Abstract
Malignancy and cancer related treatments lead to multiple symptoms. While treatments focus on cure, few research studies examined the symptoms that accompany these aggressive and complicated treatments. The purpose of the study is to evaluate the symptoms experienced by children at home. Children (n=25) and adolescents (n=33) diagnosed with cancer completed the Memorial Symptoms Assessment Scale (MSAS) during the five days at home following discharge from hospitalization. The most frequent physical symptoms were fatigue (52.1%), nausea (50.7%), lack of appetite (43.7%) and pain (42.3%). The most frequent psychological symptoms were “difficulty sleeping” (21.1%), “worrying” (18.3%), “feeling sad” (18.3%), and “feeling nervous “ (16.9%). Significant differences were found in the overall physical, psychosocial, and global distress index in patients with and without pain, fatigue, and nausea. Results indicated that physical and psychosocial symptoms as well as Global Distress Index increased as severity of pain, nausea, and fatigue increased. Children and adolescents were experiencing many symptoms at home but were often not reporting them.
Keywords: Cancer, Symptoms, Children, Adolescents, Memorial Symptoms Assessment Scale
INTRODUCTION
Children diagnosed with cancer frequently undergo invasive medical procedures and treatments as part of their care that lead to multiple symptoms (Miller et al., 2011). Previous studies have evaluated chemotherapy-related symptoms in addition to the symptoms from the underlying malignancy (Hockenberry et al., 2010; von Lützau et al., 2012; Hansson et al., 2013). While cancer treatments focus on cure, few studies have focused on the evaluation and management of the multiple symptoms accompanying these aggressive and complicated treatments in the home environment (Klaassen et al., 2010; Kaleyias et al., 2012).
Several studies reported that treatment related symptoms such as pain, nausea, and fatigue had significant effects on quality of life (Von Lützau et al., 2012; Hansson et al., 2013). In a study conducted in Australia, parents reported that their child had suffered “a lot” or “a great deal” from at least one symptom with the most common being pain, fatigue and poor appetite. Children receiving cancer-directed therapy were experiencing greater numbers of symptoms than those who were not receiving treatment (Heath, et al, 2010), Children and adolescents experiencing increased fatigue and sleep disturbances have also been found to experience depressive symptoms and behavior changes (Hockenberry, et al., 2010).
Theunissen and colleagues (2007) evaluated the physical, psychological, and social symptoms of children with cancer during palliative care. While the most frequently reported physical symptoms were pain, poor appetite, and fatigue, the most frequent psychological symptoms were sadness and having difficulty in talking with parents about illness, death, and fear of being alone. Cancer and its treatments significantly affect the psychosocial well-being and quality of life of children and adolescents hospitalized for cancer care.
Several studies have reported multiple symptoms associated with treatments during hospitalization and at the end of life. The nature of the underlying malignancy significantly influenced the prevalence of some symptoms. The majority of the literature included information on frequency of symptoms but did not include the severity and distress associated with these symptoms. Additionally, studies also focused on physical symptoms, but not psychosocial symptoms. Most studies therefore did not look at overall distress associated with all of these symptoms together. There was limited information regarding whether the physical and psychological symptoms were completely or partially resolved, and how frequent, severe, or distressful symptoms were at home following hospitalizations.
To manage symptoms effectively at home, an evaluation of symptom frequency, severity, and distress associated with these symptoms is needed. The purpose of this study was to evaluate symptom experiences at home; more specifically, we examined the frequency, severity, and distress associated with physical and psychosocial symptoms. We compared the Total Symptoms [MSAS], Physical Symptoms [PHYS], Psychological Symptoms [PSYCH], and Global Distress Index [GDI] to measure overall symptom distress in patients with and without pain, fatigue, and nausea as these were the most reported symptoms.
THEORETICAL FRAMEWORK
The Symptom Management Theory (SMT) was used as the theoretical background for the study (Humphreys et al., 2008). It consists of three interrelated components that are highly interdependent: 1) symptom experience -- the individual’s perception of the severity and distress associated with the symptoms, 2) symptom management -- strategies that are intended to “avert, delay, or minimize the symptom experience”, and 3) symptom outcomes – symptom relief or the outcomes of symptoms on functional status, emotional status, self-care, costs, quality of life, morbidity, comorbidity, and mortality (Humphreys et al., 2008). Effective symptom management requires consideration of the three components.
The SMT also includes three domains of nursing science: 1) person variables -- demographic, psychological, sociological, physiological, and developmental; 2) environment variables -- physical, cultural, and social conditions; and 3) health and illness variables -- health status, disease, injury that affect symptom experience, management, and outcomes. In this study, we investigated symptom experiences in the home (environment variable) in children (8 to 12 years) and adolescents (13 to 17 years; demographic variable) with cancer (health/illness variable). Information from this study may guide development of symptom management strategies at home and interventions aimed at alleviating distress associated with physical and psychosocial symptoms.
METHODS
Design.
We used a convenience sample of children and adolescents with cancer who were recruited prior to discharge from hospitalization. Children and adolescents were instructed to answer questions daily, during the five days at home after being discharged from the hospital. They used the Memorial Symptoms Assessment Scale [MSAS] to indicate the frequency, severity, and distress associated with daily symptoms. Only data from home was included in this report. There were some participants that reported symptoms for 3, 4, or 5 days; therefore, the average of the five days was used to calculate the symptom scores.
Setting & Sample.
Children and adolescents were recruited from the pediatric oncology acute care units of two comprehensive children’s hospitals in southwestern United States. The inclusion criteria were: 1) age 8 to 17 years, 2) anticipated discharge within 48 hours, 3) ability to speak English (MSAS was available only in English at this time), and 4) patient and parent consent. Children and adolescents were excluded if they 1) refused to participate, 2) were too ill to participate, and 3) were unable to complete the outcome measures because of prior history of neurological impairments, visual or hearing deficits, motor function deficit, or developmental delay. The study was approved by the Institutional Review Boards at the University of California, Los Angeles and the respective children’s hospitals.
Procedures.
A research assistant obtained a list of eligible participants on the pediatric oncology units from the oncology team. Information flyers were provided to eligible patients and their parents or legal guardians. Those who were interested were given more details about the study, and were given the opportunity to ask questions and express thoughts and concerns. If they agreed to participate, parents and adolescents were asked to sign the consent forms. They were enrolled after the consenting procedures, which occurred within 48 hours prior to going home.
All children and adolescents enrolled in the study were instructed to complete questions about symptoms, using the Memorial Symptoms Assessment Scale (MSAS), during the five days at home following discharge from hospitalization. Only the data that was collected at home during the five days were included in the analyses as we intended to capture only the symptom experiences at home. Because there were participants who completed 3, 4, and 5 days, the individual average of the five days were calculated and included in the analyses. The children and adolescents were informed that they may stop completing the MSAS at any time. No participants indicated that completing the MSAS daily was burdensome. In addition to the MSAS, the children and adolescents completed the Adolescent Pediatric Pain Tool (APPT), only if they had pain, and the Pediatric Quality of Life Scale (PedsQL). The MSAS and APPT took less than 10 minutes to complete. Only the pain item from the MSAS was included in the report. The pain data from the APPT was reported on another manuscript. Only pediatric cancer patients were included as the MSAS has only been validated in children and adolescents with cancer.
Instruments.
A demographic sheet was completed with information regarding age, sex, ethnicity, diagnoses, reason for hospitalizations, procedures, and treatments. The demographic information was obtained either from the parent or from the medical record.
Symptoms Measurement.
The MSAS consisted of 31 symptoms that assessed the presence, frequency, severity, and level of distress. Responses were a Likert-type scale and were quantified as follows: 1) frequency – how often did you have the symptom (0=never; 2.5=almost never; 5.0=sometimes; 7.5=a lot; 10=almost always); 2) severity – how severe was the symptom (0=none; 2.5=slight; 5.0=moderate; 7.5=severe; 10=very severe); and 3) distress – how much did it bother or distress you (0=not all; 2.5=a little bit; 5.0=somewhat; 7.5=quite a bit; 10.0=very much). The MSAS took less than five minutes to complete (Collins et al., 2000; Collins et al., 2002, Miller et al., 2011). Higher scores represented worse symptom scores.
The standard method for reporting MSAS data was to calculate the following subscale scores: 1) Total MSAS, 2) psychological symptoms (PSYCH), 3) physical symptoms (PHYS), and 4) Global Distress Index (GDI). The PHYS subscale was calculated by taking the five-day average of the 11 physical symptoms (lack of appetite, lack of energy, pain, drowsiness, constipation, dry mouth, nausea, vomiting, change in taste, weight loss, dizziness). The PSYCH subscale score was calculated by taking the five-day average of six psychological symptom scores (feeling sad, worrying, feeling irritable, feeling nervous, difficulty sleeping, difficulty concentrating). The GDI was calculated by taking the five-day average of the frequency scores reported (feeling sad, worrying, feeling irritable, and feeling nervous and the distress scores for lack of appetite, lack of energy, pain, drowsiness, constipation, dry mouth). These scoring methods represent the standardized procedures as previously reported (Collins et al., 2000; Collins et al., 2002, Miller, et al., 2011; Yeh, et al., 2009). The higher scores represent worse symptoms for PSYCH and PHYS, and worse GDI. Worse scores may include more frequent, more severe, and/or more distress associated with the symptoms.
The reliability of the PSYCH (α=0.83), PHYS (α=0.87), and GDI (α=0.85) scores were previously reported, demonstrating adequate internal consistency (Collins, et al., 2000; Collins, et al., 2002). Construct validity was demonstrated with higher symptoms and distress ratings in inpatients compared to outpatients, and in those receiving chemotherapy compared to those not receiving it (Collins, et al., 2000; Collins, et al., 2002). We also performed reliability analyses in a small sample of children (n=20; 8 to 12 years) and adolescents (n=24; 13 to 17 years) with cancer who completed at time of enrollment, on day 3 at home, and during the subsequent clinic visit in the current sample. Data showed high correlations in the hospital (r = 0.85, p < .0001), at home (r = 0.89, p < .0001), and in the clinic (r=0.94, p < .0001). For the current sample, the reliability analyses for the MSAS, PHYS, PSYCH, GDI showed good evidence of reliability with a Cronbach’s alpha of 0.97.
Data Analyses.
All data was entered into Statistical Package for Social Sciences (SPSS version 24.0 Chicago, IL), and all entries were double checked by two research assistants. Following the scoring methods previously described (Miller, et al., 2011), we quantified the 4 and 5 point Likert-type scales into a 0-10 scale as indicated above. The quantification allowed us to standardize the metrics, so that we could perform statistical tests such as t-tests (Bland & Altman, 1996). The quantification of the Likert-type responses to the 0-10 scale was selected because it is a common metric familiar to care providers (von Bayer & Hicks, 2000). We used the quantified data to calculate the five day average for the psychosocial (PSYCH), physical (PHYS), global distress index (GDI), and overall symptoms (MSAS) scores. Descriptive statistics were used to summarize demographics and the frequency, severity, and distress of physical and psychological symptoms, as well as the global distress scores. T-tests were used to examine differences between those days with and without physical symptoms a) pain, b) fatigue, and c) nausea.
RESULTS
Demographics.
Children (n=25; 10.16 ± 1.3 years) and adolescents diagnosed with cancer (n=33; 14.8 ± 1.3 years) participated in the study. About half were males (55.2%), and were predominantly Hispanic (n=32; 55.2%), with some Caucasians (n=17; 29.3%) and others (n=9; 15.5%; Table 1). The majority of the sample was diagnosed with leukemia or lymphomas (n=25; 43.1%), while the others had sarcomas (n=18; 31.0%), and other cancer diagnoses (n=15; 25.9%). They were in the hospital because of chemotherapy (n=38; 65.5%), fever, neutropenia and/or infection (n=10; 17.2%), and other reasons such as vomiting and diarrhea (n=19; 17.2%).
Table 1.
Demographics (N=58)
| n (%) | |
|---|---|
| Age | |
| Children | 25 (43.1%) |
| Mean Age | 10.16 yrs ± 1.3 |
| Adolescents | 33 (56.9%) |
| Mean Age | 14.8 yrs ± 1.4 |
| Gender | |
| Male | 33 (55.2%) |
| Female | 26 (44.8%) |
| Ethnicities | |
| Caucasian | 17 (29.3%) |
| Hispanic | 32 (55.2%) |
| Other | 9 (15.5%) |
| Cancer Diagnoses | |
| Leukemias/Lymphomas | 25 (43.1%) |
| Sarcomas | 18 (31%) |
| Other | 15 (25.9%) |
| Reasons for Hospitalization | |
| Chemotherapy | 38 (65.5%) |
| Fever & Neutropenia | 10 (17.2%) |
| Other | 10 (17.2%) |
Physical Symptoms.
The most frequent physical symptoms at home were fatigue (52.1%), nausea (50.7%), lack of appetite (43.7%) and pain (42.3%). Although “less hair than usual” was not the most frequent, it was among the most severe (7.5 ± 4.7) and more distressful (5.8 ± 4.9; Table 2). The most distressful physical symptoms (Table 2) were swallowing difficulty (9.1 ± 1.4), difficulty with urination (7.5 ± 3.5), and mouth sores (7.0 ± 3.4) on 0 to 10 scale. Other symptoms that were, among the most distressful (Table2) were shortness of breath (6.8 ± 3.4), headache (6.7 ± 2.9), and changes in food taste (6.3 ± 3.2)
Table 2.
Frequency, Severity, and Distress Associated with Physical Symptoms at Home
| Percent (%) Days with Symptoms | Severity mean ± SD | Distress mean ± SD | |
|---|---|---|---|
| GENERAL | |||
| Fatigue | 52.1 | 4.6 ± 1.8 | 3.7 ± 3.0 |
| Pain | 42.3 | 5.1 ± 1.8 | 5.9 ± 3.0 |
| RESPIRATORY | |||
| Shortness of breath | 9.9 | 5.7 ± 1.9 | 6.8 ± 3.4 |
| Cough | 0.7 | 4.0 ± 2.2 | 4.0 ± 2.2 |
| NEUROLOGICAL | |||
| Feeling drowsy | 19.7 | 5.0 ± 1.7 | 3.8 ± 2.1 |
| Headache | 16.9 | 5.8 ± 2.2 | 6.7 ± 2.9 |
| Itching | 16.9 | 4.8 ± 2.7 | 5.2 ± 3.4 |
| Dizziness | 16.9 | 5.8 ± 2.2 | 6.2 ± 3.1 |
| Numbness and tingling | 15.5 | 5.0 ± 2.7 | 4.5 ± 4.0 |
| GASTROINTESTINAL/GENITOURINARY | |||
| Nausea | 50.7 | 5.1 ± 1.6 | 5.6 ± 2.5 |
| Lack of appetite | 43.7 | 5.3 ± 2.2 | 4.7 ± 3.1 |
| Dry mouth | 33.3 | 5.0 ± 1.6 | 5.7 ± 2.9 |
| Constipation | 29.6 | 4.6 ± 2.9 | 3.7 ± 3.2 |
| Changes in food taste | 21.1 | 5.6 ± 2.6 | 6.3 ± 3.2 |
| Mouth sores | 19.7 | 5.5 ± 2.2 | 7.0 ± 3.4 |
| Vomiting | 18.3 | 5.0 ± 1.4 | 5.8 ± 2.8 |
| Diarrhea | 9.9 | 4.2 ± 1.2 | 5.4 ± 3.7 |
| Difficulty urinating | 5.6 | 4.4 ± 2.4 | 7.5 ± 3.5 |
| Swallowing difficulty | 4.2 | 5.8 ± 1.4 | 9.1 ± 1.4 |
| OTHER SIGNS/SYMPTOMS | |||
| Changes in skin | 22.5 | 4.8 ± 1.9 | 3.9 ± 3.7 |
| Less hair | 21.1 | 7.5 ± 4.7 | 5.8 ± 4.9 |
| Sweats | 11.3 | 4.7 ± 2.1 | 3.7 ± 3.2 |
| Weight loss | 12.7 | 5.3 ± 1.9 | 3.9 ± 3.3 |
| Swelling arms or legs | 2.8 | 3.8 ± 1.8 | 5.0 ± 3.5 |
Values given are mean ± SD; based on the transformed 0 to 10 Scale
Psychological symptoms.
The most frequent psychosocial symptoms at home were “difficulty sleeping” (21.1%), “wonying” (18.3%), “feeling sad” (18.3%), and “feeling of being nervous” (16.9%). Difficulty sleeping and worrying (Table 3) were not only the most severe (5.2 ±1.8 and 5.6 ± 2.3, respectively), but they were also the most distressful (6.2 ± 2.3 and 6.3 ± 3.1, respectively). Other psychological symptoms (Table 3) reported were “feeling irritable” (5.3 ± 2.3), “haring difficulty concentrating” (3.5 ± 1), and “not looking like him/herself” (4.7 ± 1.6). The majority of the psychological symptoms were reported to be moderate in severity from 3.5 ± 1.3 for difficulty concentrating to 5.6 ± 2.3 for worrying (Table 3). Values given are mean ± SD; based on the transformed 0 to 10 Scale; higher scores indicate worse in severity and worse in distress associated with the symptoms.
Table 3.
Frequency, Severity, Distress Associated with Psychological Symptoms
| Percent (%) Days with Symptoms | Severity mean ± SD | Distress mean ± SD | |
|---|---|---|---|
| Difficulty sleeping | 21.1 | 5.2 ± 1.8 | 6.2 ± 2.3 |
| Worrying | 18.3 | 5.6 ± 2.3 | 6.3 ± 3.1 |
| Feeling sad | 18.3 | 4.8 ± 1.9 | 4.4 ± 1.8 |
| Feeling nervous | 16.9 | 5.2 ± 2.2 | 5.0 ± 3.9 |
| Difficulty concentrating | 14.1 | 3.5 ± 1.3 | 2.8 ± 2.7 |
| Feeling irritable | 12.7 | 5.3 ± 2.3 | 4.4 ± 3.5 |
| I don’t look like myself | 11.3 | 4.7 ± 1.6 | 4.4 ± 3.5 |
Values given are mean ± SD; based on the transformed 0 to 10 Scale
Pain & Symptom Distress.
There was a cumulative total of 52 of 103 days when patients reported having pain. On those days when they had pain, some patients reported mild (n=8 days; 15.4%) and others were moderate to severe (n=44 days; 84.6%). No significant differences were found in the GDI, MSAS, and Physical symptoms scores on those days when patients had pain compared with those days when they did not have pain. However, there was a significant difference in the psychosocial symptoms (Figure 1A) when patients had pain (n=47 days; 3.7 ± 1.8) compared to when they did not have pain (n=45 days; 4.7 ±1.7, p = 0.009). The Total Symptoms [MSAS], Physical Symptoms [PHYS], Psychological Symptoms [PSYCH], and Global Distress Index [GDI] were worse on those days when patients had pain (Figure 1A), compared to those days when they did not have pain. The severity of Total Symptoms [MSAS], Physical Symptoms [PHYS, Psychological Symptoms [PSYCH], and Global Distress Index [GDI] were worse as the severity (Mild, Moderate, Severe) of pain increased (Figure 2A).
Figure 1.
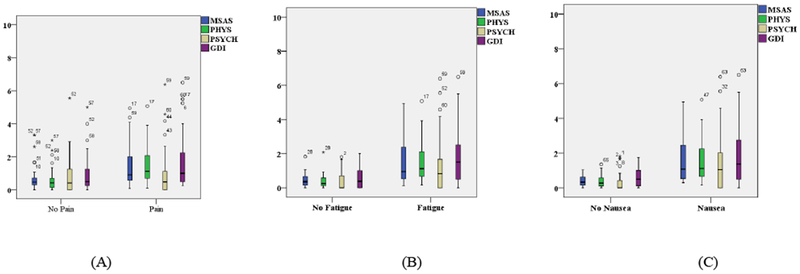
Total Symptoms [MSAS], Physical Symptoms [PHYS], Psychological Symptoms [PSYCH], and Global Distress Index [GDI] were worse on days with Pain (A), Fatigue (B), and Nausea (C), compared to the days when they did not have these symptoms.
Figure 2.
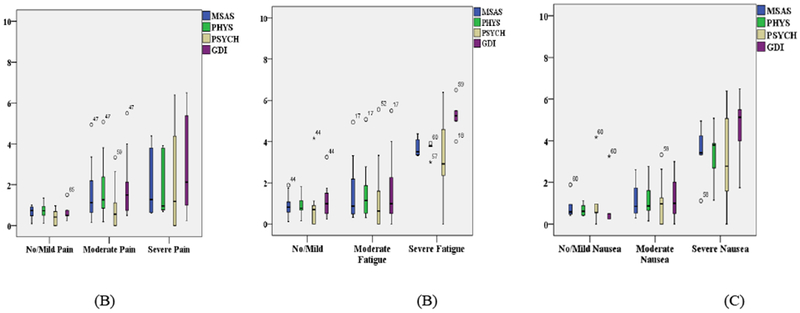
Total Symptoms [MSAS], Physical Symptoms [PHYS], Psychological Symptoms [PSYCH], and Global Distress Index [GDI] were increasing as Pain (A), Fatigue (B), and Nausea (C) increased in severity (mild, moderate, severe).
Fatigue & Symptom Distress.
There was a cumulative total of 63 of 103 days when patients reported having fatigue. On those days when they had fatigue, some patients reported “sometimes” (n=11 days; 17.5%) and others were “a lot” to “almost always” (n=52 days; 82.5%). There were significant differences in the GDI scores between days with fatigue (5.3 ± 2.3) and without fatigue (3.8 ± 2.0, p = .00089; Figure 1B). The total MSAS scores were significantly worse (5.0 ± 1.5) when they had fatigue, compared to when they did not have fatigue (3.9 ± 1.4, p = .00003). Physical symptoms (5.0 ± 1.3 vs 3.7± SD 1.4, p = .00001) and psychological symptoms (4.7 ± 1.7 vs 3.5 ± 1.6, p = .00086) were also significantly worse on days when they were experiencing fatigue, compared to when they were not experiencing fatigue (Figure 1B). The severity of Total Symptoms [MSAS], Physical Symptoms [PHYS, Psychological Symptoms [PSYCH], and Global Distress Index [GDI] were worse as the severity (Mild, Moderate, Severe) of fatigue increased (Figure 2B).
Nausea & Symptom Distress.
There was a cumulative total of 66 of 103 days when patients reported having nausea. On the days when patients reported nausea, some patients reported “sometimes” (n=17 days; 25.8%) and others reported “a lot to always” (n=49; 74.2%). The total MSAS scores were significantly worse (5.0 ±1.5) on the days when patients reported nausea, compared to when they did not report nausea (3.9 ± 1.4, p = 0.0001). Physical symptoms (4.9 ± 1.5 vs 3.8 ± 1.3 p = 0.00009) and psychosocial symptoms (3.6 ± 1.7 vs 4.7 ±1.7, p = 0.001 respectively) were also significantly worse on days when they had nausea compared to the days when they did not have nausea. The GDI scores were worse when patients had nausea (5.2 ± 2.4) compared to when they did not have nausea (4.0 ± 2.1, p = 0.009; Figure 1C). The severity of Total Symptoms [MSAS], Physical Symptoms [PHYS, Psychological Symptoms [PSYCH], and Global Distress Index [GDI] were worse as the severity (Mild, Moderate, Severe) of nausea increased (Figure 2C).
Effects of Gender, Diagnoses, Reasons for Hospitalization.
Females had significantly more severe PHYS (4.8 ± 1.4 vs 3.4 ± 1.2, p=0.0001), PSYCH (4.5 ± 1.9 vs 3.2 ± 1.0, p=0.0001), total MSAS (4.8 ± 1.5 vs 3.4 ± 1.3, p=0.0001) and GDI (5.0 ± 2.4 vs 3.4 ± 1.3, p=0.0001) compared to males (Table 4). Patients diagnosed with sarcomas also had significantly worse scores for the PHYS (5.1 ± 1.5), MSAS (5.2 ± 1.7), and GDI (5.9 ± 2.9) compared to patients diagnosed with leukemias/lymphomas and other cancer diagnoses (Table 4). Those indicating chemotherapy as primary reason for admission had significantly worse physical symptoms (4.7 ± 1.4, F=3.7, p=0.027), compared to those admitted for fever, neutropenia, infection, and other reasons (Table 4).
Table 4.
Symptoms by Sex, Diagnoses, and Reason for Hospitalization
| Symptom Scores (0 to 10 Scale) | |||
|---|---|---|---|
| SEX | |||
| Male | Female | P-value | |
| PHYS | 3.4 ± 1.2 | 4.8 ± 1.4 | 0.0001 |
| PSYCH | 3.2 ± 1.0 | 4.5 ± 1.9 | 0.0001 |
| MSAS | 3.4 ± 1.1 | 4.8 ± 1.5 | 0.0001 |
| GDI | 3.4 ± 1.3 | 5.0 ± 2.4 | 0.0001 |
| CANCER DIAGNOSES | |||
| Leukemias/Lymphomas | Sarcomas | Other | |
| PHYS | 4.5 ± 1.5 | 5.1 ± 1.5 | 3.4 ± 5.6 |
| PSYCH | 4.9 ± 1.8 | 4.0 ± 1.8 | 2.9 ± 0.9 |
| MSAS | 4.5 ± 1.4 | 5.2 ± 1.7 | 3.1 ± 0.6 |
| GDI | 4.5 ± 1.7 | 5.9 ± 2.9 | 3.0 ± 0.7 |
| REASON FOR HOSPITALIZATION | |||
| Chemotherapy | Fever/Neutropenia | Other | |
| PHYS | 4.7 ± 1.4 | 3.5 ± 1.7 | 3.5 ± 1.7 |
| PSYCH | 4.1 ± 1.8 | 3.7 ± 1.7 | 4.8 ± 1.6 |
| MSAS | 4.6 ± 1.6 | 4.0 ± 1.4 | 4.5 ± 1.3 |
| GDI | 4.9 ± 2.4 | 3.7 ± 1.9 | 4.8 ± 1.4 |
PHYS=Physical Symptoms Score; PSYCH=Psychological Symptoms Score; MSAS=Total Symptoms Score; GDI=Global Distress Index
DISCUSSION
We evaluated the symptom experiences at home in children and adolescents using MSAS. The most frequent physical symptoms (nausea, fatigue, pain) were consistent with other reports (Miller, et al., 2011; Yeh, et al., 2008; Yeh, et al., 2009; Atay, et al., 2012; Baggott, et al., 2012; Walker, et al., 2012). Most of these studies however were collected during hospitalization. Gastrointestinal symptoms (lack of appetite, nausea, mouth sores, and constipation) were also among the most frequently reported physical symptoms, similar to other reports (Atay, Conk & Bahar, 2012; Yeh, et al., 2008; Baggott, et al., 2012). A major barrier to adequate symptom management at home is underreporting (Jacob, et al., 2008). Our study is among the first to use MSAS at home and is a comprehensive tool for reporting the frequency, severity, and distress associated with symptoms. It would be useful for individually tailoring interventions that could alleviate symptoms at home.
It is important to note that pain continues to be among the most frequent, the most severe, and the most distressful symptom as previously reported by others (Collins et al., 2000; Collins et al., 2002; Miller, Jacob & Hockenberry, 2011; Walker et al., 2012). It has been estimated that about 49%-62% of children adolescents with cancer will experience pain related to the disease and/or associated invasive procedures and related treatments (Jibb et al., 2014). Pain negatively affects the patients quality of life, impedes in recovery, and is distressing to the patient and family. We found that having mouth sores was among the most distressful symptom, which may be attributed to intense chemotherapy (Atay and colleagues 2012). The use of standardized oral care protocol (preventive mouth wash—maalox, mycostatin, diphenhydramine; pain medication, lidocaine mouth swabs; chlorhexidine mouth rinses and 0.9% saline rinse) may prevent mucosal damage, or decrease the duration and severity of mouth sores (Cheng et al., 2001; De Brito Costa, 2003); however, there is little information about adherence to the multimodal oral care at home. Future studies are needed to evaluate adherence to oral care protocol treatments and effectiveness of different approaches to prevent or minimize oral mucositis, as well as other sources of pain.
While fatigue was among the most prevalent symptom, it was not reported as the most severe or distressful symptom at home, which is consistent with findings by others who used the MSAS (Walker et al., 2012; Miller, Jacob & Hockenberry, 2011). Previous studies have found that fatigue was subjective because there were differences in perceptions of fatigue, mostly attributed to developmental variations during adolescence, chemotherapy phase, or sleep patterns (Hinds et al., 2007; Walker et al., 2012). Patients reported the highest levels of fatigue one week following chemotherapy, which decreased over time (Atay et al., 2012; Baggott et al., 2012). Fatigue often goes unnoticed because it is part of various sedentary activities, such as watching TV, less frequently assessed and may not be properly addressed at home (Van Cleve et al., 2012). Increased levels of fatigue might have adverse consequences, such as sleep disturbances, depressive symptoms, and interference with social interactions, that can predominate during the maintenance phase of chemotherapy or after treatment (Walker et al., 2012). Future studies therefore, need to examine whether fatigue and depressive symptoms occur concurrently in children and adolescents with cancer at home.
Nausea was highly prevalent, which is consistent with previous reports that attributed nausea to chemotherapy (Miller, et al., 2011; Atay, Conk & Bahar, 2012). Children and adolescents receive aggressive treatment protocols with multiple chemotherapy agents that were categorized as moderately to highly emetogenic (Walker et al., 2012). Delayed nausea may be experienced two to five days after chemotherapy (Bloechl-Daum, Deuson, Mavros, Hansen, & Herrstedt, 2006; Holdsworth et al., 2006). Effectiveness of interventions for nausea has not been well established in young patients (Flank et al., 2016; Stamoulara et al., 2015). The guideline recommendation is that anticipatory nausea and vomiting may best be prevented through the use of benzodiazepines (Dupuis, et al., 2017). For example, parents may be instructed to give a prescribed lorazepam once at bedtime the night before chemotherapy, and once the next day prior to administration of chemotherapy to prevent or treat anticipatory nausea and vomiting in children and adolescents. Another recommendation is the use of behavioral therapies, such as progressive muscle relaxation training, systematic desensitization and hypnosis (Dupuis, et al., 2017). Future studies need to evaluate whether pharmacological and nonpharmacological approaches may be effective at home.
The existing literature about psychosocial symptoms at home in children and adolescents with cancer is scarce. Most reports about psychological symptoms were mostly anxiety, fear, and worries before and after procedures during hospitalizations (Baggott et al., 2012; Woodgate, 2005; Ribeiro, et al., 2009). Patients may have worries or concerns at home about treatment failure and fears of dying (Cicogna; Nascimento; Lima, 2012). Other negative emotions such as feeling sad, angry and feeling sorry for yourself have been previously reported to be associated with fatigue (Walker et al., 2010).
It is not surprising that the most severe psychosocial symptom was “less hair than usual”, as hair loss changes self-image (Heden, et al., 2013; Dupuis, et al., 2010; Cicogna, Nascimento & Lima, 2010; De Bolle, et al., 2008). Previous studies have also reported that parents considered hair loss as quintessential evidence of their child’s illness and described it as traumatizing and distressing (Sadruddin, et al., 2013; Williamson, et al., 2010). Health care providers may underestimate the importance and devastating effects of hair loss during cancer treatments. Minimizing the negative effects of hair loss and promoting positive self-image ideals need to be openly discussed with pediatric patients and parents prior to going home. Studies are needed to evaluate the effects of interventions to promote positive self-image, increase self-esteem, and self-confidence in pediatric patients with cancer (Giesbers, et al., 2010; Sidhu, et al, 2006).
Finally, we found symptoms at home varied by sex and diagnoses. Females experienced worse physical and psychosocial symptoms, which is in contrast to previous finding where males had higher reports of gastrointestinal symptoms (Van Cleve et al., 2012). The diagnoses of leukemias/lymphomas and sarcomas were associated with worse physical, psychosocial and overall distress scores. These findings are consistent with previous reports (Miller, Jacob and Hockenberry, 2011; Woodgate, 2005). Future studies are needed to develop and test specific strategies that takes into account differences in sex and cancer diagnoses to minimize distress associated with symptoms.
Limitations.
Several limitations affect the generalizability of these findings. A major limitation in this study is the small sample size. Subjects had different cancer diagnoses and treatment protocols, which may therefore influence different symptom experiences. It was not possible to make comparisons between the different diagnoses due to the small sample size of the study. However, regardless of the protocol, these symptoms need to be evaluated and managed. Future studies with a larger sample size are warranted. Also, the study was conducted in two settings which may have specific characteristics that differ from other settings. Although no participants reported difficulty in filling out all the forms, some of them could have experienced survey fatigue which could have impacted results. Children younger than 8 years and those with cognitive or neurological impairments were not included, and therefore, their symptom experiences may be different. Finally, only the English version was used, therefore those were are not able to speak, read, write, and understand English were excluded.
CONCLUSION
Understanding symptom management in the home environment is an essential area of research that could continue to improve the outcomes in children and adolescents with cancer. Pediatric Nurse Practitioners and Advanced Practice Registered Nurses (FNP/APRN) play an important role in managing the symptoms at home of their oncology patients as they develop comprehensive, therapeutic treatment plans that include both pharmacological and nonpharmacologic strategies as well as education and additional referrals. Particularly important for the APRN is to integrate and make referrals to palliative care early in the cancer trajectory for optimal symptom management. The APRN can facilitate effective teamwork and communications across settings (hospital, home, clinic) as patients transition between care providers during the cancer trajectory.
The MSAS for symptom reporting is useful for evaluating the frequency, severity, and distress associated with specific symptoms as well as the overall distress associated with multiple symptoms at home. It promotes self-monitoring that may lead to self-management. Strategies can be individually tailored not only specific to physical, but also to psychosocial symptoms experienced at home. Self-monitoring may prompt children and adolescents to contact APRNs if symptoms are not resolved, as previously done in other chronic conditions (Jacob, et al., 2012; Jacob, et al., 2013a; Jacob, et al., 2013b), instead of waiting until subsequent clinic visits (Dupuis, et al., 2012; Miller, et al., 2011). The APRN may remotely monitor symptoms using wireless technology (Jacob, et al, 2013a), facilitate communications about symptoms between patients and clinicians (Jacob, et al, 2013b), and improve timely management of symptoms (Jib, et al., 2017a; Jib, et al., 2017b).
ACKNOWLEDGEMENT
Funding was received from the Alex Lemonade Stand Foundation and the University of California Los Angeles, Center for Vulnerable Populations Research (National Institute of Nursing Research #P30NR005041). The authors would like to thank all the children and adolescents with cancer who participated in this study. They are grateful to the advanced practice registered nurses at the Children’s Hospital Los Angeles (Kellie Loera, RN, MSN, CPON, Peggy Townsend, RN, MSN, CPON) and Children’s Hospital Orange County (Sharon Bergeron, RN, BSN, CPON) who facilitated accessing participants, distributed study flyers, invited eligible participants, assisted with consenting and assenting procedures, and facilitated data collection. They also acknowledge the research assistance provided by Nicole Greenwood, BA, RN, Meredith Pelty, PsyD, and Olga Nudelman, BS, RN.
Funding for the research was provided by the UCLA Center for Vulnerable Populations Research (National Institute of Nursing Research #P30NR005041) and the Alex’s Lemonade Stand Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no conflict of interest.
Contributor Information
Vanessa Torres, Children’s Hospital Los Angeles, Children’s Center for Cancer and Blood Diseases, 4 East, 4650 Sunset Boulevard, Los Angeles, CA 90027.
Michelle Darezzo Rodrigues Nunes, Associate Professor of the Department of Maternal-Infant at State University of Rio de Janeiro, UERJ, Brazil. Address: Blvd 28 de Setembro, 157 Vila Isabel, Rio de Janeiro (RJ), Brazil 14040-902.
Fernanda Machado Silva-Rodrigues, Interunit Doctoral Program in Nursing, Ribeirao Preto College of Nursing, University of São Paulo, Bandeirantes Avenue 3900, 14040-902, Ribeirao Preto (SP), Brazil.
Lilian Bravo, University of North Carolina Chapel Hill, School of Nursing, Carrington Hall, S Columbia St, Chapel Hill, NC 27599.
Kathleen Adlard, CHOC Children’s Hospital; 1201 W La Veta Ave, Orange, CA 92868.
Rita Secola, Hematology Oncology Service, Children’s Hospital Los Angeles, Children’s Center for Cancer and Blood Diseases, 4 East, 4650 Sunset Boulevard, Los Angeles, CA 90027.
Ananda Maria Fernandes, Escola Superior de Enfermagem de Coimbra, Av. Bissaya Barreto 143, Coimbra, Portugal.
Lucila Castanheira Nascimento, Associate Professor of the Department of Maternal-Infant and Public Health Nursing at Ribeirão Preto College of Nursing, University of São Paulo. Address: Bandeirantes Avenue 3900, 14040-902, Ribeirão Preto (SP), Brazil.
Eufemia Jacob, University of California Los Angeles, USA. 700 Tiverton Avenue, Factor Building 5-942. Los Angeles, CA 90095.
REFERENCES
- Atay S, Conk Z, Bahar Z (2012). Identifying symptom clusters in pediatric cancer patients using the Memorial Symptom Assessment Scale. European Journal of Cancer Care (21): 460–468. [DOI] [PubMed] [Google Scholar]
- Baggott CR, Dodd M, Kennedy C, Marian N, Matthay KK, Cooper BA, Miaskowski C (2011). An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Support Care Cancer 19(3): 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott CR, Dodd M, Kennedy C, Marian N, Matthay KK, Cooper BA, Miaskowski C (2012). Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. Journal of Pediatric Oncology Nursing 27(6): 307–315. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG (1996). Statistics notes: Transforming data. BMJ (312) 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006). Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. Journal of Clinical Oncology 24(27): 4472–4478. [DOI] [PubMed] [Google Scholar]
- Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT (2000). The Memorial Symptom Assessment Scale Short Form (MSAS-SF) reliability and validity. Cancer (89:) 1162–1171. [DOI] [PubMed] [Google Scholar]
- Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler ΗT (2001). The adolescents with depressive and aggressive dispositions: Evidence from surveys and electronic diaries. Health Psychology 20(2): 99–111. [PubMed] [Google Scholar]
- Cicogna E de C, Nascimento LC, Zupanec S, Jones H, Stremler R (2010). Sleep habits and fatigue of children receiving maintenance chemotherapy for all and their parents. Journal of Pediatric Oncology Nursing 27(4): 217–28. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Byrnes ME, Dunkel IJ, Lapin J, Nadel T, Thaler ΗT, Portenoy RK (2000). The measurement of symptoms in children with cancer. Journal of Pain and Symptom Management 19(5): 363–377. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Devine TD, Dick GS, Johnson EA, Kilham HA, Pinkerton CR, Portenoy RK (2002). The meaurement of symptoms in young children with cancer: the validation of the Memorial Symptom Assessment Scale in children aged 7–12. Journal of Pain and Symptom Management 23(1): 10–16. [DOI] [PubMed] [Google Scholar]
- De Bolle MD, Clercq BD, Fruyt FD, Benoit Y (2008). Self- and parental perspectives on quality of life in children with cancer. Journal of Psychosocial Oncology 26: 35–47. [DOI] [PubMed] [Google Scholar]
- Dupuis LL, Milne-Wren C, Cassidy M, Barrera M, Portwine C, Johnston DL, Sung L (2010). Symptom assessment in children receiving cancer therapy: the parents’ perspective. Support Care Cancer (18): 281–99. [DOI] [PubMed] [Google Scholar]
- Dupuis LL, Marie-Chantal E, Tomlinson D, Hesser T, Sung L (2012). A systematic review of symptom assessment scales in children with cancer. BMC Cancer (12): 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis LL, Roscoe JA, Olver I, Aapro M, Molassiotis A. (2017) 2016 updated MASCC/ESMO consensus recommendations: Anticipatory nausea and vomiting in children and adults receiving chemotherapy. Support Care Cancer. January;25(1):317–321. [DOI] [PubMed] [Google Scholar]
- Flank J, Robinson PD, Holdsworth M, Phillips R, Portwine C, Gibson P, Dupuis LL (2016). Guideline for the treatment of breakthrough and the prevention of refractory chemotherapy-induced nausea and vomiting in children with cancer. Pediatric Blood and Cancer 63(7): 1144–1151. [DOI] [PubMed] [Google Scholar]
- Giesbers J, Verdonck-de Leeuw IM, Van Zuuren FJ, Kleverlaan N, Van der Linden MH (2010). Coping with parental cancer: web-based peer support in children. Psychooncology 19(8): 887–92. [DOI] [PubMed] [Google Scholar]
- Goldman A, Hewitt M, Collins GS, Childs M, Hain R (2006). Symptoms in children/young people with progressive malignant disease: United kingdom children’s cancer study group/paediatric oncology nurses forum survey. Pediatrics 117(6): e1179–86. [DOI] [PubMed] [Google Scholar]
- Hagmann C, Cramer A, Kestenbaum A, Durazo C, Downey A, Russell M, Geluz J, Ma JD, Roeland EJ. Evidence-based Palliative Care Approaches to Non-pain Physical Symptom Management in Cancer Patients. Semin Oncol Nurs. 2018. August;34(3):227–240. [DOI] [PubMed] [Google Scholar]
- Hansson H, Kjærgaard H, Johansen C, Hallström I, Christensen J, Madsen M, Schmiegelow K (2013). Hospital based home care for children with cancer: feasibility and psychosocial impact on children and their families. Pediatric Blood and Cancer 60(5): 865–872. [DOI] [PubMed] [Google Scholar]
- Heath JA, Clarke NE, Donath SM, McCarthy M, Anderson VA, Wolfe J (2010). Symptoms and suffering at the end of life in children with cancer: an australian perspective. Medical Journal of Australia 192(2): 71–5. [DOI] [PubMed] [Google Scholar]
- Heden L, Poder U, Von Essen L, Ljungman G (2013). Parents’ perception of their child symptom burden during and after cancer treatment. Journal of Pain and Symptom Management (46): 366–75. [DOI] [PubMed] [Google Scholar]
- Hinds PS, Hockenberry M, Rai SN, Zhang L, Razzouk BI, McCarthy K, Rodriguez-Galindo C (2007). Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncology Nursing Forum 34: 393–402. [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K (2010). Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncology Nursing Forum (37): 16–25. [DOI] [PubMed] [Google Scholar]
- Holdsworth MT, Raisch DW, Frost J (2006). Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer 106(4): 931–940. [DOI] [PubMed] [Google Scholar]
- Humphreys J, Lee KA, Carrieri-kohlman V, Puntillo K, Faucett J, Janson S (2008). Theory of symptom management. Middle range theory for nursing (2): 145–158. [Google Scholar]
- Jacob E, Stinson J, Duran J, Gupta A, Gerla M, Ann LM, Zeltzer L (2012). Usability testing of a smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. Journal of Pediatric Hematology/Oncology Nursing: 34(5): 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Duran J, Stinson J, Lewis MA, Zeltzer L (2013a). Remote monitoring of pain and symptoms using wireless technology in children and adolescents with sickle cell disease. Journal of the American Association of Nurse Practitioners 25(1): 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Pavlish C, Duran J, Stinson J, Lewis MA, Zeltzer L (2013b). Facilitating pediatric patient-provider communications using wireless technology in children and adolescents with sickle cell disease. Journal of Pediatric Health Care 27(4): 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Hesselgrave J, Sambuco G, Hockenberry M (2007). Variations in pain, sleep, and activity during hospitalization in children with cancer. Journal of Pediatric Oncology Nursing 24(4): 208–219. [DOI] [PubMed] [Google Scholar]
- Jacob E, McCarthy KS, Sambuco G, Hockenberry M (2008). Intensity, location, and quality of pain in spanish-speaking children with cancer. Pediatric Nursing 34(1): 45–52. [PubMed] [Google Scholar]
- Jibb LA, Stevens BJ, Nathan PC, Seto E, Cafazzo JA, Johnston DL, Hum V, Stinson JN (2017). Implementation and preliminary effectiveness of a real-time pain management smartphone app for adolescents with cancer: A multicenter pilot clinical study. Pediatric Blood Cancer 64(10). [DOI] [PubMed] [Google Scholar]
- Jibb LA, Stevens BJ, Nathan PC, Seto E, Cafazzo JA, & Stinson JN (2014). A Smartphone-Based Pain Management App for Adolescents With Cancer: Establishing System Requirements and a Pain Care Algorithm Based on Literature Review, Interviews, and Consensus. JMIR Research Protocols, 3(1), e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibb LA, Cafazzo JA, Nathan PC, Seto E, Stevens BJ, Nguyen C, Stinson JN (2017). Development of a mHealth real-time pain self-management app for adolescents with cancer: An iterative usability testing study. Journal of Pediatric Oncology Nursing 34(4): 283–294. [DOI] [PubMed] [Google Scholar]
- Kaleyias J, Manley P, Kothare SV (2012). Sleep disorders in children with cancer. Seminars in Pediatric Neurolgy 19(1): 25–34. [DOI] [PubMed] [Google Scholar]
- Klaassen RJ, Barr DB, Hughes JBA (2010). Nurses provide valuable proxy assessment of the health-related quality of life of children with hodgkin disease. Cancer 116: 1602–7. [DOI] [PubMed] [Google Scholar]
- Li HC, Williams PD, Lopez V, Chung JO, Chiu SY (2013). Relationships among therapy-related symptoms, depressive symptoms, and quality of life in Chinese children hospitalized with cancer: an exploratory study. Cancer Nursing 36(5): 346–54. [DOI] [PubMed] [Google Scholar]
- Miller E, Jacob E, Hockenberry MJ (2011). Nausea, pain, fatigue and multiple symptoms in hospitalized children with cancer. Oncology Nursing Forum 38(5). [DOI] [PubMed] [Google Scholar]
- Ribeiro CA, Coutinho RM, Araujo TF, Souza VS (2009). Vivenciando um mundo de procedimentos e preocupações: experiência da criança com Port-a-Cath. Acta Paulista de Enfermagem, 1(22). [Google Scholar]
- Sadruddin MM, Hameed-ur-Rehman M (2013). Understanding the perception of children battling cancer about self and others through drawing. South Asia Journal of Cancer (2): 113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamoulara A, Papadopoulou K, Perdikaris P, Matziou V (2015). Current management techniques of nausea and vomiting in children with cancer. Childrens Health Care 46(4): 421–438. [Google Scholar]
- Sidhu R, Passmore A, Baker D (2006). The effectiveness of a peer support camp for siblings of children with cancer. Pediatric Blood Cancer 47(5): 580–8. [DOI] [PubMed] [Google Scholar]
- Stinson JN, Stevens BI, Feldman BM, Streiner DL, McGrath PI, Dupuis A, Gill N, Petroz GC (2011) Using an electronic pain diary to better understand pain in children and adolescents with arthritis. Pain Management 1(2): 127–37. [DOI] [PubMed] [Google Scholar]
- Theunissen JΜI, Hoogerbrugge PM, Achterberg TV (2007). Symptoms in the palliative phase of children with cancer. Pediatric Blood Cancer (49): 160–5. [DOI] [PubMed] [Google Scholar]
- Van Cleve L, Muñoz CE, Savedra M, Riggs M, Bossert E, Grant M, Adlard K (2012). Symptoms in children with advanced cancer. Cancer Nursing 35(2): 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Baeyer CL, Hicks CL (2000). Support for a common metric for pediatric pain intensity scales. Pain Research and Management 5: 157–160. [Google Scholar]
- Von Lützau P, Otto M, Hechler T, Metzing S, Wolfe I, Zernikow B (2012). Children dying from cancer: parents’ perspectives on symptoms, quality of life, characteristics of death, and end-of-life decisions. Journal of Palliative Care 28(4): 274–81. [PubMed] [Google Scholar]
- Walker AJ, Gedally-Duff V, Miaskowski C, Nail L (2010a). Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. Journal Pediatric Oncology Nursing 25(5): 259–65. [DOI] [PubMed] [Google Scholar]
- Walker AJ, Johnson KP, Miaskowski C, Lee KA, Gedaly-Duff V (2010b). Sleep quality and sleep hygiene behaviors of adolescents during chemotherapy. Journal of Clinical Sleep Medicine (6)5: 439–444. [PMC free article] [PubMed] [Google Scholar]
- Wolfe J, Orellana L, Ullrich C, Cook EF, Kang TI, Rosenberg A, Geyer R, Feudtner C, Dussel V (2015). Symptoms and distress in children with advanced cancer: Prospective patient-reported outcomes from the PediQUEST Study. Journal of Clinical Oncology 33(17): 1928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates P Symptom Management and Palliative Care for Patients with Cancer. Nurs Clin North Am. 2017. March;52(1):179–191. [DOI] [PubMed] [Google Scholar]
- Yeh C, Chiang Y, Chien L, Lin L, Yang C, Chuang H (2008). Symptom clustering in older Taiwanese children with cancer. Oncology Nursing Forum 35(2): 273–281. [DOI] [PubMed] [Google Scholar]
- Yeh C, Wang C, Chiang Y, Lin L, Chien L (2009). Assessment of symptoms reported by 10 to 18-year-old cancer patients in taiwan. Journal of Pain and Symptom Management 38(5): 738–746. [DOI] [PubMed] [Google Scholar]


