Abstract
Purpose
Postresuscitation neuroprognostication is guided by neurophysiological tests, biomarker measurement, and clinical examination. Recent investigations suggest that circulating microRNAs (miRNA) may help in outcome prediction after cardiac arrest. We assessed the ability of miR-574-5p to predict neurological outcome after cardiac arrest, in a sex-specific manner.
Methods
In this substudy of the Target Temperature Management (TTM) Trial, we enrolled 590 cardiac arrest patients for which blood samples were available. Expression levels of miR-574-5p were measured by quantitative PCR in plasma samples collected 48 h after cardiac arrest. The endpoint of the study was poor neurological outcome at 6 months (cerebral performance category scores 3 to 5).
Results
Eighty-one percent of patients were men, and 49% had a poor neurological outcome. Circulating levels of miR-574-5p at 48 h were higher in patients with a poor neurological outcome at 6 months (p < 0.001), both in women and in men. Circulating levels of miR-574-5p were univariate predictors of neurological outcome (odds ratio (OR) [95% confidence interval (CI)]: 1.5 [1.26-1.78]). After adjustment with clinical variables and NSE, circulating levels of miR-574-5p predicted neurological outcome in women (OR [95% CI]: 1.9 [1.09-3.45]), but not in men (OR [95% CI]: 1.0 [0.74-1.28]).
Conclusion
miR-574-5p is associated with neurological outcome after cardiac arrest in women.
1. Introduction
Out-of-hospital cardiac arrest (OHCA) is a devastating condition, with overall survival rates lower than 10% [1]. Survival post-OHCA is associated with age, bystander cardiopulmonary resuscitation (CPR), type of first monitored rhythm, and time from cardiac arrest (CA) to the return of spontaneous circulation (ROSC) [2, 3]. Whether survival rate differs between men and women is not clear although several studies reported that women had higher survival rates than men [4].
It is well documented that the brain is highly sensitive to ischemia, and half of OHCA survivors suffer neurological damage which impacts their quality of life and survival [5]. Currently, most deaths after OHCA occur after withdrawal of life-supporting therapies in patients with severe and irreversible neurological sequelae [6]. The decision to withdraw life-supporting therapies is currently based on a multimodal approach including clinical examination, electrophysiological tests (absence of somatosensory evoked potential), electroencephalography, brain imaging, and assessment of protein biomarkers such as neuron-specific enolase (NSE) and S100b [7]. Cardiac biomarkers such as N-terminal probrain natriuretic peptide (NT-proBNP) and high-sensitive cardiac troponin T (hs-TnT) are associated with neurological outcome and death after OHCA but are not included in the guidelines [7–9]. Despite this multimodal approach, predicting outcome after OHCA, especially at an early stage and in patients with moderate brain damage, is challenging and would benefit from novel biomarkers.
MicroRNAs (miRNA) are small single-stranded RNA molecules that regulate gene expression and are involved in multiple pathophysiological processes. As they circulate in the blood and reflect disease status, they are considered promising biomarkers towards personalized medicine [10]. Several circulating miRNA have been shown to be associated with outcome after OHCA [11]. Previous studies showed associations between circulating levels of miR-21, miR-124-3p, and miR-122-5p and neurological outcome after OHCA [12–15]. The ability of brain-enriched miR-124-3p to predict outcome after OHCA has been validated in a substudy of the large TTM trial [16]. A combined use of miR-124-3p and miR-122-5p improved the outcome prediction in the same cohort [13].
We hereby aimed to extend previous investigations to novel miRNA which may provide an incremental predictive value. We focused on miR-574-5p,which is upregulated in the blood and heart tissue from patients with ischemic heart disease [17, 18] and in atrial tissues from patients with atrial fibrillation [19], two frequent causes of CA [17, 20, 21]. Of note, miR-574-5p is upregulated in the blood after intracerebral haemorrhage independently of the sex and, after ischemic stroke, specifically in men [22]. miR-574-5p is also upregulated by oestradiol treatment in breast cancer cells MCF-7 [20]. Hence, we centred our attention on sex differences, since the knowledge of the effect of sex on prediction modalities after OHCA is limited.
2. Materials and Methods
2.1. Patients
Nine hundred and thirty-nine unconscious adults admitted to an intensive care unit after an OHCA of presumed cardiac cause were enrolled in the TTM trial, in 36 recruiting centres from November 2010 to July 2013. The trial is aimed at evaluating the potential benefit of a targeted temperature management at 33°C compared to 36°C [23]. The TTM trial and collection of blood samples in participating countries was approved by ethical committees of each participating country and fulfils the declaration of Helsinki [24]. The trial is accessible at www.clinicaltrials.gov (NCT01020916), and the protocol of the trial is accessible at https://clinicaltrials.gov/ct2/show/NCT01020916?term=ttm-trial&rank=1. The design and protocol including statistical analysis, results, and interpretations of the results of the trial have been previously published [23, 25, 26]. Blood samples were collected at each site and centrally stored at the Integrated Biobank of Luxembourg, in compliance with the International Society for Biological and Environmental Repositories Best Practices and with International Organization for Standards (ISO 9001:2008, 17025:2005 and NF S96-900:2011).
2.2. Endpoints
In the present substudy, the endpoint was a poor neurological outcome at 6 months after OHCA as assessed with the cerebral performance category (CPC) scale [27]. CPC scores 1 and 2 are considered a good neurological outcome. CPC scores 3 to 5 are considered a poor neurological outcome. For each patient, the CPC score was measured as indicated in the TTM trial protocol [25].
2.3. Measurement of miRNA Levels
Samples recovered 48 h after ROSC were used to measure circulating levels of miRNA by quantitative PCR as previously described and as detailed in Supplementary Material [13, 16].
2.4. Measurement of NSE, hs-TnT, S100b, and NT-proBNP Levels
Six months after the end of the trial, a core laboratory measured NSE, hs-TnT, S100b, and NT-proBNP levels in serum samples recovered 48 h after OHCA, as previously described [8, 9, 28, 29].
2.5. Statistical Analysis
For demographic and clinical data, the Mann-Whitney test was used to compare two groups of continuous variables. The Chi-square test or the Fisher exact tests were used to compare two groups of categorical variables. A p value < 0.05 was considered statistically significant.
The Mann-Whitney test was used to compare miR-574-5p levels between two groups of patients. The Spearman correlation test on ranks was used to correlate miR-574-5p levels with age, levels of NSE, S100b, NT-proBNP, hs-TnT, miR-122-5p, and miR-124-3p.
For the prediction of neurological outcome, univariate and multivariable analysis with logistic regression allowed to estimate the association between miR-574-5p levels (log10-transformed and scaled) and neurological outcome at 6 months after OHCA, which was dichotomized: CPC 1 or 2 was considered a good outcome (0 value), and CPC 3, 4, or 5 was considered a poor outcome (1 value). 150-fold multiple imputation was used for missing values (51 values for NSE, 36 values for lactate). Odds ratio (OR) and 95% confidence intervals (CI) were computed for an increase of 1 unit for continuous variables and were centred and scaled. The Akaike information criterion (AIC) was used to estimate the prediction value of multivariable models: a low AIC indicates a better model fit. The likelihood ratio test was used to compare two AIC values. The AIC is penalized by the number of variables included in the model allowing to avoid model overfitting due to the multiplication of covariates. The incremental predictive value of miR-574-5p to the baseline model was evaluated by a decrease of AIC and the integrated discrimination improvement (IDI).
SigmaPlot version 12.5 was used for statistical analysis related to descriptive results such as the demographic and clinic feature of the patients, comparison between two groups of patients, correlations, and logistic regression. R software was used with the following packages (PredictABEL, lmtest) for univariate and multivariable analysis.
3. Results
3.1. Patient Selection and Characteristics
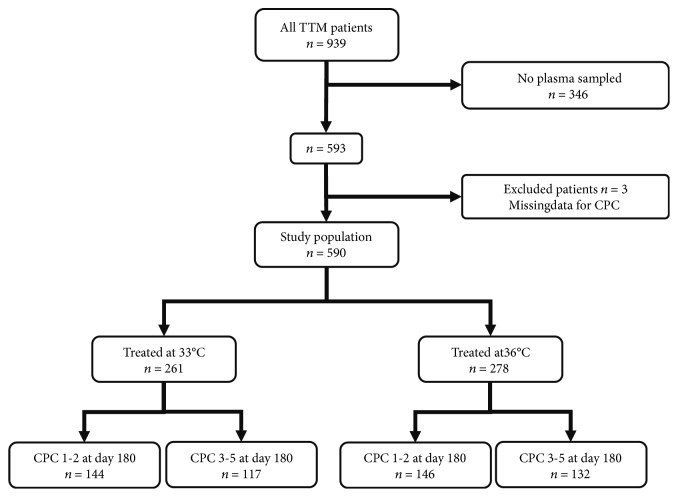
A study design chart is available (Figure 1). Among the 939 patients of the TTM trial, plasma samples were available for 593 patients enrolled in 29 of the 36 recruiting centres. Three of these patients were excluded because of missing CPC data, allowing the inclusion of 590 patients in the present substudy. There was no difference in demographic and clinical features between the whole TTM cohort and the present substudy cohort (Supplementary Table 1), apart from a higher prevalence of alcohol abuse in the TTM cohort as compared to the present substudy (4% vs. 1.9%, respectively).
Figure 1.
Study workflow.
The demographic and clinical characteristics of the study population are presented with a comparison between patients with good neurological outcome (CPC 1-2) and patients with poor neurological outcome (CPC 3-5), for all patients (n = 590; Table 1) and separately for men (n = 481) and women (n = 109; Supplementary Table 2). Eighty-one percent of patients were men, and 49% had a poor neurological outcome. Patients with a poor neurological outcome were older; had more often comorbidities, longer time between CA and ROSC, and higher initial levels of serum lactate; and less frequently had bystander CPR compared to patients with a good neurological outcome. A higher proportion of patients with poor neurological outcome presented with shock at admission and had an initial nonshockable rhythm compared to patients with a good neurological outcome (Table 1). There was no difference between men and women (Supplementary Table 2).
Table 1.
Demographic and clinical features of the 590 patients included in the present substudy.
| All patients (n = 590) |
Good outcome (n = 299) |
Poor outcome (n = 291) |
p value (Good vs. poor) |
|
|---|---|---|---|---|
| Age (years) | 65 (20-94) | 61 (20-90) | 68 (35-94) | <0.001 |
| Comorbidities | ||||
| Hypertension | 240 (41%) | 102 (34%) | 138 (47%) | 0.001 |
| Diabetes mellitus | 86 (15%) | 34 (11%) | 52 (18%) | 0.034 |
| Known ischemic heart disease | 163 (28%) | 67 (22%) | 96 (33%) | 0.005 |
| Previous MI | 118 (20%) | 48 (16%) | 70 (24%) | 0.020 |
| Heart Failure | 36 (6%) | 9 (3%) | 27 (9%) | 0.003 |
| COPD | 55 (9%) | 18 (6%) | 37 (13%) | 0.008 |
| Renal failure | 5 (1%) | 1 (0%) | 4 (1%) | 0.353 |
| Previous cerebral stroke | 50 (8%) | 19 (6%) | 31 (11%) | 0.084 |
| Alcohol abuse | 11 (2%) | 4 (1%) | 7 (2%) | 0.513 |
| First monitored rhythm | <0.001 | |||
| VF or nonperfusing VT | 467 (79%) | 276 (92%) | 191 (66%) | |
| Asystole or PEA | 104 (18%) | 16 (5%) | 88 (30%) | |
| ROSC after bystander defibrillation | 7 (1%) | 5 (2%) | 2 (1%) | |
| Unknown | 12 (2%) | 2 (1%) | 10 (3%) | |
| Witnessed arrest | 529 (90%) | 276 (92%) | 253 (87%) | 0.045 |
| Bystander CPR | 433 (73%) | 241 (81%) | 192 (66%) | <0.001 |
| Time from CA to ROSC (min) | 25 (0-170) | 20 (0-160) | 30 (0-170) | <0.001 |
| Initial serum lactate (mmol/l) | 6.1 (0.5-25) | 5.2 (0.5-20) | 6.7 (0.5-25) | <0.001 |
| Shock on admission | 76 (13%) | 27 (9%) | 49 (17%) | 0.007 |
Continuous variables are indicated as the median (range), and categorical variables are indicated as the number (frequency). CA: cardiac arrest; COPD: chronic obstructive pulmonary disease; CPR: cardiopulmonary resuscitation; MI: myocardial infarction; PEA: pulseless electric activity; ROSC: return of spontaneously circulation; VF: ventricular fibrillation; VT: ventricular tachycardia. Good outcome is CPC 1 or 2. Poor outcome is CPC 3, 4, or 5. Missing data: heart failure status for 2 patients, ischemic heart disease status for 1 patient, hypertension status for 1 patient, previous cerebral stroke status for 1 patient, diabetes mellitus status for 3 patients, alcohol abuse status for 1 patient, and lactate levels for 36 patients. p values < 0.05 were considered statistically significant and are in bold.
3.2. Association between Circulating Levels of miR-574-5p and Patient Characteristics
We first sought to determine potential associations between circulating levels of miR-574-5p measured 48 h after OHCA and the age and sex of the patients. Levels of miR-574-5p were very moderately yet statistically significantly correlated with the age of all patients (r = 0.16, p < 0.001, Supplementary Figure 1a), in men (r = 0.16, p < 0.001, Supplementary Figure 1b) but not in women (r = 0.129, p = 0.192, Supplementary Figure 1c). There was no significant difference in miR-574-5p levels between men and women (Supplementary Figure 1d).
3.3. Circulating Levels of miR-574-5p according to Neurological Outcome and Temperature
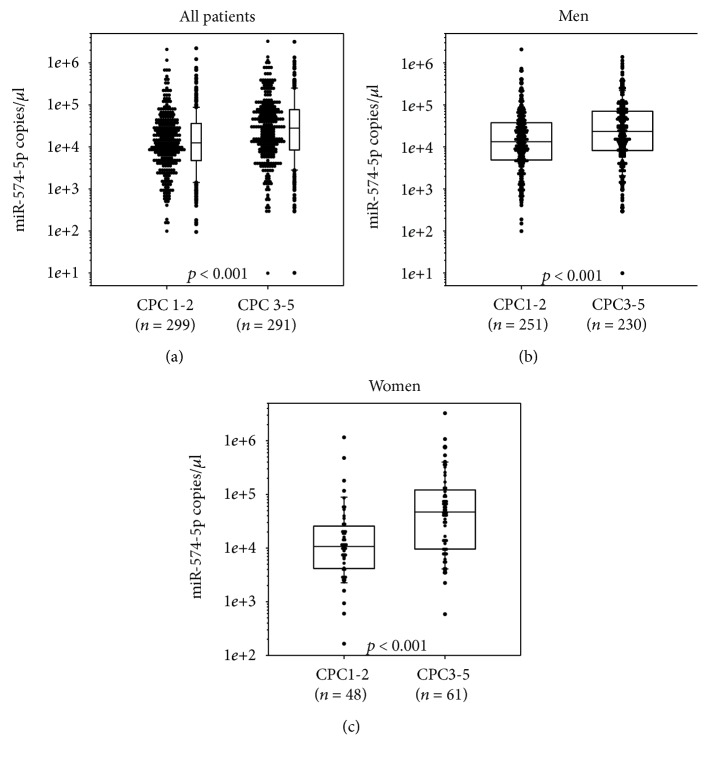
Levels of miR-574-5p were higher in patients with poor neurological outcome (CPC 3-5, Figure 2(a)), independently of sex (Figures 2(b) and 2(c)) and of the targeted temperature management regimen (33°C vs. 36°C, Supplementary Figure 2a-f). Interestingly, levels of miR-574-5p were higher in patients treated at 33°C (Supplementary Figure 2g), in both women and men (Supplementary Figure 2h-i).
Figure 2.
Plasma levels of miR-574-5p according to neurological outcome. Plasma levels of miR-574-5p were measured 48 h after the return of spontaneous circulation (ROSC) using quantitative PCR and were compared between patients with good (CPC 1-2) and poor (CPC 3-5) neurological outcomes. (a): 590 patients; (b): 481 men; (c): 109 women. Box plots represent the median and quartiles. Levels of miR-574-5p are expressed as the number of copies per microliter of plasma and are log-scaled. Plasma levels of miR-574-5p according to neurological outcome. Plasma levels of miR-574-5p were measured 48 h after the return of spontaneous circulation (ROSC) using quantitative PCR and were compared between patients with good (CPC 1-2) and poor (CPC 3-5) neurological outcomes. (a): 590 patients; (b): 481 men; (c): 109 women. Box plots represent the median and quartiles. Levels of miR-574-5p are expressed as the number of copies per microliter of plasma and are log-scaled.
3.4. Sex-Specific Association between miR-574-5p Levels and Neurological Outcome
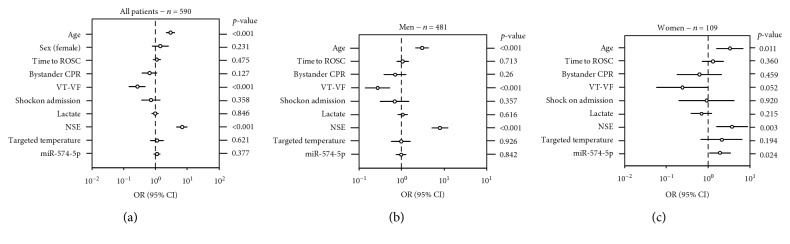
Levels of miR-574-5p measured 48 h after OHCA were univariate predictors of neurological outcome in all patients (OR [95% CI]: 1.50 [1.26-1.78], Supplementary Table. 3), in men (OR [95% CI]: 1.36 [1.13-1.64]; Supplementary Table. 3) and in women (OR [95% CI]: 2.28 [1.44-3.60]; Supplementary Table. 3). Consistent with past studies [13, 16, 28, 29], multivariable analyses included the following variables: age, sex (female), time from CA to ROSC, CPR, first monitored rhythm, circulatory shock on admission, initial serum lactate levels, NSE levels at 48 h, targeted temperature regimen, and miR-574-5p levels.
As shown in Figure 3(a), age and NSE were significant predictors of neurological outcome. After adjustment with demographic and clinical variables, miR-574-5p remained an independent predictor of neurological outcome in women (OR [95% CI]: 1.9 [1.09-3.45], Figure 3(c)) but lost significance in men (OR [95% CI]: 1.0 [0.74-1.28], Figure 3(b)) and in all patients (OR [95% CI]: 1.1 [0.87-1.42], Figure 3(a)).
Figure 3.
Sex-specific association between miR-574-5p levels and neurological outcome. Multivariable analyses (a–c) of the association between plasma miR-574-5p levels measured 48 h after OHCA and neurological outcome in all 590 patients (a), 481 men (b) and 109 women (c). Odds ratios (OR) ± 95% confidence intervals (95% CI) are shown for the prediction of poor neurological outcome (CPC 3-5) 6 months after OHCA. Variables included in the models: age, sex (female), time from cardiac arrest to return of spontaneous circulation (ROSC), bystander cardiopulmonary resuscitation (CPR), first monitored rhythm (ventricular tachycardia- (VT-) ventricular fibrillation (VF)), circulatory shock on admission, initial serum lactate levels, NSE levels at 48 h, targeted temperature regimen, and miR-574-5p levels.
We next estimated the incremental predictive value of miR-574-5p to a baseline model including all variables included in multivariable analyses. The AIC and the IDI were calculated, bearing in mind that a lower AIC and a higher IDI indicate a better predictive value. Of note, we chose to calculate the AIC instead of the area under the curve to avoid model overfitting. Adding miR-574-5p to the baseline clinical model improved the prediction of neurological outcome in women, as attested by a significant decrease of AIC (p = 0.018) and an IDI of 0.04 [0.007-0.079] (Table 2). No incremental value was found in men or in all patients (Table 2).
Table 2.
Added value of miR-574-5p to predict neurological outcome in all patients and in men and women separately.
| Models | AIC | p value | IDI [95% CI] | p value |
|---|---|---|---|---|
| All patients (n = 590) | ||||
| Baseline model | 493.7 | |||
| Baseline model + miR-574-5p | 494.9 | 0.376 (vs. baseline) | 0.0009 [-0.0016; 0.0035] | 0.465 |
| Men (n = 481) | ||||
| Baseline model | 395.2 | |||
| Baseline model + miR-574-5p | 397.1 | 0.842 (vs. baseline) | 0.0002 [-0.0005; 0.0009] | 0.644 |
| Women (n = 109) | ||||
| Baseline model | 109.3 | |||
| Baseline model + miR-574-5p | 105.7 | 0.018 (vs. baseline) | 0.0433 [0.0071; 0.0794] | 0.019 |
The baseline model includes age, sex, bystander cardiopulmonary resuscitation (CPR), first monitored rhythm, time from cardiac arrest to ROSC, initial serum lactate levels, shock on admission, NSE levels at 48 h, and targeted temperature regimen. Log10-transformed miR-574-5 p values were used in these analyses. AIC: Akaike information criteria. A lower AIC indicates a better predictive value. IDI: integrated discrimination improvement. A higher IDI indicates a better predictive value. The statistical significance was assessed using the likelihood ratio test. A p value < 0.05 was considered significant and is highlighted in bold.
3.5. Association between Circulating Levels of miR-574-5p and Markers of Neurological and Cardiac Damage
For all patients, and independently of sex, we observed a modest but significant correlation between miR-574-5p and NSE levels (r = 0.24, p < 0.001), as well as S100b levels (r = 0.29, p < 0.001, Supplementary Table 4). Circulating levels of miR-574-5p were modestly correlated with NT-proBNP levels (r = 0.17, p < 0.001) and hs-TnT levels (r = 0.20, p < 0.001; Supplementary Table 4). Interestingly, for all these markers of neurological and cardiac injury, correlations with miR-574-5p levels were slightly higher for women.
3.6. Association between Circulating Levels of miR-574-5p, miR-124-3p, and miR-122-5p
Levels of miR-574-5p were not correlated with circulating levels of miR-122-5p (r = 0.06, p = 0.19), but were positively correlated with miR-124-3p levels (r = 0.29, p < 0.001), independently of sex (Supplementary Table 4).
4. Discussion
This substudy of the TTM trial highlighted an association between circulating levels of miR-574-5p measured 48 h after OHCA and patient outcome. More specifically, we observed that this miRNA was an independent predictor of 6-month neurological outcome in women, but not in men.
We focused on miR-574-5p in the present study because it has been reported to be upregulated in plasma and cardiac samples from patients with ischemic heart disease, a frequent cause of CA [17, 18, 21]. However, circulating levels of miR-574-5p were only modestly correlated with the cardiac markers NT-proBNP and hs-TnT. This might be due to the inclusion of all OHCA of cardiac origin patients in the TTM trial, independently of the presence of ischemia.
Currently, miR-574-5p is not considered organ- or tissue-specific. It is expressed in the human heart and liver [30] and also in different cancer cell lines and adipose cells [31–33]. In mice, miR-574-5p plays different roles in the brain. It promotes the differentiation of neural progenitor cells into neurons [34], and levels of miR-574-5p were decreased in the brain of mice following injury by exposure to fine particles. Increased levels of miR-574-5p restored synaptic and cognitive impairment caused by fine particle exposure [35]. On the other hand, patients suffering of intracerebral haemorrhage showed an upregulation of circulating miR-574-5p levels, independently of sex [22]. In our study, circulating levels of miR-574-5p were weakly correlated with NSE and S100b levels. NSE is known to be expressed by neurons whereas S100b is expressed by glial cells. Both are released after brain injury following CA or in other neurological conditions such as traumatic brain injury [28, 29, 36, 37]. Our observations do not confirm that miR-574-5p only originates from the brain. The association between miR-574-5p and ischemia in different contexts (cerebral and cardiac) suggests that circulating levels of miR-574-5p may originate from different organs during or after ischemic-reperfusion injuries simultaneously after CA. Further animal experiments would be needed to test whether miR-574-5p is released from the brain and/or from other organs simultaneously after CA. Such studies would allow characterization of the tissue and cellular origin of miR-574-5p which remains poorly known.
Other miRNA have been previously studied in the context of OHCA, a number of them showing potential as prognostic indicators [11]. In the TTM cohort, both miR-124-3p and miR-122-5p showed strong associations with neurological outcome [13, 16]. miR-124-3p is enriched in the brain. The weak correlation between miR-574-5p and miR-124-3p do not support the possibility that miR-574-5p could be exclusively released from the injured brain after OHCA. Since miR-122-5p originates from the liver, the absence of correlation between miR-122-5p and miR-574-5p levels suggests that circulating levels of miR-574-5p are not only released by the liver, hence strengthening the assumption that miR-574-5p could be released simultaneously by several organs. In previous smaller-scale studies, miR-21 and miR-122-5p were also considered potential prognostic biomarkers [12, 14], although a recent small trial did not report significant associations between admission levels of miR-122-5p and all-cause mortality [15]. This lack of association might be due to the time of measurement (admission vs. 48 h post OHCA), and this highlights the need for future studies with serial assessment of miRNA, from admission to a few days after OHCA. This would determine the kinetics of miRNA release after OHCA as well as the optimal time point(s) for measurement, as studies on the kinetics of miRNA levels after OHCA are sparse.
In our study, circulating levels of miR-574-5p were higher in patients of the 33°C group. Hypothermia at 33°C induces lower clearance than hypothermia at 36°C [38], which could be involved in this upregulation of miR-574-5p. Simultaneously, Eskla et al. showed that, in HeLa cells, hypothermia at 32°C for 24h increased the expression of hypoxia-inducible factor- (HIF-) 1a [39], and another study showed that overexpression of HIF-1a led to miR-574-5p overexpression [40]. These results suggest HIF-1a may play a role in the higher levels of miR-574-5p observed in patients of the 33°C group. The exact pathway (and the clinical or neurological significance) leading to increased levels of miR-574-5p in the circulation after OHCA remains to be elucidated.
Sex disparities have been reported in the context of CA, for instance, a lower proportion of witnessed OHCA occurring in women or the fact that women have less OHCA from cardiac aetiology than men [41, 42]. Differences in treatment modalities, such as the utilization of coronary angiography, have been reported [43, 44]. Despite these, and after adjustment with confounders, no significant difference in mortality between men and women has been reported [44, 45], including in the TTM trial [41]. This is consistent with our present substudy in which sex was not associated with neurological outcome. We report here for the first time a sex-specific association between a candidate biomarker and outcome after OHCA. More specifically, we present data suggesting a prognostic value of miR-574-5p in women but not in men.
Patients after CA have higher blood levels of oestradiol than healthy controls [46] and miR-574-5p expression upregulated by oestradiol treatment in vitro [20]. This may suggest that higher levels of oestradiol in the blood may increase miR-574-5p expression, which would lead to higher circulating levels of miR-574-5p in women. Further studies are required to define a possible functional link between miR-574-5p and oestradiol which may explain the different prognostic value of miR-574-5p observed in the present study between men and women.
From a clinical point of view, predicting neurological outcome after OHCA represents a step forward towards personalized medicine. Predicting outcome at an early stage after OHCA would allow clinicians to optimize therapies in patients who would mostly likely benefit while guiding early decision making in patients with irreversible and severe neurological sequelae, thereby avoiding long and painful waiting periods for relatives [11]. Novel biomarkers will increase the accuracy of current multimodal prediction tools, and circulating miRNA may have the potential to attain an optimal predictive value. It will be important to conduct future studies in a sex-specific manner to avoid extrapolating results obtained on a mainly male population to women.
The limitations of this study are as follows: First, the choice of miR-574-5p relied on a known association with ischemic heart disease and atrial fibrillation while many other miRNA are regulated in the ischemic heart and may also deserve further investigation in the context of OHCA [10]. Second, we did not determine the cellular origin of miR-574-5p, which should be further investigated. Third, miR-574-5p was measured at a single time point. Fourth, our patient population contained only 109 women compared to 481 men, a difference which decreased the power of the study and emphasizes the need for additional validation.
5. Conclusion
We identified miR-574-5p as a female-specific predictor of neurological outcome after OHCA. Our data require further independent and large-scale testing of the ability of miR-574-5p to predict outcome after OHCA, focusing on sex disparities.
Acknowledgments
A.B. is funded by the National Research Fund of Luxembourg (grant # AFR 8832104). A.S.S. is funded by the National Research Fund of Luxembourg (grants nos. C14/BW1/8225223 and C17/BM/11613033). Y.D. is supported by the National Research Fund and the Ministry of Higher Education and Research of Luxembourg. The TTM trial and the TTM trial biobank were funded by independent research grants from the Swedish Heart Lung Foundation; Arbetsmarknadens Försäkringsaktiebolag (AFA) Insurance Foundation; Swedish Research Council; Regional research support, Region Skåne; government funding of clinical research within the Swedish NHS (National Health Services); Thelma Zoega Foundation; Krapperup Foundation; Thure Carlsson Foundation; Hans-Gabriel and Alice Trolle-Wachtmeister Foundation for Medical Research; Skåne University Hospital, Sweden; TrygFonden, Denmark; European Clinical Research Infrastructures Network; and European Critical Care Research Network. The contribution of TTM trial investigators and Cardiolinc™ network members to this work is greatly acknowledged. There was no commercial funding. We would like to thank all the staff involved in the recruitment sites of TTM trial.
Abbreviations
- AIC:
Akaike information criteria
- CA:
Cardiac arrest
- CI:
Confidence interval
- CPC:
Cerebral performance category
- HIF-1a:
Hypoxia-inducible factor-1a
- hs-TnT:
High-sensitive cardiac troponin
- IDI:
Integrated discrimination improvement
- miRNA:
MicroRNA
- NSE:
Neuron-specific enolase
- NT-proBNP:
N-terminal probrain natriuretic peptide
- OHCA:
Out-of-hospital cardiac arrest
- OR:
Odds ratio
- ROSC:
Return of spontaneous circulation
- TTM:
Targeted temperature management.
Data Availability
The demographical, clinical, and microRNA expression data used to support the findings of this study are available from the corresponding author upon request. The results of univariate and multivariable analyses are included within the article and the supplementary information file.
Disclosure
Funding organisms did not have any access to the data nor did they have any influence on their analysis or interpretation.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Supplementary Materials
Supplementary Table 1: comparison between demographic and clinical features of the patients included in the present substudy and the patients of the whole TTM cohort. Supplementary Table 2: demographic and clinical features of the 590 patients included in the present substudy separated by sex. Supplementary Table 3: univariate association between demographic and clinical characteristics, miR-574-5p levels, and neurological outcome at 6 months after CA in all 590 patients, 481 men and 109 women. Supplementary Table 4: correlation between levels of markers of neurological and cardiac damage, miR-122-5p, miR-124-3p, and miR-574-5p. Supplementary Figure 1: association between circulating levels of miR-574-5p and age and sex. Supplementary Figure 2: circulating levels of miR-574-5p according to targeted temperature management regimen and neurological outcome for all patients (a, d, g), men (b, e, h) and women (c, f, i).
References
- 1.Benjamin E. J., Blaha M. J., Chiuve S. E., et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bougouin W., Lamhaut L., Marijon E., et al. Characteristics and prognosis of sudden cardiac death in Greater Paris: population-based approach from the Paris Sudden Death Expertise Center (Paris-SDEC) Intensive Care Medicine. 2014;40(6):846–854. doi: 10.1007/s00134-014-3252-5. [DOI] [PubMed] [Google Scholar]
- 3.Perkins G. D., Jacobs I. G., Nadkarni V. M., et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation. 2015;96:328–340. doi: 10.1016/j.resuscitation.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bougouin W., Mustafic H., Marijon E., et al. Gender and survival after sudden cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2015;94:55–60. doi: 10.1016/j.resuscitation.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Gräsner J. T., Lefering R., Koster R. W., et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: A prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. doi: 10.1016/j.resuscitation.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Dragancea I., Rundgren M., Englund E., Friberg H., Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84(3):337–342. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Nolan J. P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Frydland M., Kjaergaard J., Erlinge D., et al. Usefulness of serum B-type natriuretic peptide levels in comatose patients resuscitated from out-of-hospital cardiac arrest to predict outcome. The American Journal of Cardiology. 2016;118(7):998–1005. doi: 10.1016/j.amjcard.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Gilje P., Koul S., Thomsen J. H., et al. High-sensitivity troponin-T as a prognostic marker after out-of-hospital cardiac arrest - a targeted temperature management (TTM) trial substudy. Resuscitation. 2016;107:156–161. doi: 10.1016/j.resuscitation.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Goretti E., Wagner D. R., Devaux Y. miRNAs as biomarkers of myocardial infarction: a step forward towards personalized medicine? Trends in Molecular Medicine. 2014;20(12):716–725. doi: 10.1016/j.molmed.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 11.On behalf of the Cardiolinc™ network, Devaux Y., Stammet P. What’s new in prognostication after cardiac arrest: microRNAs? Intensive Care Medicine. 2018;44(6):897–899. doi: 10.1007/s00134-017-4995-6. [DOI] [PubMed] [Google Scholar]
- 12.Gilje P., Gidlöf O., Rundgren M., et al. The brain-enriched microRNA miR-124 in plasma predicts neurological outcome after cardiac arrest. Critical Care. 2014;18(2):p. R40. doi: 10.1186/cc13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux Y., Salgado-Somoza A., Dankiewicz J., et al. Incremental value of circulating miR-122-5p to predict outcome after out of hospital cardiac arrest. Theranostics. 2017;7(10):2555–2564. doi: 10.7150/thno.19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stammet P., Goretti E., Vausort M., Zhang L., Wagner D. R., Devaux Y. Circulating microRNAs after cardiac arrest. Critical Care Medicine. 2012;40(12):3209–3214. doi: 10.1097/CCM.0b013e31825fdd5e. [DOI] [PubMed] [Google Scholar]
- 15.Gilje P., Frydland M., Bro-Jeppesen J., et al. The association between plasma miR-122-5p release pattern at admission and all-cause mortality or shock after out-of-hospital cardiac arrest. Biomarkers. 2019;24(1):29–35. doi: 10.1080/1354750X.2018.1499804. [DOI] [PubMed] [Google Scholar]
- 16.Devaux Y., Dankiewicz J., Salgado-Somoza A., et al. Association of circulating microRNA-124-3p levels with outcomes after out-of-hospital cardiac arrest: A Substudy of a Randomized Clinical Trial. JAMA Cardiology. 2016;1(3):305–313. doi: 10.1001/jamacardio.2016.0480. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J., Shao G., Chen X., et al. miRNA 206 and miRNA 574-5p are highly expression in coronary artery disease. Bioscience Reports. 2015;36(1, article e00295) doi: 10.1042/BSR20150206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooij E., Sutherland L. B., Thatcher J. E., et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J., Liang D., Zhang Y., et al. MicroRNA expression signature in atrial fibrillation with mitral stenosis. Physiological Genomics. 2011;43(11):655–664. doi: 10.1152/physiolgenomics.00139.2010. [DOI] [PubMed] [Google Scholar]
- 20.Papaioannou M. D., Koufaris C., Gooderham N. J. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) elicits estrogenic-like microRNA responses in breast cancer cells. Toxicology Letters. 2014;229(1):9–16. doi: 10.1016/j.toxlet.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Zaman S., Kovoor P. Sudden cardiac death early after myocardial infarction: pathogenesis, risk stratification, and primary prevention. Circulation. 2014;129(23):2426–2435. doi: 10.1161/CIRCULATIONAHA.113.007497. [DOI] [PubMed] [Google Scholar]
- 22.Guo D., Liu J., Wang W., et al. Alteration in abundance and compartmentalization of inflammation-related miRNAs in plasma after intracerebral hemorrhage. Stroke. 2013;44(6):1739–1742. doi: 10.1161/STROKEAHA.111.000835. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen N., Wetterslev J., Cronberg T., et al. Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest. New England Journal of Medicine. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen N., Wetterslev J., al-Subaie N., et al. Target temperature management after out-of-hospital cardiac arrest--a randomized, parallel-group, assessor-blinded clinical trial--rationale and design. American Heart Journal. 2012;163(4):541–548. doi: 10.1016/j.ahj.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen N., Winkel P., Cronberg T., et al. Detailed statistical analysis plan for the target temperature management after out-of-hospital cardiac arrest trial. Trials. 2013;14(1):p. 300. doi: 10.1186/1745-6215-14-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth C. M., Boone R. H., Tomlinson G., Detsky A. S. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291(7):870–879. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- 28.Stammet P., Collignon O., Hassager C., et al. Neuron-Specific Enolase as a Predictor of Death or Poor Neurological Outcome After Out-of-Hospital Cardiac Arrest and Targeted Temperature Management at 33°C and 36°C. Journal of the American College of Cardiology. 2015;65(19):2104–2114. doi: 10.1016/j.jacc.2015.03.538. [DOI] [PubMed] [Google Scholar]
- 29.Target Temperature Management after Out-of-Hospital Cardiac Arrest (TTM) trial investigators, Stammet P., Dankiewicz J., et al. Protein S100 as outcome predictor after out-of-hospital cardiac arrest and targeted temperature management at 33 °C and 36 °C. Critical Care. 2017;21(1):p. 153. doi: 10.1186/s13054-017-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalabus J. L., Cheng Q., Blanco J. G. MicroRNAs differentially regulate carbonyl reductase 1 (CBR1) gene expression dependent on the allele status of the common polymorphic variant rs9024. PLoS One. 2012;7(11, article e48622) doi: 10.1371/journal.pone.0048622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Lu X., Geng Z., Yang G., Shi Y. LncRNA PTCSC3/miR-574-5p Governs Cell Proliferation and Migration of Papillary Thyroid Carcinoma via Wnt/β-Catenin Signaling. Journal of Cellular Biochemistry. 2017;118(12):4745–4752. doi: 10.1002/jcb.26142. [DOI] [PubMed] [Google Scholar]
- 32.Belarbi Y., Mejhert N., Gao H., Arner P., Rydén M., Kulyté A. MicroRNAs-361-5p and miR-574-5p associate with human adipose morphology and regulate EBF1 expression in white adipose tissue. Molecular and Cellular Endocrinology. 2018;472:50–56. doi: 10.1016/j.mce.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Ji S., Ye G., Zhang J., et al. miR-574-5p negatively regulatesQki6/7to impactβ-catenin/Wnt signalling and the development of colorectal cancer. Gut. 2013;62(5):716–726. doi: 10.1136/gutjnl-2011-301083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W., Thevapriya S., Kim P. J., et al. Amyloid precursor protein regulates neurogenesis by antagonizing miR-574-5p in the developing cerebral cortex. Nature Communications. 2014;5(1, article 3330) doi: 10.1038/ncomms4330. [DOI] [PubMed] [Google Scholar]
- 35.Ku T., Li B., Gao R., et al. NF-κB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Particle and Fibre Toxicology. 2017;14(1):p. 34. doi: 10.1186/s12989-017-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sogut O., Guloglu C., Orak M., et al. Trauma scores and neuron-specific enolase, cytokine and C-reactive protein levels as predictors of mortality in patients with blunt head trauma. Journal of International Medical Research. 2010;38(5):1708–1720. doi: 10.1177/147323001003800516. [DOI] [PubMed] [Google Scholar]
- 37.Thelin E. P., Nelson D. W., Bellander B. M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochirurgica. 2017;159(2):209–225. doi: 10.1007/s00701-016-3046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjelland T. W., Klepstad P., Haugen B. O., Nilsen T., Dale O. Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug Metabolism and Disposition. 2013;41(1):214–223. doi: 10.1124/dmd.112.045567. [DOI] [PubMed] [Google Scholar]
- 39.Eskla K. L., Porosk R., Reimets R., et al. Hypothermia augments stress response in mammalian cells. Free Radical Biology and Medicine. 2018;121:157–168. doi: 10.1016/j.freeradbiomed.2018.04.571. [DOI] [PubMed] [Google Scholar]
- 40.Xie Y., Li W., Feng J., Wu T., Li J. MicroRNA-363 and GATA-1 are regulated by HIF-1α in K562 cells under hypoxia. Molecular Medicine Reports. 2016;14(3):2503–2510. doi: 10.3892/mmr.2016.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winther-Jensen M., Kjaergaard J., Wanscher M., et al. No difference in mortality between men and women after out-of-hospital cardiac arrest. Resuscitation. 2015;96:78–84. doi: 10.1016/j.resuscitation.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Herlitz J., Engdahl J., Svensson L., Young M., Ängquist K. A., Holmberg S. Is female sex associated with increased survival after out-of-hospital cardiac arrest? Resuscitation. 2004;60(2):197–203. doi: 10.1016/j.resuscitation.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Waldo S. W., Armstrong E. J., Kulkarni A., et al. Comparison of clinical characteristics and outcomes of cardiac arrest survivors having versus not having coronary angiography. The American Journal of Cardiology. 2013;111(9):1253–1258. doi: 10.1016/j.amjcard.2013.01.267. [DOI] [PubMed] [Google Scholar]
- 44.Winther-Jensen M., Hassager C., Kjaergaard J., et al. Women have a worse prognosis and undergo fewer coronary angiographies after out-of-hospital cardiac arrest than men. European Heart Journal: Acute Cardiovascular Care. 2018;7(5):414–422. doi: 10.1177/2048872617696368. [DOI] [PubMed] [Google Scholar]
- 45.Bosson N., Kaji A. H., Fang A., et al. Sex Differences in Survival From Out‐of‐Hospital Cardiac Arrest in the Era of Regionalized Systems and Advanced Post‐Resuscitation Care. Journal of the American Heart Association. 2016;5(9) doi: 10.1161/JAHA.116.004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayanan K., Havmoeller R., Reinier K., et al. Sex hormone levels in patients with sudden cardiac arrest. Heart Rhythm. 2014;11(12):2267–2272. doi: 10.1016/j.hrthm.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: comparison between demographic and clinical features of the patients included in the present substudy and the patients of the whole TTM cohort. Supplementary Table 2: demographic and clinical features of the 590 patients included in the present substudy separated by sex. Supplementary Table 3: univariate association between demographic and clinical characteristics, miR-574-5p levels, and neurological outcome at 6 months after CA in all 590 patients, 481 men and 109 women. Supplementary Table 4: correlation between levels of markers of neurological and cardiac damage, miR-122-5p, miR-124-3p, and miR-574-5p. Supplementary Figure 1: association between circulating levels of miR-574-5p and age and sex. Supplementary Figure 2: circulating levels of miR-574-5p according to targeted temperature management regimen and neurological outcome for all patients (a, d, g), men (b, e, h) and women (c, f, i).
Data Availability Statement
The demographical, clinical, and microRNA expression data used to support the findings of this study are available from the corresponding author upon request. The results of univariate and multivariable analyses are included within the article and the supplementary information file.