Abstract
Objective
To systematically evaluate the significance of squamous cell carcinoma antigen (SCC-Ag) in the prognosis of cervical cancer.
Methods
Literature from Pubmed, Embase, and Cochrane Library was retrieved to collect all English literature on the correlation between SCC-Ag and cervical cancer prognosis, and the quality of literature collected was assessed based on evaluation criteria. The heterogeneity, sensitivity, and specificity were detected using the StataSE12.0 software, and the correlation between SCC-Ag and cervical cancer prognosis as the effect variables was assessed using the hazard ratio (HR) and 95% confidence interval (CI). Moreover, the forest map and funnel plot were drawn.
Results
A total of 17 articles meeting the inclusion criteria were selected. The high expression of SCC-Ag was significantly correlated with the poor prognosis of cervical cancer (HR = 2.22, 95% CI = 1.38 − 3.57, P = 0.002). The disease-free survival (DFS) was higher in low SCC-Ag expression patients than in high SCC-Ag expression patients (HR = 2.17, 95% CI = 1.84 − 2.57, P < 0.001). The progression-free survival (PFS) was inferior in patients with a high SCC-Ag expression (HR = 2.70, 95% CI = 1.11 − 6.53, P = 0.028).
Conclusion
SCC-Ag is an important prognostic factor for cervical cancer, and its high expression is significantly correlated with a poor prognosis of the disease.
1. Introduction
Cervical cancer is the third most common cancer in females around the world and the fourth leading cause of cancer death [1]. Compared with developed countries, developing countries have a significantly higher incidence rate of cervical cancer, which is related to the intensity of cervical cancer screening [1]. More than 270,000 women die of cervical cancer every year, more than 85% of whom die in low- and middle-income countries [1]. Due to insufficient resources, cervical cancer prevention and control cannot achieve high coverage in these countries [2]. The early symptoms of cervical cancer are not obvious, so patients are often diagnosed in the advanced stages, which directly affects the quality of life and prognosis of patients. The common pathological types of cervical cancer are squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. Squamous cell carcinoma is the most common. Squamous cell carcinoma antigen (SCC-Ag) is an early tumour marker for diagnosing cervical cancer and monitoring responses to treatment in the event of relapse. It is convenient to detect, has good specificity, and is widely used in clinical practice. Studies have demonstrated that an increase in SCC-Ag concentration precedes clinical manifestations and can be used as an independent risk factor [3]. Moreover, SCC-Ag is commonly used in the detection of lung cancer, oesophageal cancer, and head and neck cancer. In the diagnosis and detecting recurrence of cervical cancer, SCC-Ag has been clearly validated. In terms of the prognosis, many studies argued that it was an independent prognostic factor. The clinical stage, depth of invasion, tumour size, and lymph node metastasis of SCC have enormous significance in the prognosis [4]. By continuous monitoring of SCC-Ag, early SCC recurrence may be detected and appropriate remedial treatment can be prescribed for early intervention, thereby improving the quality of life and prolonging survival [5]. However, there have been studies showing the different prognostic values of SSC-Ag in cervical cancer. Not all patients with recurrent cervical cancer have sustained SCC-Ag during follow-up, and some have SCC-Ag during disease progression. No specific changes have been identified that can guide the development of clinical treatment strategies for patients. This study comprehensively searched the literature on the correlation between SCC-Ag and cervical cancer prognosis and systematically analyzed appropriate publications by meta-analysis.
2. Materials and Methods
2.1. Literature Retrieval
The literature up to June 2018 was independently searched by two researchers in the library records. Pubmed, Embase, and Cochrane Library entries were retrieved. English index words included SCC-Ag, squamous cell carcinoma antigen, serum squamous carcinoma antigen, tumour, cancer, carcinoma, neoplasm, prognostic, prognosis, survival, carcinoma of uterine cervix, and cervical cancer.
2.2. Inclusion and Exclusion Criteria of Literature
Publication inclusion criteria are as follows: (1) studies based on cervical cancer patients; (2) published epidemiological studies, such as case-control studies and line-up studies; (3) studies evaluating the diagnostic value of tumour markers for cervical cancer; (4) positive values or HR and 95% CI of the case group and the control group were clearly reported, or the corresponding four-table data were provided; (5) research methods and scope were similar, and detection methods were the same; (6) the relationship between SCC-Ag expression and prognosis of cervical cancer was reported; and (7) the data and information were complete.
Publication exclusion criteria are as follows: (1) publications containing the pathological type of noncervical cancer; (2) cases that had no clear diagnostic criteria; (3) reviews or conference minutes; (4) the number of cases was less than 10; (5) publications that did not provide credible case and control sources; (6) lack of raw data to calculate the HR, 95% CI, and P value; (7) inconsistent publications; and (8) publications that lacked the results or controversial publications.
2.3. Literature Screening
Two researchers independently screened the literature according to the inclusion criteria. If there were differences or disputes in the process, they reached an agreement through discussion and study. The first step was to exclude irrelevant articles from the topic and abstract reading; the second step was to exclude publications without reference values; the third step was to exclude articles with no integrity and accuracy data; and, finally, the articles meeting the inclusion and exclusion criteria after screening were obtained.
2.4. Data Extraction
Two researchers independently screened the literature, extracted and cross-checked the data, searched the relevant publications according to the search strategy, screened the appropriate publications by looking through their titles and abstracts, and read the full text to determine whether the inclusion criteria were met. The information to be extracted mainly included the first author's name and nationality, publication period, number of patients, pathological stage, age, follow-up time, test samples, test methods, cut-off values, analysis methods of the survival rate, and survival analysis.
2.5. Statistical Analysis
The correlation between SCC-Ag expression and cervical cancer prognosis as the effect variables was evaluated by the HR value and 95% CI. If the HR and 95% CI were described in the publication, they were extracted directly. If not described, they were calculated based on available data. Data for the Kaplan-Meier survival curve were obtained by the Engauge software. A meta-analytical method was used to calculate and merge the results of the publications that met the inclusion criteria, and the Stata12.0 software was used to draw the forest map. If HR > 1, patients with a high SCC-Ag expression had a worse prognosis; if HR < 1, patients with a high SCC-Ag expression had a better prognosis; and if HR = 1, the high expression of SCC-Ag was not related to the prognosis of patients. The heterogeneity of this study was investigated by I2 and P. If I2 > 50% and P < 0.05, the heterogeneity among studies was strong and the data were combined by a random effects model; if I2 < 50% and P > 0.05, there was no heterogeneity among studies and the data were combined using a fixed effects model. The Stata12.0 software was also used to draw the funnel plot to find potential publication bias.
3. Results
3.1. Literature Retrieval Results
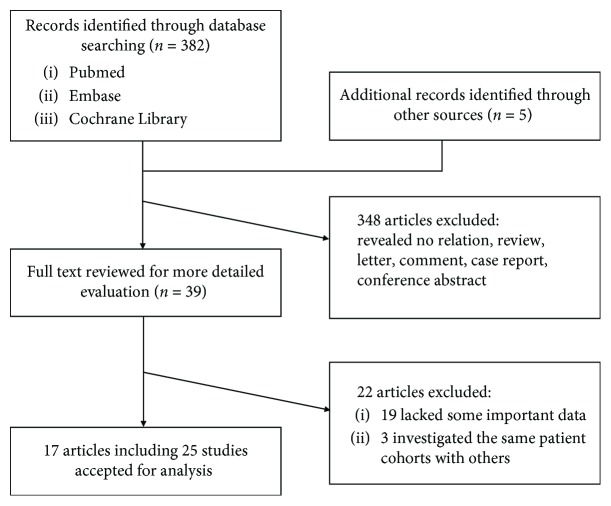
A total of 387 publications related to SCC-Ag expression and the prognosis of cervical cancer were retrieved. Of these, 348 publications unrelated to the study were excluded by carefully reading the title and abstract, and the remaining 39 publications were further examined by screening the full text. Finally, 17 publications were studied, including 4 that simultaneously analyzed overall survival (OS) and progression-free survival (PFS) as prognostic indicators, 4 that analyzed OS and disease-free survival (DFS) as prognostic indicators, 5 studied with DFS alone, 3 articles with the most endpoints of OS alone, and 1 studied with PFS alone (Figure 1) [6–22]. The publication period of the literature was between 2003 and 2017. A total of 3308 patients from different countries were included. Patients mostly had pathological stages II-III. The average age ranged from 42.7 to 68 years. The minimum follow-up time was 34 months, and 60 months was the longest follow-up period. The cut-off value of each article was selected according to the research needs. The test samples extracted from the patients' serum in 18 articles were analyzed by ELISA. The basic characteristics of the included literature are shown in Table 1.
Figure 1.
Flow diagram of the study selection process.
Table 1.
Main characteristics of all studies included in the meta-analysis.
| Author | Country | Year | Sample number | FIGO stage I/II/III/IV | Age | Follow-up months | Detected sample | Test way | Cut-off value (ng/mL) | Multivariate analysis | Survival analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ouyang et al. [20] | China | 2017 | 155 | 101/54/0/0 | Median 47 | Over 60 | Serum | ELISA | 1.5 | Yes | OS |
| Jiang et al. [21] | China | 2017 | 182 | IB-IIA153/IIB-IIIB29 | Mean 53 | Over 60 | Serum | ELISA | 4 | Yes | OS |
| Kim et al. [22] | Korea | 2016 | 48 | 9/28/6/5 | Median 52 | Median 34 | Serum | ELISA | 2 | Yes | PFS |
| Salvatici et al. [7] | Italy | 2016 | 197 | 158/39/0/0 | Median 43.9 | Over 60 | Serum | ELISA | 1.5 | Yes | OS, PFS |
| Ryu et al. [8] | Korea | 2016 | 154 | 53/84/9/8 | Median 59 | Median 41 | Serum | ELISA | 1.86 | Yes | DFS |
| Kotowicz et al. [6] | Poland | 2016 | 138 | 38/40/57/3 | Median 54 | Over 60 | Serum | ELISA | 1.6/1.8 | Yes | OS, DFS |
| Li et al. [9] | China | 2015 | 286 | IB1-IIA135/IIB-IIIB151 | Median 45 | Over 60 | Serum | ELISA | 3.5 | No | OS, DFS |
| Binbin et al. [10] | China | 2014 | 172 | 0/119/50/3 | Median 49 | Median 54.5 | Serum | ELISA | 3 | Yes | OS, PFS |
| Shimura et al. [11] | Japan | 2013 | 167 | 22/66/45/34 | Median 58 | Over 60 | Serum | ELISA | 2 | No | DFS |
| Kawaguchi et al. [12] | Japan | 2013 | 116 | IIB33/IIIA6/IIIB64/IVA13 | Median 68 | Median 58.6 | Serum | ELISA | 1.15 | No | OS, PFS |
| Hirakawa et al. [13] | Japan | 2008 | 108 | IB-2/II-57/III-46/IV-3 | Median 50 | Median 48 | Serum | ELISA | 1.5 | Yes | DFS |
| Hsu et al. [14] | Taiwan | 2007 | 38 | IB-32/IIA-6 | Median 50.8 | Median 29.8 | Serum | ELISA | NR | No | DFS |
| Qgino et al. [15] | Japan | 2006 | 352 | IIB-99/III-239/IVA-14 | Median 61 | Median 53 | Serum | ELISA | 6 | Yes | DFS |
| Molina et al. [19] | Spain | 2005 | 156 | 47/65/39/5 | NR | Median 44 | Serum | ELISA | 2 | No | OS, DFS |
| Huang et al. [16] | Taiwan | 2012 | 188 | 22/125/37/4 | Median 56.5 | Median 58.2 | Serum | ELISA | 10 | No | OS |
| Lee et al. [17] | Korea | 2011 | 788 | 505/219/50/14 | Median 51 | Median 53.4 | Serum | ELISA | 1.6 | Yes | OS, PFS |
| Ohno et al. [18] | Japan | 2003 | 63 | 2/21/29/11 | Median 66 | Over 48 | Serum | ELISA | 1.5 | Yes | OS, DFS |
NR: no report; OS: overall survival; PFS: progression-free survival; DFS: disease-free survival.
3.2. Meta-analysis
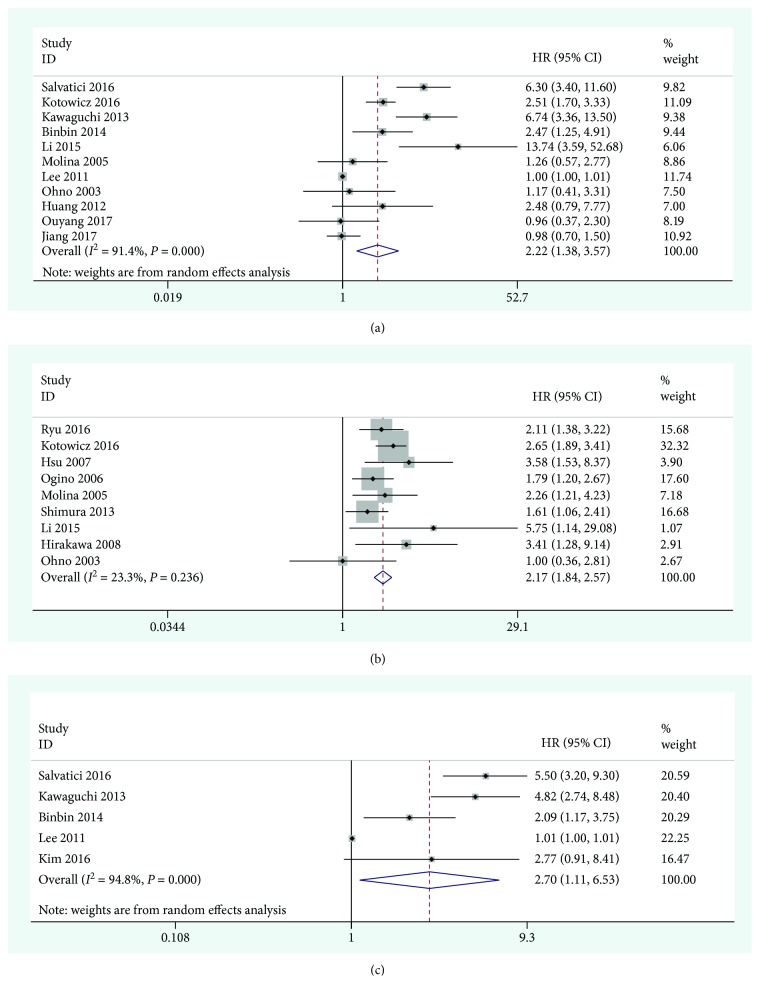
A total of 11 publications including 2441 patients provided the data for the OS analysis. Meta-analysis using the Stata12.0 software showed statistically significant heterogeneity in the study of OS (I2 = 91.4%, P < 0.001). The random effects model was used to combine the HRs. The pooled results showed that the high expression of SCC-Ag was significantly associated with the poor prognosis of cervical cancer (combined HR = 2.22, 95% CI = 1.38 − 3.57, P = 0.002). The forest graph is shown in Figure 2(a). In addition, the studies evaluating DFS and PFS were summarized and analyzed. The results showed that there was no statistical heterogeneity in the study of DFS (I2 = 23.3%, P = 0.236), and the combined effects model was used to merge the HRs (HR = 2.17, 95% CI = 1.84 − 2.57, P < 0.001) (Figure 2(b)). There was significant heterogeneity in the study of PFS (I2 = 94.8%, P < 0.001), and HR was combined using a random effects model (HR = 2.70, 95% CI = 1.11 − 6.53, P = 0.028) (Figure 2(c)). The cause of heterogeneity may be related to race and the small number of articles included.
Figure 2.
Forest plot of the relationship between high SCC-Ag and OS (a), DFS (b), and PFS (c) in cervical cancer.
3.3. Subgroup Analysis
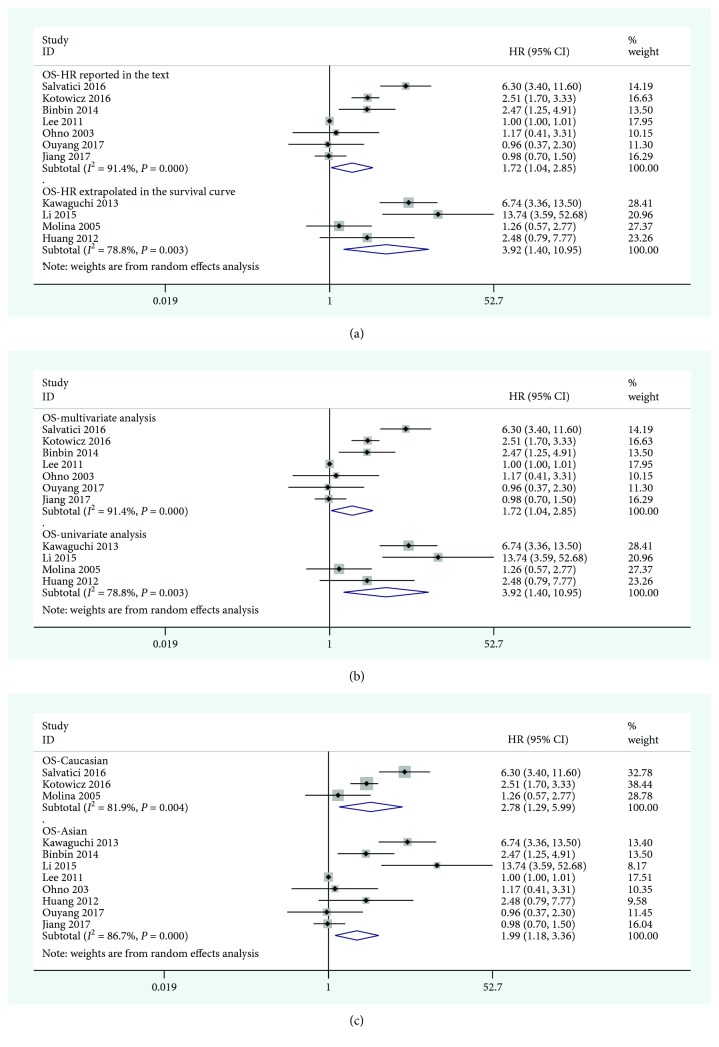
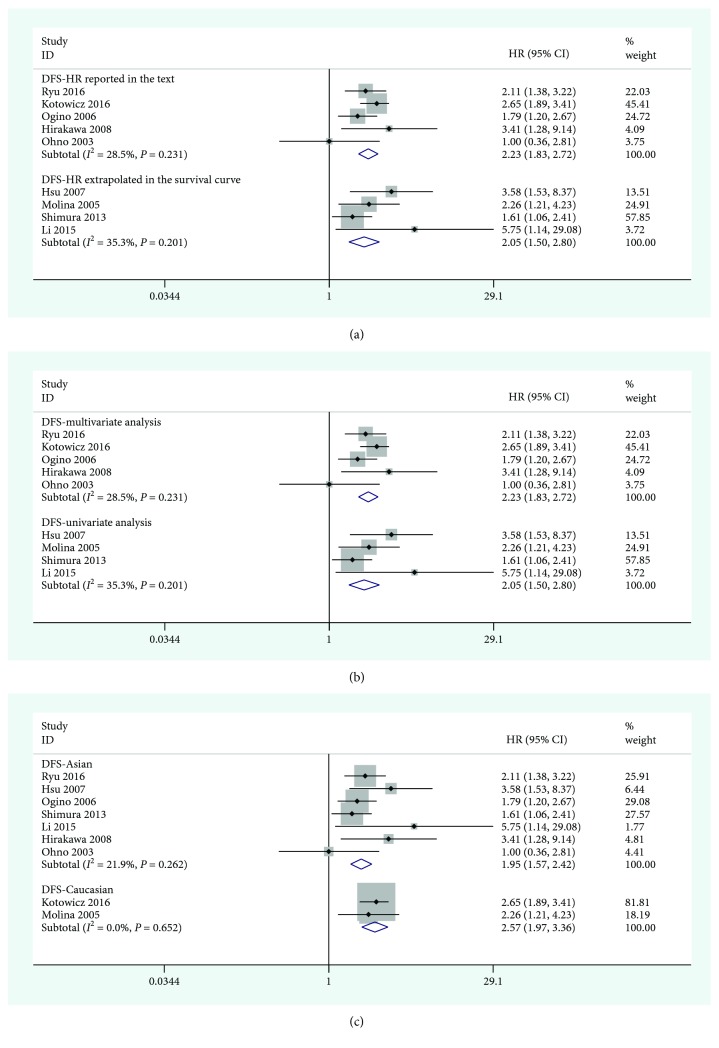
According to the clinical characteristics and the results of the meta-analysis, the studies providing OS (Figure 3) and DFS (Figure 4) data were separately analyzed in subgroups. The results are shown in Table 2. In the ethnic subgroup analysis, the high expression of SCC-Ag was a poor prognostic factor for cervical cancer. In the other subgroup analyses, such as the data acquisition group and the factor analysis group, there were also statistical significance. In the prognostic OS subgroup analysis, the results for the HR reported in the text group were HR = 1.72 (95% CI = 1.04 − 2.85, P = 0.038) and those for the HR-extrapolated group were HR = 3.92 (95% CI = 1.40 − 10.95, P = 0.009). In the racial analysis, the results for East Asian were HR = 1.99 (95% CI = 1.18 − 3.36, P = 0.009) and those for Caucasian race were HR = 2.78 (95% CI = 1.29 − 5.99, P = 0.010). Multivariate analysis of prognostic DFS subgroups resulted in HR = 2.23 (95% CI = 1.83 − 2.72, P < 0.001), whereas the univariate analysis results were HR = 2.05 (95% CI = 1.50 − 2.80, P < 0.001); the results for East Asian were HR = 1.95 (95% CI = 1.57 − 2.42, P < 0.001), and those for Caucasian race were HR = 2.57 (95% CI = 1.97 − 3.36, P < 0.001).
Figure 3.
Forest plot of the relationship between high SCC-Ag and OS in the HR obtain method subgroup (a), the analysis-type subgroup (b), and the ethnicity subgroup (c).
Figure 4.
Forest plot of the relationship between high SCC-Ag and DFS in the HR obtain method subgroup (a), the analysis-type subgroup (b), and the ethnicity subgroup (c).
Table 2.
The pooled associations between different situations of SCC-Ag expression and the prognosis of patients with cervical cancer.
| Outcome subgroup | No. of patients | No. of studies | HR (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I 2 | P | |||||
| OS | 2441 | 11 | 2.22 (1.38-3.57)b | 0.002 | 91.4 | <0.001 |
| HR obtain method | ||||||
| Reported in the text | 1918 | 7 | 1.72 (1.04-2.85)b | 0.038∗ | 91.4 | <0.001 |
| Data extrapolated | 523 | 4 | 3.92 (1.40-10.95)b | 0.009 | 78.8 | 0.003 |
| Analysis type | ||||||
| Multivariate | 1918 | 7 | 1.72 (1.04-2.85)b | 0.038∗ | 91.4 | <0.001 |
| Univariate | 523 | 4 | 3.92 (1.40-10.95)b | 0.009 | 78.8 | 0.003 |
| Ethnicity | ||||||
| Asian | 1950 | 8 | 1.99 (1.18-3.36)b | 0.009∗ | 96.7 | <0.001 |
| Caucasian | 491 | 3 | 2.78 (1.29-5.99)b | 0.010∗ | 81.9 | 0.004 |
| DFS | 1462 | 9 | 2.17 (1.84-2.57)a | <0.001 | 23.3 | 0.236 |
| HR obtain method | ||||||
| Reported in the text | 815 | 5 | 2.23 (1.83-2.72)a | <0.001 | 28.5 | 0.231 |
| Data extrapolated | 647 | 4 | 2.05 (1.50-2.80)a | <0.001 | 35.3 | 0.201 |
| Analysis type | ||||||
| Multivariate | 815 | 5 | 2.23 (1.83-2.72)a | <0.001 | 28.5 | 0.231 |
| Univariate | 647 | 4 | 2.05 (1.50-2.80)a | <0.001 | 35.3 | 0.201 |
| Ethnicity | ||||||
| Asian | 1348 | 7 | 1.95 (1.57-2.42)a | <0.001 | 21.9 | 0.262 |
| Caucasian | 294 | 2 | 2.57 (1.97-3.36)a | <0.001 | 0 | 0.652 |
| PFS | 1321 | 5 | 2.70 (1.11-6.53)b | 0.028 | 94.8 | <0.001 |
OS: overall survival; DFS: disease-free survival; PFS: progression-free survival; HR: hazard ratio; CI: confidence interval. aFixed effects model. bRandom effects model.
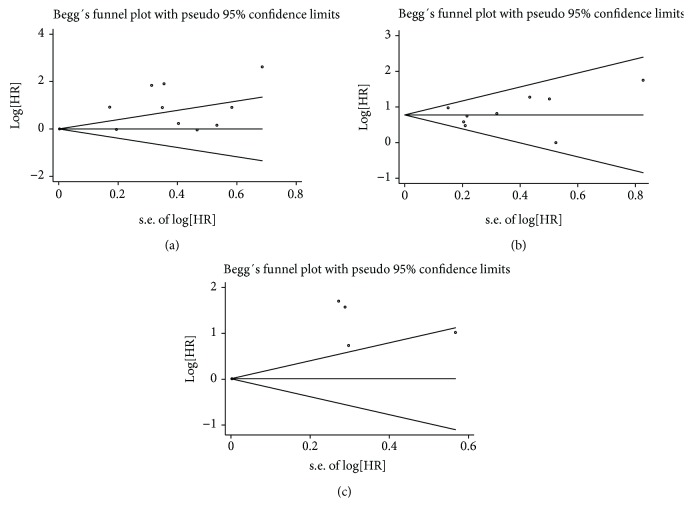
3.4. Publication Bias
The publication bias was determined by Egger's test and Begg's test, and the resulting funnel plot was asymmetrical, as shown in Figure 5, indicating that the study had a publication bias.
Figure 5.
Sensitivity analysis for meta-analysis between high SCC-Ag and OS (a), DFS (b), and PFS (c) in cervical cancer.
4. Discussion
SCC-Ag is a glycoprotein, a subtype of the tumour-associated antigen TA-4, with a molecular weight of approximately 45 kDa. It is a clinically important tumour marker. Kato and Torigoe invented a radioimmunoassay for simple and convenient detection [23]. It has advantages over imaging tests, such as B-ultrasound, CT, and MRI. SCC-Ag can act as a serine/cysteine protease inhibitor with components involved in the degradation of extracellular matrices and tumour invasion and metastasis [24]. SCC-Ag is expressed in a normal squamous epithelium and can be used clinically for the diagnosis and monitoring responses to treatment in the event of cervical, lung, oesophageal, and head and neck cancer but especially in the diagnosis of cervical cancer, with specificity higher than 80%. Therefore, it has been widely used in recent years and is recognized as a highly reliable serum cervical cancer tumour marker [25]. Many scholars have conducted research on this tumour marker. For example, Cox analysis indicated that SCC-Ag is an independent prognostic factor for OS and DFS, as shown by Kotowicz et al. [6]. Ryu et al. showed by single-factor and multivariate analyses that the high SCC-Ag expression and poor DFS were statistically significantly correlated [8]. However, some studies have demonstrated that SCC-Ag is not a completely reliable prognostic factor. For example, a previous study indicated that SCC-Ag was statistically significant in the univariate analysis of the DFS prognosis but not in the multivariate analysis [14]. Additionally, in the study by Molina et al., Kaplan-Meier survival curves showed that the high expression of SCC-Ag did not indicate poor OS [19]. In addition, SCC-Ag combined with other tumour markers, such as CEA, CA 19-9, and CYFRA21-1, had significance in the diagnosis and prognosis of squamous cell carcinoma. At the same time, some studies have noted the value of SCC-Ag and IL-6, VEGF, and TNFα in diagnosis [26]. Cervical cancer is mainly treated with surgery, radiotherapy, and chemotherapy. Synchronous chemoradiotherapy is often used in patients with advanced disease. SCC-Ag can also be used as an independent predictor of OS, disease-specific survival, and distant metastasis during treatment [27, 28]. In 1998, Maruo et al. showed a positive correlation between the EGF receptor and SCC antigen expression levels. Thus, an increase in the level of SCC antigen can indicate the amount of cell proliferation that occurs in cervical cancer [29]. Serum SCC-Ag indicates a certain tumour burden in patients with cervical cancer. As the disease progresses to the later pathological stages of cervical cancer, the depth of infiltration is deeper, the tumour volume is larger, and SCC-Ag enters the tumour lymphatics. This increases the likelihood that the tumour cells will enter the bloodstream, and as more cancer antigens are produced and released into serum, the higher levels of SCC-Ag are detected in the venous blood of patients with cervical cancer. This antigen is involved in the development of tumours and thus is related to the treatment and prognosis of tumours, with a certain regulatory effect in the process of apoptosis.
In the meta-analysis, it was found that the high expression of SCC-Ag was significantly associated with the poor OS of patients with cervical cancer (HR = 2.73, 95% CI = 1.48 − 5.05, P = 0.001). However, the cut-off values used in these studies were all different. The most used cut-off value was 1.5 ng/mL, which may be a criterion for predicting prognosis, but it needs to be further verified, and there may be errors in some data extraction processes. In addition, the follow-up time of each study was different, and some patients were lost to follow-up or were false positives. Moreover, the literature included a retrospective study that may affect the accuracy and authenticity of the data. Therefore, the reasons for the obvious heterogeneity of this meta-analysis may be the inclusion of English literature, the small number of included publications, tumour stage, cut-off value, follow-up time, and other factors. Finally, although this is the only marker assessed in the study, we should realize its limitations that other factors may be more sensitive. Future research needs to focus on the combination between SCC and other important factors of cervical cancer. In the clinical treatment of cervical cancer, it is necessary to accurately diagnose the patient, analyze the patient's pathological stage and lymphatic metastasis, dynamically observe the changes in the patient's SCC-Ag, and implement remedial measures in advance to improve the patient's quality of life and reduce their pain.
5. Conclusion
This meta-analysis summarizes all studies of SCC-Ag and cervical cancer prognosis, indicating that the high expression of SCC-Ag is significantly correlated with poor OS in cervical cancer. Considering the limitations of the study, further prospective and large-sample studies are needed to confirm the prognostic value of SCC-Ag in cervical cancer and to provide evidence for a better investigation of treatment strategies.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Z.H.L. and H.T.S. conceived and designed the study and helped to draft the manuscript. Z.H.L. performed the statistical analysis. Z.H.L. and H.T.S. performed the data collection. All authors have read and approved the content and agreed to submit the article for consideration for publication in the journal.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Cui T., Du L., et al. The correlation between the serum squamous carcinoma antigen and the prognosis of recurrent cervical squamous carcinoma. Journal of Clinical Laboratory Analysis. 2017;31(1, article e22020) doi: 10.1002/jcla.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K. C., Kim H. J., Sung K., et al. The predictive value of tumor size, volume, and markers during radiation therapy in patients with cervical cancer. International Journal of Gynecologic Cancer. 2016;27(1):123–130. doi: 10.1097/IGC.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 4.Molina R., Filella X., Lejarcegui J. A., et al. Prospective evaluation of squamous cell carcinoma and carcinoembryonic antigen as prognostic factors in patients with cervical cancer. Tumor Biology. 2003;24(3):156–164. doi: 10.1159/000073846. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y., Liang L. Z., Zheng M., Wei M., Shen Y. Correlation of serum squamous cell carcinoma antigen with clinico-pathological features and prognosis of squamous cell carcinoma of uterine cervix. Zhonghua Fu Chan Ke Za Zhi. 2007;42(1):29–33. [PubMed] [Google Scholar]
- 6.Kotowicz B., Fuksiewicz M., Jonska-Gmyrek J., Bidzinski M., Kowalska M. The assessment of the prognostic value of tumor markers and cytokines as SCCAg, CYFRA 21.1, IL-6, VEGF and sTNF receptors in patients with squamous cell cervical cancer, particularly with early stage of the disease. Tumor Biology. 2016;37(1):1271–1278. doi: 10.1007/s13277-015-3914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvatici M., Achilarre M. T., Sandri M. T., Boveri S., Vanna Z., Landoni F. Squamous cell carcinoma antigen (SCC-Ag) during follow-up of cervical cancer patients: role in the early diagnosis of recurrence. Gynecologic Oncology. 2016;142(1):115–119. doi: 10.1016/j.ygyno.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Ryu H. K., Baek J. S., Kang W. D., Kim S. M. The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstetrics & Gynecology Science. 2015;58(5):368–376. doi: 10.5468/ogs.2015.58.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Zhou J., Huang K., et al. The predictive value of serum squamous cell carcinoma antigen in patients with cervical cancer who receive neoadjuvant chemotherapy followed by radical surgery: a single-institute study. PLoS One. 2015;10(4, article e0122361) doi: 10.1371/journal.pone.0122361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binbin T., Lingying W., Manni H., Jusheng A., Ning L. Outcomes and prognostic factors of advanced squamous cervical cancer after concurrent chemoradiotherapy. Zhonghua Fu Chan Ke Za Zhi. 2014;49(5):348–354. [PubMed] [Google Scholar]
- 11.Shimura K., Mabuchi S., Yokoi T., et al. Utility of serum squamous cell carcinoma antigen levels at the time of recurrent cervical cancer diagnosis in determining the optimal treatment choice. Journal of Gynecologic Oncology. 2013;24(4):321–329. doi: 10.3802/jgo.2013.24.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi R., Furukawa N., Kobayashi H., Asakawa I. Posttreatment cut-off levels of squamous cell carcinoma antigen as a prognostic factor in patients with locally advanced cervical cancer treated with radiotherapy. Journal of Gynecologic Oncology. 2013;24(4):313–320. doi: 10.3802/jgo.2013.24.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirakawa M., Nagai Y., Inamine M., et al. Predictive factor of distant recurrence in locally advanced squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy. Gynecologic Oncology. 2008;108(1):126–129. doi: 10.1016/j.ygyno.2007.08.091. [DOI] [PubMed] [Google Scholar]
- 14.Hsu K. F., Huang S. C., Shiau A. L., et al. Increased expression level of squamous cell carcinoma antigen 2 and 1 ratio is associated with poor prognosis in early-stage uterine cervical cancer. International Journal of Gynecologic Cancer. 2007;17(1):174–181. doi: 10.1111/j.1525-1438.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogino I., Nakayama H., Okamoto N., Kitamura T., Inoue T. The role of pretreatment squamous cell carcinoma antigen level in locally advanced squamous cell carcinoma of the uterine cervix treated by radiotherapy. International Journal of Gynecologic Cancer. 2006;16(3):1094–1100. doi: 10.1136/ijgc-00009577-200605000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Huang E. Y., Huang Y. J., Chanchien C. C., et al. Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix. Radiation Oncology. 2012;7(1):p. 13. doi: 10.1186/1748-717X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y. Y., Choi C. H., Sung C. O., et al. Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecologic Oncology. 2012;124(1):92–97. doi: 10.1016/j.ygyno.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Ohno T., Nakayama Y., Nakamoto S., et al. Measurement of serum squamous cell carcinoma antigen levels as a predictor of radiation response in patients with carcinoma of the uterine cervix. Cancer. 2003;97(12):3114–3120. doi: 10.1002/cncr.11453. [DOI] [PubMed] [Google Scholar]
- 19.Molina R., Filella X., Auge J. M., et al. CYFRA 21.1 in patients with cervical cancer: comparison with SCC and CEA. Anticancer Research. 2005;25(3A):1765–1771. [PubMed] [Google Scholar]
- 20.Ouyang F., Liu J., Xia M., et al. GINS2 is a novel prognostic biomarker and promotes tumor progression in early-stage cervical cancer. Oncology Reports. 2017;37(5):2652–2662. doi: 10.3892/or.2017.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W., Pan J. J., Deng Y. H., Liang M. R., Yao L. H. Down-regulated serum microRNA-101 is associated with aggressive progression and poor prognosis of cervical cancer. Journal of Gynecologic Oncology. 2017;28(6):p. e75. doi: 10.3802/jgo.2017.28.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D. H., Kim W. T., Bae J. S., et al. Maximum standardized uptake value of pelvic lymph nodes in [18F]-fluorodeoxyglucose positron emission tomography is a prognostic factor for para-aortic lymph node recurrence in pelvic node-positive cervical cancer treated with definitive chemoradiotherapy. International Journal of Gynecological Cancer. 2016;26(7):1274–1280. doi: 10.1097/IGC.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 23.Strauss H. G., Laban C., Lautenschlager C., Buchmann J., Schneider I., Koelbl H. SCC antigen in the serum as an independent prognostic factor in operable squamous cell carcinoma of the cervix. European Journal of Cancer. 2002;38(15):1987–1991. doi: 10.1016/S0959-8049(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 24.Ogino I., Nakayama H., Kitamura T., Okamoto N., Inoue T. The curative role of radiotherapy in patients with isolated para-aortic node recurrence from cervical cancer and value of squamous cell carcinoma antigen for early detection. International Journal of Gynecologic Cancer. 2005;15(4):630–638. doi: 10.1136/ijgc-00009577-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Micke O., Bruns F., Schafer U., Prott F. J., Willich N. The impact of squamous cell carcinoma (SCC) antigen in patients with advanced cancer of uterine cervix treated with (chemo-)radiotherapy. Anticancer Research. 2005;25(3A):1663–1666. [PubMed] [Google Scholar]
- 26.Jeong B. K., Huh S. J., Choi D. H., Park W., Bae D. S., Kim B. G. Prognostic value of different patterns of squamous cell carcinoma antigen level for the recurrent cervical cancer. Cancer Research and Treatment. 2013;45(1):48–54. doi: 10.4143/crt.2013.45.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lekskul N., Charakorn C., Lertkhachonsuk A. A., Rattanasiri S., Ayudhya N. I. N. The level of squamous cell carcinoma antigen and lymph node metastasis in locally advanced cervical cancer. Asian Pacific Journal of Cancer Prevention. 2015;16(11):4719–4722. doi: 10.7314/APJCP.2015.16.11.4719. [DOI] [PubMed] [Google Scholar]
- 28.Yoon S. M., Shin K. H., Kim J. Y., et al. Use of serum squamous cell carcinoma antigen for follow-up monitoring of cervical cancer patients who were treated by concurrent chemoradiotherapy. Radiation Oncology. 2010;5(1):p. 78. doi: 10.1186/1748-717X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang E. Y., Wang C. J., Chen H. C., et al. Multivariate analysis of para-aortic lymph node recurrence after definitive radiotherapy for stage IB-IVA squamous cell carcinoma of uterine cervix. International Journal of Radiation Oncology • Biology • Physics. 2008;72(3):834–842. doi: 10.1016/j.ijrobp.2008.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.