Abstract
Background
Diabetes mellitus type II (DMT-2) is a widely spread metabolic disorder both in developed and developing countries. The role of oxidative stress is well established in DMT-2 pathogenesis. The synthetic drugs for DMT-2 are associated with serious side complications. Antioxidant and α-glucosidase inhibitory actions of phytochemicals from various plant species are considered as an alternative to synthetic drugs for DMT-2 management. The present study aimed to evaluate the antioxidant activity, α-glucosidase inhibitory potential and phytochemical profiling of Hyophorbe lagenicaulis.
Methods
The total phenolic and flavonoid contents, in vitro antioxidant activity (α, α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging and phosphomolybdenum method) and α-glucosidase inhibition of ultrasonicated hydroethanolic H. lagenicaulis leaf extracts were determined spectrophotometrically. The results of DPPH assay and α-glucosidase inhibition were reported in terms of IC50 value. The phytochemical profiling was accomplished by UHPLC-Q-TOF/MS/MS technique.
Results and Discussion
Findings leaped 60% ethanolic extract as rich fraction regarding total phenolic and flavonoid contents. The 60% ethanolic fraction was a promising source of natural antioxidants and α-glucosidase inhibitory agents as indicated by anti-radical and enzyme inibitory activities. Kaempferol, rutin, hesperetin 5-O-glucoside, kaempferol-coumaroyl-glucoside, luteolin 3-glucoside, Isorhamnetin-3-O-rutinoside, trimethoxyflavone derivatives and citric acid were identified by UHPLC-Q-TOF-MS/MS. These compounds were believed to be responsible for the strong antioxidant and enzyme inhibitory activity of plant extracts. The extensive metabolite profiling of H. lagenicaulis was carried out the first time as never reported previously. The H. lagenicaulis might be an appropriate choice to manage diabetes mellitus in an alternate way. The findings may be further exploited extensively for toxicity evaluation to proceed with functional food development having antidiabetic attributes.
Keywords: Antioxidant, Antidiabetic, UHPLC-Q-TOF-MS/MS, Hyophorbe lagenicaulis, Diabetes mellitus
Introduction
Diabetes is one of the leading non-infectious diseases with multiple side complications characterized by persistent hyperglycemia due to reduced insulin secretion or action (Schwartz et al., 2016). More than 90% diabetic patients are suffering from diabetes mellitus type 2 (DMT-2) and it is estimated that by 2,035 the expansion of DMT-2 will result in 592 million diabetic patients worldwide (Guariguata et al., 2014). The huge expansion rate of DMT-2 is now considered a socio-economic burden and covers about 10% of the total health care expenditures in many countries (Pari & Saravanan, 2007). The modern lifestyle and dietary habits are among the key factors responsible for the DMT-2 progression. These factors are associated with the production of reactive oxygen species (ROS) which in excess may generate the state of oxidative stress. The role of ROS and oxidative stress in DMT-2 progression is evident from scientific studies (Charokopou et al., 2016; Sami et al., 2017). The mechanism of action through which oxidative stress contributes to DM pathogenesis is not fully clear. However, the hyperglycemia is reported to produce free radicals which impair the function of antioxidant enzymes in plasma (Asmat, Abad & Ismail, 2016). The oxidative stress also impairs the insulin secretion, alteration in glucose uptake, abnormal glucose release form liver and mediation of metabolic pathways (Akash et al., 2011). The natural antioxidants in the body are mainly enzymes which include superoxide dismutase, glutathione peroxidases and reductases. The vitamin A and E are also important endogenous antioxidants which substantially reduce the level of oxidative stress (Madhikarmi & Murthy, 2014). The glycation of antioxidant enzymes also alters the structure based function of enzymes to increase the chances of damage by ROS (Singh et al., 2014). The lipid oxidation and glutathione metabolism impairments are used as biomarkers for DM and oxidative stress usually alters this to initiate DMT-2. Antioxidants are believed to encounter ROS to reduce level of oxidative stress to prevent development of DMT-2. High levels of ROS are involved in glycation of proteins, lipid peroxidation and glucose oxidation and these collectively impart in DMT-2 development and related disorders (Asmat, Abad & Ismail, 2016). The elimination of ROS or reduction in the level of oxidative stress may diminish the chances of DMT-2 pathogenesis and prolongation by improving the intra cellular antioxidant defense (Ceriello & Testa, 2009). The antioxidants based therapy is considered as promising approach to treat DMT-2 as antioxidants effectively scavenge the free radicals and ROS to prevent DM pathogenesis and related complications (Rahimi-Madiseh et al., 2016).
Many synthetic drugs are available to manage diabetes but the side effects associated with these compounds are a matter of keen concern (Chaudhury et al., 2017). Health related drawbacks of synthetic medicines emphasize on the need to develop alternate treatments for DMT-2. Plants are a rich source of natural, safe and potent phytochemicals of medicinal attributes to treat chronic ailments. The many natural bioactive ingredients in plants are also associated with α-glucosidase inhibition. The α-glucosidase inhibitors restrict the hydrolysis of carbohydrates (Oligosaccahrides, trisaccharides and disaccharides) in the intestine and slow down their absorption, hence limit the postprandial glucose level (Rouzbehan et al., 2017). Plants also contain natural antioxidants which encounter the exceeding ROS levels in body to reduce the risks of DMT-2 pathogenesis and progression (Zaid et al., 2015). Therefore search for α-glucosidase inhibitors from plants for DMT-2 management is workable, safe, reliable and cost effective approach.
Family Arecaceae contains more than 189 genera, 3,000 species and some species are very rich in antioxidants and other functional molecules. Plants from family Arecaceae have been studied for their potential medicinal use however many species are still needed to explore for their hidden biological and pharmacological role (Govaerts & Dransfield, 2005). Hyophorbe lagenicaulis of family Arecaceae is among such plants which are not completely studied for their potential biological activities (Elgindi et al., 2016). Inspite of effective role of H. lagenicaulis in folk medicines, no scientific evidence is available on biological activities and phytochemical distribution in this plant. The current work was performed to evaluate the in vitro antioxidant and antidiabetic potential of H. lagenicaulis. The metabolite profiling of leaf extract of H. lagenicaulis was also carried out.
Material and Methods
Collection of plant material
The plant material was collected from Lahore, Pakistan and was identified from the Department of Botany, GC University Lahore.
Green extract preparation
Quenching of fresh leaves was done in liquid nitrogen and grinded to a fine powder to enhance the surface area. The obtained powder was lyophilized using a freeze-dryer (Christ Alpha 1-4 LD; Osterode am Harz, Germany) and subjected to hydroethanolic solvent compositions (Ethanol, 100%, 80%, 60%, 40%, 20%) for 48 h. Mixtures were sonicated at soniprep 150 disintegrator below 10 °C. Samples were shaken for 2 h and filtered. The excess solvent from the filtrate was removed under the vacuum on rotary evaporator at 40 °C. The extracts were again freeze dried for 48 h. Extract yields (%) were calculated and extracts were stored at −80 °C till further use.
Determination of total phenolic and flavonoid contents
Total phenolic contents (TPC) of freeze dried leaf extracts were determined by Folin Ciocalteu reagent method with slight modification in previously reported scheme (Zhishen, Mengcheng & Jianming, 1999). The plant extract (one mg) was dissolved in methanol (one mL) and 0.25 μL of this was added to one mL of Folin Ciocalteu reagent. Then two mL of 10% solution of Na2CO3 followed by addition of two mL distilled water. The resultant mixture was stayed for 120 min at ambient conditions of temperature. The absorbance was noted at 765 nm. Standard curve of gallic acid was also drawn. Results were expressed as gallic acid equivalent (GAE) mg/g dried extract (Zengin et al., 2010).
Total flavonoid contents (TFC) were determined by AlCl3 colorimetric method. The 0.1 mg of plant extract was dissolved in methanol (two mL) and added by five mL of distilled water. Then 0.5 mL of NaNO2 (5%) was added to the mixture followed by the addition of 10% AlCl3 solution. After 10 min NaOH (1 molar) was added to resultant mixture and after vigorous shaking, the absorbance was measured at 510 nm. The results were expressed as rutin equivalent mg/g dried extract (RE mg/DE) (Zhishen, Mengcheng & Jianming, 1999)
Antioxidant activities
Antioxidant potential of extracts was determined by DPPH scavenging assay as reported previously with little modification (Fki, Allouche & Sayadi, 2005). The one mg plant extract was dissolved in methanol. The 50–200 ppm of these dilutions were added to 10 mL of DPPH solution (0.001 molar). After 30 min incubation in the dark at room temperature, absorbance was measured at 520 nm. Butylated hyroxyanisole (BHA) was used as standard for comparison. All the measurements were carried out in triplicate and standard deviation was applied.
Phosphomolybdenum complex formation method was used as per previously reported method with minute modifications (Prieto, Pineda & Aguilar, 1999). Initially, 250 μg/mL of each extract was mixed with a solution composed of sulphuric acid (0.6M), ammonium molybdate (four mM) and 28 mM sodium phosphate. The mixtures and blank solution both were incubated at 95 °C for 90 min at water bath. After cooling, absorbance was noted at 695 nm wavelength. Ascorbic acid standard curve was drawn and butylated hyroxyanisole was used as standard antioxidant. The results were represented as ascorbic acid equivalent per gram dried plant extract (AE/g DE).
Anti-α-glucosidase activity
Inhibition potential of extracts against α-glucosidase was measured to evaluate in vitro antidiabetic potential. Various concentrations of extarcts (200 ppm) were added to phosphate buffer (70 μL of 50 mM) followed by addition of α-glucosidase (one unit/mL). After 10 min incubation at 37 °C, five mM of ρ-nitrophenolglucopyranoside was added and absorbance was noted at 405 nm after 30 min. Acarbose was used as standard reference and results were represented as IC50 (μg/mL) values for each extract (Jabeen et al., 2013).
Where, Ab is absorbance of blank, As is absorbance of Sample.
The α-amylase inhibition activity
The 250 μL of each extract (1.0–10 mg/mL) were added to 0.02M sodium phosphate buffer containing porcine α-amylase (0.5 mg/mL). The reaction mixture was incubated for 10 min at 25 °C. The dinitrosalicylic acid was added to the mixture to stop the reaction. The reaction mixtures were further incubated for a time period of 5 min and diluted with distilled water to note absorbance at 540 nm. A control (no extract) was also run and acarbose was used as standard enzyme inhibitory substance (Kazeem, Adamson & Ogunwande, 2013). The % inhibition was calculated by following relationship;
Where, Ab is absorbance of blank, As is absorbance of Sample.
Results were represented as IC50 (μg/mL) values for each extract.
UHPLC-Q-TOF-MS/MS analysis
Plant extract was extracted with suitable solvent and filtered with plastic filter having 0.45 μm pore size. The filtered extract sample was subjected to UHPLC-Q-TOF-MS/MS (AB Sciex 5600-1, equipped with Eksigent UHPLC system). The scanning range of 50–1,200 m/z for MS/MS (negative ionization mode), column Thermo Hypersil Gold (100 mm× 2.1 mm × 3 μm), gradient mobile phase composition (water and acetonitrile) each having 0.1% formic acid and five mM ammonium formate was used. Gradient programming started from 10% acetonitrile to 90% acetonitrile with mobile phase flow rate of 0.25 mL/min. Desolvation temperature (TEM) was 500 °C and ion spray voltage was −4,500 V.
Statistical analysis
The experimental findings were evaluated for statistical significance by using Statistix 10.0 software. Analysis of variance was used to compare variations in treatment means to assess efficacy of treatments.
Results
Extract yields (%), TPC and TFC
The extract yields, TPC and TFC with respect to solvent system used for extraction are given in Table 1. It was revealed by the findings that solvent influenced extract yields significantly. Maximum extract yield 20.46% ± 0.25a% was given by 60% ethanol and second highest yield 18.05% ± 0.13b% was obtained with 80% ethanol. Solvent composition not only affected the extract yields but also TPC and TFC. Maximum TPC of 178.56 ± 1.47a mg GAE/g DE were recovered with 60% ethanol. Similarly highest TFC of 133.96 ± 1.19a mg Rutin/g DE were extracted with 60% ethanol. Efficiency of 60% ethanolic extract was significantly higher than other solvent fraction for extract yield, TPC and TFC respectively (ρ < 0.05).
Table 1. Extract yields, TPC and TFC from leaves of H. lagenicaulis fractions.
| Solvent composition | Extract yield (%) | TPC in mg GAE/g DE | TFC in mg Rutin/g DE |
|---|---|---|---|
| 20% ethanol | 14.31 ± 0.2de | 78.09 ± 1.36d | 68.94 ± 1.61e |
| 40% ethanol | 16.33 ± 0.32c | 109.63 ± 1.67c | 92.02 ± 1.72d |
| 60% ethanol | 20.46 ± 0.25a | 178.56 ± 1.47a | 133.96 ± 1.19a |
| 80% ethanol | 18.05 ± 0.13b | 144.67 ± 2.31b | 115.51± 0.90b |
| 100% ethanol | 15.10 ± 0.15d | 109.62 ± 0.44c | 100.90 ± 1.59c |
Note:
Results were represented with standard deviation values (±) and significant level was indicated by letter as superscript.
Antioxidant activities
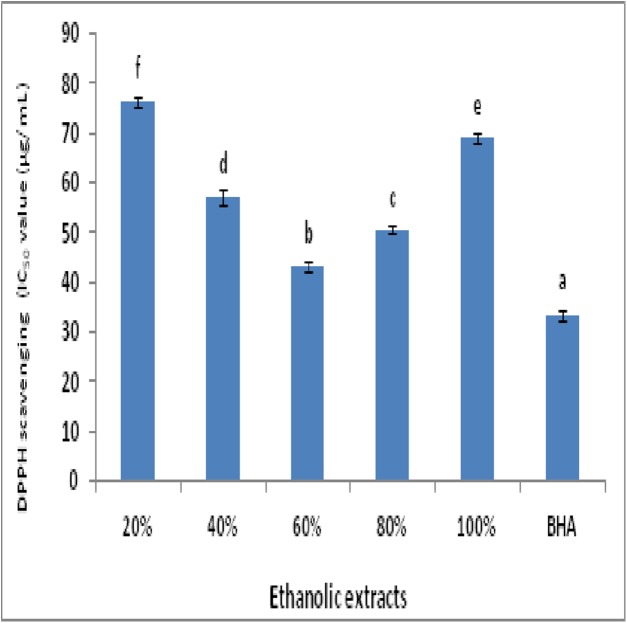
The DPPH radical scavenging in terms of IC-50 value (μg/mL) by plant extracts in comparison with BHA is represented as Fig. 1. Maximum radical scavenging among extracts was exhibited by 60% ethanolic extract with minimum IC-50 value of 43.11 ± 0.96 μg/mL.
Figure 1. DPPH radicals scavenging activity in terms of IC-50 value for plant extracts and BHA (a, reference; b–f, ascending order of IC-50).
Total antioxidant power of extracts was determined by phosphomolybdenum methods. This method involved the reduction of Mo (VI) to Mo (V) with characteristic color change due to complex formation. This assay is widely used to evaluate the total antioxidant power of plant extracts and compounds (Prieto, Pineda & Aguilar, 1999; Rani et al., 2018).
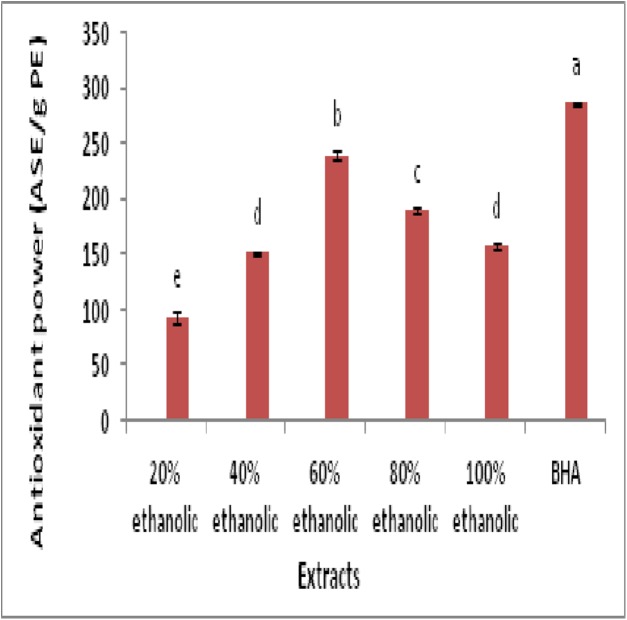
The results of assay were represented in Fig. 2. Findings unveiled that 60% ethanolic fraction exhibited maximum antioxidant power with value of 239.33 ± 3.78b (ASE/g PE) followed by 80% ethanolic extract (189.33 ± 2.51c ASE/g DE). Statistical analysis indicated that antioxidant power of BHA was significantly higher than all extracts (ρ < 0.05). However 60% ethanolic extract was most potent among all plant extracts (ρ < 0.05) regarding antioxidant potential.
Figure 2. Antioxidant power assay (ASE/g PE) for determination of antioxidant activity of plant extracts and BHA (a, reference; b–e, descending order).
Anti-α- glucosidase activity
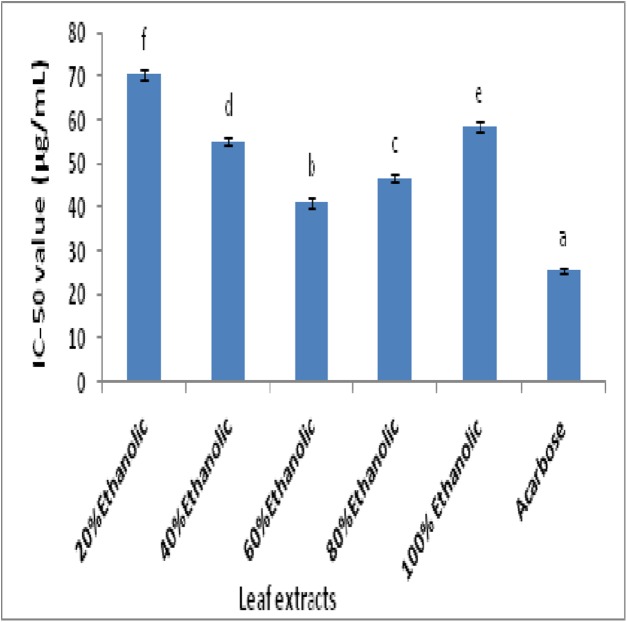
Inhibition of α-glucosidase enzyme reflects the in vitro antidiabetic potential which is determined spectrophotometrically. The IC-50 values (μg/mL) of plant extracts and standard drug acarbose are represented in Fig. 3. The comparison of extracts showed that 60% ethanolic extract among all fractions possessed maximum α-glucosidase inhibition with IC-50 value of 41.25 ± 1.25 μg/mL. The standard drug acarbose exhibited lowest IC-50 value of 25.50 ± 0.45 μg/mL which was significantly lower than all extracts (ρ < 0.05). Statistical comparison further indicated that 60% ethanolic extract was significantly better than remaining extracts (ρ < 0.05).
Figure 3. The IC-50 values for α-glucosidase inhibitory potential of extract fractions and acarbose (a, reference; b–f, ascending order of IC-50).
The α- amylase inhibition
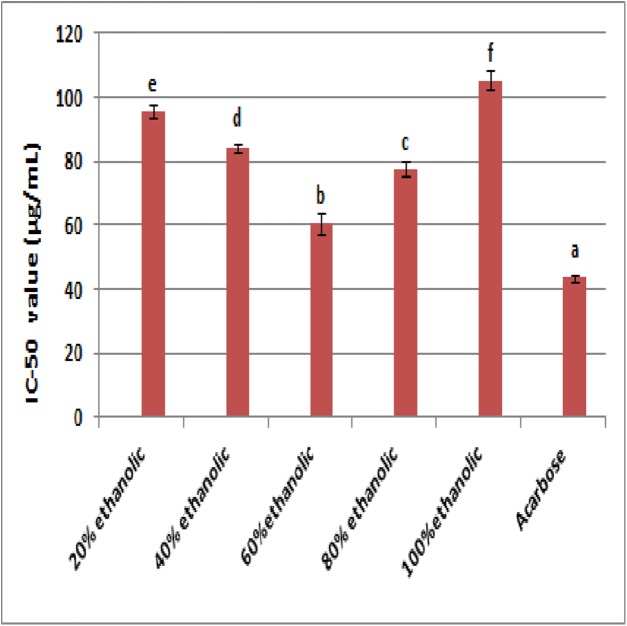
The results of α-amylase inhibition are given in Fig. 4. The 60% ethanolic extract (IC-50 60.58 ± 3.24 μg/mL) exhibited highest α-amylase inhibition followed by 80% ethanolic extract with IC-50 value of 77.57 ± 2.25 μg/mL. The 20% ethanolic extract exhibited least inhibition of enzyme activity as indicated by IC-50 value (114.00 ± 1.88 μg/mL). The standard compound acarbose showed highest α-glucosidase inhibition with IC-50 value of 43.37 ± 0.75 μg/mL. Statistical analysis revealed that 60% ethanolic extract was the most potent α-glucosidase inhibitory fraction.
Figure 4. The IC-50 values for α-amylase inhibitory potential of extract fractions and acarbose (a, reference; b–f, ascending order of IC-50).
UHPLC-Q-TOF-MS/MS analysis
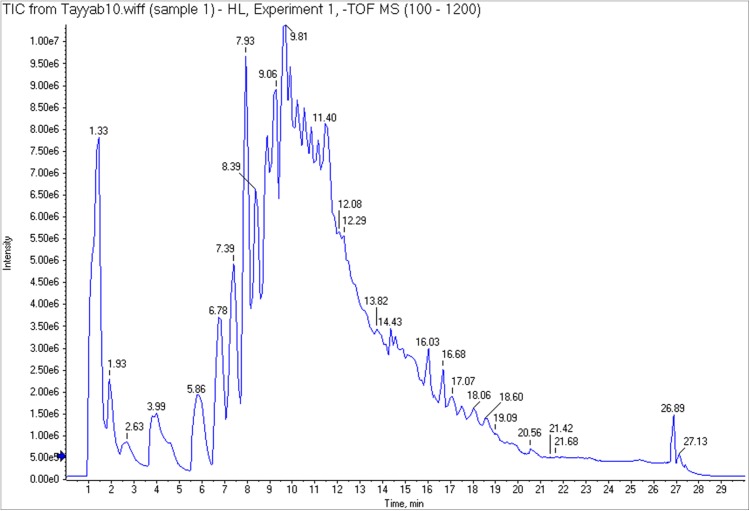
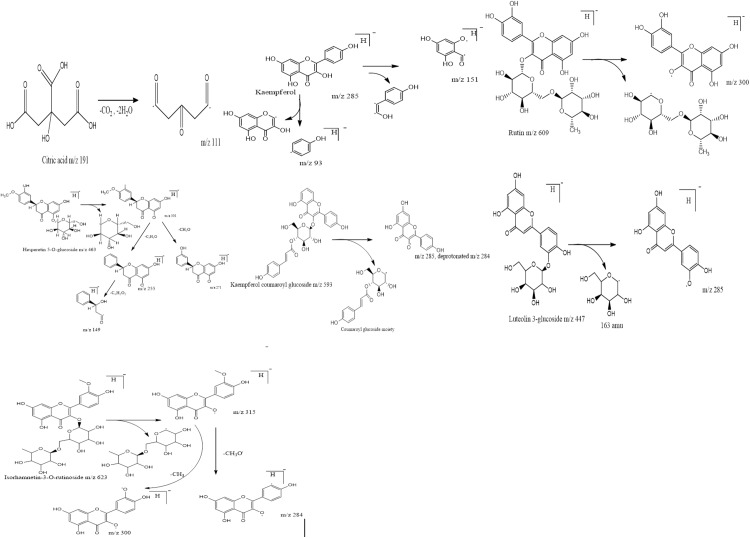
The efficacy of 60% ethanolic extract regarding antioxidant and α-glucosidase inhibition emphasized to explore this fraction for metabolite identification. So the 60% ethanolic extract was subjected to UHPLC-Q-TOF-MS/MS analysis and identified compounds along with their retention time (Rt), fragment ions and molecular formula are listed in Table 2. The main chromatogram of UHPLC separation is shown as Fig. 5. The fragmentation pattern of identified compounds is represented in Fig. 6. Citric acid appeared at Rt 1.603 min with characteristic parent ion peak at m/z 191[M-H]− and daughter ion peak at m/z 111[M-CO2-H2O]−. Trimethoxy flavone derivative arrived at Rt 8.972 min with peaks at m/z 635, m/z 609 and m/z 300. Kaempferol was recorded at Rt 9.110 min with m/z 285. The fragment ion peaks at m/z 151 and m/z 93 due to removal of fragment of 134 amu and phenoyl moiety respectively. Rutin was recorded at Rt 9.27 min with parent ion peak m/z 609. The fragment ion having m/z 300 appeared due to removal of moiety of mass 309 amu. The fragment ion of m/z 271 was produced due to removal of 29 amu from fragment ion m/z 300. Peak at Rt 9.689 was identified as kaempferol-coumaroyl-glucoside with parent peak at m/z 593. The removal of coumaroyl glucoside produced fragment ion peak at m/z 285. Leuteolin 3-glucoside was identified at Rt 9.724 with parent ion peak at m/z 447. Removal of glucose from parent ion generated leuteolin characteristic peak at m/z 285. Hesperetin 5-O-glucoside was identified at Rt 9.433 with parent peak at m/z 463[M-H]− and fragment ions at m/z 301[M-glucose-H]−, m/z 271[m/z 301-CH2O-H]− m/z 255[m/z 301-C2H2O-H]−, m/z 149[m/z 255-C6H2O2-H]− respectively. Isorhamnetin 3-O-rutinoside appeared at Rt 9.995 with m/z 623. The further collision resulted in fragment ions m/z 315[M-318-H amu]−, m/z 300 [m/z 315-CH3-H]− and m/z 284[m/z 315-CH3O-H]−.
Table 2. Peak assignments for identified compounds by UHPLC-MS/MS in negative mode.
| Sr. No | Name of compound | Rt (min) | Molecular ion peak (m/z) | Main fragments ion (m/z) | Molecular formula |
|---|---|---|---|---|---|
| 1 | Citric acid | 1.603 | 191 | 111 | C6H8O7 |
| 2 | Trimethoxy flavone derivative | 8.972 | 773 | 635, 609, 300 | C40H38O16 |
| 3 | Kaempferol | 9.110 | 285 | 151, 93 | C15H10O6 |
| 4 | Rutin | 9.27 | 609 | 300, 271 | C27H30O16 |
| 5 | Hesperetin 5-O-glucoside | 9.433 | 463 | 301, 300, 271, 97 | C22H24O11 |
| 6 | Kaempferol-coumaroyl-glucoside | 9.689 | 593 | 285, 284, 255 | C31H30O12 |
| 7 | Luteolin 3-glucoside | 9.724 | 447 | 285, 284, 255, 227 | C21H20O11 |
| 8 | Isorhamnetin-3-O-rutinoside | 9.995 | 623 | 543, 527, 427, 315, 314 | C21H36O21 |
Figure 5. Main chromatogram of H. lagenicaulis (UHPLC) indicating the peaks of eluted compounds.
Figure 6. Fragmentation pattern of identified compounds with respective m/z values.
Discussion
The polarity of the solvent system used for extraction might be the decisive factor for enhanced productivity (Chew et al., 2011). Phenolic and flavonoid compounds present in plants are associated with medicinal properties. Higher concentration of both phenolics and flavonoids triggers the pharmaceutical and biological attributes of a particular plant (Baba & Malik, 2015; Shreshtha et al., 2017). The discrimination in TPC and TFC was probably due to solvent polarity interaction with heterogeneous structural features of phytochemicals. The promising antioxidant activity (DPPH and phosphomolybdenum complex method) was exerted by extracts. The proton transfer from phenolic compound to DPPH free radical was reported as the possible mechanism in free radical scavenging process (Liang & Kitts, 2014). The proton and electron transfer from antioxidants to Mo(VI) resulted in loss of blue coloration to decrease the intensity of absorbance. The reduction in absorbance was used to assess the antioxidant potential (Prieto, Pineda & Aguilar, 1999). The antioxidant potential of plant extracts is directly proportional to the concentration and quality of phytochemicals including phenolics and flavonoids (Liew et al., 2018). The high α-glucosidase inhibitory action of 60% ethanolic extract was probably due to its high phenolic and flavonoid contents. Many plant based bioactive ingredients are well known α-glucosidase inhibitors and this potential may be exploited to manage DMT-2 (Yin et al., 2014). The 60% ethanolic extract might be a rich source of natural α-glucosidase inhibitors. The anti α-glucosidase activity exhibited by natural inhibitors was probably due to active site occupation by a particular inhibitor molecule to restrict the mode of enzymatic action by structural modification (Martinez-Gonzalez et al., 2017). Citric acid and other identified compounds of flavonoid origin are well known antioxidants (Rostamzad et al., 2011). The antioxidant activities of these identified compounds were due to the interaction of phenolic groups and related structural features with ROS and other free radicals (Aadesariya, Ram & Dave, 2017). The antioxidant activities of plants are known as a decisive factor to control ROS and to eliminate state of oxidative stress. The reduction in oxidative stress can improve the physiological status to avoid DM pathogenesis and prolongation (Singh, Parasuraman & Kathiresan, 2018). The identified compounds in addition to antioxidant potential, were also reported to be associated with antidiabetic potential. The kaempferol and rutin, both flavonoids were proved to inhibit the α-glucosidase activity to control glucose homeostasis (Pereira et al., 2011). Another study reported the comparative evaluation of anti- α-glucosidase activity of kaempferol and quercetin. The findings revealed that kaempferol due to low IC-50 value was more efficient α-glucosidase inhibitor than quercetin (Dewi & Maryani, 2015). Rutin is a widely distributed polyphenolic flavonoid of plants. Previous reports also highlighted the effective contribution of rutin against α-glucosidase activity, diabetes and obesity (Jo et al., 2009; Habtemariam & Lentini, 2015). Luteolin and its derivatives were reported to have promising anti- α-glucosidase inhibition potential even higher than acarbose suggesting it as a functional tool to control postprandial hyperglycemia (Kim, Kwon & Son, 2000). Isorhamnetin 3-O-rutinoside a flavonoid, was reported as a perfect α-glucosidase inhibitor with significantly low IC-50 values (Yin et al., 2014). The α-glucosidase inhibitory activity of acarbose is well established but some gastrointestinal problems also lie with it (Van De Laar, 2008). The acarbose was proved as a competitive inhibitor of α-glucosidase while plant extracts having phenolics were reported to possess non-competitive inhibition of dietary enzyme. The non-competitive mode provided multiple site interactions of phenolics with α-glucosidase rather than limited binding as in case of acarbose. In contrast to acarbose, α-glucosidase inhibition by phenolics in plant extracts does not depend upon the substrate concentration (Zhang et al., 2015). A recent study evaluated the phenolic contents, flavonoids, antioxidant and antidiabetic activities of hydroethanolic leaf extract of Conocarpus erectus. The study supported the linkage between polyphenol based antioxidant activity and hypoglycemic potential of extract (Raza et al., 2018). Another investigation revealed that phytochemicals from plants not only reduce the blood glucose level during diabetes but also improves the hematological parameters (Sudasinghe & Peiris, 2018). The antioxidant and anti-α-glucosidase potentials of H. lagenicaulis extracts were probably due to synergic behavior commonly observed with biologically functional plant extracts (Adamska-Patruno et al., 2018). The presence of high value bioactives in plants supports the efforts being made in search of safe and healthy therapeutic approaches for DMT-2 management. The study confirmed the antioxidant and antidiabetic potential of H. lagenicaulis leaves. The findings may be helpful to move for the reduction in socioeconomic burden, build by DMT-2.
Conclusion
The promising antioxidant activity and α-glucosidase inhibition by H. lagenicaulis plant extracts were probably due to the presence of kaempferol, rutin, isorhamnetin and luteolin derivative. The findings provided us with leads to proceed for functional food development having antidiabetic attributes. Further in vivo studies may be carried out to support the findings of current study and to evaluate the toxicity.
Supplemental Information
DPPH assay, TPC and TFC.
Funding Statement
The authors received no funding for this work.
Contributor Information
Peter John, Email: peterjohn@gcu.edu.pk.
Muhammad Waseem Mumtaz, Email: muhammad.waseem@uog.edu.pk.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
James William performed the experiments, contributed reagents/materials/analysis tools, approved the final draft, arrangement of equipment, calibration.
Peter John analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Muhammad Waseem Mumtaz conceived and designed the experiments.
Ayoub Rashid Ch performed the experiments, contributed reagents/materials/analysis tools.
Ahmad Adnan conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Hamid Mukhtar analyzed the data, authored or reviewed drafts of the paper.
Shahzad Sharif analyzed the data, prepared figures and/or tables.
Syed Ali Raza performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, statistical analysis, instrument handling, editting.
Muhammad Tayyab Akhtar conceived and designed the experiments, analyzed the data.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Aadesariya, Ram & Dave (2017).Aadesariya MK, Ram VR, Dave PN. Evaluation of antioxidant activities by use of various extracts from Abutilon pannosum and Grewia tenax leaves in the kachchh region. MOJ Food Processing & Technology. 2017;17(1):359. [Google Scholar]
- Adamska-Patruno et al. (2018).Adamska-Patruno E, Billing-Marczak K, Orlowski M, Gorska M, Krotkiewski M, Kretowski A. A synergistic formulation of plant extracts decreases postprandial glucose and insulin peaks: results from two randomized, controlled, cross-over studies using real-world meals. Nutrients. 2018;10(8):956. doi: 10.3390/nu10080956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akash et al. (2011).Akash M, Rehman K, Rasool F, Sethi A, Abrar M, Irshad A, Abid A, Murtaza G. Alternate therapy of type 2 diabetes mellitus (T2DM) with Nigella (Ranunculaceae) Journal of Medicinal Plants Research. 2011;5(31):6885–6889. [Google Scholar]
- Asmat, Abad & Ismail (2016).Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharmaceutical Journal. 2016;24(5):547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba & Malik (2015).Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Journal of Taibah University for Science. 2015;9(4):449–454. doi: 10.1016/j.jtusci.2014.11.001. [DOI] [Google Scholar]
- Ceriello & Testa (2009).Ceriello A, Testa R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care. 2009;32(suppl_2):S232–S236. doi: 10.2337/dc09-s316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charokopou et al. (2016).Charokopou M, Sabater F, Townsend R, Roudaut M, McEwan P, Verheggen B. Methods applied in cost-effectiveness models for treatment strategies in type 2 diabetes mellitus and their use in Health Technology Assessments: a systematic review of the literature from 2008 to 2013. Current Medical Research and Opinion. 2016;32(2):207–218. doi: 10.1185/03007995.2015.1102722. [DOI] [PubMed] [Google Scholar]
- Chaudhury et al. (2017).Chaudhury A, Duvoor C, Dendi R, Sena V, Kraleti S, Chada A, Montales MT. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Frontiers in Endocrinology. 2017;8:6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew et al. (2011).Chew K, Khoo M, Ng S, Thoo Y, Wan Aida W, Ho C. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. International Food Research Journal. 2011;18(4):1427–1435. [Google Scholar]
- Dewi & Maryani (2015).Dewi RT, Maryani F. Antioxidant and α-glucosidase inhibitory compounds of Centella Asiatica. Procedia Chemistry. 2015;17:147–152. doi: 10.1016/j.proche.2015.12.130. [DOI] [Google Scholar]
- Elgindi et al. (2016).Elgindi MR, Singab AE-NB, Aly SH, Mahmoud II. Phytochemical investigation and antioxidant activity of Hyophorbe verschaffeltii (Arecaceae) Journal of Pharmacognosy & Phytochemistry. 2016;5(2):39–46. [Google Scholar]
- Fki, Allouche & Sayadi (2005).Fki I, Allouche N, Sayadi S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: a potential alternative to synthetic antioxidants. Food Chemistry. 2005;93(2):197–204. doi: 10.1016/j.foodchem.2004.09.014. [DOI] [Google Scholar]
- Govaerts & Dransfield (2005).Govaerts R, Dransfield J. World checklist of palms: Royal Botanic Gardens. Vol. 2. Deurne: MIM; 2005. p. 66. [Google Scholar]
- Guariguata et al. (2014).Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research & Clinical Practice. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Habtemariam & Lentini (2015).Habtemariam S, Lentini G. The therapeutic potential of rutin for diabetes: an update. Mini-Reviews in Medicinal Chemistry. 2015;15(7):524–528. doi: 10.2174/138955751507150424103721. [DOI] [PubMed] [Google Scholar]
- Jabeen et al. (2013).Jabeen B, Riaz N, Saleem M, Naveed MA, Ashraf M, Alam U, Jabbar A. Isolation of natural compounds from Phlomis stewartii showing α-glucosidase inhibitory activity. Phytochemistry. 2013;96:443–448. doi: 10.1016/j.phytochem.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Jo et al. (2009).Jo S, Ka E, Lee H, Apostolidis E, Jang H, Kwon Y. Comparison of antioxidant potential and rat intestinal a-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. International Journal of Applied Research. 2009;62(4):52–60. [Google Scholar]
- Kazeem, Adamson & Ogunwande (2013).Kazeem M, Adamson J, Ogunwande I. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Research International. 2013;2013:527570. doi: 10.1155/2013/527570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Kwon & Son (2000).Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Bioscience, Biotechnology, & Biochemistry. 2000;64(11):2458–2461. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- Madhikarmi & Murthy (2014).Madhikarmi NL, Murthy KRS. Antioxidant enzymes and oxidative stress in the erythrocytes of iron deficiency anemic patients supplemented with vitamins. Iranian Biomedical Journal. 2014;18(2):82–87. [PMC free article] [PubMed] [Google Scholar]
- Liang & Kitts (2014).Liang N, Kitts DD. Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules. 2014;19(11):19180–19208. doi: 10.3390/molecules191119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew et al. (2018).Liew SS, Ho WY, Yeap SK, Sharifudin SAB. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. PeerJ. 2018;6:e5331. doi: 10.7717/peerj.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez et al. (2017).Martinez-Gonzalez AI, Alvarez-Parrilla E, Díaz-Sánchez ÁG, De la Rosa LA, Núñez-Gastélum JA, Vazquez-Flores AA, Gonzalez-Aguilar GA. In vitro inhibition of pancreatic lipase by polyphenols: a kinetic, fluorescence spectroscopy and molecular docking study. Food Technology & Biotechnology. 2017;55(4):519–530. doi: 10.17113/ftb.55.04.17.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari & Saravanan (2007).Pari L, Saravanan R. Beneficial effect of succinic acid monoethyl ester on erythrocyte membrane bound enzymes and antioxidant status in streptozotocin–nicotinamide induced type 2 diabetes. Chemico-Biological Interactions. 2007;169(1):15–24. doi: 10.1016/j.cbi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Pereira et al. (2011).Pereira DF, Cazarolli LH, Lavado C, Mengatto V, Figueiredo MSRB, Guedes A, Silva FRMB. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition. 2011;27(11–12):1161–1167. doi: 10.1016/j.nut.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Prieto, Pineda & Aguilar (1999).Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Rahimi-Madiseh et al. (2016).Rahimi-Madiseh M, Malekpour-Tehrani A, Bahmani M, Rafieian-Kopaei M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pacific Journal of Tropical Medicine. 2016;9(9):825–831. doi: 10.1016/j.apjtm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Rani et al. (2018).Rani R, Arora S, Kaur J, Manhas RK. Phenolic compounds as antioxidants and chemopreventive drugs from Streptomyces cellulosae strain TES17 isolated from rhizosphere of Camellia sinensis. BMC Complementary and Alternative Medicine. 2018;18(1):82. doi: 10.1186/s12906-018-2154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza et al. (2018).Raza SA, Chaudhary AR, Mumtaz MW, Ghaffar A, Adnan A, Waheed A. Antihyperglycemic effect of Conocarpus erectus leaf extract in alloxan-induced diabetic mice. Pakistan Journal of Pharmaceutical Sciences. 2018;31(2):637–642. [PubMed] [Google Scholar]
- Rostamzad et al. (2011).Rostamzad H, Shabanpour B, Kashaninejad M, Shabani A. Antioxidative activity of citric and ascorbic acids and their preventive effect on lipid oxidation in frozen Persian sturgeon fillets. Latin American Applied Research. 2011;41(2):135–140. [Google Scholar]
- Rouzbehan et al. (2017).Rouzbehan S, Moein S, Homaei A, Moein MR. Kinetics of α-glucosidase inhibition by different fractions of three species of Labiatae extracts: a new diabetes treatment model. Pharmaceutical Biology. 2017;55(1):1483–1488. doi: 10.1080/13880209.2017.1306569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami et al. (2017).Sami W, Ansari T, Butt NS, Ab Hamid MR. Effect of diet on type 2 diabetes mellitus: a review. International Journal Health Sciences. 2017;11(2):65–71. [PMC free article] [PubMed] [Google Scholar]
- Schwartz et al. (2016).Schwartz SS, Epstein S, Corkey BE, Grant SFA, Gavin JR, Aguilar RB. The time is right for a new classification system for diabetes: rationale and implications of the β-cell–centric classification schema. Diabetes Care. 2016;39(2):179–186. doi: 10.2337/dc15-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreshtha et al. (2017).Shreshtha S, Anushi J, Joshi AN, Joshi N, Anupma H. Study of total phenol, flavonoid contents and phytochemical screening of methanolic crude extracts of two weed plants. Annals of Plant Sciences. 2017;6(6):1645–1648. doi: 10.21746/1651-1654. [DOI] [Google Scholar]
- Singh et al. (2014).Singh M, Jha A, Kumar A, Hettiarachchy N, Rai AK, Sharma D. Influence of the solvents on the extraction of major phenolic compounds (punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. Journal of Food Science and Technology. 2014;51(9):2070–2077. doi: 10.1007/s13197-014-1267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, Parasuraman & Kathiresan (2018).Singh J, Parasuraman S, Kathiresan S. Antioxidant and antidiabetic activities of methanolic extract of Cinnamomum cassia. Pharmacognosy Research. 2018;10(3):237–242. doi: 10.4103/pr.pr_162_17. [DOI] [Google Scholar]
- Sudasinghe & Peiris (2018).Sudasinghe HP, Peiris DC. Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. PeerJ. 2018;6:e4389. doi: 10.7717/peerj.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Laar (2008).Van De Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vascular Health Risk & Management. 2008;4(6):1189–1195. doi: 10.2147/vhrm.s3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2014).Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Science and Human Wellness. 2014;3(3–4):136–174. doi: 10.1016/j.fshw.2014.11.003. [DOI] [Google Scholar]
- Zaid et al. (2015).Zaid H, Saad B, Mahdi AA, Tamrakar AK, Haddad PS, Afifi FU. Medicinal plants and natural active compounds for diabetes and/or obesity treatment. Evidence Based Complementary & Alternative Medicine. 2015;2015:469762. doi: 10.1155/2015/469762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin et al. (2010).Zengin G, Cakmak YS, Guler GO, Aktumsek A. In vitro antioxidant capacities and fatty acid compositions of three Centaurea species collected from Central Anatolia region of Turkey. Food and Chemistry Toxicology. 2010;48(10):2638–2641. doi: 10.1016/j.fct.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang H, Wang G, Beta T, Dong J. Inhibitory properties of aqueous ethanol extracts of propolis on alpha-glucosidase. Evidence-Based Complementary and Alternative Medicine. 2015;2015:587383. doi: 10.1155/2015/587383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhishen, Mengcheng & Jianming (1999).Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1999;64(4):555–559. doi: 10.1016/s0308-8146(98)00102-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DPPH assay, TPC and TFC.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.