Abstract
Objective:
The aim was to describe the early phases of the progressive development of a lifestyle treatment for sustained remission of the metabolic syndrome (MetS) using the ORBIT model for behavioral treatment development as a guide.
Methods:
Early discovery and design phases produced a 3 component (diet, physical activity, stress), group-based lifestyle treatment with an intensive 6-month phase followed by monthly, participant-led maintenance meetings. In the proof-of-concept phase, 26 participants with the MetS (age 53 ±7 years, 77% female, and 65% ethnic minority) were recruited in a quasi-experimental design to determine if treatment could achieve the pre-specified benchmark of MetS remission in ≥ 50% at 2.5 years. Exploratory outcomes focused on MetS components, weight, and patient-centered benefits on energy/vitality and psychosocial status.
Results:
MetS remission was achieved in 53.8% after a median of 2.5 years. At 2.5 years, an increase of +15.4% reported eating ≥3 servings of vegetables/day, +7.7% engaged in ≥150 minutes of moderate-to-vigorous physical activity/week, and +11.5% reported experiencing no depression in the past 2 weeks. Weight loss ≥ 5% was achieved by 38.5% and energy/vitality, negative affect, and social support improved. Median group attendance over 2.5 years was 73.8%.
Conclusions:
It is plausible that this lifestyle program could produce a remission in the MetS, sustained through 2.5 years. After refinements to enhance precision and strength, progression to feasibility pilot testing and a randomized clinical trial will determine its efficacy as a cost-effective lifestyle option for managing the MetS in the current health care system.
Keywords: metabolic syndrome, lifestyle, intervention development, proof-of-concept, ORBIT model
Introduction
The metabolic syndrome (MetS) is diagnosed by the co-occurrence of at least 3 out of 5 cardio-metabolic risk factors: abdominal obesity, hypertension, hyperglycemia, hypertriglyceridemia, and low high-density lipoprotein cholesterol (Alberti, Eckel, Grundy, Zimmet, Cleeman et al., 2009). Affecting one-third of American adults,(Moore, Chaudhury, & Akinyemiju, 2017) the MetS increases total health care costs by 60% (Boudreau, Malone, Raebel, Fishman, Nichols et al., 2009), quintuples the risk of diabetes, doubles the risk of cardiovascular disease (Go, Mozaffarian, & Roger, 2014), and is considered a state of prediabetes (Grundy, 2012) and Stage A of heart failure (Hunt, Abraham, Chin, Feldman, Francis et al., 2009).
As a subclinical precursor to serious and costly clinical conditions, the MetS is an ideal target for prevention. There are no medications to induce MetS remission. Instead, each component is managed separately, giving rise to questions about the utility of a syndrome relative to a mix of separately treated phenotypes (Kahn, 2007). However, there is widespread agreement that metabolic abnormalities co-occur more often than expected by chance and share a common etiology in lifestyle (Alberti et al., 2009; Edwardson, Gorely, Davies, Gray, Khunti et al., 2012; Kahn, 2007; Kaur, 2014). In 93% of cases, obesity sets the stage for the MetS (Bradshaw, Monda, & Stevens, 2013; Ervin, 2009). A lifestyle of imbalance between energy intake and output promotes both obesity and the MetS (Edwardson et al., 2012; Kaur, 2014).
If lifestyle change is to be considered a viable target for remission of the MetS, it must be sustained over time. Six trials to date have assessed the impact of sustained lifestyle change on MetS remission at ≥1 year (Babio, Toledo, Estruch, Ros, Martínez-González et al., 2014; Chirinos, Goldberg, Llabre, Gellman, Gutt et al., 2016; Esposito, Marfella, Ciotola, Di Palo, Giugliano et al., 2004; Gomez-Huelgas, Jansen-Chaparro, Baca-Osorio, Mancera-Romero, Tinahones et al., 2015; Ilanne-Parikka, Eriksson, Lindström, Peltonen, Aunola et al., 2008; Orchard, Temprosa, Goldberg, Haffner, Ratner et al., 2005)). These trials produced wide variation in remission, ranging from 3–31% at 1 year (5 studies, 1,540 participants), 10–47% at 2 years (2 studies, 639 participants), and 9–31% at 3 or more years (4 studies, 3,406 participants).
All of these studies targeted primarily diet or diet plus physical activity. Another viable target for remission is chronic psychosocial stress which promotes the MetS directly by triggering the release of glucocorticoids (Rosmond, 2005) and indirectly by interfering with efforts to adopt healthy behaviors (Bergmann, Gyntelberg, & Faber, 2014). The value of intervening simultaneously on diet, physical activity, and stress has been demonstrated in patients with coronary artery disease who underwent a regression of atherosclerosis (Ornish, Brown, Scherwitz, Billings, Armstrong et al., 1990). A three-component lifestyle intervention has never been evaluated for its ability to promote a sustained remission of the MetS.
To develop such an intervention, guidance was provided by the ORBIT model for developing behavioral treatments for chronic diseases (Czajkowski, Powell, Adler, Naar-King, Reynolds et al., 2015). As a parallel to the widely-accepted drug development model (Turner, 2010), early behavioral treatment development features a discovery phase aimed at identifying a significant health need for which a behavioral treatment could provide a solution, and any basic science research that could illuminate the drivers of behavioral risk which, if altered, would strengthen the treatment’s efficacy. In a define phase, the basic elements of the treatment are assembled culminating in a hypothesized pathway that presents the components, their implementation targets, the behavioral risk factors they aim to improve, the primary clinical endpoint, and ambitious, clinically significant, quantitative milestones for judging the treatment’s success. In a proof-of-concept phase, the aim is to conduct a preliminary test to prove the concept that it is plausible that a fixed treatment package can produce a clinically significant improvement on a primary outcome. Here the focus is on the outcome, the design is often quasi-experimental, samples are small and accessible, success is judged by clinical rather than statistical significance, and the results determine whether the treatment merits evaluation in a more rigorous randomized design. The ORBIT model is progressive but not linear, providing a bi-directional framework for decisions to refine, abandon, or progress to more conclusive testing.
This paper applies the ORBIT model to the early phases of developing a lifestyle treatment to produce a sustained remission in the MetS. Small studies become significant when framed within the context of a structured process. Greater visibility of this process can produce stronger behavioral treatments and avoid costly trials of those that are underdeveloped.
Method
Discovery
Significant clinical question.
The MetS is a prevalent and increasing cause of high health care utilization. It frequently progresses to diabetes, which accounts for 1 in 10 health care dollars (American Diabetes Association, 2013), and heart failure with projected 2030 costs of $70 billion/year (Heidenreich, Albert, Allen, Bluemke, Butler et al., 2013). Drugs treat individual MetS components, are adhered to as prescribed in less than 50% of patients (Choudhry, Avorn, Glynn, Antman, Schneeweiss et al., 2011; World Health Organization, 2003), are needed for a lifetime, and costly, accounting for a 37% increase in drug costs (Boudreau et al., 2009). Lifestyle options can be justified if any short-term investment in a program reduces short- and long-term health care utilization.
Application of basic behavioral science.
The conventional approach to achieving sustained lifestyle change is to engage the goal-directed cognitive learning system in the frontal cortex to fortify intention by teaching self-management skills (Hall & Marteaux, 2014). However, neuroscience studies have shown that self-destructive food choices and opportunistic eating may not only be lapses in self-control but also well-entrenched automatic habits that engage the dorsal and lateral striatum, the center in the brain for automatic behaviors (Foerde, Steinglass, Shohamy, & Walsh, 2015; Guo, Simmons, Herscovitch, Martin, & Hall, 2014). Stressors and negative emotions promote a shift from goal-directed cognitive learning systems to stimulus-directed automatic habit-learning systems (Schwabe & Wolf, 2009). Thus, in the presence of stress and negative emotions, habit, rather than intention, influences action. This suggests that a treatment aimed at promoting sustained change could be potentiated by augmenting self-management skills training with the development of new habits that could be invoked in the presence of negative emotions. Experimental studies of habit formation emphasize repeated real-time pairing of repeating daily stimuli with new responses (Lally & Gardner, 2011). If a lifestyle treatment could mimic ecologically valid activities where old habits germane to diet, physical activity, and stress were likely to emerge (e.g., discomfort associated with physical activity; opportunistic eating triggered by food availability; emotional reactivity in response to an interpersonal stressor), it would create the opportunity to replace them with new alternatives (e.g., noticing without judgement heart rate response to activity; putting a pause between an emotional trigger and one’s response to it). If new, automatic habits can be formed, they operate at the subconscious level, thus obviating the need for ongoing, consciously-mediated motivation and support to sustain change.
Define Basic Elements
Basic principles.
Basic principles were developed over the course of 1 year by a multi-disciplinary team of behavioral and medical experts who met weekly and drew upon clinical experience, theoretical models of sustained behavior change, and basic and applied research to answer the question of what it would take to produce a sustained improvement in lifestyle. The group converged upon three guiding principles. (1) Diet, physical activity, and chronic stress are interrelated and independent in the risk they exert on cardiometabolic factors and therefore should be targeted simultaneously. (2) Real-time patient discovery of healthy behaviors during the course of ecologically valid settings fosters sustained change. (3) Immediate benefits are more powerful patient-centered motivators than distal negative consequences. Since integration into the healthcare system requires referrals from medical providers, their involvement in supporting patient progress was deemed to be essential.
Behavior change techniques.
Techniques were informed by two theories of sustained behavior change which emphasize ongoing social support, objective feedback, and behavioral self-management skills (Bandura, 1986; Ryan & Deci, 2000); principles of habit formation that emphasize real-time pairing of repeating daily cues with new habits in the service of creating new automatic responses (Lally & Gardner, 2011); and mindfulness practice from which a subset of mindfulness-based behaviors is linked to weight and reduction of cardio-metabolic risk (Camilleri, Méjean, Bellisle, Hercberg, & Péneau, 2015; Loucks, Schuman-Olivier, Britton, Fresco, Desbordes et al., 2015). In the taxonomy of behavior change techniques, self-monitoring and one or more additional self-management techniques maximized improvement in eating, physical activity, and weight in the obese over an average follow-up of 6 months (Dombrowski, Sniehotta, Avenell, Johnston, MacLennan et al., 2012; Michie, Abraham, Whittington, McAteer, & Gupta, 2009).
Treatment targets.
The lifestyle treatment was called ELM (Eat Love Move) and treatment targets were translated into nine specific habits, called “ELM leaves” for ease of patient recall. The dietary component was operationalized as two habits. “Perfect Plate” encouraged half of every plate at lunch and dinner to be comprised of vegetables (ChooseMyPlate.gov). The aim was to simplify by targeting one dietary component that would have a ripple effect (Mata, Silva, Vieira, Carraça, Andrade et al., 2009) on other potential targets (e.g., reduction in calories, saturated fat, dietary cholesterol, simple sugars, and sodium, as well as an increase in intake of fruits, whole grains, and fish) (Grundy, 2012). “HALT” aimed to reduce emotional eating by pausing, taking a deep breath, considering whether one was Hungry, Angry, Lonely, or Tired/Bored, and recognizing the difference between hunger and emotional triggers (Loucks et al., 2015; O’Reilly, Cook, Spruijt-Metz, & Black, 2014).
The physical activity component was operationalized as three habits. “Play” aimed to restructure perceptions of physical activity from work to enjoyment and to notice experiences within the body and the environment during activity (Kennedy & Resnick, 2015; Ulmer, Stetson, & Salmon, 2010). “Use It or Lose It” encouraged maintenance of muscle mass with moderate-to-vigorous physical activity for 30 minutes on most days (Eckel, Jakicic, Ard, Miller, Hubbard et al., 2014). “Waste Energy” encouraged ≥ 10,000 steps/day by finding ways to get more, rather than less, steps into a day (Tudor-Locke, Craig, Aoyagi, Bell, Croteau et al., 2011).
The stress component aimed to integrate mindfulness behaviors with conventional health behaviors. “Discover This Moment” encouraged nonjudgemental sensitivity to bodily sensations, thoughts, emotions and sensory experiences while exercising, preparing food, eating, and managing stressors (Daubenmier, Kristeller, Hecht, Maninger, Kuwata et al., 2011; Loucks et al., 2015; Younge, Gotink, Baena, Roos-Hesselink, & Hunink, 2015). “Recognize Hooks” aimed to put time between occurrence of a stressor and one’s emotional reaction to it by restructuring the stressor as a “hook;” an inevitable hassle of daily life (Powell, 1996). “Smile First” encouraged practice in kindness to self and to others (Hutcherson, Seppala, & Gross, 2008). “Important Trumps Urgent” aimed to develop a daily “ELM Hour” habit by reallocating time from urgent/unimportant activities to those that are nonurgent/important (Covey, 1989).
Hypothesized treatment pathway.
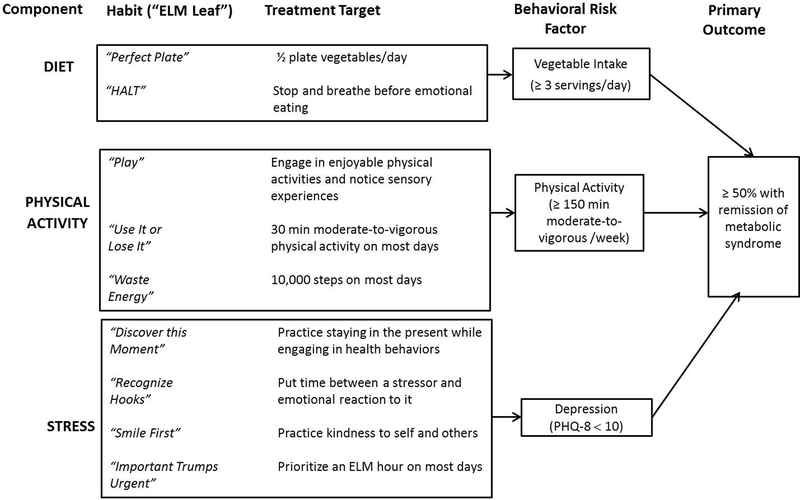
The pathway by which the intervention was hypothesized to influence the primary outcome is presented in Figure 1. This prespecified pathway links the three treatment components to their associated targets for implementation. The milestone sought for each behavioral risk factor target of treatment is the clinically significant cutpoint at which cardiometabolic risk is decreased (Centers for Disease Control and Prevention, 2010; Eckel et al., 2014; Kroenke, Strine, Spitzer, Williams, Berry et al., 2009). The clinically significant target for the primary outcome of MetS remission is the same level of success as that achieved by (1) adherence to drug therapy, which is less than 50% regardless of the drug class (Choudhry et al., 2011; World Health Organization, 2003), and (2) the most successful lifestyle study which achieved a MetS remission of 46.7% at 2 years (Esposito et al., 2004). This 50% milestone exceeds the ≥35% threshold for success in weight loss interventions defined by the Food and Drug Administration (Colman, 2012). Interpretation of results is enhanced by linking success of the intervention to success at targeting each element of this pathway.
Figure 1.
Hypothesized treatment pathway. ELM = Eat Love Move; PHQ-8 = Patient Health Questionnaire-8.
Proof-of-Concept
Design.
The aim was to determine whether it is plausible that this newly developed treatment could achieve a prespecified, clinically significant milestone in MetS remission in a meaningful percentage of a small, accessible sample (Czajkowski et al., 2015). The design was a quasi-experimental, pre-post evaluation of success at reaching the clinically significant target.
Participants.
Twenty-six men and women without locomotor limitations were recruited serially from two primary care clinics and one preventive cardiology clinic between January 2010 and January 2011. At the time of the patient’s regular medical visit, the chart was reviewed to assess diagnosis of MetS, age, and presence of medical exclusions. MetS was diagnosed when at least three of five diagnostic criteria were met (Alberti et al., 2009): (1) abdominal obesity (waist girth ≥102 cm in men; ≥88 cm in women); (2) high blood pressure (≥130/85 mmHg or anti-hypertensive treatment); (3) low high-density lipoprotein cholesterol (<1.0 mmol/L in men; <1.3 mmol/L in women); (4) fasting hypertriglyceridemia (≥1.70 mmol/L); or (5) fasting hyperglycemia (≥ 5.56 mmol/L). Eligible age was between 35 and 65 years. Medical exclusions included diabetes, coronary heart disease, upcoming surgery, pregnancy, cognitive impairment, psychiatric co-morbidities, substance abuse, eating disorder, or current smoking. Of 1,745 individuals screened, 571 (32.7%) did not have MetS, 479 (27.5%) did not fall within the age range, 590 (33.8%) met a medical exclusion criterion, 37 (2.1%) were unable to be screened, and 105 (6.0%) had the MetS and were eligible. These 105 patients were invited for an interview to learn about the study, enhance understanding of the time commitment, explore motivation to change behavior, and explore comfort with group treatment. Of these, 57 (54.3%) were not interested in undergoing an interview, 5 (4.8%) no longer had the MetS, 4 (3.8%) had a logistical problem, 13 (12.4%) decided against participation, and 26 (24.8%) were enrolled. Thus, 26% of the 100 eligible MetS patients were enrolled (approximately 1 out of every 4 MetS-eligible patients). Study procedures were approved by the Rush University Medical Center Institutional Review Board before obtaining informed consent.
Intervention.
The intervention featured a 6-month intensive phase followed by a 24-month, participant-led maintenance phase. During the intensive phase, three cohorts of 8–10 participants met in groups on a weekly (first 3 months) and then biweekly (second 3 months) basis. Each group was facilitated by a dietitian and one of three participating health psychologists (LHP, BMA, JCO). During maintenance, the three groups merged and met on a monthly basis around an agenda they developed. Individual telephone coaching was available for participants who requested it. Group treatment was featured because of its efficacy, cost-effectiveness, and ability to foster peer modeling (Bandura, 1986).
Group sessions lasted approximately 2 hours, and included 30 minutes of physical activity, 30 minutes of meal preparation led by two participants, and 60 minutes of sharing the prepared meal together with “dinner conversation” that focused on new content presented via demonstrations, feedback, successes, challenges, problem-solving and goal-setting. Habit change was implemented by real-time recognition, by participants and coaches, of the emergence of an old habit during the course of exercising, preparing food, eating, or conversing, and experimenting with a healthier alternative. Participants received pedometers (Omron model HJ-720ITC) to self-monitor their steps. Between sessions, the “ELM leaves” reminded participants of themes/goals and lifestyle diaries recorded each day’s achievement of treatment implementation goals. Participants received reports on their MetS status at baseline, 6 months, and 30 months. Because the program was embedded within medical care, participants were encouraged to share reports with their physicians for support, advice, and possible medication adjustments.
Measurements.
Assessments were conducted at baseline, at the end of the 6-month intensive phase, and at the final follow-up, which was a median of 2.5 years, depending upon when the participant was recruited. After 7 years, a qualitative survey was conducted.
Primary Outcome.
MetS remission was diagnosed when participants met criteria for fewer than three diagnostic components without pharmacotherapy. A standard protocol was used to assess blood pressure and waist girth (Pickering, Hall, Appel, Falkner, Hill et al., 2005; World Health Organization, 2011). Blood was collected after a 10-hour fast and sent to a commercial laboratory for lipid and glucose analyses. A standardized medication questionnaire collected information about medication use.
Treatment Targets.
The target of the diet component was vegetable intake over the past month, assessed using the All-Day version of the Fruit and Vegetable Screener (Thompson, Subar, Smith, Midthune, Radimer et al., 2002). Average daily servings of non-starchy vegetables and sugar-sweetened beverages were scored separately. The target of the physical activity component was moderate-to-vigorous physical activity assessed via accelerometer using 7-day monitoring with a Sensewear Pro3 ArmBand™, (BodyMedia, Inc, Pittsburgh, PA) (Miller, Jakicic, Rejeski, Whit-Glover, Lang et al., 2013). Assessments with at least 4 valid days (≥10 h/day of non-sleep wear time) were required but 82–95% of the assessments used 6 or 7 days of monitoring. The accelerometer is valid for assessment of daily steps and moderate-to-vigorous physical activity at ≥3.0 metabolic exercise equivalents for ≥10 min bouts (Drenowatz & Eisenmann, 2011). The target of the stress component was depression in the past 2 weeks, chosen because physical activity improves depressed affect (Dunn, Trivedi, & O’Neal, 2001) and assessed using the Patient Health Questionnaire Depression Scale (PHQ-8) (Kroenke et al., 2009) where a score ≥ 10 is consistent with depression (range of possible scores: 0–24).
Exploratory Outcomes.
A sex- and race/ethnic-specific MetS Severity Score quantified total burden of risk associated with components of the MetS (Gurka, Lilly, Oliver, & DeBoer, 2014). Energy and vitality over the past 30 days was assessed with the four-item Energy and Vitality Index, a subscale of the Medical Outcomes Study (MOS) Short Form Health Survey (McHorney, Ware, & Raczek, 1993) where higher scores reflected greater energy and vitality (range of possible scores: 0–100). Social support was assessed using the overall social support index from the MOS Social Support Survey where higher scores indicate more support (range of possible scores: 0–100) (Sherbourne & Stewart, 1991). Negative affect was assessed over the past 7 days by the Positive and Negative Affect Schedule (PANAS) where higher scores indicate more negative affect (range of possible scores: 10–50) (Watson, Clark, & Tellegen, 1988).
Statistical Analyses
To explore changes in the hypothesized pathway and exploratory outcomes, baseline, 6-month, and 2.5-year comparisons were made using percentages (with ns) and means (with SDs). To explore the relationship between remission of the MetS at 2.5 years and the hypothesized pathway, the sample was stratified into remission versus no-remission subgroups, and status on treatment targets was compared. Because weight was a strong correlate of MetS remission, stratification was also conducted by achievement of clinically significant weight loss at 2.5 years. Missing data were replaced with the baseline value (the worst-case scenario, assuming no change). There were no missing data at 6 months; five participants missed the 2.5-year exam. Since the priority was on clinical significance and exploration, no sample size calculations or statistical tests were conducted (Lancaster, Dodd, & Williamson, 2004).
Results
Table 1 summarizes baseline characteristics. Participants were on average 53 years (range 38–65), 77% were women, 65% were ethnic minorities, and all were overweight or obese. The median attendance was 31 (73.8%) of the 42 group sessions: 16 (88.9%) of the 18 sessions in the intensive phase and 15 (62.5%) of the 24 sessions in the maintenance phase. No serious adverse events were reported and one participant developed Type 2 diabetes.
Table 1.
Baseline characteristics in 26 participants with the metabolic syndrome
| Socio-Demographic | Age, years, mean (sd)* | 53.3 (7.2) |
| Women, n (%)** | 20 (76.9) | |
| Race/Ethnicity, n (%) African American Hispanic Caucasian |
15 (57.7) 2 (7.7) 9 (34.6) |
|
| Education < College, n (%) | 16 (61.5) | |
| Income, n (%) <$35,000 $35,000 – $49,999 $50,000 - $75,999 ≥ $75,000 |
7 (26.9) 6 (23.1) 4 (15.4) 9 (34.6) |
|
| Health | Weight (kg) Body Mass Index, mean (sd) Categoriesa, n (%) Overweight Obesity, Class 1 Obesity, Class 2 Obesity, Class 3 Number of metabolic syndrome components, n (%) 3 4 5 Number of medications, mean (sd) |
97.2 (19.5) 35.2 (7.3) 5 (19.2) 10 (38.5) 6 (23.1) 5 (19.2) 18 (69.2) 7 (26.9) 1 (3.8) 2 (1.2) |
Body Mass Index (BMI) categories: overweight = BMI 25.0 −29.9 kg/m2; Obesity Class 1 = BMI 30.0 – 34.9 kg/m2; Obesity Class 2 = BMI 35.0 – 39.9 kg/m2; Obesity Class 3 = BMI ≥ 40 kg/m2
sd: standard deviation;
n: number of participants
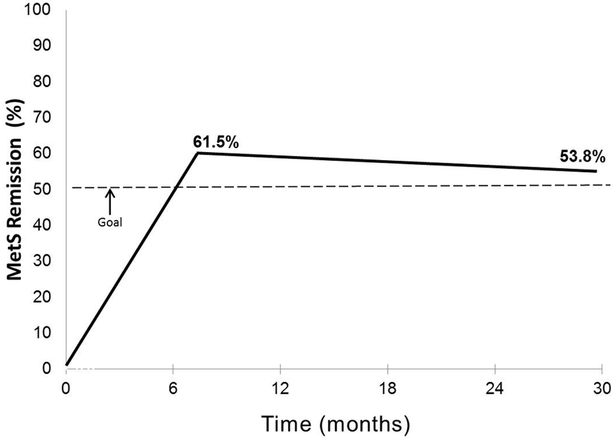
Figure 2 presents the results on the primary outcome. MetS remission was achieved in 16 (61.5%) at 6 months and sustained at 2.5 years in 14 (53.8%), exceeding the goal of 50%.
Figure 2.
Incidence of remission of the metabolic syndrome relative to the clinically significant goal of ≥ 50%.
Table 2 presents data on achievement of the lifestyle targets. Comparing percent at goal at baseline and 2.5 years, achievement of the dietary target increased from 30.8% to 46.2%, the physical activity target from 23.1% to 30.8%, and the depression target from 84.6% to 96.1%.
Table 2.
Percent achieving lifestyle treatment targets at baseline, 6 months, and 2.5 years, N=26
| Baseline N (%) |
6 Months N (%) |
2.5 years N (%) |
Change: Baseline- 2.5 Years |
|
|---|---|---|---|---|
| ≥ 3 Vegetable Servings/daya | 8 (30.8) | 12 (46.2) | 12 (46.2) | +15.4% |
| Moderate-to-Vigorous Physical Activity, ≥150 min/weekb | 6 (23.1) | 10 (38.5) | 8 (30.8) | +7.7% |
| No Depression in past 2 weeks, PHQ-8 Score <10c |
22 (84.6) | 26 (100.0) | 25 (96.1) | +11.5% |
The National Cancer Institute Fruit and Vegetable Screener
Accelerometry
The Patient Health Questionnaire (PHQ-8)
Table 3 presents exploratory outcomes. A clinically significant weight loss of 5% was achieved in 38.5% at 2.5 years. Among those who were on antihypertensive medication at baseline, one-quarter no longer needed it at 2.5 years. Daily steps, energy/vitality, and social support increased and sugary beverages, depressive symptoms and negative affect decreased.
Table 3.
Exploratory outcomes at baseline, 6 months, and 2.5 years, N=26
| Baseline | 6 Months | 2.5 years | ||
|---|---|---|---|---|
| Metabolic Syndrome Components, n(%) |
Abdominal Obesity: ≥102 cm (men); ≥ 88cm (women) |
25 (96.2) | 20 (76.9) | 23 (88.5) |
| Blood Pressure: ≥130/85 mmHg or on antihypertensive drugs |
23 (88.5) | 19 (73.1) | 21 (80.8) | |
| HDL Cholesterol <1.0 mmol/L (men); <1.3 mmol/L (women) |
14 (53.8) | 7 (26.9) | 8 (30.8) | |
| Triglycerides: ≥1.70 mmol/L |
10 (38.5) | 4 (15.4) | 4 (15.4) | |
| Glucose: ≥5.56 mmol/L |
15 (57.7) | 13 (50.0) | 7 (26.9) | |
| Metabolic Syndrome Severity, mean(sd) |
0.8 (0.6) | 0.4 (0.7) | 0.2 (0.9) | |
| Weight | Weight Loss ≥ 5%, n(%) | N/A | 12 (46.2) | 10 (38.5) |
| Weight Loss, kg, mean(sd) |
N/A | −5.7 (6.5) | −3.0 (7.6) | |
| Body Mass Index, mean(sd) |
35.2 (7.3) | 33.1 (7.4) | 34.1 (7.0) | |
| Medication Use | Anti-hypertensivesa, n(%) | 20 (100.0) | 14 (70.0) | 15 (75.0) |
| Lifestyle | Vegetable Servings/day, mean(sd) |
3.1 (2.7) | 3.9 (3.3) | 4.6 (3.6) |
| Sugar Sweetened Beverages/dayc, mean(sd) |
1.4 (2.2) | 0.9 (2.1) | 1.3 (2.3) | |
| Moderate-to-Vigorous Physical Activity min/wkd, mean(sd) |
112.5 (152.1) | 165.3 (164.1) | 152.9 (206.5) | |
| Daily Stepsd, mean(sd) | 6253.9 (2330.8) | 7542.9 (2922.8) | 7149.3 (3149.8) | |
| Energy and Vitalityb, mean(sd) |
60.6 (15.8) | 68.8 (21.4) | 72.3(17.4) | |
| Psychosocial | Social Supporte, mean(sd) |
71.3 (26.8) | 77.8 (21.0) | 82.8 (23.2) |
| Depressive Symptomsf, mean(sd) |
4.5 (4.4) | 2.4 (2.3) | 2.9 (3.0) | |
| Negative Affectg, mean(sd) | 15.0 (7.4) | 13.6 (5.6) | 13.4 (5.7) |
In the 20 participants who had treated hypertension at baseline
Medical Outcomes Study (MOS) Short Form Health Survey
The National Cancer Institute Fruit and Vegetable Screener
Accelerometer
Medical Outcomes Study Social Support Scale
The Patient Health Questionnaire (PHQ-8)
The Positive and Negative Affect Schedule (PANAS)
Table 4 explores active treatment components by comparing MetS status at 2.5 years with achievement of lifestyle targets alone and in combination at 2.5 years. The biggest differences in remission status were in the dietary target of ≥ 3 servings of vegetables/day (remission: 57.1%; no remission: 33.3%) and the exploratory goal of loss of ≥5% of baseline weight (remission: 57.1%; no remission: 16.7%). Stratifying by success at weight loss at 2.5 years, 60% of those successful, and 12.5% of those not successful, achieved the physical activity goal.
Table 4.
Percent achieving lifestyle target(s) of treatment by metabolic syndrome status and weight change at 2.5 years, N=26
| METABOLIC SYNDROME | ||
|---|---|---|
| Remission N (%) |
No Remission N (%) |
|
| Total | 14 (53.8) | 12 (46.2) |
| Achieved Target on: Vegetable Intakea Physical Activityb Depressionc Vegetable Intake and Physical Activity Vegetable Intake and Depression Physical Activity and Depression All 3 Targets Weight Loss ≥ 5% of Baseline Weight |
8 (57.1) 4 (28.6) 14 (100.0) 2 (14.3) 8 (57.1) 4 (28.6) 2 (14.3) 8 (57.1) |
4 (33.3) 4 (33.3) 11 (91.7) 2 (16.7) 4 (33.3) 4 (33.3) 2 (16.7) 2 (16.7) |
| WEIGHT LOSS ≥ 5% OF BASELINE WEIGHT | ||
| Yes N (%) |
No N (%) |
|
| Total Achieved Target on: Vegetable Intake Physical Activity Depression |
10 (38.5) 6 (60.0) 6 (60.0) 10 (100.0) |
16 (61.5) 6 (37.5) 2 (12.5) 15 (93.8) |
≥ 3 servings of vegetables/day, NCI Fruit and Vegetable Screener
≥ 150 minutes/week of moderate-to-vigorous physical activity by accelerometer
< 10 on PHQ-8
Table 5 presents the results of a qualitative survey conducted 7 years from baseline. Of the 10 participants who were successful in achieving clinically significant weight loss at 2.5 years, 70.0% self-reported no weight regain 4.5 years later. Among the 14 participants who sustained remission of the MetS at 2.5 years, 93% endorsed the value of nutrition education, 71% endorsed the value of physical activity, and 86% endorsed the value of non-reacting to daily stressors. Sustained connections with friends made during ELM occurred in 43% of those who were, and 25% of those who were not, in MetS remission.
Table 5.
Qualitative survey results after 7 years (N=26)*
| Metabolic Syndrome Status at 2.5 years |
||
|---|---|---|
| Remission N (%) |
No Remission N (%) |
|
|
Total Weight Lost or stayed the same |
14 (53.8) 7 (50.0) |
12 (46.2) 5 (41.7) |
|
What did you find most helpful about ELM? Nutrition education Physical activity Comprehensive Lifestyle Change Access to a dietitian Accountability Cooking demonstration Group support |
13 (92.8) 10 (71.4) 8 (57.1) 8 (57.1) 8 (57.1) 7 (50.0) 4 (28.6) |
6 (50.0) 5 (41.7) 5 (41.7) 4 (33.3) 3 (25.0) 6 (50.0) 5 (41.7) |
|
What did you find least helpful about ELM? Everything was helpful |
8 (57.1) |
4 (33.3) |
|
Which ELM Leaves (habits) were most helpful? ”Recognize Hooks” (Non-reacting) ”Perfect Plate” (1/2 plate vegetables) ”Use It or Lose It” (Physical activity on most days) |
12 (85.7) 8 (57.1) 4 (28.6) |
8 (66.7) 8 (66.7) 6 (50.0) |
|
Which habits were most difficult to break? Not exercising Which apps do you use for your health? Fitbit None |
10 (71.4) 6 (42.9) 4 (28.6) |
6 (50.0) 4 (33.3) 5 (41.7) |
|
Health Network I connect with health conscious friends ≥ once a week I connect with ELM friends |
8 (57.1) 6 (42.9) |
6 (50.0) 3 (25.0) |
Conducted 4.5 years after the end of maintenance and the cessation of any contact. For simplicity of presentation, only responses endorsed by ≥ 35% of the total sample are presented.
Discussion
The essential features of the ORBIT model for developing behavioral treatments aligns well with the widely accepted process of drug development. The development of a new drug is a multidisciplinary progression from discovery, to exploration, and then to confirmation in Phase III efficacy trials (Turner, 2010). Early phase, low-cost explorations offset high failure rates, which drive the cost of bringing a drug to market to approximately $1.3 billion (Schuler & Buckley, 2015). Because the failure rate for behavioral treatments is also high but support is significantly less, it is important to minimize the cost of failure by a progressive process starting with simple tests on small numbers of accessible subjects (Czajkowski et al., 2015; Turner, 2010)). If the cost of failure is low, persistence evolving into innovation will be enhanced.
The most fundamental product from the early discovery and design phases was the creation of a hypothesized treatment pathway that quantified how the lifestyle treatment would translate into remission of the MetS. The a priori articulation of this hypothesis guided priorities for assessment measures, facilitated interpretation of results, prevented post hoc claims of success from unexpected findings, and placed emphasis on the level of change needed to affect disease rather than whether the sample was large enough to detect change statistically.
The preliminary testing phase featured a small, proof-of-concept test of the plausibility that the complete treatment as originally designed would produce a remission of the MetS. This is a movement in the ORBIT model from the design phase (Ia) to the preliminary testing phase (IIa). Although the model provides a refine phase (Ib) to enhance efficiency, it does not recommend an optimum timing for refinement. A building design evaluates promising components individually before settling on a fixed protocol (Collins, Dziak, & Li, 2009). A dismantling design tests the efficacy of the best possible full multicomponent intervention and, if successful, then identifies components that are most active (Knowler, Barrett-Connor, Fowler, Hamman, Lachin et al., 2002). This investigation featured the latter approach but tested plausibility rather than efficacy of the full treatment. This saved time and resources, provided a justification for proceeding to a more rigorous evaluation, and provided a benchmark against which any efficacy trial or dismantling study could be judged.
This proof-of-concept test showed that over 50% of those medically eligible remained in MetS remission after a median of 2.5 years with minimal maintenance. This level of success is comparable to the 50% adherence achieved with drugs (Choudhry et al., 2011; World Health Organization, 2003). The Diabetes Prevention Program, widely judged to be the greatest success a lifestyle treatment has achieved to date, reported a 2-year MetS remission rate of 12.0% (Orchard et al., 2005). The two best European trials of the MetS both targeted the Mediterranean diet alone. The Spanish PREvención con DIeta MEDiterránea (PREDIMED) trial achieved 14.5% MetS remission at 1 year and 28.2% MetS remission at 4.8 years (Babio et al., 2014). A small Italian trial featuring an intensive treatment aimed at studying mechanisms achieved 46.7% remission at 2 years (Esposito et al., 2004). All 3 of these trials focused on diet with or without physical activity, self-management techniques to change them, and active treatment throughout the follow-up to sustain it. ELM is unique in elevating stress and emotions as a key target, expanding behavior change techniques to include habit modification and mindfulness practice, and offering a minimal maintenance program initiated and sustained by participants.
The proof-of-concept test showed that a refinement of the hypothesized pathway was needed. Clinically significant weight loss was not in the original hypothesized pathway but appears to be a key driver of success at MetS remission. But weight loss does not appear to be sufficient unto itself since more participants underwent remission (53.8%) than underwent successful weight loss (38.5%). Attention to the emotional side of lifestyle change may have contributed, but it was difficult to detect benefit from alleviation of depression since most participants were not depressed at entry and a ceiling effect resulted. This was a costly mistake. Thus, a revision of the hypothesized pathway will include weight loss as a surrogate endpoint, and identify a measure to evaluate the stress component that is linked tightly to treatment goals, has baseline variability, and is associated with the MetS.
Because the goal of the ELM program is a sustained change in lifestyle, a 7-year follow-up provided a depth of perspective on what helped participants change. The value of a 3-component treatment was supported by the 14 participants in MetS remission at 2.5 years whose top three ratings of helpfulness were diet (93%), physical activity (71%), and nonreacting to stressors (86%). These successful participants also appeared to have maintained a health network where 43% were still connecting with ELM friends and 57% were connecting at least weekly with a health-conscious friend. Replacement of the pedometer with a Fitbit will provide the opportunity to designate people as friends, give each other daily support for successes and lapses, and possibly enhance the development of a health network. Expansion of the function of wearable technology from daily self-monitoring to daily support makes it possible to extend reach beyond the group setting (Jakicic, Davis, Rogers, King, Marcus et al., 2016).
A strength of ELM is the excellent attendance of 88.9% achieved in the intensive phase, possibly resulting from the positive reinforcers at each session. Because the program is intended for integration into the healthcare system, subsequent studies will examine the nature of interactions that occur between participants and their providers around the ELM reports.
The proof-of-concept design has obvious limitations. Its small, select sample of primarily women limits the generalizability of results. It has no control group leaving unanswered the question of whether participants would get better over time on their own. No statistical tests were performed, making it possible that any changes observed were due to chance alone. It did not study mechanisms of benefit, leaving unanswered such questions as whether it was intake of vegetables or sugary beverages that accounted for effects. But the goal of this design was not to answer any of these questions. It was simply to determine whether or not there was any merit in going forward with a more rigorous study to answer them.
In summary, it is plausible that this lifestyle intervention can achieve MetS remission and sustain it over 2.5 years in half of the participants treated. With minor refinements to the hypothesized pathway and the use of more sophisticated wearable technology, progression to feasibility pilot testing and, conditional on results, a randomized efficacy trial is justified.
Acknowledgements
We appreciate enormously the ELM participants who made this study possible. We would like to thank the medical providers and staff of collaborating clinics. We are sincerely grateful to Sue Ginn (in memoriam), the CEO of the McGowan Charitable Fund, for her inspirational leadership and belief that prevention is better than cure.
Funding sources: This work was supported by the William G. McGowan Charitable Fund and P50HL105189, R56HL118343 from the National Heart, Lung, and Blood Institute.
References
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, … the International Association for the Study of Obesity. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; the International Association for the Study of Obesity. Circulation, 120, 1640–1645. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. (2013). Economic costs of diabetes in the U.S. in 2012. Diabetes Care, 36, 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babio N, Toledo E, Estruch R, Ros E, Martínez-González MA, Castañer O, … PREDIMED Study Investigators. (2014). Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. Canadian Medical Association Journal, 186, E649–E657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A (1986). Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Bergmann N, Gyntelberg F, & Faber J (2014). The appraisal of chronic stress and the development of the metabolic syndrome: A systematic review of prospective cohort studies. Endocrine Connections, 3, R55–R80. 10.1530/EC-1514-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, … Okamoto LJ (2009). Health care utilization and costs by metabolic syndrome risk factors. Metabolic Syndrome Related Disorders, 7, 305–314. [DOI] [PubMed] [Google Scholar]
- Bradshaw PT, Monda KL, & Stevens J (2013). Metabolic syndrome in healthy obese, overweight, and normal weight individuals: The Atherosclerosis Risk in Communities Study. Obesity, 21, 203–209. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri GM, Méjean C, Bellisle F, Hercberg S, & Péneau S (2015). Association between mindfulness and weight status in a general population from the NutriNet-Santé Study. PLoS One, 10, e0127447. doi: 0127410.0121371/journal.pone.0127447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2010). State-specific trends in fruit and vegetable consumption among adults --- United States, 2000–2009. Morbidity and Mortality Weekly Report, 59, 1125–1130. [PubMed] [Google Scholar]
- Chirinos DA, Goldberg RB, Llabre MM, Gellman M, Gutt M, McCalla J, … Schneiderman N (2016). Lifestyle modification and weight reduction among low-income patients with the metabolic syndrome: The CHARMS randomized controlled trial. Journal of Behavioral Medicine, 39, 483–492. [DOI] [PubMed] [Google Scholar]
- Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, … Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial. (2011). Full coverage for preventive medications after myocardial infarction. New England Journal of Medicine, 365, 2088–2097. [DOI] [PubMed] [Google Scholar]
- Collins LM, Dziak JJ, & Li R (2009). Design of experiments with multiple independent variables: A resource management perspective on complete and reduced factorial designs. Psychological Methods, 14, 202–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman E (2012). Food and Drug Administration’s Obesity Drug Guidance Document: A short history. Circulation, 125, 2156–2164. [DOI] [PubMed] [Google Scholar]
- Covey S (1989). The seven habits of highly effective people. New York, NY: Simon and Schuster. [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, … Charlson M (2015). From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, … Epel E (2011). Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. Journal of Obesity, 2011.651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, MacLennan G, & Araújo-Soares V (2012). Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: A systematic review. Health Psychology Review, 6, 7–32. [Google Scholar]
- Drenowatz C, & Eisenmann JC (2011). Validation of the SenseWear Armband at high intensity exercise. European Journal Applied Physiology, 111, 883–887. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, & O’Neal HA (2001). Physical activity dose-response effects on outcomes of depression and anxiety. Medicine and Science in Sports and Exercise, 33, S587–S597. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Jakicic JM, Ard JD, Miller NH, Hubbard VS, Nonas CA, … Yanovski SZ (2014). 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 129(supp 2), S76–S799.24222015 [Google Scholar]
- Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, … Biddle SJ (2012). Association of sedentary behaviour with metabolic syndrome: A meta-analysis. PLoS One, 7, e34916. doi: 34910.31371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin RB (2009). Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. National Health Statistics Reports, 5, 1–7. [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, … Giugliano D (2004). Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. Journal of the American Medical Association, 292, 1440–1446. [DOI] [PubMed] [Google Scholar]
- Foerde K, Steinglass JE, Shohamy D, & Walsh BT (2015). Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nature Neuroscience, 18, 1571–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, & Roger VL (2014). Heart disease and stroke statistics - 2014 update: A report from the American Heart Association. Circulation, 129, e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Huelgas R, Jansen-Chaparro S, Baca-Osorio AJ, Mancera-Romero J, Tinahones FJ, & Bernal-López MR (2015). Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. European Journal of Internal Medicine, 26, 317–323. [DOI] [PubMed] [Google Scholar]
- Grundy SM (2012). Pre-diabetes, metabolic syndrome, and cardiovascular risk. Journal of the American College of Cardiology, 59, 635–643. [DOI] [PubMed] [Google Scholar]
- Guo J, Simmons WK, Herscovitch P, Martin A, & Hall KD (2014). Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Molecular Psychiatry, 19, 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurka MJ, Lilly CL, Oliver MN, & DeBoer MD (2014). An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism, 63, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, & Marteaux TM (2014). Executive function in the context of chronic disease prevention: Theory, research and practice. Preventive Medicine: An International Journal Devoted to Practice and theory, 68, 44–50. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, … Stroke Council. (2013). Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circulation Heart Failure, 6, 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, … Yancy CW (2009). 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation, 119, e391–e479. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Seppala EM, & Gross JJ (2008). Loving-kindness meditation increases social connectedness. Emotion, 8, 720–724. [DOI] [PubMed] [Google Scholar]
- Ilanne-Parikka P, Eriksson JG, Lindström J, Peltonen M, Aunola S, Hämäläinen H, … Finnish Diabetes Prevention Study Group. (2008). Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care, 31, 805–807. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, … Belle SH (2016). Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. Journal of the American Medical Association, 316, 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R (2007). Metabolic syndrome: is it a syndrome? Does it matter? Circulation, 115, 1806–1810. [DOI] [PubMed] [Google Scholar]
- Kaur J (2014). A comprehensive review on metabolic syndrome. Cardiology Research and Practice, 21 pages. doi: 10.1155/2014/943162 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kennedy AB, & Resnick PB (2015). Mindfulness and physical activity. American Journal of Lifestyle Medicine, 9, 221–223. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, … Diabetes Prevention Program Research Group. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, & Mokdad AH (2009). The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders, 114, 163–173. [DOI] [PubMed] [Google Scholar]
- Lally P, & Gardner B (2011). Promoting habit formation. Health Psychology Review, 7, S137–S158. [Google Scholar]
- Lancaster GA, Dodd S, & Williamson PR (2004). Design and analysis of pilot studies: Recommendations for good practice. Journal of Evaluation Clinical Practice, 10, 307–312. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Schuman-Olivier Z, Britton WB, Fresco DM, Desbordes G, Brewer JA, & Fulwiler C (2015). Mindfulness and cardiovascular disease risk: State of the evidence, plausible mechanisms, and theoretical framework. Current Cardiology Reports, 17, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Silva MN, Vieira PN, Carraça EV, Andrade AM, Coutinho SR, … Teixeira PJ (2009). Motivational “spill-over” during weight control: Increased self-determination and exercise intrinsic motivation predict eating self-regulation. Health Psychology, 28, 709–716. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JEJ, & Raczek AE (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31, 247–263. [DOI] [PubMed] [Google Scholar]
- Michie S, Abraham C, Whittington C, McAteer J, & Gupta S (2009). Effective techniques in healthy eating and physical activity interventions: A meta-regression. Health Psychology, 28, 690–701. [DOI] [PubMed] [Google Scholar]
- Miller GD, Jakicic JM, Rejeski WJ, Whit-Glover MC, Lang W, Walkup MP, & Hodges ML (2013). Effect of varying accelerometry criteria on physical activity: The Look Ahead Study. Obesity, 21, 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JX, Chaudhury N, & Akinyemiju T (2017). Metabolic syndrome by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Preventing Chronic Disease, 14:160287.DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly GA, Cook L, Spruijt-Metz D, & Black DS (2014). Mindfulness-based interventions for obesity-related eating behaviours: A literature review. Obesity Reviews, 15, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, … the Diabetes Prevention Program Research Group. (2005). The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program randomized trial. Annals of Internal Medicine, 142, 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, … Gould KL (1990). Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet, 336, 129–133. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BEG,J, Hill MN, Jones DW, … Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. (2005). Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension, 45, 142–161. [DOI] [PubMed] [Google Scholar]
- Powell LH (1996). The hook: A metaphor for gaining control of emotional reactivity In Allen R & Scheidt S (Eds.), Heart and mind: The emergence of cardiac psychology (pp. 313–327). Washington, DC: American Psychological Association. [Google Scholar]
- Rosmond R (2005). Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology, 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Ryan RM, & Deci EL (2000). Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist, 55, 68–78. [DOI] [PubMed] [Google Scholar]
- Schuler P, & Buckley B (2015). Re-engineering clinical trials. Best practices for streamlining drug development Cambridge, MA: Academic Press. [Google Scholar]
- Schwabe L, & Wolf OT (2009). Stress prompts habit behavior in humans. Journal of Neuroscience, 29, 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, & Stewart AL (1991). The MOS social support survey. Social Science and Medicine, 32, 705–714. [DOI] [PubMed] [Google Scholar]
- Thompson FE, Subar AF, Smith AF, Midthune D, Radimer KL, Kahle LL, & Kipnis V (2002). Fruit and vegetable assessment: Performance of 2 new short instruments and a food frequency questionnaire. Journal of the American Dietetic Association, 102, 1764–1772. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, … Blair SN (2011). How many steps/day are enough? For older adults and special populations. International Journal of Behavioral Nutrition and Physical Activity, 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR (2010). New drug development: An introduction to clinical trials (2nd ed.). New York, NY: Springer. [Google Scholar]
- Ulmer CS, Stetson BA, & Salmon PG (2010). Mindfulness and acceptance are associated with exercise maintenance in YMCA exercisers. Behaviour Research and Therapy, 48, 805–809. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2003). Adherence to long-term therapies: Evidence for action. Retrieved from http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf.
- World Health Organization. (2011). Waist circumference and waist–hip ratio: Report of a WHO Expert Consultation. Geneva, 8–11 December 2008. Retrieved from http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf. [Google Scholar]
- Younge JO, Gotink RA, Baena CP, Roos-Hesselink JW, & Hunink MG (2015). Mind-body practices for patients with cardiac disease: A systematic review and meta-analysis. European Journal of Preventive Cardiology, 22, 1385–1398. [DOI] [PubMed] [Google Scholar]