Abstract
Background
Malnutrition among children remains one of the most important causes of morbidity and mortality in the world. In Ethiopia, malnutrition is one of the most serious public health problem and the biggest in the world. This study aimed to assess the prevalence of malnutrition and associated factors among under-five children in pastoral communities of Afar Regional state, Northeast Ethiopia.
Methods
A community-based cross-sectional study was conducted on 840 children aged 6–59 months from March 01–25, 2017. A multistage cluster sampling method was used to select the study participants. A structured questionnaire was used and anthropometric measurements were taken to collect data. EPI Data 3.1 and SPSS version 20.0 were used for data entry and analysis, respectively. Bivariate and multivariable logistic regression analysis was used to identify the factors associated with malnutrition. The statistical significance was declared at p value < 0.05 with 95% confidence intervals in the final model.
Result
The study found the prevalence of wasting, stunting, and underweight was 16.2% (95% CI: 13.8–18.8%), 43.1% (95% CI: 39.8–46.5%), and 24.8% (95% CI: 21.9–27.8%), respectively. Family size (AOR = 2.72, 95% CI: 1.62–4.55), prelacteal feeding (AOR = 3.81, 95% CI: 1.79–5.42), and diarrhoea in the past two weeks (AOR = 4.57, 95% CI: 2.56–8.16) were associated with wasting. And sex of child (AOR = 1.98, 95% CI: 1.46–2.72), age of child ((12–23 months: AOR = 3.44, 95% CI: 2.24–5.29); (24–35 months: AOR = 3.58, 95% CI: 2.25–5.69); and (36–59 months: AOR = 4.42, 95% CI: 2.79–6.94)), and immunization status of child (AOR = 3.34, 95% CI: 1.31–4.81) were predictors for stunting. Moreover, mother's education (AOR = 4.06, 95% CI: 2.01–8.19), sex of child (AOR = 1.83, 95% CI: 1.29–2.94), prelacteal feeding (AOR = 2.81, 95% CI: 1.64–3.72), and immunization status of child (AOR = 3.17, 95% CI: 2.14–4.99) were significantly associated with underweight.
Conclusions
This study indicated that child malnutrition was high among under-five children. Family size of five and above, receiving prelacteal feeding, and diarrhoea in the past two weeks were positively associated with wasting. Male child, increasing age of child, and not fully immunized child were positive predictors for increasing stunting. Maternal illiteracy, male child, prelacteal feeding, and not fully immunized child were factors affecting underweight. Promoting use of family planning, preventing diarrhoeal diseases, and vaccinating children integrated with the access of nutrition education programs are vital interventions to improve nutritional status of the children.
1. Background
Adequate nutrition is vital for healthy growth and development during childhood [1]. Malnutrition refers a pathological state resulting from relative or absolute deficiency or excess of one or more essential nutrients [2]. Wasting, stunting, and underweight are among those anthropometric indicators commonly used to measure under nutrition in a population of under-five children [3]. According to the World Health Organization (WHO), wasting, stunting, and underweight are defined as Z-scores less than −2 standard deviations of weight for height, height for age, and weight for age, respectively [4]. Wasting and stunting reflect acute and chronic exposures for nutritional deficiency, respectively. In addition, underweight reflects both acute and chronic exposures for nutritional deficiency [5, 6].
Malnutrition among children is one of the most important causes of morbidity and mortality in the world, particularly in developing countries [7]. It is the most important risk factor for the burden of disease causing about 300,000 deaths per year directly or indirectly responsible for more than half of the all deaths in children [8]. Globally, approximately 60 million and 13 million of children are affected with moderate and severe acute malnutrition, respectively [9]. Worldwide reports show that 21.9%, 13.4%, and 7.3% of under five years of age are stunted, underweight, and wasted, respectively [10]. The WHO also estimated that about 5.4 million under-five children die each year with 2.7 million deaths occurred in Sub-Saharan African countries including Ethiopia [11].
Many studies reported the health and physical consequences of child malnutrition include delaying their physical growth and motor development, lower intellectual quotient (IQ), greater behavioral problems, deficient social skills, and susceptibility to contracting diseases. Child malnutrition may also lead to higher levels of chronic illnesses in adult life which may have intergenerational effects, as malnourished females are more likely to give birth to low-weight babies [12, 13].
Malnutrition is not a simple problem with a single and simple solution. Multiple and hierarchically interrelated determinants are involved in causing malnutrition [14]. The most immediate determinants are inadequate dietary intake and disease which are themselves caused by a set of underlying factors: household food insecurity, poor maternal/child caring practices, and lack of access to basic health services including lack of safe water supply and unhealthy living environment such as open defecation [15, 16]. In turn, these underlying causes themselves are influenced by economic, political, and sociocultural conditions; national and global contexts; capacity, resources, environmental conditions, and governance [17].
The Ethiopian government has been implementing a number of strategies such as the 2004 National Strategy for IYCF practices, the 2005/2006 National Nutrition Strategy, and the 2008 National Nutrition Program [18–20]. Furthermore, the government has planned to reach the zero-level under nutrition by 2030 [21]. As a result, the country has demonstrated a promising progress in reducing child malnutrition over the past decades. Though the problem of under nutrition has decreased in the country, it still continues to be one of the countries with the highest burden of malnutrition among under-five children in the world causing a significant obstacle to achieving better child health outcomes [22]. The prevalence of underweight and stunting among young children are the highest among Sub-Saharan African countries. Moreover, 51% of under-five children's deaths are associated with malnutrition where its causes are multifaceted [21, 22].
According to the Ethiopian Demographic and Health Survey (EDHS) 2016 report, about 9.7% of the children were wasted, 28.7% of the children were underweight, and 44.4% of the children were stunted with wide regional variations [17, 22]. In Afar Regional State, the prevalence of wasting, stunting, and underweight among under-five children were 19.5%, 50.2%, and 40.2%, respectively, which is the highest as compared to the national average and across other regions [22]. Other earlier studies from other specific regions and localities of the country also indicated the prevalence of wasting in the range 11%–24%, stunting 35–49%, and underweight 21–48% [7, 23–27].
Although the prevalence of child malnutrition is relatively well documented among agrarian communities and urban dwellers of Ethiopia, evidence on the nature, prevalence, and factors affecting for child malnutrition in pastoralist communities is limited. And, national estimates are also usually not a reflection of the local estimate of child malnutrition. Afar regional state, where pastoralist communities mainly resides, has been identified as one of the hotspot regions in the country with high food insecurity, higher child malnutrition rates, and recurrent onset of droughts [28]. This indicted that investigating the problem and identifying its causative factors within this context is an important step to design appropriate strategies to mitigate the problem. Therefore, with this background in mind, this study aimed to assess the prevalence and associated factors with malnutrition among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia.
2. Methods
2.1. Study Design and Setting
The study was conducted in Dubti district located at a distance of 850 km from the capital, Addis Ababa, and 80 km from the regional capital, Semera, in Northeast direction of Ethiopia. The district has 13 kebeles (the smallest administrative units). The total population of the district was estimated to be 65,314, of which 34,870 and 30,444 are males and females, respectively, as projected from the 2007 census of the district [29].
Most of the pastoral community's income depends on animal breeding. The district has two public health centers, ten health posts, and two private clinics providing health services. The most common childhood illnesses in the district are malnutrition, diarrhoeal diseases, malaria, pneumonia, and measles [28]. A community-based cross-sectional study was conducted from March 01–25, 2017 in the district to assess the prevalence and associated factors with malnutrition among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, through door-to-door visits. Each child had 6–59 months of age and his/her mother/care giver was chosen by systematic random sampling method and involved in the study.
2.2. Study Populations
All children aged 6–59 months old, and their mothers were the target for the study, whereas the study population consisted of a sample of all households with 6–59 months old children who were residing in the randomly selected kebeles. Those study participants who were residents of the study area for less than 6 months, children's mother who was seriously ill and difficulty to communicate, and children with physical deformities that hinder height measurements at the time of data collection were excluded from the study.
2.3. Sample Size and Sampling Procedure
Sample size was determined based on a single proportion population formula using z2 × p × q/d2 considering the following assumptions: 95% confidence level, estimated proportion (P) of wasting (19.5%), stunting (50.2%), and underweight (40.2%) taken from the previous EDHS report for Afar Regional State [22] and margin of error of 5%. Accordingly, the calculated sample size for prevalence of stunting was relatively largest (n = 384) and was taken as the sample size for this study. Considering design effect of 2% and 10% nonresponse rates, the final sample size was 844.
A multistage cluster sampling method was used to enroll the study participants from the pastoral communities. First, six out of the thirteen kebeles were selected by lottery method. Next, the total number of 6–59 months old children in the selected kebeles was taken from the respective households using the registration at health posts. Then, the calculated sample size (844) was proportionally allocated to the selected kebeles based on the total number of households with 6–59 months children in each kebele. Finally, participants were selected using systematic random sampling technique after identifying the first household randomly and proceeded to the second participant based on the Kth interval. Whenever there were two or more 6–59 months old children, the youngest child was selected to avoid recall bias.
2.4. Data Collection Procedures
The questionnaire was developed from the Ethiopian Demographic and Health Survey (EDHS) [22] and other relevant literatures based on the study objectives. The questionnaire was translated in to the local language, Afaraff, for data collection. The questionnaire consisted of socioeconomic and demographic factors, child feeding and caring practices, maternal health factors, environmental health related characteristics, and anthropometrics measurements.
A structured-interviewer-administered questionnaire in a face-to-face manner was used to collect the data from mothers of children 6–59 months of age. Six health-extension workers for data and two BSc nurse supervisors who are fluent speakers of the local languages (Afaraff) including the principal investigator were involved in the data collection process. Prior to the interview, verbal informed consent was obtained from all participants after explaining about the objective of the study, and it was confirmed that the information will be kept confidential.
2.5. Measurements
Anthropometric measurements such as weight and height of children were taken using the standard anthropometric measurement procedures outlined in the measurement guide prepared by the Food and Nutrition Technical Assistance (FANTA) project in 2007 [30]. Body weight was measured using a weighing scale in light clothing with no jackets or coats, shoes, and additional clothing to the nearest 0.1 kg on a new calibrated portable scale.
Height of children was measured using a portable stadiometer with no shoes; the shoulders, buttocks, and the heels touched the vertical stand with the head in Frankfurt's position to the nearest 0.1 cm. For children with 6–23 months of age, recumbent length and for children 24–59 months of age, standing height to the nearest 0.1 cm were measured. MUAC was measured by marking midway between shoulder tip and the elbow tip on the vertical axis of the upper arm with the arm bent at right angle and between the lateral and medial surface of the left arm [30, 31]. Age of each child was also collected from the mother and counter-checked using vaccination cards or other forms of informal recording. All anthropometric measurements were taken twice, and the average of the two measurements was calculated and recorded.
The 2006 WHO Anthro 3.2.1 software was used to convert weight, height, and age of child (months) into height-for-age (HAZ), weight-for-age (WAZ), and weight-for-height (WHZ) Z-scores to assess malnutrition taking sex in to consideration. Anthropometric classifications were based on global standards: <−3 SD, <−2 SD, and ≥−2 SD. Children with HAZ, WAZ, and WHZ below −2 SD of the median of reference population were considered as stunted, underweight and wasted, respectively. Children with HAZ, WAZ, and WHZ below −3 SD were also considered as severely stunted, wasted, and underweight, respectively.
Moreover, these variables were considered as the dependent variables during statistical analysis. The dichotomous variables stunting, underweight, and wasting were defined as 1 = for stunted and 0 = for not stunted, 1 = for underweight and 0 = for not underweight, and 1 = for wasted and 0 = for not wasted, respectively [16, 32].
Child feeding practices such as exclusive breastfeeding (EBF) were understood as feeding a child only breast milk without anything else for the first six months of life, with the exception of medicines for therapeutic purpose [33]. A 24-hour recall method (from sun rise to sun rise) was used to assess dietary diversity practices. This was based on the mother's recall of foods given to her child in the previous 24 hours prior to the interview date. Then, minimum dietary diversity was estimated using information collected from the 24-hour dietary recall.
Minimum dietary diversity was fulfilled if a child had received food from four or more food groups from the seven WHO food groups in the last 24 hours preceding the survey [34]. The seven food groups used included were grains, roots, and tubers; legumes and nuts; dairy products (milk, yogurt, and cheese); flesh foods (meat, fish, poultry, and liver/organ meats); eggs; vitamin-A rich fruits and vegetables; and other fruits and vegetables. Moreover, minimum meal frequency was fulfilled if breastfed child with the of age 6–8 months and 9–59 months received a minimum of two or three meals with one to two snacks and three or four meals with one to two snacks per day, respectively [33, 34].
Food security status of the households was determined based on nine standard Household Food Insecurity Access Scale (HFIAS) questions that were developed for this purpose by Food and Nutrition Technical Assistance (FANTA). The respondents were asked about the amount and variety of meal eaten and the occurrence of food shortage for the household members, causing them not to eat the whole day or eat at night only, in the past four weeks before the survey. Then, food-secure households were coded “1” and food-insecure ones “0” for further analysis [35, 36]. Child immunization status of children (full, partial, or never) was also checked by observing the immunization card, and if not available, mothers were asked to recall it. BCG vaccination was checked by observing scar on right (also left) arm.
2.6. Data Quality Control
To ensure data quality, the English version questionnaire was translated into the local language Afaraff and then back to English to maintain its consistency. Pretest was conducted on 42 subjects (5% of the sample) in kebeles not in the study for necessary modification. Two-day training was given to the data collectors and supervisors before the actual date of data collection. Continuous supervision was done by the supervisors and principal investigator on daily bases.
2.7. Statistical Analysis
Data entry and analysis was done using EPI data 3.1 and SPSS version 20.0, respectively. Anthropometric indices were calculated using the 2006 WHO Anthro 3.2.1 Software. Descriptive analysis was used to describe the percentages and frequency of sociodemographic characteristics and other relevant variables in the study. Bivariate and multivariable logistic regression analysis was used to identify the factors associated with child malnutrition. Both crude and adjusted odds ratios together with their corresponding 95% confidence intervals were computed to see the strength of association between the outcome and independent variables.
All independent variables that were associated with the outcome variables (wasting, stunting, and underweight) in bivariate analysis (with p value < 0.20) were included in the final multivariable logistic analysis. A p value of < 0.05 was considered to declare the result as statistically significant. The Hosmer–Lemeshow test was performed for model fitness in the final model, and p value > 0.05 was considered a good fit. The result was presented in text, tables, and graphs based on the types of data.
2.8. Ethical Considerations
Ethical clearance was obtained from the College of Health Sciences of Samara University, Research and Ethical Review Committee (RERC). Then, officials at different levels in the study area were communicated through letters from Afar National Regional Health Bureau. Letters of permission were obtained from district administrative and health offices. Verbal informed consent was obtained from each participant prior to the interview after explaining the purpose of the study.
3. Results
3.1. Demographic and Socioeconomic Characteristics
In this study, the final analysis included 840 mothers with their children aged 6–59 months, making the response rate of 99.5%. Of the total respondents, 806 (96.0%) and 804 (95.7%) of them were Afar in their ethnicity and Muslims in their religion, respectively. Majority of respondents, 758 (90.2%), were currently married. 726 (86.4%) of respondents were illiterate, and 678 (79.8%) of them were housewives by occupation (Table 1).
Table 1.
Demographic and socioeconomic characteristics of respondents in Dubti district, Afar Regional State, Northeast Ethiopia, May, 2017 (n = 840).
| Characteristics | Categories | Frequency (n) | Percent (%) |
|---|---|---|---|
| Mother's age (in years) | 15–19 | 76 | 9.0 |
| 20–24 | 226 | 26.9 | |
| 25–29 | 340 | 40.5 | |
| 30–34 | 110 | 13.1 | |
| 35–39 | 47 | 5.6 | |
| ≥40 | 41 | 4.9 | |
|
| |||
| Mother's marital status | Currently married | 758 | 90.2 |
| Divorced | 30 | 3.5 | |
| Widowed | 35 | 4.1 | |
| Others | 19 | 2.2 | |
|
| |||
| Mother's ethnicity | Afar | 806 | 96.0 |
| Others∗∗ | 34 | 4.0 | |
|
| |||
| Mother's religion | Muslim | 804 | 95.7 |
| Others∗∗ | 36 | 4.2 | |
|
| |||
| Mother's education | Illiterate | 726 | 86.3 |
| Primary (grade 1–8) | 71 | 8.5 | |
| Secondary (grade 9–12) | 29 | 3.5 | |
| Above secondary (grade 12+) | 14 | 1.7 | |
|
| |||
| Mother's occupation | Housewife | 678 | 80.7 |
| Herding livestock | 92 | 10.9 | |
| Government employee | 38 | 4.5 | |
| Others | 32 | 3.9 | |
|
| |||
| Head of household | Father | 624 | 74.3 |
| Mother | 216 | 25.7 | |
|
| |||
| Total family size | <5 | 249 | 29.6 |
| ≥5 | 591 | 70.4 | |
|
| |||
| Number of under-five children | 1 | 326 | 38.8 |
| 2 | 424 | 50.5 | |
| ≥3 | 90 | 10.7 | |
|
| |||
| Family monthly income (ETB) | <500 | 665 | 79.2 |
| 500–1000 | 103 | 12.3 | |
| >1000 | 72 | 8.5 | |
|
| |||
| Household food security | Secured | 267 | 31.8 |
| Not secured | 573 | 68.2 | |
|
| |||
| Ownership of livestock | Yes | 724 | 86.2 |
| No | 116 | 13.8 | |
Others include ∗Amhara, Tigray, Oromo; ∗∗Orthodox, Protestant. ETB = Ethiopian birr.
Majority, 624 (74.3%) of households were headed by fathers, whereas 421 (67.5%), 123 (19.7%), and 49 (7.8%) of them were pastoralists, agropastoralists, and employed, respectively. Of the total interviewed households, about 724 (86.2%) of them owned livestock. 591 (70.5%) the households had more than five family size, whereas half of the households, 424 (50.5%), had at least two under-five children.
3.2. Characteristics and Caring Practices of Children
Among the children participated in this study, 476 (56.7%) and 364 (43.3%) were males and females, respectively, where 486 (57.8%) fell in the age group of 6–23 months. The mean age ± SD of children were 17.6 ± 5.3 months. Of the studied children, 352 (41.9%) of them started breastfeeding immediately after birth within one hour. About 352 (41.9%) of children received prelacteal feeding; animal milk was the most predominant prelacteal food given, which accounts for 262 children (74.4%) followed by butter, 69 (19.6%) (Table 2).
Table 2.
Characteristics and caring practices of under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, May 2017 (n = 840).
| Characteristics | Categories | Frequency (n) | Percent (%) |
|---|---|---|---|
| Child's sex | Male | 476 | 56.7 |
| Female | 364 | 43.3 | |
|
| |||
| Child age in months | 6–11 | 254 | 30.2 |
| 12–23 | 232 | 27.6 | |
| 24–35 | 154 | 18.3 | |
| 36–47 | 104 | 12.4 | |
| 48–59 | 96 | 11.4 | |
|
| |||
| Birth order | 1 | 94 | 11.2 |
| 2–3 | 422 | 50.2 | |
| 4–5 | 194 | 23.1 | |
| ≥6 | 130 | 15.5 | |
|
| |||
| Initiation of breastfeeding | Within one hour | 352 | 41.9 |
| Hours later | 290 | 34.5 | |
| Days later | 198 | 23.6 | |
|
| |||
| Received prelacteal feeding | Yes | 352 | 41.9 |
| No | 488 | 58.1 | |
|
| |||
| Type of prelacteal food (n = 352) | Animal milk | 262 | 74.4 |
| Butter | 69 | 19.6 | |
| Water | 15 | 4.3 | |
| Others | 6 | 1.7 | |
|
| |||
| Avoiding colostrum | Yes | 384 | 45.7 |
| No | 456 | 54.3 | |
|
| |||
| Exclusive breastfeeding | Yes | 245 | 29.9 |
| No | 595 | 70.1 | |
|
| |||
| Currently breastfeeding | Yes | 286 | 34.0 |
| No | 554 | 66.0 | |
|
| |||
| Timely complementary feeding started | Yes | 105 | 12.5 |
| No | 735 | 87.5 | |
|
| |||
| Materials used to feed | Spoon | 388 | 46.2 |
| Cup | 172 | 20.5 | |
| Hand | 242 | 28.8 | |
| Bottle | 38 | 4.5 | |
|
| |||
| Dietary diversity score | <4 | 138 | 16.4 |
| ≥4 | 296 | 35.2 | |
|
| |||
| Child immunization | Yes | 252 | 30.0 |
| No | 588 | 70.0 | |
|
| |||
| Diarrhoea preceding two weeks | Yes | 108 | 12.9 |
| No | 732 | 87.2 | |
|
| |||
| Fever preceding two weeks | Yes | 100 | 11.9 |
| No | 740 | 88.1 | |
In addition, 245 (29.9%) of studied children were exclusively breastfed for six months. About, 105 (12.5%) children started complementary feeding at 6 months and 328 (39.0%) of children feed more than three times per day. Concerning vaccination, 588 (70.0%) of the children did not receive full vaccine, 108 (12.9%) and 100 (11.9%) of children had diarrhoea and fever, respectively, in the past two weeks prior to the study period.
3.3. Maternal and Environmental Health Characteristics
Among the total mothers interviewed, 494 (58.8%) of them attended antenatal care visit child and 357 (42.5%) of mothers delivered at health institution for their index child. The source of drinking water for 410 (48.8%) of households was protected source (Table 3).
Table 3.
Maternal and environmental health characteristics of respondents in Dubti district, Afar Regional State, Northeast Ethiopia, May 2017 (n = 840).
| Characteristics | Categories | Frequency (n) | Percent (%) |
|---|---|---|---|
| Antenatal care follow-up (index child) | Yes | 494 | 58.8 |
| No | 346 | 41.2 | |
|
| |||
| Place of delivery for (index child) | Home | 483 | 57.5 |
| Health institution | 357 | 42.5 | |
|
| |||
| Hand washing practice of mother∗ | After latrine use | 280 | 33.3 |
| Before preparing food | 720 | 85.7 | |
| Before serving food | 224 | 26.7 | |
|
| |||
| Source of drinking water | Unprotected source | 410 | 48.8 |
| Protected source | 430 | 51.2 | |
|
| |||
| Presence of latrine | Yes | 362 | 43.1 |
| No | 478 | 56.9 | |
|
| |||
| Solid waste disposal | Open field | 766 | 91.2 |
| In a pit | 74 | 8.8 | |
∗Multiple responses in %.
With regard to the presence of latrine, 362 (43.1%) of households had latrine, whilst 766 (91.2%) of households used open field to dispose solid waste. Moreover, 720 (85.7%) of respondents were washed their hand before preparing food, followed by 280 (33.3%) after latrine use.
3.4. Prevalence of Malnutrition among Children
The analysis of the three anthropometric indices revealed that the prevalence of wasting, stunting, and underweight were 16.2% (95% CI: 12.9%–19.9%), 24.8% (95% CI: 20.8%–29.1%), and 43.1% (95% CI: 38.4%–47.9%), respectively. Moreover, the prevalence of severe wasting, stunting, and underweight among the children was 5.7%, 16.7%, and 13.1%, respectively (Table 4).
Table 4.
Overall prevalence of malnutrition among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, 2017 (n = 840).
| Anthropometric indices | Categories | Frequency (n) | Percent (%) |
|---|---|---|---|
| Weight for height (wasting) | Normal (≥−2WHZ score) | 704 | 83.8 |
| Moderate wasting (−3 ≤ WHZ score < −2) | 88 | 10.5 | |
| Severe wasting (<−3WHZ score) | 48 | 5.7 | |
| Overall wasting (<−2WHZ score) | 136 | 16.2 | |
|
| |||
| Height for age (stunting) | Normal (≥−2HAZ score) | 478 | 56.9 |
| Moderate stunting (−3 ≤ HAZ score < −2) | 222 | 26.4 | |
| Severe stunting (<−3HAZ score) | 140 | 16.7 | |
| Overall stunting (<−2HAZ score) | 362 | 43.1 | |
|
| |||
| Weight for age (underweight) | Normal (≥−2WAZ score) | 632 | 75.2 |
| Moderate underweight (−3 ≤ WAZ score < −2) | 98 | 11.7 | |
| Severe underweight (<−3WAZ score) | 110 | 13.1 | |
| Overall underweight (<−2WAZ score) | 208 | 24.8 | |
The prevalence of stunting was recorded in 234 (27.9%) male children, which was higher compared to females, 128 (15.2%). Similarly, underweight was higher among males, 149 (17.8%), than females, 59 (7.0%). However, wasting was slightly higher in males, 83 (9.9%) than in females, 53 (6.3%) (Figure 1).
Figure 1.
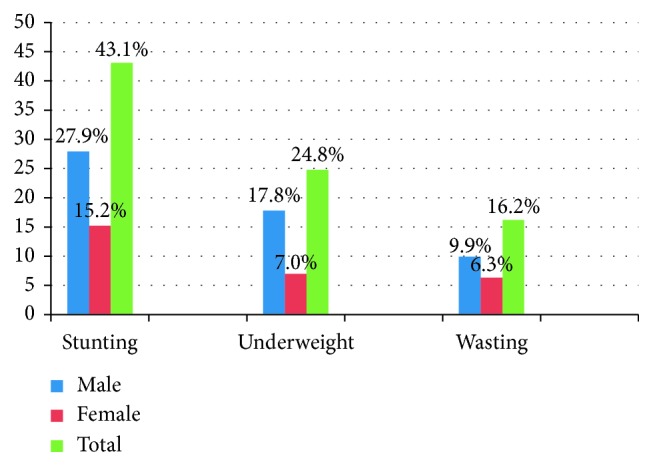
Prevalence of malnutrition by sex among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, 2017 (n = 840).
Comparing age groups, the highest prevalence of wasting was seen among children aged 12–23 months with prevalence of 4.9% and the lowest among children aged 48–59 months with prevalence of 1.3%. Similarly, the highest prevalence of stunting, 13.3%, was seen among children aged 12–23 months and the lowest prevalence, 6.2%, in children aged 48–59 months. Moreover, the prevalence of underweight was lower for younger children and increases around 6–11 months of age making the highest in children aged between aged 12–23 months (Figure 2).
Figure 2.
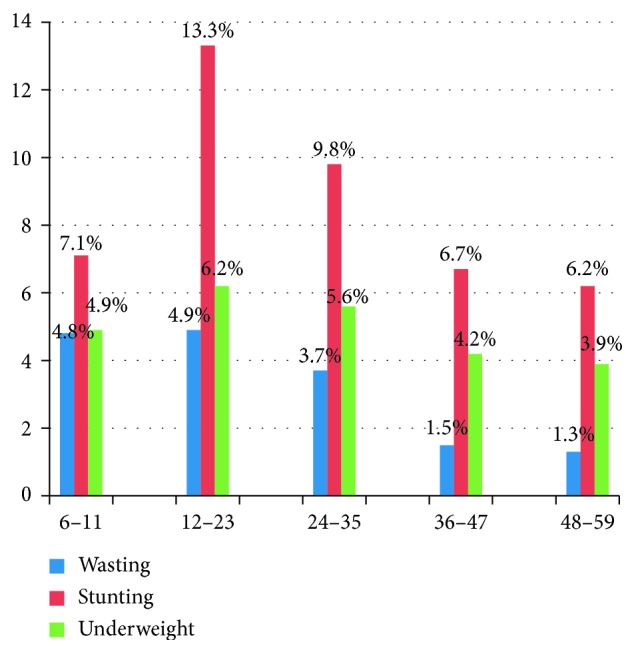
Prevalence of malnutrition by age group (months) among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, 2017 (n = 840).
3.5. Factors Associated with Malnutrition of Children
3.5.1. Factors Associated with Wasting
Among the variables entered into bivariate logistic regression analysis, mother's occupation, family size, place of delivery, initiation of breastfeeding, prelacteal feeding, diarrhoea in the last two weeks, and presence of latrine were associated with wasting at p value < 0.25. After controlling for potential confounders, the final multivariable logistic regression analysis revealed that family size (AOR = 2.72, 95% CI: 1.62–4.55), prelacteal feeding (AOR = 3.81, 95% CI: 1.79–5.42), and presence of diarrhoea in the past two weeks prior to the study (AOR = 4.57, 95% CI: 2.56–8.16) were the independent predictors for child wasting (Table 5).
Table 5.
Factors associated with wasting among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, 2017 (n = 840).
| Variables | Categories | Wasting | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Yes (%) | No (%) | ||||
| Mother's occupation | Housewife | 103 (15.2) | 575 (84.8) | 1 | 1 |
| Working | 33 (20.4) | 129 (79.6) | 1.43 (1.12–2.65) | 1.34 (0.84–2.14) | |
|
| |||||
| Family size | <5 | 25 (10.0) | 224 (90.0) | 1 | 1 |
| ≥5 | 111 (18.8) | 480 (81.2) | 2.07 (1.31–3.29) | 2.72 (1.62–4.55)∗ | |
|
| |||||
| Place of delivery | Home | 90 (18.6) | 393 (81.4) | 1.55 (1.05–2.28) | 1.17 (0.76–2.79) |
| Health facility | 46 (12.9) | 311 (87.1) | 1 | 1 | |
|
| |||||
| Prelacteal feeding | Yes | 77 (21.9) | 275 (78.1) | 2.04 (1.41–2.95) | 3.81 (1.79–5.42)∗ |
| No | 59 (12.1) | 429 (87.9) | 1 | 1 | |
|
| |||||
| Initiation of breastfeeding | Within 1 hour | 49 (13.9) | 303 (86.1) | 1 | 1 |
| Hours later | 87 (17.8) | 401 (82.2) | 1.75 (0.51–1.09) | 0.98 (0.61–3.58) | |
|
| |||||
| Diarrhoea in the last two weeks | Yes | 30 (27.8) | 78 (72.2) | 2.27 (1.42–3.63) | 4.57 (2.56–8.16)∗ |
| No | 106 (14.5) | 626 (85.5) | 1 | 1 | |
|
| |||||
| Presence of latrine | Yes | 51 (14.1) | 311 (85.9) | 1 | 1 |
| No | 85 (17.8) | 393 (82.2) | 1.32 (0.90–1.92) | 1.65 (0.98–3.65) | |
∗Significant at p < 0.05; COR = crude odd ratio; AOR = adjusted odd ratio; CI = confidence interval.
Therefore, children living in households with greater than or equal to five family members were 2.72 times more likely to be wasted than those children living in households with less family members (AOR = 2.72, 95% CI: 1.62–4.55). Children who received prelacteal feeding at the time of birth were 3.8 times more likely to be wasted than those children who did not receive prelacteal feeding at the time of birth (AOR = 3.81, 95% CI: 1.79–5.42). Children who had diarrhoea in the past two weeks prior to the study were 4.6 times more likely to be wasted than those children without diarrhoeal disease (AOR = 4.57, 95% CI: 2.56–8.16).
3.5.2. Factors Associated with Stunting
In the bivariate logistic regression model, sex and age of child, prelacteal feeding, child immunization status, timely complementary feeding, diarrhoea in the last two weeks, and presence of latrine were associated with stunting. However, sex of index child (AOR = 1.98, 95% CI: 1.46–2.72), age of index child ((AOR = 3.44, 95% CI: 2.24–5.29), (AOR = 3.58, 95% CI: 2.25–5.69), and (AOR = 4.42, 95% CI: 2.79–6.94)), and child immunization (AOR = 3.34, 95% CI: 1.31–4.81) were independent predictors for child stunting in the final multivariable logistic regression analysis (Table 6).
Table 6.
Factors associated with stunting among under five children in Dubti district, Afar Regional State, Northeast Ethiopia, 2017 (n = 840).
| Variables | Category | Stunting | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Yes (%) | No (%) | ||||
| Sex of child | Male | 234 (49.2) | 242 (50.8) | 1.78 (1.35–2.36) | 1.98 (1.46–2.72)∗ |
| Female | 128 (35.2) | 236 (64.8) | 1 | 1 | |
|
| |||||
| Age of child | 6–11 | 60 (23.6) | 194 (76.4) | 1 | 1 |
| 12–23 | 112 (48.3) | 120 (51.7) | 3.02 (2.05–4.45) | 3.44 (2.24–5.29)∗ | |
| 24–35 | 82 (53.2) | 72 (46.8) | 3.68 (2.39–5.66) | 3.58 (2.25–5.69)∗ | |
| 36–59 | 108 (54.0) | 92 (46.0) | 3.79 (2.54–5.67) | 4.42 (2.79–6.94)∗ | |
|
| |||||
| Timely complementary feeding started | Yes | 34 (32.4) | 71 (67.6) | 1 | 1 |
| No | 328 (44.6) | 407 (55.4) | 1.68 (1.09–2.59) | 1.87 (0.97–3.53) | |
|
| |||||
| Child immunization | Yes | 54 (21.4) | 198 (78.6) | 1 | 1 |
| No | 308 (52.4) | 280 (47.6) | 4.03 (2.87–5.68) | 3.34 (2.31–4.81)∗ | |
|
| |||||
| Diarrhoeal in the last two weeks | Yes | 55 (50.8) | 53 (49.1) | 1.44 (0.96–2.15) | 1.45 (0.91–3.31) |
| No | 307 (41.9) | 440 (58.1) | 1 | 1 | |
|
| |||||
| Fever in the last two weeks | Yes | 109 (49.8) | 110 (50.2) | 1.44 (1.06–1.96) | 1.37 (0.94–2.98) |
| No | 253 (40.7) | 368 (59.3) | 1 | 1 | |
|
| |||||
| Presence of latrine | Yes | 141 (39.0) | 221 (61.0) | 1 | 1 |
| No | 221 (46.2) | 257 (53.8) | 1.35 (1.02–2.18) | 1.16 (0.84–2.59) | |
∗Significant at p < 0.05; COR = crude odd ratio; AOR = adjusted odd ratio; CI = confidence interval.
Therefore, male children had 1.9 times higher risk to develop stunting as compared to female children (AOR = 1.98, 95% CI: 1.46–2.72). It was also observed that children in the age ranges of 12–23, 25–34, and 35–59 months old were 3.4, 3.6, and 4.4 times more likely to be stunted than those of children in 6–11 months of age ranges, respectively, ((AOR = 3.44, 95% CI: 2.24–5.29), (AOR = 3.58, 95% CI: 2.25–5.69), and (AOR = 4.42, 95% CI: 2.79–6.94)). And, not fully immunized children were 3.3 times more likely to be stunted than fully immunized children (AOR = 3.34, 95% CI: 1.31–4.81).
3.5.3. Factors Associated with Underweight
The bivariate logistic regression analysis showed that mother's education, mother's occupation, family monthly income, sex of index child, initiation of breastfeeding; prelacteal feeding and child immunization status were associated with underweight. However, after controlling for potential confounders, the final multivariable logistic regression model analysis showed that mother's education (AOR = 4.06, 95% CI: 2.01–8.19), sex of index child (AOR = 1.83, 95% CI: 1.29–2.94), prelacteal feeding (AOR = 2.81, 95% CI: 1.64–3.72), and child immunization (AOR = 3.17, 95% CI: 2.14–4.99) were independent predictors for underweight (Table 7).
Table 7.
Factors associated with underweight among under-five children in Dubti district, Afar Regional State, Northeast Ethiopia, 2017 (n = 840).
| Variables | Category | Underweight | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Yes (%) | No (%) | ||||
| Mother's occupation | Housewife | 156 (23.0) | 522 (77.0) | 1 | 1 |
| Working | 52 (32.1) | 110 (67.9) | 1.48 (1.08–2.30) | 1.68 (0.87–3.38) | |
|
| |||||
| Mother education | Illiterate | 197 (27.1) | 529 (72.9) | 3.49 (1.83–6.63) | 4.06 (2.01–8.19)∗ |
| Educated | 11 (9.6) | 103 (90.4) | 1 | 1 | |
|
| |||||
| Child's sex | Male | 149 (31.3) | 327 (68.7) | 2.36 (1.67–3.31) | 1.83 (1.29–2.94)∗ |
| Female | 59 (16.2) | 305 (83.8) | 1 | 1 | |
|
| |||||
| Initiation of breastfeeding | Within one hour | 77 (21.9) | 275 (78.1) | 1 | 1 |
| Hours later | 131 (26.8) | 357 (73.2) | 1.31 (0.95–1.81) | 0.82 (0.57–2.23) | |
|
| |||||
| Child immunization | Yes | 29 (11.5) | 223 (88.5) | 1 | 1 |
| No | 179 (30.4) | 409 (69.6) | 3.37 (2.00–5.15) | 3.17 (2.14–7.99)∗ | |
|
| |||||
| Ownership of livestock | Yes | 171 (23.6) | 553 (76.4) | 1 | 1 |
| No | 37 (31.9) | 79 (68.1) | 1.52 (0.99–2.32) | 1.78 (0.92–4.54) | |
|
| |||||
| Prelacteal feeding | Yes | 126 (35.8) | 226 (64.2) | 2.76 (1.04–3.81) | 2.81 (1.64–3.72)∗ |
| No | 82 (16.8) | 406 (83.2) | 1 | 1 | |
∗Significant at p < 0.05; COR = crude odd ratio; AOR = adjusted odd ratio; CI = confidence interval.
Therefore, the risk of developing underweight among children who had illiterate mothers was 4.1 times higher than that of children whose mothers were literate (AOR = 4.06, 95% CI: 2.01–8.19). Male children were 1.8 times more likely to be underweight than female children (AOR = 1.83, 95% CI: 1.29–2.94). It was also observed that the likelihood of being underweight among children who received prelacteal feeding at the time of birth was 2.8 times higher than that of children who did not receive prelacteal feeding at the time of birth (AOR = 2.81, 95% CI: 1.64–3.72). And, not fully immunized children had about 3.2 times higher risk to develop underweight as compared to fully immunized children (AOR = 3.17, 95% CI: 2.14–4.99).
4. Discussions
Child malnutrition continues to be a major public health problem in developing countries including Ethiopia. Children are most vulnerable to malnutrition because of low dietary intakes, infectious diseases, lack of appropriate care, and inequitable distribution of food within the household in developing countries [37, 38]. Therefore, the current study aimed to assess the prevalence and associated factors with malnutrition (wasting, stunting, and underweight) among under-five children in Dubti district, one of the pastoralist communities of Afar Regional State, Northeast Ethiopia.
The current study revealed that 16.2% (95% CI: 13.8–18.8%), 43.1%(95% CI: 39.8–46.5%) and 24.8% (95% CI: 21.9–27.8%) of the under-five children were wasted, stunted and underweight, respectively in the study area, which were found to be very high according to WHO classification [33]. Regarding the associated factors of malnutrition, analysis of this study indicated family size, prelacteal feeding practice and diarrhoeal morbidity in the past two weeks were significantly associated with wasting. Furthermore, sex of child, age of child and child immunization status were the independent predictors for stunting. According to this finding, mother's education, sex of child, prelacteal feeding, and child immunization status were significantly associated with underweight in the study community.
In this study, the prevalence of wasting (16.2%) was similar with the regional figure, 19.5% [22] and Hidabu Abote district, Ethiopia, 16.7% [12]. But, it was much higher than the national figure, 9.7% [22], and various studies done in Ethiopia like Damot district, 9% [14], Metekele zone, 10.2% [39], Haramaya district, 10.7% [24], and other countries including Kenya, 2.6% [40], and Nigeria, 8.8% [41], which was an alarming case to increase risk of deaths among children. Furthermore, it also was lower than the study done in the pastoral communities of Dollo Ado district, Eastern Ethiopia, 42.3% [26]. Wasting is an indicator of acute malnutrition that can occur due to recent infection or weight loss due to periodical variation of food supply. The possible explanation for this difference might be due to a difference in the socioeconomic, recurrent drought, agroecology, and feeding habits of the study population. An additional explanation could also be that the study was conducted during drought season, when milk production, the most food in the community was scarce.
In this study, the prevalence of stunting (43.1%) was similar to the national figure, 44.4% [22]. But, it was much higher than other studies done in Ethiopia like Somali region, 22.9% [31], Dollo Ado district, 34.4% [26], Gumbrit district, 24.0% [42], and other countries including Kenya, 28.9% [43], Sudan, 24.9% [44], and Mongolia [45]. It was also lower than the regional figure, 50.2% [22], Libo-kemekem, Ethiopia, 49.4% [46], and India, 74.2% [47]. Stunting showed a failure to get adequate food over long period and affected through infections. Despite little improvements from 2016 EDHS report, the current prevalence of stunting is still a public health problem of the area. The possible explanation for this difference in prevalence could be due to a difference in the socioeconomic, agricultural productivity, food insecurity at household level, and nomadic nature of the population. An additional explanation for this could also be due to a difference in cultural and child feeding habits, study setting, and periods of the study.
In this study, the prevalence of underweight (24.8%) was found to be lower than the national figure, 28.7%, and regional figure, 40.2%, of EDHS report [22] and other studies done in Ethiopia such as West Gojam zone, 49.2% [23], Dollo Ado district, 47.7% [26], Sudan, 52.0% [48], India, 32.4% [49], and Pakistan, 39.5% [50]. However, it was also relatively similar with the findings from Haramaya, district, Ethiopia, 21.0% [24], Bahir Dar, Ethiopia, 22.1% [51], and Nigeria, 24.6% [41], and higher than the results in Kenya, 11.8% [40]. The possible explanation for the improvement in weight for age could be due to current efforts of the health sector to improve health intervention through health extension workers at the community level. An additional explanation could also be due to the fact that the Ethiopian health extension program has included underweight as indicator for child growth monitoring and promotion at the community level.
In the current study, mother's educational status was a strong predictor for high prevalence of underweight. And, the risk of developing underweight among children who had illiterate mothers was 4.1 times higher than children whose mothers were literate which showed that as mother's educational level increased, child nutritional well-being also increases. This might be due to the fact that educated mothers would have proper management of resources, practice better health promoting behaviors, and might develop better children centered caring practices. This result is in line with other studies conducted in Hawassa Zuria, Ethiopia [25], Dollo Ado district, Ethiopia [26], Rwanda [52], and Iran [53].
In the current study, sex of index child was strongly associated with both stunting and underweight. The likelihood of being both stunted and underweight among male children were 1.9 and 1.8 times higher when compared to female children. This could partially be explained due to the fact that boys are more vulnerable to health inequalities than their female counterparts in the same age groups [26]. Many studies in Ethiopia, Dollo Ado district [26], Bule Hora district [54], and Benna Tsemay district [55], and other developing countries, Kenya [40], Indonesia [56], and Pakistan [50], reported similar results. A systematic review conducted in Ethiopia and other developing countries also showed that male children were highly vulnerable to malnutrition when compared with female children due a difference in the frequency of eating, energy expenditure, and exposure to health problems than female children [57, 58].
In the current study, household family size was associated with wasting among study children. Children living in households with greater than or equal to five family members had 2.7 times higher risk to be wasted than children living in households with less than five family members. This might be due to fact that families with more children might experience more economic strain for food consumption; hence, they might be more likely to suffer from poor nutritional status since the available food is shared by all members. This is in agreement with other studies done in Ethiopia, Libo-Kemekem district [46] and Benna Tsemay district [55], and other countries like Bangladesh [9].
In the current study, age of the child was another factor associated with stunting. Children in the age range of 12–23, 25–34, and 35–59 months were about 3.4, 3.6, and 4.4 times, respectively, higher risk to be stunted than children of 6–11 months of age ranges. This might due to the fact that stunting is a cumulative process that can begin in utero and continues to about five years after birth [23]. The other possible reason also might be caring practices usually tend to decrease when children grown-up than infants and shifted to adult foods. Similar results have been reported from Libo-kemekem district, Ethiopia [46], Hawassa Zuria, Ethiopia [25], Kenya [40], and India [59]. However, an opposite result was still reported from studies done in Shey Bench district, Southwest Ethiopia [37], West Gojam zone, Ethiopia [23], and Vietnam [60] indicated that highest risk of stunting occurs in the youngest age group.
In the current study, prelacteal feeding was another factor strongly associated with both wasting and underweight. This study showed that wasting and underweight among children who received prelacteal feeding at the time of birth were 3.8 and 3.2 times, respectively, higher than children who did not receive prelacteal feeding at the time of birth. This might due to its negative impact on breastfeeding, and when children are not breastfed appropriately, they are at higher risk of developing undernourishment [23]. Studies conducted in Bule Hora district, Ethiopia [54], Mai-Aini Eritrean Refugees' Camp, Ethiopia [61], and Hidabu Abote district, Ethiopia [12] have revealed consistent with current study.
In the current study, immunization status of children had a significant association with increased risk of high prevalence of stunting and underweight. Nonimmunized children were 3.3 and 3.8 times, respectively, more likely to be stunted and underweight than immunized children. This could partially be explained that nonimmunized children could be at risk of many vaccine preventable diseases such as diarrhoea and respiratory infections, which might result in depleting nutrients from the body. This finding was in line with other studies conducted in Ethiopia, Metekele Zone [39], Shinille district [62], EDHS report [22], and other countries such as Zambia [63].
In the current study, presence of diarrhoeal disease was significantly associated with increasing prevalence of wasting. Children who had diarrhoeal disease in the past two weeks prior to the study were 4.6 times more likely to be wasted than those children without diarrhoeal disease. This might be due to the fact that diarrhoea may result in lower appetite, poor digestion, and malabsorption which leads to malnutrition. The other possible reason also might be that malnourished children would have more diarrhoeal episodes and a child with diarrhoea losses weight and can quickly become malnourished. This result was in line with other findings conducted in Damot district, Ethiopia [16], and Vadodara, India [49].
4.1. Limitation of the Study
As the study involved a single cross-sectional design, causal inference might not be strong between the dependent and independent variables. There might also be the possibility of recall and reporting bias in some infant and young child feeding (IYCF) indicators such as breastfeeding patterns, dietary diversity, and child's history of illness events happening in the past.
5. Conclusions and Recommendations
The present study revealed that the prevalence of wasting, stunting, and underweight were about 16.2% (95% CI: 13.8–18.8%), 43.1% (95% CI: 39.8–46.5%), and 24.8% (95% CI: 21.9–27.8%), respectively, in the study area. This result indicated the prevalence of child malnutrition (wasting, stunting, and underweight) was a serious public health problem in the pastoral community according to the WHO classification for public health significance. According to analysis of independent variables with the outcome variables, households having a family size of five or more, receiving prelacteal feeding, and presence of diarrhoeal disease in the past two weeks were the independent predictors for increasing wasting. Being male child, increasing age child, and not fully immunized child were the independent predictors for increasing stunting.
Moreover, the independent predictors positively y associated with increasing underweight were being illiterate mother, being male child, prelacteal feeding practices, and not fully immunized child. Promoting use of family planning, preventing diarrhoeal diseases, and vaccinating children integrated with the access of nutrition education programs are vital interventions to improve nutritional status of the children. A due emphasis should also be given to strengthen the health extension program to improve and provide participatory nutrition education to create awareness and to develop behavior change communication for better child feeding and caring practices in the pastoral community.
Acknowledgments
The authors would like to thank the Samara University, Dubti District Administrative and Health Office and Kebele Administrative, study participants, data collectors, and supervisors for their cooperation in the study.
Abbreviations
- EBF:
Exclusive breastfeeding
- EDHS:
Ethiopian Demographic and Health Survey
- FANTA:
Food and Nutrition Technical Assistance
- HAZ:
Height-for-age Z score
- HFIAS:
Household Food Insecurity Access Scale
- IQ:
Intellectual quotient
- IYCF:
Infant and young child feeding
- RERC:
Research and Ethical Review Committee
- SD:
Standard deviation
- WAZ:
Weight-for-age Z score
- WHO:
World Health Organization
- WHZ:
Weight-for-height Z score.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The ethical approval was obtained from Samara University, College of Health Sciences, Research and Ethical Review Committee (RERC). An informed consent was obtained from the participants' parent before being enrolled in the study. In addition, participants were assured about the aim, possible risk if any, and confidentiality of the study using their own local language (Afaraff).
Conflicts of Interest
The authors declared that they have no conflicts of interest.
Authors' Contributions
YS, AG, and AM conceived the study, developed the tool, conducted the research, carried out the statistical analysis, and wrote manuscript. PSR and MK also participated in the design of the study and tool development, performed statistical analysis, and drafted the manuscript. All authors read and approved the final manuscript.
References
- 1.Black R. E., Allen L. H., Bhutta Z. A., et al. Maternal and child undernutrition: global and regional exposures and health consequences. The Lancet. 2008;371(9608):243–260. doi: 10.1016/s0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 2.Pavani Varma M., Prasad K. S. V. Malnutrition and its related factors among children 0–5 years in rural Shamirpet mandal, Ranga Reddy district, India. International Journal of Bioassays. 2017;6(4):5340–5342. doi: 10.21746/ijbio.2017.04.002. [DOI] [Google Scholar]
- 3.Zemenu Y. K., Tsigereda B., Alemu M., Mesfine T., Sintayehu Y. Malnutrition and associated factors among under five children (6–59 months) at Shashemene referral hospital, West Arsi zone, Oromia, Ethiopia. Current Pediatric Research. 2017;21(1):172–180. [Google Scholar]
- 4.World Health Organization. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: Joint Statement by the World Health Organization and the United Nations Children’s Fund. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 5.UN. Levels and Trends in Child Mortality. New York, NY, USA: United Nations Inter-Agency Group for Child Mortality Estimation; 2011. [Google Scholar]
- 6.Janevic T., Petrovic O., Bjelic I., Kubera A. Risk factors for childhood malnutrition in Roma settlements in Serbia. BMC Public Health. 2010;10(1):p. 509. doi: 10.1186/1471-2458-10-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amsalu S., Tigabu T. Risk factors for severe acute malnutrition in children under the age of five: a case-control study. Ethiopian Journal of Health Development. 2008;22(1):21–25. doi: 10.4314/ejhd.v22i1.10058. [DOI] [Google Scholar]
- 8.Alemu M., Nicola J., Belele T. Tackling Child Malnutrition Ethiopia; Young Lives Project Working Paper No. 19. London, UK: Save the Children; 2009. [Google Scholar]
- 9.Islam M. M., Alam M., Tariquzaman M., et al. Predictors of the number of under-five malnourished children in Bangladesh: application of the generalized poisson regression model. BMC Public Health. 2013;13(1):p. 11. doi: 10.1186/1471-2458-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Onis M., Brown D., Blossner M., Borghi E. Levels and Trends in Child Malnutrition. UNICEF-WHO-The World Bank Joint Child Malnutrition Estimates. New York, NY, USA: UNICEF; 2018. [Google Scholar]
- 11.Hug L., Sharrow D., You D. Levels & Trends in Child Mortality: Report. Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. New York, NY, USA: United Nations Inter-Agency Group for Child Mortality Estimation; 2017. [Google Scholar]
- 12.Mengistu K., Alemu K., Destaw B. Prevalence of malnutrition and associated factors among children aged 6–59 months at Hidabu Abote district, North Shewa, Oromia regional state. Journal of Nutritional Disorders & Therapy. 2013;3(3) doi: 10.4172/2161-0509.T1-001. [DOI] [Google Scholar]
- 13.Darsene H., Ayele G., Abebaw G., Solomon M. Magnitude and predictors of undernutrition among children aged six to fifty nine months in Ethiopia: a cross sectional study. Archives of Public Health. 2017;75(1):p. 29. doi: 10.1186/s13690-017-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bantamen G., Belaynew W., Dube J. Assessment of factors associated with malnutrition among under-five years age children at Machakel Woreda, Northwest Ethiopia: a case control study. Journal of Nutrition and Food Sciences. 2014;4(1):p. 256. doi: 10.4172/2155-9600.1000256. [DOI] [Google Scholar]
- 15.Berhanu G., Mekonnen S., Sisay M. Prevalence of stunting and associated factors among preschool children: a community based comparative cross sectional study in Ethiopia. BMC Nutrition. 2018;4(1):p. 28. doi: 10.1186/s40795-018-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abera L., Tariku D., Tariku L. Prevalence of malnutrition and associated factors in children aged 6–59 months among rural dwellers of Damot Gale district, South Ethiopia: community based cross sectional study. International Journal for Equity in Health. 2017;16(1):p. 111. doi: 10.1186/s12939-017-0608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsedeke W., Tefera B., Debebe M. Prevalence of acute malnutrition (wasting) and associated factors among preschool children aged 36–60 months at Hawassa Zuria, South Ethiopia: a community based cross sectional study. Journal of Nutrition & Food Sciences. 2016;6(2):p. 466. doi: 10.4172/2155-9600.1000466. [DOI] [Google Scholar]
- 18.Federal Ministry of Health. National Strategy for Infant and Young Child Feeding. Addis Ababa, Ethiopia: Federal Ministry of Health; 2004. [Google Scholar]
- 19.Federal Ministry of Health. National Nutrition Strategy. Addis Ababa; Ethiopia: Federal Ministry of Health; 2005-2006. [Google Scholar]
- 20.Federal Ministry of Health. National Nutrition Program. Addis Ababa; Ethiopia: Federal Ministry of Health; 2008. [Google Scholar]
- 21.Federal Democratic Republic of Ethiopia. Seqota Declaration; A Commitment to End Child Undernutition in Ethiopia by the Year 2030. Addis Ababa; Ethiopia: Ethiopia Federal Ministry of Health; 2016. [Google Scholar]
- 22.Central Statistical Agency. Ethiopia Demographic and Health Survey. Addis Ababa, Ethiopia: Central Statistical Agency; 2016. [Google Scholar]
- 23.Teshome B., Kogi-Makau W., Getahun Z., Taye G. Magnitude and determinants of stunting in children under-five years of age in food surplus region of Ethiopia. The case of West Gojam Zone. Ethiopian Journal of Health Development. 2009;23(2):98–106. doi: 10.4314/ejhd.v23i2.53223. [DOI] [Google Scholar]
- 24.Yisak H., Gobena T., Mesfin F. Prevalence and risk factors for under nutrition among children under five at Haramaya district, Eastern Ethiopia. BMC Pediatrics. 2015;15(1):p. 212. doi: 10.1186/s12887-015-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debeko D. D., Goshu A. T. Nutritional status of under-five children in Hawassa Zuria district, Southern Ethiopia. American Journal of Health Research. 2015;3(5):286–292. doi: 10.11648/j.ajhr.20150305.14. [DOI] [Google Scholar]
- 26.Demissie S., Worku A. Magnitude and factors associated with malnutrition in children 6–59 months of age in pastoral community of Dollo Ado district, Somali region, Ethiopia. Science Journal of Public Health. 2013;1(4):175–183. doi: 10.11648/j.sjph.20130104.12. [DOI] [Google Scholar]
- 27.Tesfamariam K., Yilma D. Prevalence of stunting and its associated factors among children under 5 age in Holeta town, West Shoa zone, Oromia region, Ethiopia, 2017. EC Nutrition. 2017;12(2):90–98. [Google Scholar]
- 28.Afar Regional Health Bureau. Annual Health Profile. Semera, Ethiopia: Afar Regional Health Bureau; 2017. [Google Scholar]
- 29.The 2007 Population and Housing Census of Ethiopia Results for Afar Regional state at Kebele Level. Adddis Ababa, Ethiopia: 2015. [Google Scholar]
- 30.Bruce C. Anthropometric Indicators Measurement Guide. Washington, DC, USA: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 31.Fekadu Y., Mesfin A., Haile D., Stoecker B. J. Factors associated with nutritional status of infants and young children in Somali region, Ethiopia: a cross-sectional study. BMC Public Health. 2015;15(1):p. 846. doi: 10.1186/s12889-015-2190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 33.Yalew B. M., Amsalu F., Bikes D. Prevalence and factors associated with stunting, underweight and wasting: a community based cross sectional study among children age 6–59 months at Lalibela town, Northern Ethiopia. Journal of Nutritional Disorders & Therapy. 2014;4(2):p. 147. doi: 10.4172/2161-0509.1000147. [DOI] [Google Scholar]
- 34.World Health Organization. Indicators for Assessing Infant and Young Child Feeding Practices: Part 1: Definitions: Conclusions of a Consensus Meeting Held 6–8 November 2007. Washington, DC, USA: World Health Organization; 2008. [Google Scholar]
- 35.Coates J., Swindale A., Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3) Washington, DC, USA: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 36.Egata G., Berhane Y., Worku A. Predictors of acute undernutrition among children aged 6 to 36 months in east rural Ethiopia: a community based nested case—control study. BMC Pediatrics. 2014;14(1):p. 91. doi: 10.1186/1471-2431-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teferi M. B., Hassen H. Y., Kebede A., Adugnaw E., Gebrekrstos G., Guesh M. Prevalence of stunting and associated factors among children aged 06–59 months in Southwest Ethiopia: a cross-sectional study. Journal of Nutritional Health & Food Science. 2016;4(6):1–6. doi: 10.15226/jnhfs.2016.00180. [DOI] [Google Scholar]
- 38.Victora C. G., de Onis M., Hallal P. C., Blossner M., Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 39.Wuneh M. T., Guracho Y. D. Nutritional status and feeding practice of children 6–59 months old, Metekele zone of Benishangul-Gumuz region, Northwest Ethiopia. Science Journal of Clinical Medicine. 2017;6(6):120–129. doi: 10.11648/j.sjcm.20170606.15. [DOI] [Google Scholar]
- 40.Olack B., Burke H., Cosmas L., et al. Nutritional status of under-five children living in an informal urban settlement in Nairobi, Kenya. Journal of Health, Population, and Nutrition. 2011;29(4):357–363. doi: 10.3329/jhpn.v29i4.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andy E., Juliet N. O., Adetunji O. E., Hosea G. K., Partience K. R. Malnutrition and associated factors among under-five in a Nigeria local government area. International Journal of Contemporary Medical Research. 2016;3(6):1766–1768. [Google Scholar]
- 42.Edris M. Assessment of nutritional status of preschool children of Gumbrit, North West Ethiopia. Ethiopian Journal of Health Development. 2007;21(2):125–129. doi: 10.4314/ejhd.v21i2.10039. [DOI] [Google Scholar]
- 43.Dennis M., Anzelimo M., Isaac K., et al. Prevalence of malnutrition among preschool children (6–59 months) in Western Province, Kenya. Journal of Public Health and Epidemiology. 2014;6(11):398–406. doi: 10.5897/jphe2014.0660. [DOI] [Google Scholar]
- 44.Musa T. H., Musa H. H., Ali E. A., Musa N. E. Prevalence of malnutrition among children under five years old in Khartoum state, Sudan. Polish Annals of Medicine. 2014;21(1):1–7. doi: 10.1016/j.poamed.2014.01.001. [DOI] [Google Scholar]
- 45.Otgonjargal D., Woodruff B. A., Batjargal J., Gereljarga B., Davaalkham D. Nutritional status of under-five children in Mongolia. Journal of Medicine and Medical Sciences. 2012;3(5):341–349. [Google Scholar]
- 46.Geberselassie S. B., Abebe S. M., Melsew Y. A., Mutuku S. M., Wassie M. M. Prevalence of stunting and its associated factors among children 6–59 months of age in Libo-Kemekem district, Northwest Ethiopia; a community based cross sectional study. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0195361.e0195361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amritanshu K., Banerjee D. P., Ranjan B., Manali K. Nutritional status of children attending OPD at tertiary care hospital in Katihar, Bihar. International Journal of Medical Science and Public Health. 2013;2(4):853–856. doi: 10.5455/ijmsph.2013.260620131. [DOI] [Google Scholar]
- 48.Eptihag A., Kamal A., Sharafeldeen I., Aboshora W. Assessment of maternal knowledge of under-five year’s children regard malnutrition and related factors in Kosti and Tandalty hospital, White Nile state, Sudan. BAOJ Nutrition. 2017;3(1):1–3. [Google Scholar]
- 49.Popat C., Chaudhari A., Mazumdar V., Patel S. A cross sectional study to measure the prevalence of malnutrition and factors associated with malnutrition among under five children of an urban slum of Vadodara city. Journal of Research in Medical and Dental Science. 2014;2(3):59–64. doi: 10.5455/jrmds.20142313. [DOI] [Google Scholar]
- 50.Khan G. N., Turab A., Khan M. I., et al. Prevalence and associated factors of Malnutrition among children under-five years in Sindh, Pakistan: a cross-sectional study. BMC Nutrition. 2016;2(1):p. 69. doi: 10.1186/s40795-016-0112-4. [DOI] [Google Scholar]
- 51.Demilew Y. M., Abie D. D. Under nutrition and associated factors among 24–36-month-old children in slum areas of Bahir Dar city, Ethiopia. International Journal of General Medicine. 2017;10:79–86. doi: 10.2147/ijgm.s126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Habyarimana F., Zewotir T., Ramroop S. Key determinants of malnutrition of children under five years of age in Rwanda: simultaneous measurement of three anthropometric indices. African Population Studies. 2016;30(2) doi: 10.11564/30-2-836. [DOI] [Google Scholar]
- 53.Sharifzadeh G., Mehrjoofard H., Raghebi S. Prevalence of malnutrition in under 6-year olds in South Khorasan, Iran. Iranian Journal of Pediatrics. 2010;20(4):435–441. [PMC free article] [PubMed] [Google Scholar]
- 54.Asfaw M., Wondaferash M., Taha M., Dube L. Prevalence of under nutrition and associated factors among children aged between six to fifty nine months in Bule Hora district, South Ethiopia. BMC Public Health. 2015;15(1):p. 41. doi: 10.1186/s12889-015-1370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tadesse A., Hailu D., Bosha T. Nutritional status and associated factors among pastoralist children aged 6–23 months in Benna Tsemay Woreda, South Omo zone, Southern Ethiopia. International Journal of Nutrition and Food Sciences. 2018;7(1):11–23. doi: 10.11648/j.ijnfs.20180701.13. [DOI] [Google Scholar]
- 56.Ramli, Agho K. E., Inder K. J., Bowe S. J., Jacobs J., Dibley M. J. Prevalence and risk factors for stunting and severe stunting among under-fives in North Maluku province of Indonesia. BMC Pediatrics. 2009;9(1):p. 64. doi: 10.1186/1471-2431-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdulahi A., Shab-Bidar S., Rezaei S., Djafarian K. Nutritional status of under five children in Ethiopia: a systematic review and meta-analysis. Ethiopian Journal of Health Sciences. 2017;27(2):175–188. doi: 10.4314/ejhs.v27i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tebeje N. B., Bikes G. A., Abebe S. M., Yesuf M. E. Prevalence and major contributors of child malnutrition in developing countries: systematic review and meta-analysis. Journal of Childhood Obesity. 2017;2(4):2572–5394. doi: 10.21767/2572-5394.100037. [DOI] [Google Scholar]
- 59.Kumar D., Goel N. K., Mittal P. C., Misra P. Influence of infant-feeding practices on nutritional status of under-five children. Indian Journal of Pediatrics. 2006;73(5):417–421. doi: 10.1007/bf02758565. [DOI] [PubMed] [Google Scholar]
- 60.Nakamori M., Xuan Ninh N., Cong Khan N., et al. Nutritional status, feeding practice and incidence of infectious diseases among children aged 6 to 18 months in Northern mountainous Vietnam. Journal of Medical Investigation. 2010;57(1-2):45–53. doi: 10.2152/jmi.57.45. [DOI] [PubMed] [Google Scholar]
- 61.Kelati H., Mengiste B., Alemayehu T., Damtew B. Prevalence of acute malnutrition and its associated factors among children aged 6–59 months in Mai-Aini Eritrean refugees’ camp, Northern Ethiopia. International Journal of Food Sciences and Nutrition. 2015;5(1):p. 336. doi: 10.4172/2155-9600.1000336. [DOI] [Google Scholar]
- 62.Ma’alin A., Birhanu D., Melaku S., Tolossa D., Mohammed Y., Gebremicheal K. Magnitude and factors associated with malnutrition in children 6–59 months of age in Shinille Woreda, Ethiopian Somali regional state: a cross-sectional study. BMC Nutrition. 2016;2(1):p. 44. doi: 10.1186/s40795-016-0079-1. [DOI] [Google Scholar]
- 63.Nzala S. H., Siziya S., Babaniyi O., Songolo P., Muula A. S., Rudatsikira E. Demographic, cultural and environmental factors associated with frequency and severity of malnutrition among Zambian children less than five years of age. Journal of Public Health and Epidemiology. 2011;3(8):362–370. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


