Abstract
Normal aging is associated with decline of the sensorimotor mechanisms that support movement function in the human brain. In this study, we used behavioral and event-related potential (ERP) recordings to investigate the effects of normal aging on the motor preparatory mechanisms of speech production and limb movement. The experiment involved two groups of older and younger adults who performed randomized speech vowel vocalization and button press motor reaction time tasks in response to temporally predictable and unpredictable visual stimuli. Behavioral results revealed age-related slowness of motor reaction time only during speech production in response to temporally unpredictable stimuli, and this effect was accompanied by increased pre-motor ERP activities in older vs. younger adults during the speech task. These results indicate that motor preparatory mechanisms of limb movement during button press are not affected by normal aging, whereas the functional capacity of these mechanisms is reduced in older adults during speech production in response to unpredictable sensory stimuli. These findings suggest that the aging brain selectively compromises the motor timing of speech and recruits additional neural resources for motor planning and execution of speech, as indexed by the increased pre-motor ERP activations in response to temporally unpredictable vs. predictable sensory stimuli.
Keywords: Normal aging, Speech production, Limb movement, Motor timing, Reaction time, Event-related potentials
1. Introduction
In humans and many animal species, the central nervous system has developed highly specialized mechanisms to generate precisely timed and fine-tuned movements for interaction with the environment and reaching the goals of a wide range of behaviorally relevant tasks. Although the underlying neural mechanisms of movement timing processing are not fully understood, recent theories have proposed that the brain can learn and simulate the temporal patterns of sensory stimuli and establish an internal model to predict the neural representations of motor timing and their expected sensory feedback (Wolpert 1997; Wolpert and Flanagan 2001). This mechanism forms the basis of skilled motor behavior through establishing an internal temporal predictive code for estimating the next state of movements and their upcoming sensory consequences even before the actual sensory feedback has become available. This enhanced functional capacity plays a key role in optimized motor behavior with relevance to timing of current and upcoming sensory stimuli. However, an important question remains as to how normal aging affects the neural and behavioral mechanisms of motor timing processing and control.
Findings of previous studies in young adults have shown that the internal predictive mechanisms are modulated by the inherent temporal characteristics of external sensory stimuli (Bertelson and Boons 1960; Bevan et al. 1965; Vallesi et al. 2009a; Koppe et al. 2014; Berchicci et al. 2015; Behroozmand et al. 2016). This effect has been suggested to account for increased accuracy of the temporal predictive codes in response to predictable vs. unpredictable sensory stimuli, and subsequently faster motor reaction times in response to stimuli with predictable timing patterns (Klemmer 1956; Karlin 1959; Li et al. 2005; Koppe et al. 2014; Johari and Behroozmand 2017b; Johari and Behroozmand 2017a).
The underlying neural mechanisms of internal predictive codes have been investigated using neurophysiological recordings from the visual (Samaha et al. 2015), auditory (Lange 2009), and somatosensory (van Ede et al. 2011; Haegens et al. 2012; van Ede et al. 2014) systems. Findings of these studies have highlighted the role of the alpha and beta band neural oscillations in generating internal predictive codes and suggested that the timing of external stimuli can enhance such top-down predictive mechanisms and subsequently facilitate neural processing of incoming sensory information.
An important proposal of the internal forward model theory (Wolpert 1997; Wolpert and Flanagan 2001) is that temporal information processing is not only mediated by anticipatory mechanisms in the sensory system, but this process utilizes predictive coding mechanisms in the motor system that can further enhance temporal information processing during movement production. This notion has been supported by previous studies on limb movement (Johansson and Westling 1988; Bard et al. 1992; Blakemore et al. 1998; Witney et al. 1999) and speech production (Behroozmand et al. 2011; Chen et al. 2012; Kotz and Schmidt-Kassow 2015; Behroozmand et al. 2016), demonstrating that when sensory stimuli arise from self-produced motor actions, the internal forward model predicts the temporal relationships between motor commands and their sensory consequences. Findings of these studies have indicated that temporally predictable patterns can be learned by the internal forward model to modulate perceptual sensations arising from self-generated motor actions. During limb movement, the modulation of perceptual sensations has been shown to be reflected in attenuation of sensory responses to self-produced motor responses (Blakemore et al. 1998; Blakemore et al. 2000), which is hypothesized to be caused by central cancellation of sensory responses by the efference copies of the motor commands. In addition, studies have shown that the neural correlates of limb motor movement are differentially modulated by predictable vs. unpredictable stimuli (Alegre et al. 2003; Schwartze et al. 2012; Koppe et al. 2014), indicating that the internal predictive mechanisms are affected by temporal dynamics of incoming sensory stimuli. In the speech modality, studies have also demonstrated that neural responses to alterations in speech auditory feedback are differentially modulated in response to temporally predictable vs. unpredictable sensory stimuli, with greater motor-induced suppression in response to predictable feedback alteration stimuli (Chen et al. 2012; Behroozmand et al. 2016).
Single neuron recordings in primates have further corroborated the notion of an internal predictive mechanism during vocal production and motor control by showing that neurons in the primates’ auditory cortex were suppressed prior to the onset of self-produced vocalizations (Eliades and Wang 2003). This effect was suggested to reflect top-down predictive mechanisms (i.e. efference copies) that fine-tune sensory neural representations through motor-induced suppression of cortical auditory neurons before the onset of self-produced vocalizations. Further insights into the neural bases of temporal predictive mechanisms have been provided by recent neuroimaging studies in humans showing increased activation of a network involving the supplementary motor area (SMA) (Thickbroom et al. 2000), right dorsolateral (DLFPC) and ventrolateral (VLPFC) prefrontal cortex (Vallesi et al. 2007; Vallesi et al. 2009a), and the left inferior parietal cortex (IPC) (Coull et al. 2016) during movement initiation in response to temporally unpredictable vs. predictable stimuli. These findings support the key role of a frontoparietal network in differential neural processing of motor timing in response to predictable vs. unpredictable sensory stimuli. This latter notion was further supported by event-related potential (ERP) recordings revealing distinct patterns of neural activities during speech and limb motor responses to temporally predictable vs. unpredictable stimuli in young healthy adults (Alegre et al. 2003; Berchicci et al. 2015; Johari and Behroozmand 2017a; Johari and Behroozmand 2018). Findings of these studies suggest that pre-motor ERPs serve as a biomarker of temporal predictive coding during the planning phase of movement by showing that these neural activities were significantly suppressed in response to predictable vs. unpredictable stimuli, and that this suppression was correlated with faster motor reaction times in response to temporally predictable sensory stimuli (Johari and Behroozmand 2017a; Johari and Behroozmand 2018).
Despite the existing evidence supporting the notion of temporal predictive mechanisms during movement production, our understanding about the effect of normal aging on these mechanisms has remained limited. Normal aging is associated with functional decline in the temporal processing mechanisms of movement production, as indexed by age-related slowness of motor reaction time in response to externally presented sensory stimuli (Bherer and Belleville 2004; Sterr and Dean 2008; Balci et al. 2009; Seidler et al. 2010; Diersch et al. 2016). Such reduced capacity for motor timing processing has been suggested to result from declined internal temporal predictive mechanisms in older adults (Vieweg et al. 2015), and their reduced accuracy in predicting the timing of movement sequences during action occlusion tasks (Diersch et al. 2012; Diersch et al. 2013; Wolpe et al. 2016).
The age-related decline in the neural mechanisms of temporal predictive coding were characterized by decreased power of the alpha and increased power of the beta band neural oscillations in older adults during the planning phase of limb movement (Zanto et al. 2011; Vaden et al. 2012; Deiber et al. 2013). In other studies, neural deficits during the planning phase of limb movement in older adults were characterized by age-related increase in the amplitude of ERPs prior to the onset of movement, which was associated with the slowness of motor reaction time responses (Haaland et al. 1993; Yan et al. 1998; Berchicci et al. 2012). In addition, age-related modulation of ERP activation was identified as a neural correlate of diminished predictive coding mechanisms during speech production under altered auditory feedback in older adults (Li et al. 2018). Moreover, evidence from neuroimaging studies has suggested that older adults exhibit difficulties in incorporating temporal information from external sensory stimuli for motor timing coordination, and exhibit slower reaction times compared with their younger adult counterparts (Vallesi et al. 2009b; Zanto et al. 2011). The neural substrates of such age-related changes have been identified by showing that areas within the right dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) were less activated in older vs. younger adults during movement initiation in response to temporally unpredictable sensory stimuli (Vallesi et al. 2009b). These findings have indicated an age-related selective deterioration in sensory processing and motor timing coordination in response to stimuli with unpredictable temporal dynamics.
Although previous studies have provided new insights into the effects of normal aging on temporal predictive mechanisms of movement (Vallesi et al. 2009b; Seidler et al. 2010; Zanto et al. 2011; Diersch et al. 2013; Diersch et al. 2016), most of these studies have focused on the limb motor system (primarily limb movement), and therefore, less is known about the effects of age-related changes in motor timing processing during speech production. Evidence from previous research has suggested possible anatomical overlaps between neural substrates implicated in speech and limb movement tasks by showing concurrent activation of the left inferior frontal gyrus (i.e. Broca’s area) during tasks involving speech and limb movement (Binkofski et al. 1999; Gentilucci and Volta 2008; Gentilucci et al. 2009).
The present study was motivated by the question of how normal aging would affect motor timing processing of speech and limb movement in response to temporally predictable and unpredictable sensory stimuli. By using a classical motor reaction time paradigm combined with ERP recordings, we aimed to conduct a systematic investigation to determine the effects of normal aging on the behavioral and neural correlates of temporal predictive mechanisms in the speech and limb motor systems. Based on findings of previous studies (Bherer and Belleville 2004; Sterr and Dean 2008; Balci et al. 2009; Seidler et al. 2010; Diersch et al. 2016; Johari et al. 2018), we hypothesized that older adults would exhibit greater decline in motor timing processing of temporally unpredictable compared to predictable sensory stimuli, as indexed by slowed motor reaction times during speech production and limb movement. In addition, previous research has led to the identification of pre-motor ERP activities over the frontal and parietal areas that were modulated by temporal characteristics of sensory stimuli (Walter et al. 1964; Alegre et al. 2003; Pfeuty et al. 2005; Nobre et al. 2007; Coull et al. 2016; Johari and Behroozmand 2017b; Johari and Behroozmand 2018), and it was shown that these neural responses were increased in older adults for tasks involving speech production and limb movement (Haaland et al. 1993; Yan et al. 1998; Berchicci et al. 2012). Based on these data, we focused on examining the pre-motor ERP correlates of speech and limb movement and hypothesized that older adults would exhibit stronger neural activities within a fronto-parietal network, reflecting their need for access to additional neural resources for motor planning and execution of movement during motor reaction time tasks. In addition, we predicted to observe differential modulation of ERPs in response to temporally predictable vs. unpredictable visual cues in younger vs. older adults, which would reflect age-related changes in the temporal predictive mechanisms that extract timing information to drive speech and limb motor reaction time responses to externally presented sensory stimuli.
2. Materials and methods
2.1. Participants
Fifteen younger (20 – 30 years old; mean age: 23; 7 males) and fifteen older (50 to 80 years old; mean age: 63; 8 males) adults who were native speakers of English participated in the present study. All subjects reported no history of psychiatric, neurological or speech disorder, and had normal hearing and normal (or corrected) vision. Handedness of subjects was assessed using the Edinburgh handedness inventory (Oldfield 1971), and all were right handed (score rage 72–100). All study procedures, including recruitment, data acquisition and informed consent were approved by the University of South Carolina Institutional Review Board, and subjects were monetarily compensated for their participation.
2.2. Experimental design
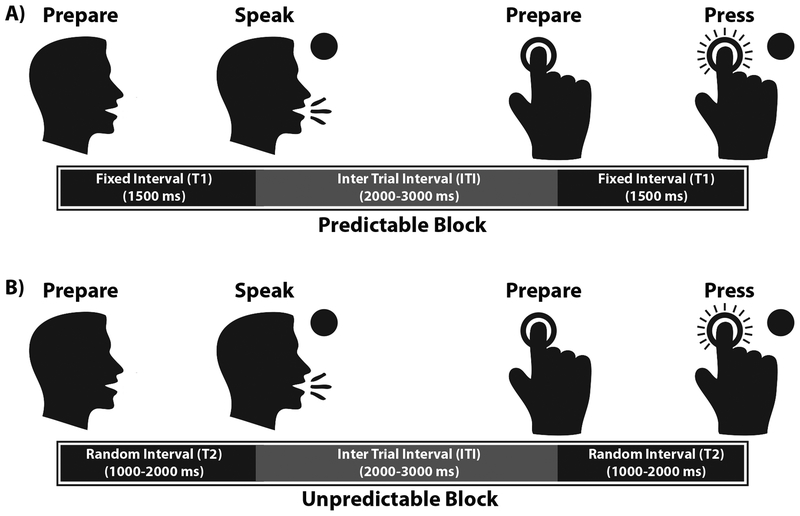
The experiment was conducted in a sound attenuated booth in which subjects performed the speech and limb movement tasks while EEG signals were recorded. Note that in this study, the terms speech production and limb movement are used to refer to vowel vocalization and button press tasks, respectively. During each task, subjects were instructed to prepare for the cued movement and start vocalizing a steady speech vowel sound /a/ or pressing a button with the index finger of their dominant (i.e. right) limb after a circle (go signal) appeared on the screen and to stop when the circle disappeared (Figure 1). We designed two counterbalanced blocks within which subjects performed the speech and limb movement tasks in a randomized order: 1) a temporally-predictable block, in which there was a fixed time interval of 1500 ms between the onset of the visual cue and go signal and 2) a temporally-unpredictable block in which the time internal between visual cue and go signal was randomized between 1000–2000 ms using a linear distribution. During each block, a total number of 220 trials were collected, with approximately 110 trials for speech and 110 trials for limb movement. The inter-trial-interval (ITI) was 2–3 seconds in each block and subjects took a 5-minute break between two blocks. All the experimental parameters, including the order of the tasks, conditions, visual cues, go/stop signals, and the stimulus timing intervals were controlled by a custom-made program implemented in Max/Msp 5.0 program (Cycling ‘74). Subjects’ responses including speech vowel sound vocalizations and button presses were recorded at 44.1 KHz on a laboratory computer for the analysis of the motor reaction times and time-locked averaging of the ERP responses in each experimental condition.
Figure 1.
Experimental design of the motor reaction time task for A) temporally predictable and B) unpredictable blocks. In each block, subjects were presented with a task-relevant visual cue (limb or speech) and were instructed to prepare to press a button or vocalize the vowel /a/ after a circle (go signal) appeared on the screen and stop after it disappeared. In this figure, T indicates the time interval between “Preparation” and “Go” in either button press or vocalization task. For the predictable block, the time interval (T1) was fixed at 1500 ms, whereas for the unpredictable block, the time interval (T2) was randomized between 1000–2000 ms. ITI represents the inter-trial-interval which was about 2–3 seconds for both predictable and unpredictable conditions.
2.3. Behavioral and EEG data acquisition
The speech signal was picked up using a head-mounted AKG condenser microphone (model C520), amplified by a Motu Ultralite-MK3, and delivered to subjects through Etymotic insert earphones (model ER-1). The onset of speech vowel vocalizations were detected using a voice onset detector algorithm in Max/Msp, and the onset of button presses were registered at the time when subjects pressed the button in response visual cue stimuli. The onsets of speech and limb movement triggered TTL pulses that were generated by Max/Msp, and these TTL pulses were simultaneously recorded in the EEG file for time-locked averaging of ERP activities in response to the onset of speech and limb movement. The EEG signals were recorded from 64 electrodes on the subjects’ scalp using the Brain Vision active electrode system (Brain Products GmbH, Germany) placed on a standard electrode cap (Easy-Cap GmbH, Germany). The electrode placement on the cap followed the standard 10–20 montage and the EEG signals were recorded using a common reference. A BrainVision actiCHamp amplifier (Brain Products GmbH, Germany) on a computer utilizing Pycorder software recorded the EEG signals at 1 kHz sampling rate after applying a low-pass anti-aliasing filter with 200 Hz cut-off frequency.
2.4. Reaction time analysis
A custom-made MATLAB code was used to obtain measures of reaction time during speech production and limb movement for both younger and older adults. Reaction times for speech production and limb movement were calculated by the time difference between the onset of the “Go” cues and the initiation of speech and limb movement responses, respectively. We verified that the error rates of inconsistent motor responses (e.g., pressing a button instead of vocalizing or vice versa) were below 5% for both younger and older adults, and those erroneous trials we excluded from data analysis. For statistical analysis, measures of speech and limb motor reaction times were submitted to a mixed ANOVA model with the group age (older vs. younger adults) as a between-subjects factor, and stimulus timing (predictable vs. unpredictable) and modality (speech vs. limb) as within-subjects factors.
2.5. EEG analysis
The EEGLAB toolbox (Delorme and Makeig 2004) was used to analyze the recorded EEG signals in order to extract ERPs time-locked to the onset of speech production and limb movement during temporally predictable and unpredictable conditions for both age groups. The recorded EEGs were first band-pass filtered using a standard EEGLAB FIR filter with cut-off frequencies set to 1 and 30 Hz (−24 dB/oct). Independent Component analysis (ICA) was applied to remove eye movement, blinks, muscle, and line noise artefacts. Following ICA, the EEG signals were segmented into epochs ranging from −500 ms before and 500 ms after the onset of speech production and limb movement. Since the choice of the band-pass filter with its high-pass cut-off at 1 Hz would automatically remove DC offsets from EEG data and would make baseline correction obsolete (Widmann et al. 2015; Maess et al. 2016), we did not implement a separate baseline correction procedure. This approach was specifically helpful to analyze EEG data in the pre-motor time window without artificially aligning EEG activities to a pre-defined baseline period before the onset of speech and limb movement responses. The extracted epochs were then averaged across all trials separately for each condition (predictable vs. unpredictable) to obtain ERP responses for speech and limb movement onset during predictable and unpredictable blocks for both age groups separately. A minimum number of 100 trials for each condition were used to calculate ERP responses for each individual subject. The extracted ERP profiles were then averaged across all subjects to calculate the grand-average ERP responses.
The extracted ERP components in response to speech and limb movement initiation were separately analyzed within 6 regions of interests (ROIs) that included electrodes over the frontal (F), frontocentral (FC), frontotemporal (FT), central (C), centroparietal (CP), parietal (P) areas. In our previous studies (Johari and Behroozmand 2017a; Johari and Behroozmand 2018), we found that 50 ms time windows are sensitive enough to capture the dynamic nature of ongoing motor timing processing of sensory stimuli during the preparatory phase of speech and limb movement. Therefore, in the present study, ERP amplitudes were extracted for 10 pre-motor time windows from −500 to 0 ms time windows with 50 ms duration. For each time window, neural responses were measured as the mean amplitude of ERP responses in two electrodes in the left (e.g., left frontocentral: FC1and FC5) and two electrodes in the contralateral right side for each ROIs (e.g., right frontocentral: FC2 and FC6). In each pre-motor time window, mixed ANOVA models were performed using SPSS v.24 for each ROI to examine the effects of age group (young vs. old adults) as a between-subjects factor, and stimulus timing (predictable vs. unpredictable), modality (speech vs. limb), and laterality (left vs. right) as within-subjects factors on pre-motor ERP activities. The p-values were adjusted for the number of time windows using Bonferroni’s correction for multiple comparisons. The partial eta squared (η2) was used to report effect size for the main effects and interactions.
3. Results
3.1. Motor reaction time
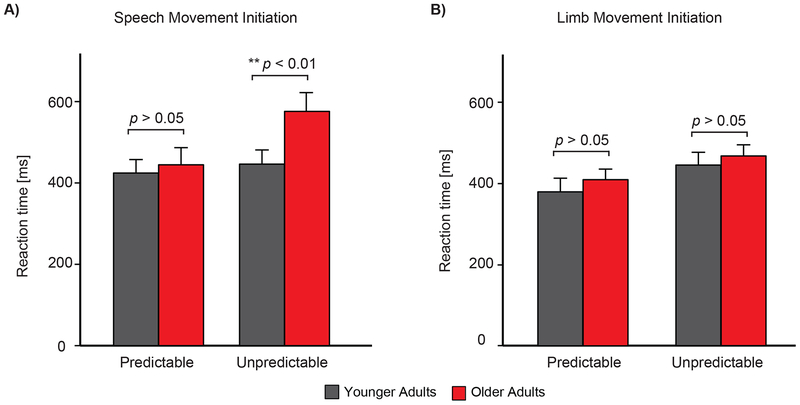
The bar plot representation of the behavioral measures of motor reaction time are shown in Figure 2. The statistical analysis yielded significant main effects of group (F(1,28) = 4.27, p < 0.05, partial η2 = 0.13), timing (F(1,28) = 14.67, p < 0.001, partial η2 = 0.34), and modality (F(1,28) = 15.76, p < 0.001, partial η2 = 0.36), and these effects were qualified by a significant group × timing × modality interaction (F(1,28) = 4.60, p = 0.04, partial η2 = 0.14). Follow-up analysis for speech movement revealed significant main effects of timing (F(1,28) = 8.17, p < 0.01, partial η2 = 0.22), group (F(1,28) = 7.12, p < 0.05, partial η2 = 0.20), and timing × group interaction (F(1,28) = 6.06, p < 0.05, partial η2 = 0.17). Post-hoc analysis revealed that older adults were significantly slower than younger adults during speech production in response to temporally unpredictable stimuli (t(28) = 3.23, p < 0.01), but no such effect was observed for the predictable stimuli (t(28) = 0.26, p = 0.79). Follow-up analysis for limb movement revealed a significant effect of timing (F1,28) = 7.89, p < 0.01, partial η2 = 0.22) with faster motor reaction times in response to temporally predictable compared with unpredictable sensory stimuli. However, there was no significant effect of group (F(1,28) = 0.70, p = 0.40, partial η2 = 0.02), nor a timing × group interaction (F(1,28) = 0.01, p = 0.90, partial η2 = 0.001) on motor reaction times during limb movement.
Figure 2.
Illustrates the motor reaction times in younger and older adult for initiation of A) speech and B) limb movement initiation in response to temporally predictable and unpredictable stimuli.
3.2. ERP results
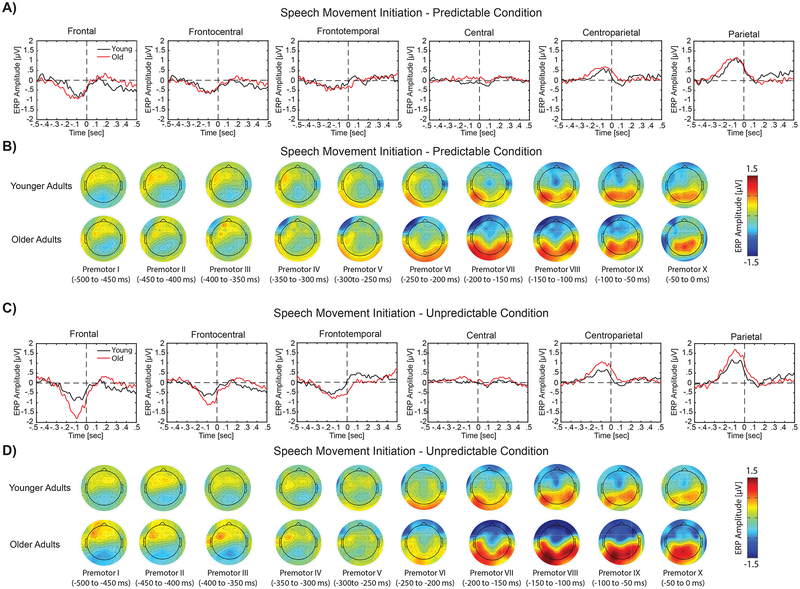
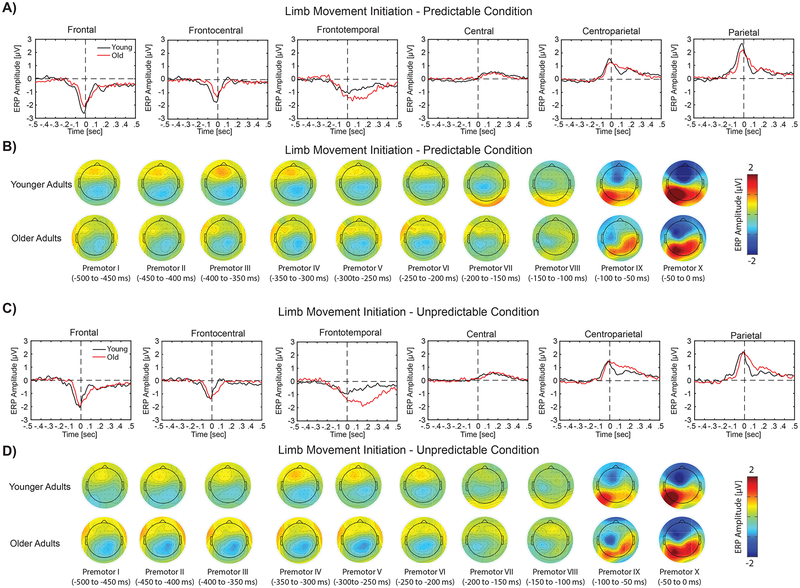
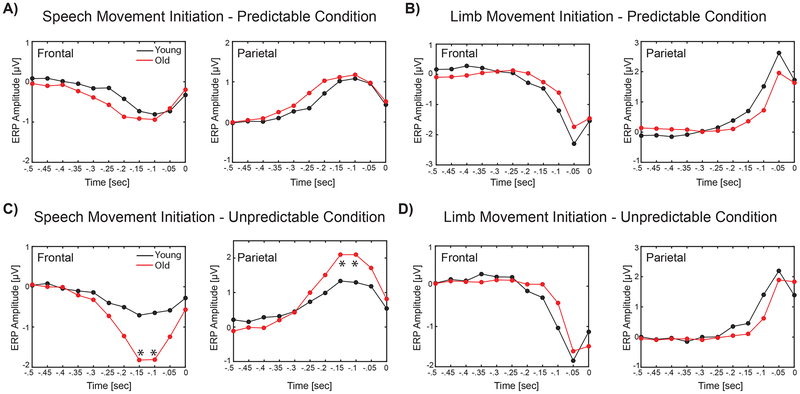
Results of the analysis for ERP responses to temporally predictable and unpredictable stimuli are shown in Figures 3 and 4, respectively for speech production and limb movement. In these figures, the overlaid profiles of ERP activities for younger vs. older adults are shown in panels A and C for temporally predictable and unpredictable conditions, respectively. The topographical distribution maps are plotted for 64 electrodes within 10 time windows from −500 to 0 ms prior to the onset of speech and limb movement in panels B and D for temporally predictable and unpredictable stimuli, respectively. For both speech and limb movement, prominent ERP activities were identified over the bilateral frontal and parietal areas in response to temporally predictable and unpredictable sensory stimuli. Statistical analysis for pre-motor ERP activities revealed a significant group × timing × modality interaction over the frontal (F(1,28) > 6.58 p < 0.01, partial η2 > 0.21) and parietal electrodes (F(1,28) > 5.60, p < 0.02, partial η2 > 0.19) within two time windows from −150 to −50 ms. Follow-up analysis for these time windows revealed significant timing × group interactions for speech production over the frontal (F(1,28) > 7.67, p < 0.01, partial η2 > 0.23) and parietal (F(1,28) > 6.45, p < 0.02, partial η2 > 0.20) areas. Post-hoc analysis showed that pre-motor ERP activities before the onset of speech were significantly larger for older vs. younger adults in response to unpredictable stimuli (t(28) > 2.5, p < 0.03), but no such effect was observed for predictable stimuli (t(28) < 0.68, p > 0.5) (Figure 5, Panels A and C). However, follow-up analysis for limb movement did not revealed a significant timing × group interaction over the frontal and parietal areas (F(1,28) < 0.88, p > 0.36, partial η2 < 0.03) (Figures 5, panels B and D). In addition, we found a significant main effect of laterality, indicating stronger pre-motor ERP activities in the left vs. right hemisphere for limb movement over the parietal area within time windows from −100 to 0 ms (F(1,28) > 7.87, p < 0.01, partial η2 > 0.23).
Figure 3.
Panels A and C display the overlaid temporal profiles of ERPs for older (red line) vs. younger (black line) adults during speech motor reaction time task in response to temporally predictable and unpredictable conditions, respectively. In these plots, ERP responses are shown for six different regions of interests in time windows spanning −500 ms before to 500 ms after the onset of speech movement initiation. Panels B and D show the topographical scalp distribution maps of pre-motor ERP activities for younger (top row) and older (bottom row) adults for speech motor responses to temporally predictable and unpredictable stimuli, respectively. In these plots, topographical distribution maps are shown in 10 time windows from −500 to 0 ms before the onset of speech movement initiation (each window at 50 ms).
Figure 4.
Panels A and C display the overlaid temporal profiles of ERPs for older (red line) vs. younger (black line) adults during limb motor reaction time task in response to temporally predictable and unpredictable conditions, respectively. In these plots, ERP responses are shown for six different regions of interests in time windows spanning −500 ms before to 500 ms after the onset of limb movement initiation. Panels B and D show the topographical scalp distribution maps of pre-motor ERP activities for younger (top row) and older (bottom row) adults for limb motor responses to temporally predictable and unpredictable stimuli, respectively. In these plots, topographical distribution maps are shown in 10 time windows from −500 to 0 ms before the onset of limb movement initiation (each window at 50 ms).
Figure 5.
Profiles of the the mean amplitude of ERPs across older (red line) and younger (black line) adults (n = 15 per group) in 10 different time windows before the onset of speech and limb movement in response to temporally predictable and unpredictable stimuli for electrodes over the frontal and parietal areas. In these plots, each circle represents the mean amplitude of ERPs for a 50 ms time window. Significant between-groups differences (p < 0.05, Bonferroni corrected) are marked by asterisks (*) in each panel.
4. Discussion
In the present study, we conducted a systematic investigation to determine the effects of normal aging on the temporal predictive mechanisms in the motor system by examining pre-motor ERP components of speech and limb movement in response to temporally predictable and unpredictable sensory (i.e. visual) stimuli. Previous studies in younger adults have shown that temporal predictability of sensory stimuli can modulate ERP activities prior to the onset of speech and limb movement (Alegre et al. 2003; Johari and Behroozmand 2017a; Johari and Behroozmand 2018). These pre-motor neural activities have been suggested as neurophysiological biomarkers of the temporal predictive code in the motor system that plays a critical role in extracting timing information from sensory stimuli to drive behaviorally relevant motor responses (Johari and Behroozmand 2017a; Johari and Behroozmand 2018). In this study, we utilized a motor reaction time paradigm to address the question as to how normal aging may affect the behavioral and neural correlates of the temporal predictive mechanisms for speech production and limb movement in two groups of older and younger adults. Results of our analysis revealed a temporal and modality-specific decline in the preparatory mechanisms of movement in older adults by showing age-related increases in pre-motor ERP activities for speech production (but not limb movement) only in response to temporally unpredictable sensory stimuli. Our data also showed that such age-related modulation of ERP activities were associated with increased (slower) motor reaction times for speech responses to unpredictable stimuli. These findings suggest that motor timing processing of speech is compromised in older adults and that the aging brain calls for the engagement of additional neural mechanisms to prepare and execute motor commands for speech production in response to sensory stimuli with unpredictable timing intervals.
4.1. Effects of normal aging on movement reaction time
Our behavioral findings revealed that in response to temporally unpredictable stimuli, motor responses were significantly slower (longer reaction times) in older vs. younger adults only during speech production but not limb movement initiation. In contrast, for temporally predictable stimuli, motor response reaction times were not significantly different in older vs. younger adults during both speech and limb movement initiation. These findings confirmed our hypothesis that older adults would exhibit greater decline in motor timing processing of temporally unpredictable sensory stimuli, and further validated similar findings of previous studies in the speech and limb motor systems (Chauvin et al. 2016; Johari et al. 2018). These data support the notion that motor timing processing mechanisms of speech and limb movement are spared in normal aging in response to sensory stimuli with predictable temporal patterns.
For motor reaction time responses to temporally unpredictable stimuli, our data showed a modality-specific effect of normal aging as indexed by slower speech movement initiating responses in older vs. younger adults, but no such effect was observed during limb movement. This modality-specific decline in motor timing processing of unpredictable sensory stimuli may be accounted for by the inherent differences between the underlying mechanisms of movement production in the speech and limb motor systems. While pressing a button in our experimental paradigm required activation of a group of muscles for limb movement, performing the speech vowel vocalization task was mediated by the sequential, precisely timed, and coordinated activation of a larger group of muscles in multiple functionally independent systems such as the respiratory, laryngeal, and articulatory mechanisms. In addition, retrieving the phonological representation of the vowel sound before generating the motor representation may call for more cognitive resources during the planning phase of speech compared with limb movement. Such inherent differences may potentially lead to higher demands on cognitive and sensorimotor resources for speech production vs. limb movement during the button press task. Therefore, an older brain with limited capacity may selectively compromise motor timing of speech in response to sensory stimuli with lower temporal expectancy (i.e. unpredictable stimuli), as these require more neural resources for processing than those in response to temporally predictable cues. The reduced capacity for processing timing information in unpredictable sensory stimuli and a diminished ability for translating it into a temporal predictive code may explain why motor timing processing of speech is deteriorated in older adults, who exhibited slower reaction times during vowel vocalizations than their younger counterparts. Further supporting evidence for age-related decline of temporal processing mechanisms is provided by findings of previous studies showing an increased error rate during temporal estimation, discrimination, motor reproduction, and judgement of unpredictable timing intervals in older vs. younger adults (Balci et al. 2009; Zanto et al. 2011).
4.2. Effects of normal aging on neural correlates of movement preparation
Results of our analysis on ERP responses showed that the pre-motor ERP activities before speech and limb movement onset were not different in older vs. younger adults in response to temporally predictable sensory stimuli. In line with our behavioral data, this latter evidence at the neural level further supports the notion that the underlying neural mechanisms of motor timing processing are spared in normal aging when sensory stimuli are temporally predictable. However, when movement was generated in response to unpredictable stimuli, the amplitude of the pre-motor ERP activities was significantly increased in older vs. younger adults for speech production, though no such effect was observed during limb movement. In conjunction with our behavioral data, this latter evidence at the neural level corroborated the notion that normal aging is associated with modality-specific decline of speech motor timing processing in response to unpredictable stimuli, as indexed by an age-related increase in pre-motor ERPs in older vs. younger adults.
Previous studies on the mechanisms of timing processing during a wide range of memory, cognitive, and action observation or prediction tasks have identified the “Contingent Negative Variation” (CNV) component, which is an ERP activity elicited before the onset of an imperative signal that reflects how the brain encodes the timing of an upcoming sensory stimulus for establishing a temporal predictive coding mechanism (Walter et al. 1964; Pfeuty et al. 2005; Diersch et al. 2013; Nobre et al. 2007). Since the pre-motor ERP activities elicited before the onset of speech and limb movement in the present and previous studies (Alegre et al. 2003; Kuhn et al. 2004; Johari and Behroozmand 2017a; Johari and Behroozmand 2018) share common characteristics with the CNV response component (e.g., latency, amplitude, and topographical morphology), it is reasonable to propose that these observed pre-motor ERP responses reflect a similar temporal predictive coding mechanism that extracts timing information from sensory stimuli and prepares and drives motor actions (e.g., speech or limb movement) in response to events with predictable or unpredictable temporal characteristics. Our current data provide supporting evidence for this proposal by showing that age-related modulation of pre-motor ERPs were associated with age-related decline in preparatory neural mechanisms of motor timing processing in response to externally presented sensory stimuli. In this context, results of our study are indicative of modality-specific decline of neural mechanisms that support temporal predictive coding of unpredictable sensory stimuli during speech production, leading to slower motor reaction time responses in older adults.
A possible account of the age-related increase in pre-motor ERP activations in our study is that an older brain may recruit additional neural resources to compensate for the decline of the cognitive and sensorimotor mechanisms of speech motor timing processing. As suggested by our data, such an age-related effect was reflected in the slowed motor reaction times in response to unpredictable sensory stimuli, accompanied by increased pre-motor ERP activations during speech production in older adults. Studies in patients with Parkinson’s disease (PD) have reported that multiple brain regions including the premotor/motor cortex, supplementary motor area (SMA), dorsolateral prefrontal cortex (DLPFC), and cerebellum are overactivated, especially during speech, to compensate for deficits in dopamine-dependent mechanisms of motor timing processing as a result of basal ganglia pathology (Liotti et al. 2003; Wu and Hallett 2005; Yu et al. 2007; Sachin et al. 2008; Narayana et al. 2009). The significant role of the basal ganglia network and its underlying dopamine transmission mechanisms have been emphasized in fine-tuned regulation of movement timing in previous studies (Matell and Meck 2004; Coull et al. 2011; Tomassini et al. 2015).
Although not as extensive as that in PD, studies on neurologically intact older adults have demonstrated atrophy of dopaminergic neurons in fronto-basal ganglia networks (Volkow et al. 1998; Rubin 1999; Bäckman et al. 2000; Mozley et al. 2001; Balci et al. 2009; Merchant et al. 2013). Based on findings of these previous studies, we suggest that normal aging is associated with recruiting compensatory neural mechanisms similar to those in PD to counteract age-related decline of motor timing processing. In the context of the temporal compensation theory (Turgeon et al. 2016), older adults are able to perform low-demand (i.e. simple) motor timing tasks similar to what is performed by their younger counterparts. However, for high-demand tasks that require processing beyond the level of available neural resources, the older brain can use compensatory mechanisms to ameliorate age-related decline in temporal processing of sensory stimuli during movement production. In this study, we found that pre-motor ERP activities over the frontal areas were increased in older vs. younger adults when subjects produced speech movement in response to temporally unpredictable sensory stimuli. This age-related modulation of frontal ERPs during speech production may be a neural indicator of compensatory mechanisms for fronto-basal ganglia dysfunctions in older adults. This notion is further corroborated by results of a recent neuroimaging study showing overactivation of BOLD responses in the right motor cortex in older vs. younger adults during speech motor timing tasks (Tremblay et al. 2017), suggesting that older adults may recruit additional neural resources to compensate for functional decline during speech production. In addition, Tremblay et al. (Tremblay et al. 2017) showed that overactivation of the right posterior cingulate cortex in older adults was indicative of compensatory mechanisms and the need for allocating higher levels of cognitive resources to counteract age-related decline during speech production tasks. In the present study, we found a consistent pattern of increased ERP activation in the frontal regions, which may similarly highlight the neural signatures of such cognitive-related compensatory mechanisms during speech production in older adults. However, our data showed that recruiting such compensatory mechanisms at the neural level may not necessarily translate into boosting the behavioral performance and improving speech motor reaction times in older adults in response to temporally unpredictable sensory stimuli. As discussed earlier, this effect may be due to the older adults’ potential inability to recruit sufficient neural resources even after activating compensatory mechanisms to perform a high-demand speech task that requires coordinated movement of a large group of muscles in multiple functionally independent systems (e.g., respiratory, laryngeal, and articulatory) in response to sensory stimuli with unpredictable temporal patterns.
The absence of behavioral and ERP differences between older and younger adults during the button press task in the present study was not consistent with findings of previous studies that showed slower motor reaction time (Vallesi et al. 2009b; Zanto et al. 2011) and reduced activation of neural responses during limb movement in older vs. younger adults (Loveless and Sanford 1974; Barrett et al. 1986; Yordanova et al. 2004; Stewart et al. 2014). This inconsistency may partially be attributable to the differences between the experimental tasks implemented in the present compared with previous studies. In this study, the limb motor reaction time task involved a button press condition that was simpler to perform than the motor selection and limb movement tasks used in previous studies (Dirnberger et al. 2000; Yordanova et al. 2004; Stewart et al. 2014). For example, Stewart et al. (Stewart et al. 2014) showed that diminished behavioral performance during an action selection task was associated with deactivation of the primary motor cortex in older adults, but no such effect was examined in younger adults as a control group. In addition, the timing intervals between the warning and imperative signals were not similar in the present and those previous studies, and we also used two blocks of predictable and unpredictable conditions in which subjects responded to visual cues during speech and limb motor reaction time tasks. Furthermore, the present study used different age groups than those used in previous studies for examining the behavioral and neural correlates of movement timing in older and younger adults. Altogether, the differences in the experimental paradigm and characteristics of recruited subjects may explain inconsistencies related to the effect of normal aging on the behavioral and neural mechanisms of speech production and limb movement in older vs. younger adults between the present and previous studies.
In addition to the pre-motor ERP modulation over the frontal areas, our data revealed a similar effect of normal aging on pre-motor ERP activities over the parietal areas during speech responses to temporally unpredictable sensory stimuli. In line with this finding, previous fMRI studies have identified neural mechanisms within the parietal cortex that are involved in differential neural processing of temporally predictable vs. unpredictable sensory stimuli (Nobre et al. 2007; Coull et al. 2016). Based on findings of these previous studies, it has been proposed that the parietal cortex subserves a dual-mode processing mechanism in which the brain establishes a temporal expectancy model for estimating the timing of upcoming predictable sensory stimuli, and for temporally unpredictable stimuli, it recruits a hazard function in which the likelihood of occurrence for an upcoming sensory stimulus increases as time elapses. In the context of this dual-mode processing model, we suggest that the absence of a difference between pre-motor ERPs over the parietal areas in older vs. younger adults in this study indicates that the neural mechanisms of temporal expectancy are unaffected by normal aging during speech production and limb movement in response to temporally predictable sensory stimuli. However, increased pre-motor ERP activities in older adults over the parietal area suggests an age-related decline of the neural mechanisms underlying the hazard function in normal aging, which may subsequently lead to less accurate estimation of timing information in response to unpredictable stimuli and slowed motor reaction times, particularly during speech production.
4.3. Limitations
A potential limitation of the present study is that it did not probe the effects of gender-specific differences on age-related changes in motor preparatory mechanisms of speech production and limb movement. In one previous study (Li et al. 2018), it has been shown that males generate stronger N1 and P2 ERP components compared with females during speech production, however, females were shown to generate faster N1 ERP responses compared to male speakers. While we did not include gender as a factor of interest for data analysis in the present study, it is important to note that inherent gender-specific characteristics may have differential effects on age-related changes in the behavioral and neural mechanisms of speech and limb movement. Therefore, further research is warranted to conduct systematic examination on the effect of gender on the mechanisms of speech and limb movement in normal aging.
Another limitation of the present study is the lack of control conditions for ruling out the effect of visual-evoked neural responses to the “go” cues (i.e. the onset of the black circles on the screen) from the pre-motor time windows. However, examination of our data suggests that the observed differences in pre-motor neural activities are not accounted for by differences in neural processing of the “go” visual cue stimuli as the ERP responses are qualitatively different between motor conditions, with characteristics consistent with responses associated with speech versus limb movements, while the visual “go” cue signal (i.e. the onset of a black circle on the screen) remains constant between age groups or predictable vs. unpredictable timing conditions. This is verified by comparing pre-motor neural activities for speech vs. limb movements in response to predictable stimuli. Since the measures of motor reaction time were not significantly different for these conditions within age groups, we can directly compare them and it is reasonable to hypothesize that if the calculated ERPs were reflective of visual-evoked activities, such neural responses would be elicited with nearly identical response profiles for speech and limb movement because in that case the stimulus in both conditions was the onset of a black circle (“go” cue) that appeared ~400 ms before the onset of the motor response. However, as shown in our data, time-locked ERP responses to the onset of speech vs. limb movement show different patterns of neural activations that are indexed by the differences in latency, amplitude, and the overall spatio-temporal profiles of neural activation patters for these different conditions. In general, pre-motor ERP responses to speech movement emerged earlier than responses to limb movement and represented a more smooth deflection of potentials with smaller amplitudes compared with the sharp and large amplitude pattern of deflection for limb movement. In addition, topographical distribution maps of these responses follow the pattern of pre-motor rather than visual evoked potentials and suggest the presence of a hypothetical dipole in pre-motor and motor cortex with a negative polarity component over the fronto-central electrodes and its inverted (positive) polarity over the parietal area (as compared with visual-related dipoles with potentials over the posterior occipital electrodes). Moreover, since the ERP responses were calculated time-locked to the onset of speech and limb movement, the inherent trial-by-trial jitter in the measures of motor reaction time will likely have led to the cancellation of out-of-phase visual evoked responses in the pre-motor time window examined in this study. This notion is further corroborated by the observation that the pre-motor responses to the onset of speech and limb movement are preceded by a relatively flat baseline activity at latencies ~400 ms before the onset of pre-motor ERP activities. Based on these observations, we argue that the observed differences in neurophysiological responses to speech vs. limb movement are in fact driven by differences in pre-motor neural processing mechanisms underlying these different motor functions, rather than by differences in visual evoked responses to the onset of the “go” cues presented on the screen. Since the current study was primarily motivated by the question as to how normal aging affects the pre-motor mechanisms of motor timing during speech production and limb movement, limiting our analysis to the pre-cue time window was not possible because the inherent trial-by-trial jitter in motor reaction time will have led to the cancellation of pre-motor responses that were elicited prior to the onset of movement. Therefore, we aimed to examine ERP response profiles that were time-locked to the onset of motor responses to temporally predictable and unpredictable visual cue stimuli between the young and old adults during speech production and limb movement tasks.
Lastly, although previous studies have shown that modulation of band-specific power of neural oscillations (e.g., alpha or beta) are reflective of top-down predictive coding mechanisms, examining the effects of normal aging on these neural oscillatory mechanisms was beyond the scope of the present study and its hypotheses. Future studies are warranted to investigate the age-related modulation of band-specific neural responses and their association with predictive coding mechanisms during speech and limb motor reaction time tasks in response to sensory stimuli with predictable and unpredictable temporal characteristics.
5. Conclusion
Our findings indicate that timing processing mechanisms of speech and limb motor systems are spared in normal aging when older adults generate movement in response to temporally predictable sensory stimuli. In contrast, we found age-related decline in motor timing processing of speech in response to unpredictable stimuli, as indexed by slower motor reaction times and increased amplitude of pre-motor ERP activities in older vs. younger adults. We conclude that the aged brain relies on compensatory neural mechanisms to offset age-related functional decline in motor timing processing of speech in response to unpredictable sensory stimuli. However, due to limitations imposed by task demands and reduced capacity of cognitive and sensorimotor resources, recruiting such compensatory mechanisms at the neural level may not immediately translate into improved behavioral performance of speech motor timing processing in older adults. To our knowledge, this is the first study to systematically investigate the behavioral and neural correlates of normal aging effects on speech and limb motor timing processing in a unified framework. Future studies will further elucidate the effects of normal aging by using advanced techniques to map out the brain networks involved in neural processing of motor timing in the speech and limb modalities.
10. Acknowledgements
The authors wish to thank Drs. Chris Rorden and Allen Montgomery for their feedback on this manuscript.
9. Funding
This research was supported by a grant from the NIH/NIDCD, Grant Number: K01-DC015831-01A1 (PI: Behroozmand), and by the Graduate Scholar Award for Aging Research received by Karim Johari from the University of South Carolina. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Ethics Statement
This study was carried out in accordance with the recommendations of the University of South Carolina Institutional Review Board, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the University of South Carolina Institutional Review Board.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial, financial, or non-financial relationships that could be construed as a potential conflict of interest.
11. References
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Malanda A, Artieda J (2003) Alpha and beta oscillatory changes during stimulus-induced movement paradigms: effect of stimulus predictability. Neuroreport 14:381–385 doi: 10.1097/01.wnr.0000059624.96928.c0 [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Wahlin T-BR, Wahlin Å, Halldin C, Farde L (2000) Age-related cognitive deficits mediated by changes in the striatal dopamine system. American Journal of Psychiatry 157:635–637 [DOI] [PubMed] [Google Scholar]
- Balci F, Meck WH, Moore H, Brunner D (2009) Timing deficits in aging and neuropathology In: Animal models of human cognitive aging. Springer, pp 1–41 [Google Scholar]
- Bard C, Paillard J, Lajoie Y, Fleury M, Teasdale N, Forget R, Lamarre Y (1992) Role of afferent information in the timing of motor command: A comparative study with a deafferented patient. Neuropsychologia 30:201–206 [DOI] [PubMed] [Google Scholar]
- Barrett G, Shibasaki H, Neshige R (1986) Cortical potentials preceding voluntary movement: evidence for three periods of preparation in man. Electroencephalography and clinical neurophysiology 63:327–339 [DOI] [PubMed] [Google Scholar]
- Behroozmand R, Liu H, Larson CR (2011) Time-dependent neural processing of auditory feedback during voice pitch error detection. Journal of Cognitive Neuroscience 23:1205–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozmand R, Sangtian S, Korzyukov O, Larson CR (2016) A temporal predictive code for voice motor control: Evidence from ERP and behavioral responses to pitch-shifted auditory feedback. Brain Res 1636:1–12 doi: 10.1016/j.brainres.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchicci M, Lucci G, Pesce C, Spinelli D, Di Russo F (2012) Prefrontal hyperactivity in older people during motor planning. Neuroimage 62:1750–1760 [DOI] [PubMed] [Google Scholar]
- Berchicci M, Lucci G, Spinelli D, Di Russo F (2015) Stimulus onset predictability modulates proactive action control in a Go/No-go task. Frontiers in Behavioral Neuroscience 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelson P, Boons J-P (1960) Time uncertainty and choice reaction time. Nature 187:531–532 [DOI] [PubMed] [Google Scholar]
- Bevan W, Hardesty DL, Avant LL (1965) Response Latency with Constant and Variable Interval Schedules. Perceptual and Motor Skills 20:969–972 [DOI] [PubMed] [Google Scholar]
- Bherer L, Belleville S (2004) Age-related differences in response preparation: The role of time uncertainty. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 59:P66–P74 [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund H-J (1999) A parieto-premotor network for object manipulation: evidence from neuroimaging. Experimental Brain Research 128:210–213 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Wolpert D, Frith C (2000) Why can’t you tickle yourself? Neuroreport 11:R11–R16 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Wolpert DM, Frith CD (1998) Central cancellation of self-produced tickle sensation. Nature neuroscience 1:635. [DOI] [PubMed] [Google Scholar]
- Chauvin JJ, Gillebert CR, Rohenkohl G, Humphreys GW, Nobre AC (2016) Temporal orienting of attention can be preserved in normal aging. Psychology and aging 31:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Chen X, Liu P, Huang D, Liu H (2012) Effect of temporal predictability on the neural processing of self-triggered auditory stimulation during vocalization. BMC neuroscience 13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K, Meck WH (2011) Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Cotti J, Vidal F (2016) Differential roles for parietal and frontal cortices in fixed versus evolving temporal expectations: Dissociating prior from posterior temporal probabilities with fMRI. NeuroImage 141:40–51 [DOI] [PubMed] [Google Scholar]
- Deiber M-P, Ibañez V, Missonnier P, Rodriguez C, Giannakopoulos P (2013) Age-associated modulations of cerebral oscillatory patterns related to attention control. Neuroimage 82:531–546 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods 134:9–21 [DOI] [PubMed] [Google Scholar]
- Diersch N, Cross ES, Stadler W, Schütz-Bosbach S, Rieger M (2012) Representing others’ actions: the role of expertise in the aging mind. Psychological research 76:525–541 [DOI] [PubMed] [Google Scholar]
- Diersch N, Jones AL, Cross ES (2016) The timing and precision of action prediction in the aging brain. Hum Brain Mapp 37:54–66 doi: 10.1002/hbm.23012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diersch N, Mueller K, Cross ES, Stadler W, Rieger M, Schutz-Bosbach S (2013) Action prediction in younger versus older adults: neural correlates of motor familiarity. PLoS One 8:e64195 doi: 10.1371/journal.pone.0064195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger G, Lalouschek W, Lindinger G, Egkher A, Deecke L, Lang W (2000) Reduced activation of midline frontal areas in human elderly subjects: a contingent negative variation study. Neuroscience letters 280:61–64 [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X (2003) Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. Journal of neurophysiology 89:2194–2207 [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Campione GC, Dalla Volta R, Bernardis P (2009) The observation of manual grasp actions affects the control of speech: A combined behavioral and Transcranial Magnetic Stimulation study. Neuropsychologia 47:3190–3202 [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Volta RD (2008) Spoken language and arm gestures are controlled by the same motor control system. Quarterly Journal of Experimental Psychology 61:944–957 [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Grice JW (1993) Effects of aging on planning and implementing arm movements. Psychology and Aging 8:617. [DOI] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O (2012) Somatosensory anticipatory alpha activity increases to suppress distracting input. Journal of cognitive neuroscience 24:677–685 [DOI] [PubMed] [Google Scholar]
- Johansson R, Westling G (1988) Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Experimental brain research 71:59–71 [DOI] [PubMed] [Google Scholar]
- Johari K, Behroozmand R (2017a) Premotor neural correlates of predictive motor timing for speech production and hand movement: evidence for a temporal predictive code in the motor system. Experimental brain research 235:1439–1453 [DOI] [PubMed] [Google Scholar]
- Johari K, Behroozmand R (2017b) Temporal predictive mechanisms modulate motor reaction time during initiation and inhibition of speech and hand movement. Human movement science 54:41–50 [DOI] [PubMed] [Google Scholar]
- Johari K, Behroozmand R (2018) Functional Dissociation of Temporal Processing Mechanisms during Speech Production and Hand Movement: An ERP Study. Behavioural brain research [DOI] [PubMed] [Google Scholar]
- Johari K, den Ouden D-B, Behroozmand R (2018) Effects of aging on temporal predictive mechanisms of speech and hand motor reaction time. Aging clinical and experimental research:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin L (1959) Reaction time as a function of foreperiod duration and variability. Journal of experimental psychology 58:185. [DOI] [PubMed] [Google Scholar]
- Klemmer ET (1956) Time uncertainty in simple reaction time. Journal of experimental psychology 51:179. [DOI] [PubMed] [Google Scholar]
- Koppe G, Gruppe H, Sammer G, Gallhofer B, Kirsch P, Lis S (2014) Temporal unpredictability of a stimulus sequence affects brain activation differently depending on cognitive task demands. Neuroimage 101:236–244 doi: 10.1016/j.neuroimage.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Kotz SA, Schmidt-Kassow M (2015) Basal ganglia contribution to rule expectancy and temporal predictability in speech. Cortex 68:48–60 [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, et al. (2004) Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain 127:735–746 doi: 10.1093/brain/awh106 [DOI] [PubMed] [Google Scholar]
- Lange K (2009) Brain correlates of early auditory processing are attenuated by expectations for time and pitch. Brain and cognition 69:127–137 [DOI] [PubMed] [Google Scholar]
- Li C-SR, Krystal JH, Mathalon DH (2005) Fore-period effect and stop-signal reaction time. Experimental brain research 167:305–309 [DOI] [PubMed] [Google Scholar]
- Li J, Hu H, Chen N, Jones JA, Wu D, Liu P, Liu H (2018) Aging and Sex Influence Cortical Auditory-Motor Integration for Speech Control. Frontiers in neuroscience 12:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Ramig L, Vogel D, et al. (2003) Hypophonia in Parkinson’s disease neural correlates of voice treatment revealed by PET. Neurology 60:432–440 [DOI] [PubMed] [Google Scholar]
- Loveless NE, Sanford AJ (1974) Effects of age on the contingent negative variation and preparatory set in a reaction-time task. Journal of Gerontology 29:52–63 [DOI] [PubMed] [Google Scholar]
- Maess B, Schröger E, Widmann A (2016) High-pass filters and baseline correction in M/EEG analysis. Commentary on:“How inappropriate high-pass filters can produce artefacts and incorrect conclusions in ERP studies of language and cognition”. Journal of neuroscience methods 266:164–165 [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH (2004) Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cognitive brain research 21:139–170 [DOI] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, Meck WH (2013) Neural basis of the perception and estimation of time. Annual review of neuroscience 36:313–336 [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE (2001) Striatal dopamine transporters and cognitive functioning in healthy men and women. American Journal of Psychiatry 158:1492–1499 [DOI] [PubMed] [Google Scholar]
- Narayana S, Jacks A, Robin DA, et al. (2009) A noninvasive imaging approach to understanding speech changes following deep brain stimulation in Parkinson’s disease. American Journal of Speech-Language Pathology 18:146–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Correa A, Coull JT (2007) The hazards of time. Current opinion in neurobiology 17:465–470 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Pfeuty M, Ragot R, Pouthas V (2005) Relationship between CNV and timing of an upcoming event. Neuroscience letters 382:106–111 [DOI] [PubMed] [Google Scholar]
- Rubin DC (1999) Frontal-striatal circuits in cognitive aging: evidence for caudate involvement. Aging, Neuropsychology, and Cognition 6:241–259 [Google Scholar]
- Sachin S, Kumaran SS, Singh S, Goyal V, Shukla G, Mahajan H, Behari M (2008) Functional mapping in PD and PSP for sustained phonation and phoneme tasks. Journal of the neurological sciences 273:51–56 [DOI] [PubMed] [Google Scholar]
- Samaha J, Bauer P, Cimaroli S, Postle BR (2015) Top-down control of the phase of alpha-band oscillations as a mechanism for temporal prediction. Proceedings of the National Academy of Sciences 112:8439–8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartze M, Rothermich K, Kotz SA (2012) Functional dissociation of pre-SMA and SMA-proper in temporal processing. Neuroimage 60:290–298 [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, et al. (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733 doi: 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Dean P (2008) Neural correlates of movement preparation in healthy ageing. Eur J Neurosci 27:254–260 doi: 10.1111/j.1460-9568.2007.05975.x [DOI] [PubMed] [Google Scholar]
- Stewart JC, Tran X, Cramer SC (2014) Age-related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults. Neuroimage 86:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman E, Ritter W, Vaughan HG Jr (1998) Predictability of stimulus deviance and the mismatch negativity. Neuroreport 9:4167–4170 [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Sacco P, Ghosh S, Morris IT, Mastaglia FL (2000) The role of the supplementary motor area in externally timed movement: the influence of predictability of movement timing. Brain Research 874:233–241 doi: Doi 10.1016/S0006-8993(00)02588-9 [DOI] [PubMed] [Google Scholar]
- Todorovic A, van Ede F, Maris E, de Lange FP (2011) Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. Journal of Neuroscience 31:9118–9123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini A, Ruge D, Galea JM, Penny W, Bestmann S (2015) The role of dopamine in temporal uncertainty. Journal of cognitive neuroscience [DOI] [PubMed] [Google Scholar]
- Tremblay P, Sato M, Deschamps I (2017) Age differences in the motor control of speech: An fMRI study of healthy aging. Human brain mapping 38:2751–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon M, Lustig C, Meck WH (2016) Cognitive aging and time perception: roles of Bayesian optimization and degeneracy. Frontiers in aging neuroscience 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden RJ, Hutcheson NL, McCollum LA, Kentros J, Visscher KM (2012) Older adults, unlike younger adults, do not modulate alpha power to suppress irrelevant information. Neuroimage 63:1127–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi A, McIntosh AR, Shallice T, Stuss DT (2009a) When time shapes behavior: fMRI evidence of brain correlates of temporal monitoring. Journal of Cognitive Neuroscience 21:1116–1126 [DOI] [PubMed] [Google Scholar]
- Vallesi A, McIntosh AR, Stuss DT (2009b) Temporal preparation in aging: A functional MRI study. Neuropsychologia 47:2876–2881 [DOI] [PubMed] [Google Scholar]
- Vallesi A, Shallice T, Walsh V (2007) Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cerebral Cortex 17:466–474 [DOI] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E (2011) Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha-and beta-band oscillations. Journal of Neuroscience 31:2016–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Szebényi S, Maris E (2014) Attentional modulations of somatosensory alpha, beta and gamma oscillations dissociate between anticipation and stimulus processing. Neuroimage 97:134–141 [DOI] [PubMed] [Google Scholar]
- Vieweg P, Stangl M, Howard LR, Wolbers T (2015) Changes in pattern completion--a key mechanism to explain age-related recognition memory deficits? Cortex 64:343–351 doi: 10.1016/j.cortex.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang G-J, et al. (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. American Journal of psychiatry 155:344–349 [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge V, McCallum W, Winter A (1964) Contingent negative variation: an electric sign of sensori-motor association and expectancy in the human brain. Nature 203:380–384 [DOI] [PubMed] [Google Scholar]
- Widmann A, Schröger E, Maess B (2015) Digital filter design for electrophysiological data–a practical approach. Journal of neuroscience methods 250:34–46 [DOI] [PubMed] [Google Scholar]
- Witney AG, Goodbody SJ, Wolpert DM (1999) Predictive motor learning of temporal delays. Journal of Neurophysiology 82:2039–2048 [DOI] [PubMed] [Google Scholar]
- Wolpe N, Ingram JN, Tsvetanov KA, et al. (2016) Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nature communications 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM (1997) Computational approaches to motor control. Trends in cognitive sciences 1:209–216 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR (2001) Motor prediction. Current biology 11:R729–R732 [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M (2005) A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 128:2250–2259 [DOI] [PubMed] [Google Scholar]
- Yan JH, Thomas JR, Stelmach GE (1998) Aging and rapid aiming arm movement control. Experimental aging research 24:155–168 [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Hohnsbein J, Falkenstein M (2004) Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor‐generation processes: evidence from high‐resolution event‐related potentials. Brain 127:351–362 [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35:222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Pan P, Liu H, Bollinger J, Nobre AC, Gazzaley A (2011) Age-related changes in orienting attention in time. Journal of Neuroscience 31:12461–12470 [DOI] [PMC free article] [PubMed] [Google Scholar]