However, no correlation was found in another study between the apnea-hypopnea index and a painful sickle cell crisis during sleep (Salles, et al., 2009). In yet another study, restless leg syndrome or periodic limb movements were found to be the cause of sleep disruption in 12.5% of children with SCD (Rogers, et al., 2011). Evidence suggests that pain causation in youth with SCD is multi-factorial and is responsible for disruptions in sleep.
Studies of pain related to gender and age differences suggest that girls experience more restrictions in their life from pain than boys. Girls reported more diminished quality of life on most functioning scales related to pain (Dampier et al., 2010; Roth-Isigkeit, Thyen, Stoven, Swartzenberger, & Schmucker, 2005). More chronic pain with greater intensity was reported for girls (4–18 years) than boys (Perquin, et al., 2000). Lynch, Kashikar-Zuck, Goldschneider, & Jones (2007) suggested that greater pain intensity (also chronicity and multiple pain locations) in girls 8–12 years of age may be the result of the interactive role that exists between pain and biological (hormonal) and psychosocial influences.
The intensity of pain but not the duration and frequency of pain predicted restrictions in daily activities among healthy children and adolescents (Roth-Isigkeit, Thyen, Stoven, Swartzenberger & Schmucker, 2005). Further, the intensity of pain and duration was predictive of health care utilization; however, the frequency of pain had no effect. Pain intensity was handled by children with SCD with distraction strategies, such as engaging in TV or the movies, thus experiencing less pain (Reid, Gilbert & McGrath, 1998). These children reported feeling less distressed and disabled emotionally.
While positive distraction strategies were reported to lessened pain intensity, negative thinking was found to compromise an adolescent’s ability to cope with pain (Barakat, Schwartz, Simon & Radcliffe, 2007). Fletcher (2000) also found that the more negative thoughts during a pain crises, the poorer the coping strategies and adjustment to painful episodes. Conversely, positive patient coping with pain correlated with positive family functioning and less health care utilization (Mitchell, Lemanek, Palermo, Crosby, Nichols & Powers, 2007). When comparing coping strategies between children with parental assistance and those without this assistance, children were found to develop their own coping strategies with less parental involvement, which included massage, prayer and positive thinking (Oliver-Carpenter, Barach, Crosby, Vanenzuela, & Mitchell, 2011).
Coping strategies were significantly found to be related to gender differences, with girls preferring social support to cope with pain or sometimes emotion-focused avoidance with ruminations, such as freely expressing negative emotions (Thomsen, Compas. Colletti, Stanger, Boyer & Konik, 2002). Boys tended to engage in behavioral distraction techniques, such as watching sports on television, playing electronic games, and other more enjoyable activities (Lynch, Kashikar-Zuck, Goldschneider & Jones, 2007). The Lynch study also found a significant negative correlation for girls between pain coping efficacy and internalizing/catastrophizing, which refers to a lack of effort to regulate emotions and feelings when in pain. Internalizing/catastrophizing has been linked to more pain, poorer adjustment and delayed physical recovery (Reid, Gilbert & McGrath, 1998). No significant correlations were found for pain intensity or frequency and coping strategies among the girls; however, a positive relationship for boys was found between average pain intensity and information seeking to attain pain relief (Lynch et al., 2007).
An age comparison in coping strategies between children (8–12 years of age) and adolescents (13–18 years of age) were made from participants (n = 272) in a pediatric chronic pain clinic (Lynch et al., 2007). Further, age played a role in the manner in which patients coped with pain, with adolescents more likely to pray and hope, while children tended to ignore the pain or alleviate it with heat or cold. Roth-Isigkeit and colleagues (2005) also found that restrictions of daily living, self-reported medication use and doctor’s visits increased with the age of children over 10 years, which supports the finding that pain increases as children age (LeResche, Manel, Drangsholt, Saunders & Korff, 2005). Surprisingly, although comfortable with online resources, few adolescents searched the Internet for information related to coping with pain during the experience (Henderson, Keogh, & Eccleston, 2013).
A comparison of coping strategy stability over an 18 month period of time was made between young children, adolescents and adults by Gil and colleagues (1997), who found that while adults were stable in coping styles, responses of youth with SCD were variable. Adolescents scored higher than young children in negative thinking and illness focused strategies. One explanation is that younger children still hope their pain issues will improve but adolescents become more realistic about the course of the disease and nature of painful episodes (Reid, Gilbert & McGrath, 1998). These findings among children and adolescents speak to the need for early assessments and interventions before maladaptive coping becomes ingrained.
Palermo and Kiska (2005) found that sleep disturbances in adolescents with SCD were associated with a lower health related quality of life (HRQOL) and functional disability. They further determined that in adolescents with chronic pain, there is a relationship between mood and sleep. Valrie, Gil, Redding-Lallinger and Daeschner (2007) also found complex interrelationships among sleep, pain and mood in children and adolescents with SCD with daytime dysfunction from lack of sleep greater in younger than in older children.
Pain and complications of SCD may put children at risk for psychological disorders, especially depression and anxiety (Benton, Boyd, Ifeagwu, Feldtmose, & Smith-Whitley, 2011, Simon, Barakat, Patterson & Dampier, 2009). In a study by Jerrell, Tripathi & McIntyre. 2011), 48% of 2,194 children and adolescents with SCD were diagnosed with a depressive disorder. This cohort reported more pain and complications which caused organ damage. Children who experienced over 10 pain episodes a year reported higher levels of depression (Unal, Toros, Kutuk, & Uyaniker, 2011). Similar to children, adolescents who experienced more frequent pain episodes, frequency and intensity had increased symptoms of depression as well as anxiety (Barakat, Swartz, Simon & Radcliffe, 2007; Gil, et al., 2003).
While pain is a major recurring event in SCD, there is conflicting information about pain and pain coping strategies and how pain is associated with sleep disturbances in children and adolescents with SCD. Understanding the relationships among pain, pain coping and sleep and contributing factors of age, gender, frequency and intensity of pain could help health care professionals plan care that meets the physical and psychosocial needs of children and adolescents with SCD.
Methods
Design
A cross sectional design was used to collect data from children and adolescents with SCD who completed questionnaires that included measures related to pain, pain coping, and sleep.
Setting and Sample
Children and adolescents with SCD were recruited from the population served by the Sickle Cell Disease Foundation of California (SCDFC) who assisted with the distribution and mailing of study flyers. Participants were eligible if they were a) between 10 and 17 years, b) spoke English and c) were able to use a computer. They were excluded if they had physical or cognitive impairments that precluded completion of the study procedures as determined by parental information given the special projects coordinator
Procedures
After approval was received from the Institutional Review Board of the University of California at Los Angeles, children and adolescents were scheduled by the special projects coordinator at the Sickle Cell Disease Foundation (SCDFC) for information sessions. After consenting procedures, they completed questionnaires related to pain, pain coping, and sleep.
Measures
Parents completed a questionnaire using a paper and pen with items related to demographics (age, gender, and ethnicity) and medical information: hemoglobin genotype, and history of SCD related events (acute chest syndrome, splenic sequestrations, iron overload, stroke, avascular necrosis, priapism, leg ulcers, asthma, cholecystectomy, pulmonary hypertension and other). Parents were also asked about the number of acute pain episodes the child had that required hospitalization during the past year.
Pain intensity
Pain intensity was measured using an electronic version of the visual analog scale (eVAS; Jacob, et al., 2012). Patients used the computer cursor to mark on the eVAS to indicate their overall pain the previous month. A 0 to 10 score was generated and imported into an Excel file stored in a server that was purchased specifically for the study (Jacob, et al., 2012). Inter-rater agreement among 30 patients with SCD who participated in the usability testing indicated 80 to 100% between the paper VAS and eVAS versions in their rating of three SCD pain case scenarios (Jacob, et al., 2012). The VAS was previously validated for hospitalized and healthy children 8 to 17 years; convergent validity ranged from r= 0.68 to 0.97, p<0.001, and test-retest reliability was r=0.91 (Savedra, Holzemer, Tesler & Wilkie, 1993).
Pediatric Pain Coping Questionnaire (PCQ)
Pain coping was measured using the Pediatric Pain Coping Questionnaire (Reid, Gilbert & McGrath, 1998; Thastum, Herlin & Zachariae, 2005). The PCQ is a self-report instrument that consisted of 39 statements related to pain coping strategies in children and adolescents. They used each of 39 statements to respond to the prompt, “When I am hurting or in pain for a few hours or days, I….” Reid and colleagues (1998) identified the eight subscales using factor analysis, and found the three higher-order pain coping categories (approach, problem-focused avoidance, emotion-focused avoidance). The approach coping strategy comprised of items from three subscales (information seeking, problem solving, seeking social support) and measures direct attempts to deal with the pain and the use of active methods to regulate feelings when in pain. Examples of statements from the approach coping strategy are 1) .....I ask a nurse or doctor questions (information seeking subscale); 2) …I think about what needs to be done to make things better (problem solving subscale); and 3) …I talk to a friend about how I feel (seeking social support subscale). The problem-focused avoidance coping strategy comprised of items from three subscales (positive self-statements, behavioral distraction, cognitive distraction) and measures attempts to disengage from the pain. Examples of statements from the problem-focused coping strategy are 1)…I say to myself to be strong (positive self-statements; 2) …I go and play (behavioral distraction); and 3) …I try to forget it (cognitive distraction). The emotion-focused avoidance coping strategy is comprised of the externalizing and internalizing/catastrophizing subscales and measures strategies in which emotions are freely expressed and strategies that reflect a lack of effort to regulate feelings when in pain. Examples of items are ….I say mean things to people (externalizing) and ….I worry that I will always be in pain (internalizing/catastrophizing). Respondents selected one of five response options (1 = never, 2 = hardly ever, 3 = sometimes, 4 = often, 5 = very often) indicating the degree to which they experience each statement. Higher scores indicate greater use of the coping strategy.
The PCQ was validated in a healthy sample of children in Grades 3 to 12 and has been administered to children age 8 years or older with recurrent pain, such as headaches and arthritis (Reid, Gilbert & McGrath, 1998; Thastum, et al, 2005). Reid and colleagues (1998) reported convergent validity data with significant correlations between positive coping strategies and the related constructs of pain controllability (r=0.57, p<0.001) and coping effectiveness (r=0.37, P<0.001). Emotion-focused avoidance was negatively correlated with pain controllability (r=−0.30, p<0.001) and coping effectiveness (r=−0.52, p<0.001). Discriminant validity was supported with negative correlations between unrelated constructs of pain intensity (r=−0.19, p<0.01) and distress (r=−0.33, P<0.001). Emotion-focused avoidance was positively related to pain intensity (r=0.21, p<0.01) and distress (r=0.57, P<0.001). Internal consistency reliability indicated Chronbach’s alpha of 0.79 – 0.94 (Reid, et al., 1994). Reid and colleagues estimated the reading level to be Grade 3 (Flesch-Kincaid index), comparable to the youngest children for whom the measure was designed. On average the items were 6.3 words long and the average word was 1.28 syllables, which were analyzed using the Grammatik computer program (Novell, 1994, Orem, UT, USA). For the sample in the current study, internal consistency reliability was supported for positive approach (alpha=0.907), problem-focused avoidance (alpha=0.884), and emotion-focused avoidance (alpha=0.887). The children and adolescents were able to complete the PSQI in 10 minutes.
Pittsburg Sleep Quality Index (PSQI)
The PSQI is a self-report measure with 19 items related to sleep quality and sleep disturbance the previous month (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989; Carpenter & Andrykowski, 1998). Sleep quality and sleep disturbance were quantified based on the responses to the questions and the scoring instructions provided with the PSQI (Buysse, et al, 1989). There were seven subscales: 1) sleep quality was scored based on the question “How would you rate your sleep quality overall” (responses were “very good, fairly good, fairly bad, very bad”); 2) sleep latency was scored based on a combination of responses to “how long has it usually taken you to sleep at night” (response was in minutes), and “how often have you had trouble sleeping because you….” (responses were “not during the month, less than once a week, once or twice a week, three or more times a week”) for each of corresponding reasons; 3) sleep duration was scored based on response to “how many hours of actual sleep did you get at night” (response was in hours); 4) sleep disturbance was scored based on a combination of responses related to the number of reasons, and “how often have you had trouble sleeping because” of the corresponding reasons; 5) sleep efficiency was scored following specified instructions on calculating percentage based on responses to “what time you usually went to bed at night”(clock time), “how long it usually take you to fall asleep at night” (minutes), and “how many hours did you usually get at night (hours).” 6) day dysfunction due to sleepiness was scored based on a combination of responses to “how often have you had trouble staying awake while eating meals, or participating in social activity” (responses were “not during the month, less than once a week, once or twice a week, three or more times a week”) and “how much of a problem has it been for you to do the things you normally do” (responses were “no problem at all to a very big problem”); and 7) use of medications was scored based on response to “how often have you taken medicine to help you sleep” (responses were “not during the month, less than once a week, once or twice a week, three or more times a week”). Each component of the subscale items is scored from 0 to 3, with 3 having the highest difficulty. A global PSQI score of specific components is calculated that ranges from 0 to 21, with higher scores indicating a lower quality of sleep. A PSQI score above 5 indicates poor sleep or evidence of sleep disturbance.
Carpenter and colleagues (1998) reported evidence of convergent and discriminant validity. PSQI global scores were moderately to highly correlated with sleep problems, r=0.65 to 0.74, p<0.001, which support convergent validity. PSQI global scores were poorly correlated with unrelated constructs, such as mood symptoms and depression (r=0.22 – 0.65), nausea (r=0.17 to 0.37, p<0.05), and vomiting (r=0.08 to 0.26), which support discriminant validity. Evidence of internal consistency reliability was supported with Cronbach’s alpha coefficients of 0.80 for the global PSQI and its components; range 0.70 to 0.78 for the sleep disturbance component. The component that was most highly correlated with the PSQI global scores was subjective sleep quality (r=0.79 to 0.83, p<0.001). The test-retest reliability was 0.87 for the PSQI global score (Backhaus, Junghanns, Broocks, Riemann & Hohagen, 2002) For the current study a group of experts (nurse, physician, advanced practice registered nurse, research associate with doctorate in psychology) provided support for face validity, readability, and age appropriateness of the items for children and adolescents 10 – 17 years. For the sample in the current study, internal consistency reliability indicated Chronbach’s alpha of 0.729 and 0.803 based on standardized items. The components that was most highly correlated with the PSQI global scores were sleep disturbance (r=0.728, p<0.0001) and day dysfunction due to sleepiness (r=0.735, p<0.0001). The global PSQI Scores above 5 resulted in a sensitivity of 98.7% and specificity of 84.4% to persons with sleep disturbances when compared to controls (Backhaus, et al., 2002). The children and adolescents were able to complete the PSQI in 5 to 10 minutes.
Data Analyses
The Statistical Package for Social Sciences (SPSS version 20.0, Armonk, NY) was used during data entry and data analyses. To describe demographics, pain (eVAS), pain coping (PCQ) and sleep (PSQI), descriptive statistics (frequencies, means, standard deviations) were used. Pearson correlations were used to examine the relationships among pain, pain coping, and sleep scores. Bivariate and multivariate analyses were used to examine factors (age, gender, number and severity of pain episodes) that may have effects on pain, pain coping, and sleep.
Results
Demographics
Data were collected from 66 participants, 39 children (mean age 11.9 ± 1.1years) and 27 adolescents (mean 15.5 ± 0.9 years). There were 31 (47%) boys and 35 (53%) girls (Table 1). The hemoglobin phenotype was predominantly Hgb SS (n=31, 47%) or Hgb SC (n=16, 24.2%); others were unknown (n=19, 28.8%). The majority (n=63; 95.5%) were African Americans. Although 21(31.8%) participants reported no pain requiring hospitalization the past year, the majority (n=30, 45.5%) reported acute pain episodes one to three times per year requiring hospitalization (Table 1). Some (n=8; 12.1%) reported more than three pain episodes per year and others (n=7; 10.6%) did not provide this information. The majority had SCD related events (Table 2) such as acute chest syndrome (n=32, 48.5%), asthma (n=14, 21.2%), iron overload (n=9. 13.6%) and splenectomy (n=8, 12.1%).
Table 1.
Demographics [N= 66]
| N (%) | |
|---|---|
| Age | |
| Children | 39 (59.09%) |
| Mean | 11.9 ± 1.1 years |
| Range | 10 to 12 years |
| Adolescents | 27 (40.0%) |
| Mean | 15.5 ± 0.9 years |
| Range | 13 to 17 years |
| Gender | |
| Males | 31 (47%) |
| Females | 35 (53%) |
| Hgb Phenotype | |
| Hgb SS | 31 (47%) |
| Hgb SC | 16 (24.2%) |
| Unknown | 19 (28.8%) |
| Ethnicity | |
| African-American | 63 (95.5%) |
| Hispanic/Caucasian | 3 (4.5%) |
| Number of Acute Pain Episodes** | |
| 0 | 21 (31.8%) |
| 1 to 3 per year | 30 (45.5%) |
| ≥3 per year | 8 (12.1%) |
| Unknown | 7 (10.6%) |
Number of acute pain episodes requiring hospitalizations
Table 2.
History of SCD Related Complications & Other Illnesses [N= 66]
| N (%) | |
|---|---|
| Acute Chest Syndrome | 32 (48.5%) |
| Asthma | 14 (21.2%) |
| Iron Overload | 9 (13.6%) |
| Splenectomy | 8 (12.1%) |
| Stroke | 5 (7.6%) |
| Avascular Necrosis | 4 (6.1%) |
| Leg Ulcers | 4 (6.1%) |
| Priapism | 3 (4.5%) |
| Splenic Sequestration | 3 (4.5%) |
| Cholecystectomy | 2 (3.0%) |
| Pulmonary Hypertension | 2 (0.3%) |
| Other Illnesses (not stated) | 15 (22.7%) |
Some patient may have more than 1 response; therefore the totals exceed 100%.
Pain
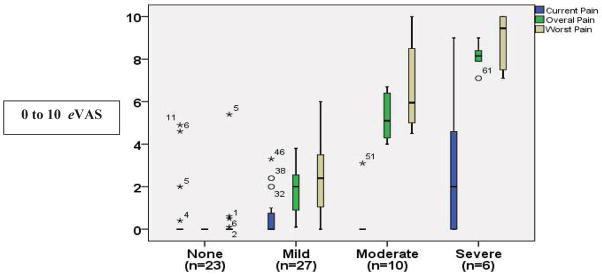
About one third (n=23; 34.8%) did not report having pain the previous month. The others reported their overall pain as mild (n=27; 40.9%) with a mean rating of 1.8 ± 1.1; moderate (n=10; 15.1%) with a mean rating of 5.3 ± 1.0; or severe (n=6; 9.0%) with a mean rating of 8.1 ± 0.6 (Figure 1).
Figure 1.
Overall pain the previous month in children with SCD.
Pain by Age & Gender
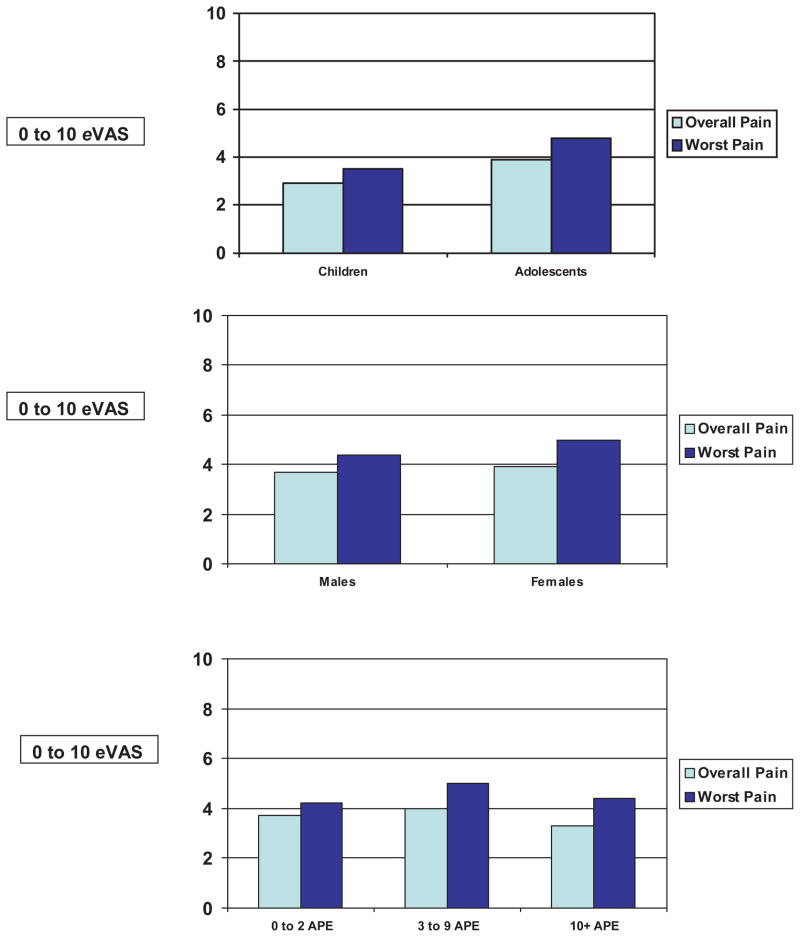
As illustrated in Figure 2, no significant differences were found in overall pain between children (2.9 ± 2.3) and adolescents (3.9 ± 2.0, p=0.27), and in worst pain (3.5 ± 2.2 versus 4.8 ± 2.5, p=0.25, respectively). Similarly, no significant differences were found in overall pain (Figure 2) between males (3.7 ± 2.6) and females (3.9 ± 2.2, p=0.75), and in worst pain (4.4 ± 2.7 versus 5.0 ± 2.6, p=0.40, respectively).
Figure 2.
No differences in overall and worst pain (0 to 10 eVAS) by age, gender, and pain frequency [APE: Number of pain episodes previous 12 months].
Pain by Number of Acute Pain Episodes
No significant differences were found in overall pain regardless of the number of acute pain episodes the previous year: 1) 0 to 2 episodes (n=11; 3.7 ± 1.6); 2) 3 to 9 episodes (n=16; 4.0 ± 2.6), and 3) 10 or more episodes (n=14; 3.3 ± 1.5, F=0.46, p=0.63) (Figure 2). Similarly, no significant differences were found in worst pain the previous month regardless of number of acute pain episodes: 1) 0 to 2 episodes (n=11; 4.2 ± 2.2, 2) 3 to 9 episodes (n=16; 5.0 ± 2.9), and 3) 10 or more episodes (n=14; 4.4 ± 2.3, F=0.41, p=0.67) (Figure 2).
Pain Coping
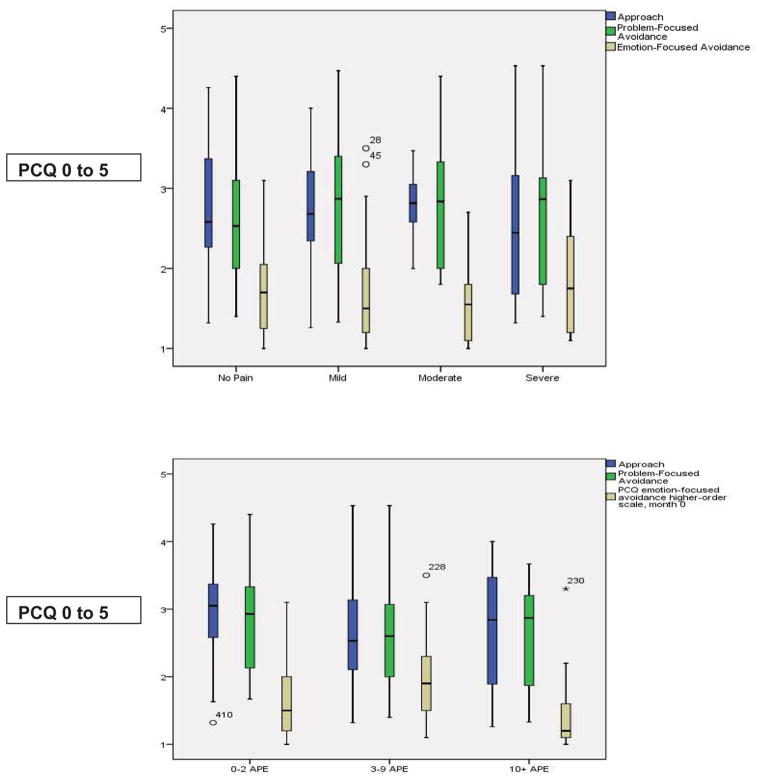
Participants used different pain coping strategies: 1) positive approach (2.8 ± 0.8 on 0 to 5 scale), such as seeking information and support for pain controllability, 2) problem-focused avoidance (2.7 ± 0.8), such as behavioral and cognitive distraction, and 3) emotion-focused avoidance (1.7 ± 0.6), such as internalizing and externalizing. No significant differences in pain coping were found by age or gender (Table 3). Pain frequency and pain severity did not have significant effects on pain coping (Figure 3). Participants were less likely to use emotion focused avoidance; 75% of the participants indicated “hardly ever” or “never” used emotional focused avoidance (Figure 3).
Table 3.
Pain Coping by Age and Gender
| Children (10 to 12 years) | Adolescents (13 to 17 years) | |||
|---|---|---|---|---|
|
| ||||
| Males (n=15) | Females (n=14) | Males (n=16) | Females (n=21) | |
| APPROACH | ||||
| Information seeking | 2.7 ± 0.8 | 2.9 ± 1.0 | 2.3 ± 1.0 | 2.5 ± 0.9 |
| Problem solving | 3.4 ± 0.8 | 3.4 ± 1.2 | 2.9 ± 1.2 | 3.4 ± 0.7 |
| Seeking social support | 2.8 ± 0.9 | 2.6 ± 0.8 | 1.8 ± 0.8 | 2.6 ± 0.9 |
| Positive self-statements | 2.5 ± 1.0 | 3.1 ± 1.0 | 2.4 ± 0.9 | 2.8 ± 1.1 |
|
| ||||
| PROBLEM-FOCUSED AVOIDANCE | ||||
| Behavioral distraction | 2.5 ± 1.0 | 2.5 ± 0.8 | 3.3 ± 1.0 | 2.4 ± 1.1 |
| Cognitive distraction | 2.2 ± 0.8 | 2.8 ± 0.9 | 3.1 ± 1.2 | 2.8 ± 1.0 |
|
| ||||
| EMOTION-FOCUSED AVOIDANCE | ||||
| Externalizing | 1.5 ± 0.6 | 1.5 ± 0.8 | 1.7 ± 0.9 | 1.3 ± 0.3 |
| Internalizing/catastrophizing | 2.2 ± 0.8 | 2.1 ± 0.7 | 1.8 ± 0.8 | 2.0 ± 0.9 |
Values represent frequency of use of the coping strategy: 1= Never; 2=Hardly Ever, 3=Sometimes, 4=Often, 5=Very Often
Figure 3.
Pain coping strategies by severity and frequency (APE: Number of Acute Pain Episodes previous 12 months)
There were no significant correlations between worst pain severity the previous month and pain coping. While there were significant negative correlations in males between worst pain severity and the subscale for behavioral distraction (r= −0.432, p = 0.015) and the subscale for internalizing/catastrophizing (r= −0.457, p = 0.049), there were no significant correlations in females.
Sleep
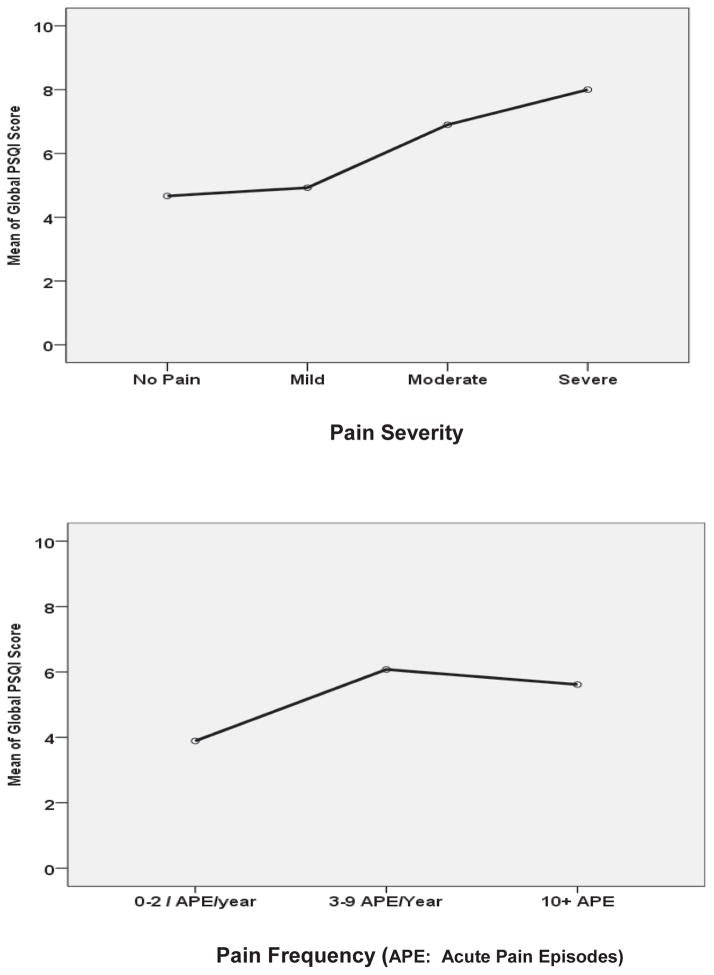
Scores obtained for each component of the PSQI in children and adolescents with SCD are shown in table 4. The majority had mild (n=38; 57.6%) or moderate (n=22; 33.3%) sleep disturbance; few were severe (n=2; 3%). Some (n=12; 18.2%) required the use of sleeping medication three or more times a week. Ten participants (15.1%) reported “fairly bad” to “very bad” sleep quality. Some (n=13; 19.7%) reported day dysfunction due to sleepiness. There were no significant differences in age or gender in the PSQI. The Global PSQI was 5.4 ± 3.3, indicating poor sleep or sleep disturbance. There were no significant differences in PSQI by pain severity and pain frequency (Figure 4: children and adolescent data combined).
Table 4.
Sleep
| Sleep Quality | |
| Very Good | 31 (47.0%) |
| Fairly Good | 25 (37.9%) |
| Fairly Bad | 8 (12.1%) |
| Very Bad | 2 (3.0%) |
| Sleep Latency | |
| ≤ 15 minutes | 24 (36.4%) |
| 16 to 30 minutes | 23 (34.8%) |
| 31 to 60 minutes | 16 (24.2%) |
| > 60 minutes | 3 (4.5%) |
| Sleep Duration | |
| > 7 hours | 57 (86.4%) |
| 6–7 hours | 7 (10.6%) |
| ≤ 5 hours | 2 (3.0%) |
| Sleep Efficiency | |
| >85% | 47 (71.2%) |
| 75–84% | 11 (16.7%) |
| 65–74% | 4 (6.1%) |
| <65% | 4 (6.1%) |
| Sleep Disturbance | |
| Not During the past month | 4 (6.1%) |
| Less than once a week | 38 (57.6%) |
| Once or twice a week | 22 (33.3%) |
| Three or more times a week | 2 (3.0%) |
| Use of Sleeping Medication | |
| Not during past month | 40 (60.6%) |
| Less than 1x/week | 7 (10.6%) |
| 1x or 2x/week | 7 (10.6%) |
| 3 or more times/week | 12 (18.2%) |
| Day Dysfunction Due to Sleepiness | |
| Never | 34 (51.5%) |
| 1 or 2x/month | 19 (28.8%) |
| 1 or 2x/week | 10 (15.2%) |
| 3 or more times/week | 3 (4.5%) |
| Global PSQI | 5.4 ±3.3 |
Figure 4.
Relationship between PSQI and pain frequency (A) and severity (B).
DISCUSSION
This study examined the pain, pain coping and sleep experience reported by children and adolescents with SCD. The hypothesis predicting that more pain is associated with more emotion-based coping skills and more problems with sleep was supported. There was a significant negative correlation between worst pain severity in boys and pain coping strategies for positive behavioral distraction and negative emotion-based internalizing/catastrophizing. Negative emotions impair coping skills and are related to higher levels of pain. The boys’ responses may be related to cultural norms which may have shifted in a manner that being masculine is no longer equated with being stoic when in pain, as suggested by Reid, Gilbert & McGrath (1998).
The majority of participants had mild to severe sleep disturbances, with some requiring sleeping medication three or more times a week. The Global PSQI score indicated poor sleep or sleep disturbance. However, there were no significant differences in age, gender, pain frequency and intensity related to pain and sleep. The imperative to include sleeping patterns as part of an assessment of children with SCD is clear. In addition, because depression and anxiety may cause sleep disturbances, screening for depression and anxiety is also warranted (Benton, Ifeagwu & Smith-Whitley, 2007). Studies reporting the relationship between depression and sleep disturbances demonstrated that sleep disturbance may be associated with depression. For example, Palermo and Kiska (2005) reported that after controlling for the effect of all other demographic, pain, and functional impact variables, depressive symptoms were predictive of the severity of sleep disturbances in children with chronic pain.
Participants in this study used a variety of active, positive coping strategies to regulate feelings when in pain, which included information seeking, problem solving, seeking social support and positive self-statements. Problem focused avoidance, a method to disengage from pain which included behavioral and cognitive distraction, such as watching a movie or engaging with friends, was also used occasionally. Negative emotion focused avoidance was seldom used. There were no significant differences in coping by age, frequency or intensity of pain.
More than half of the participants experienced acute pain episodes that required hospitalization, with some experiencing more than three episodes a year. This number exceeds previous findings that reported about one third of the children with SCD required hospitalizations for acute pain episodes (Jacob, Hockenberry, Mueller, Coates, & Zeltzer, 2008) One contributing factor for hospitalizations in this study may be that nearly half of the participants reported their major health diagnosis was acute chest syndrome, one of the more serious complications of SCD. There were also several participants who experienced asthma, another potentially acute crises, which often results in emergency care.
Limitations
A major limitation in the study was the convenience sampling; therefore our findings may not be generalizable to all children with SCD. Another limitation is that information about confounding psychiatric or physical problems were not reported, which could affect perceptions of pain, sleep and the participant’s ability to cope. A third limitation is that data were only collected at one time point; longitudinal study following children from grade school through high school would provide more information about pain coping strategies that might be modifiable early rather than later trying to reverse maladaptive coping strategies. A fourth limitation is that a questionnaire for measurement of sleep was used. An actigraph to measure sleep patterns and circadian rhythms, in addition to a diary to record sleep quality daily, may improve collection of sleep data. Finally, data were collected only from children, and not from parents or hospital records, thereby introducing the possibility of recall and memory bias. However, both recall and memory bias for validity of past pain experiences was established by Koutanji, Pearce, Oajley, & Feinmann (1999). A pain-specific memory bias for children with chronic pain was found by their faster recall and processing times for pain-related material, even with depression and/or anxiety, when compared with a control group. In addition, recall of acute pain was found to be more accurate than chronic pain (Erskine, Morley & Pearce, 1990).
Implications for nursing
The bi-directional relationship of co-existing pain and sleep indicates that health care providers must address sleep when implementing a pain intervention. Pain and sleep management strategies which can support youth with SCD include an interdisciplinary team with pain and sleep experts, an environment conducive to sleeping, and use of nonpharmacological pain and sleep interventions.
An interdisciplinary team, which would include nurses and doctors with expertise in pain and sleep, therapists with certifications in nonpharmacological pain and sleep interventions, and others as needed in collaboration with the family and school personnel, is needed for more comprehensive interventions that promote sleep in children with SCD pain.
Parents can be taught that a sleeping environment can be established for children early, because SCD is usually diagnosed soon after birth, and bedtime routines conducive to sleep can be emphasized through adolescence. A bedroom should be free of distractions such as television sets, computers, cell phones and any other electronic devices. A consistent room temperature at night protects against disruptions of sleep from a child with SCD being too hot or too cold. Children usually adapt to established routines at bedtime that ideally would carry through to adolescence.
Although very little information is available for nonpharmacological pain and sleep interventions for use in children with SCD, interventions such as music-assisted relaxation, cognitive behavioral therapy, progressive muscle relaxation, and relaxation training have been demonstrated to have moderate to large effects that improve sleep quality (De Niet, Tiemens, Kloos, & Hutschemaekers, 2009). For example, cognitive behavioral therapy addresses sleep onset latency and maintenance of sleep by education regarding exercise, stimulus control (caffeine, nicotine and alcohol), sleep schedule, sleep habits and maintenance of a sleep diary. Previous studies showed that cognitive behavioral therapy improves sleep, energy, productivity and a general feeling of well-being (Manber, Bwenert, Nowakowski, & Sieben, 2011).
CONCLUSION
Children and adolescents with SCD experience pain that affects their sleep patterns and the way they cope with pain. The more severe the pain, the more negative coping strategies were used. Health care practitioners need to assess pain, coping and sleep during acute painful episodes and promote positive coping strategies such as providing social support, promoting the use of positive self-statements and providing information. In addition, children and adolescents need to be assessed and screened for anxiety and depression as sleep disturbance is associated with anxiety and depression. The patient’s perception of his/her health and ability to function are factors affecting attendance in school, participation in sports or hobbies, and interaction at social or family functions. Health care providers need to have knowledge of medications that alleviate pain, their side effects and possible long term effects in order to educate patients and family. Future research is needed to design strategies for teaching children how to cope with pain and promote sleep. Future studies in SCD that examine the effects of cognitive behavioral therapy, especially for insomnia, and other nonpharmacological interventions are recommended in children with SCD pain.
Acknowledgments
Funding was received from the National Institute of Health, National Heart, Blood, & Lung Institute, American Recovery & Reinvestment Act Grant #1RC1 HL100301-01. We thank all the children, adolescents and parents from the Sickle Cell Disease Foundation of California (SCDFC) who participated in the study. We are grateful to Mary Brown at the SCDFC and her staff, particularly Tara Ragin, who facilitated accessing participants, screening and recruitment, scheduling of participants, and making private room arrangements during enrollment and follow-up procedures.
Contributor Information
J. Kelly Graves, UCLA Adjunct Assistant Professor.
Eufemia Jacob, UCLA Assistant Professor.
References
- Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database of Systemic Reviews. 2012;15(2):1–2. doi: 10.1002/14651888.CD001916. [DOI] [PubMed] [Google Scholar]
- Backhaus, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Barakat LP, Schwartz LA, Simon K, Radcliffe J. Negative thinking as a coping strategy mediator of pain and internalizing symptoms in adolescents with sickle cell disease. Journal of Behavioral Medicine. 2007;30(3):199–208. doi: 10.1007/s10865-007-9103x. [DOI] [PubMed] [Google Scholar]
- Benjamin LJ. Pain management in sickle cell disease: Palliative care begins at birth. ASH Education Book Dow. 2008 doi: 10.1182/asheducation-2008.1.466. [DOI] [PubMed] [Google Scholar]
- Benton TD, Boyd R, Ifeagwu J, Feldtmose E, Smith-Whitley K. Psychiatric diagnosis in adolescents with sickle cell disease: A preliminary report. Current Psychiatric Reports. 2011;13(2):111–115. doi: 10.1007/s11920-011-0177-3. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Yanni EA, Creary MS, Olney RS. Health status and healthcare use in a national sample of children with sickle cell disease. American Journal of Preventive Medicine. 2010;38(4):528–535. doi: 10.1016/j.amepre.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Junghaenel D/U, Schneider S, Pilosi JJ, Stone AA. Pittsburgh and Epworth Sleep Scale items: Accuracy of ratings across different reporting periods. Behavioral Sleep Medicine. 2013;2(3):173–188. doi: 10.1080/15402002.2012.654549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatric Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. Journal of Psychosomatic Research. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Sickle cell anemia. 2011 Retrieved from http://www/cdc/gov/scd.
- Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Wang W. Health-related quality of life in children with sickle cell disease: A report from the comprehensive sickle cell centers clinical trial consortium. Pediatric Blood & Cancer. 2010;55(3):485–94. doi: 10.1002/phc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Niet GJ, Tiemens BG, Kloos MW, Hutschemaekers GJ. Review of systematic reviews about the efficacy of non-pharmacological interventions to improve sleep quality in insomnia. International Journal of Evidence-Based Healthcare. 2009;7(4):233–42. doi: 10.1111/j.1744-1609.2009.00142.x. [DOI] [PubMed] [Google Scholar]
- Erskine A, Morley S, Pearce S. Memory for pain: A review. Pain. 1990;41(3):255–265. doi: 10.1016/0304-3959(90)90002-U. [DOI] [PubMed] [Google Scholar]
- Fletcher C. Appraisal and coping with vaso-occlusive crisis in adolescents with sickle cell disease. Pediatric Nursing. 2000;26(3):319–324. [PubMed] [Google Scholar]
- Gil KM, Wilson JJ, Edens JL, Workman E, Ready J, Sedway J, Redding-Lallinger R, Daeschner CW. Cognitive coping skills training in children with sickle cell disease pain. International Journal of Behavioral Medicine. 1997;4(4):364–377. doi: 10.1207/s15327558ijbm0404_7. [DOI] [PubMed] [Google Scholar]
- Gil KM, Carson JW, Porter LS, Ready J, Valrie C, Redding-Lallinger R, Daeschner C. Daily stress and mood and their association with pain, health-care use, and school activity in adolescents with sickle cell disease. Journal of Pediatric Psychology. 2003;28(5):363–73. doi: 10.1093/jpepsy/jsg026. [DOI] [PubMed] [Google Scholar]
- Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101(3):846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- Henderson EM, Keogh E, Eccleston C. Why go online when you have pain? A qualitative analysis of teenagers’ use of the Internet Pain Management Service. Child Care Health and Development. 2013;5 doi: 10.1111/cch.12072. [DOI] [PubMed] [Google Scholar]
- Jacob E, Hockenberry M, Mueller BM, Coates T, Zeltzer L. Analgesic Response to Morphine in Children with Sickle Cell Disease. Journal of Pain Management. 2008;2(1):179–190. [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Stinson J, Duean J, Grupta A, Gerla M, Lewis M, Zeltzer L. Usability testing of a Smartphone for accessing a wed-based e-diary for self- monitoring of pain and symptoms in sickle cell disease. Journal of Pediatric Hematology and Oncology. 2012;34(5):326–335. doi: 10.1097/MPH.0b013e318257a13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrell JM, Tripathi A, McIntyre RS. Prevalence and treatment of depression in children and adolescents with sickle cell disease: A retrospective cohort study. Primary Care Companion for CNS Disorders. 2011;13(2) doi: 10.4088/PCC.10m01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle cell disease. Lancet. 2001;357(9269):1656–1659. doi: 10.1016/S0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- Koutanje M, Pearce SA, Oajley DA, Feinmann C. Children in pain: An investigation of selective memory for pain and psychological adjustment. Pain. 1999;81(3):237–244. doi: 10.1016/S0304-3959(99)00020-2. [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl LA, Drangsholt MT, Saunders K, Korff MV. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118(1–2):201–209. doi: 10.1016/j.pain.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. Journal of Developmental and Behavioral Pediatrics. 1999;20:244–252. doi: 10.1097/00004703-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Long AC, Krishnamurthy V, Palermo TM. Sleep disturbances in school-age children with chronic pain. Journal of Pediatric Psychology. 2008;33(3):258–268. doi: 10.1093/jpepsy/jsm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Sex and age differences in coping styles among children with chronic pain. Journal of Pain and Symptom Management. 2007;33(2):208–216. doi: 10.1016/j.jpainsymman.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: Adherence and clinical outcomes. Journal of Clinical Sleep Medicine. 2011;7(6):645–652. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MJ, Lemanek K, Palermo TM, Crosby LE, Nichols A, Powers SW. Parent perspectives on pain management, coping and family functioning in pediatric sickle cell disease. Clinical Pediatrics. 2007;46(4):311–319. doi: 10.1177/0009922806293985. [DOI] [PubMed] [Google Scholar]
- Oliver-Carpenter G, Barach I, Crosby LE, Vanenzuela J, Mirchell MJ. Disease management, coping and functional disability in pediatric sickle cell disease. Journal of National Medical Association. 2011;103(2):131–137. doi: 10.1016/s0027-9684(15)30262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: Relationship to daily functioning and quality of life. The Journal of Pain. 2005;6(3):201–207. doi: 10.1093/jpepsy/jsm129. [DOI] [PubMed] [Google Scholar]
- Perquin CW, Hazebroek-Kampschreur AA, Hunfield JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J. Pain in children and adolescents: A common experience. Pain. 2000;87(1):51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Pieh C, Popp R, Geisler P, Hajak G. Sleep and pain: Bi-directional relation? Psychiatric Praxis. 2011;38(4):166–170. doi: 10.1055/s-0030-1265949. [DOI] [PubMed] [Google Scholar]
- Reid GJ, Gilbert CA, McGrath PJ, Chipuer HM, Ellerton ML, Ritchie JA. Development of a pediatric pain coping checklist. Third International Symposium on Pediatric pain; Philadelphia, PA. 1994. [Google Scholar]
- Reid GL, Gilbert CA, McGrath PJ. The pain coping questionnaire: Preliminary validation. Pain. 1998;76:83–96. doi: 10.1016/s0304-3959(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Rogers VE, Marcus CL, Jawad AF, Smith-Whitley K, Ohene-Frempong K, Bowdre C, Mason TB. Periodic limb movements and disrupted sleep in children with sickle cell disease. Sleep. 2011;34(7):889–908. doi: 10.5665/SLEEP.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Isigkeit A, Thyen U, Stoven H, Swartzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115(2):152–162. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- Salles C, Ramos RT, Daktro C, Barral A, Marinho JM, Matos MA. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. Journal Brasileiro de Pneumologia. 2009;35(11):1075–1083. doi: 10.1590/s1806-37132009001100004. [DOI] [PubMed] [Google Scholar]
- Sanders KA, Labott SM, Molokie R, Shelby SR, Desimone J. Pain, coping and health care utilization in younger and older adults with sickle cell disease. Journal of Health Psychology. 2010;15(1):131–137. doi: 10.1177/1359105309345554. [DOI] [PubMed] [Google Scholar]
- Savedra MC, Holzemer WL, Tesler MD, Wilkie DJ. Assessment of postoperation pain in children and adolescents using the adolescent pediatric pain tool. Nursing Research. 1993;42(1):5–9. [PubMed] [Google Scholar]
- Simon K, Barakat LP, Patterson CA, Danpier C. Symptoms of depression and anxiety in adolescents with sickle cell disease: The role of intrapersonal characteristics and stress processing variables. Child Psychiatry and Human Development. 2009;49(2):317–330. doi: 10.1007/s10578-009-0129-x. [DOI] [PubMed] [Google Scholar]
- Smaldone A, Honig JC, Byrne MW. Sleepless in America: Inadequate sleep and relationships to health and the well-being of our nation’s children. Pediatrics. 2007;119(Suppl 1):29–37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- SPSS. Statistics for Wndows Version 20.0. Armonk, N.Y: IBM Corporation; [Google Scholar]
- Thastum M, Herlin T, Zachcariae R. Relationship of pain-coping strategies and pain-specific beliefs to pain experience in children with juvenile idiopathic arthritis. Arthritis & Rheumatism. 2005;53(2):178–184. doi: 10.1002/art.21081. [DOI] [PubMed] [Google Scholar]
- Thomsen AH, Compas BE, Colletti RB, Stanger C, Boyer MC, Konik BS. Parent reports of coping and stress responses in children with recurrent abdominal pain. Journal of Pediatric Psychology. 2002;27(3):215–226. doi: 10.1093/ipepsy/27.3.215. [DOI] [PubMed] [Google Scholar]
- Unal S, Toros F, Kutuk MO, Uyaniker MG. Evaluation of the psychological problems in children with sickle cell anemia and their families. Pediatric Hematology Oncology. 2011;28(4):321–328. doi: 10.3109/08880018.2010.540735. [DOI] [PubMed] [Google Scholar]
- Unruh AM. Gender variation in clinical pain experiences. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Valrie CR, Gil KM, Redding-lallinger R, Daeschner C. Brief report: Daily mood as a mediator or moderator of the pain-sleep relationship in children with sickle cell disease. Journal of Pediatric Psychology. 2007;32(7):857–861. doi: 10.1093/jpepsy/jsmO16. [DOI] [PubMed] [Google Scholar]