Abstract
This commentary begins by presenting a simplified way of making the diagnosis of myalgic encephalomyelitis [ME/CFS] using the 1994 CDC case definition. The format used can easily be modified for other case definitions. The commentary then discusses whether ME/CFS is the same or a different illness from fibromyalgia [FM]. Because overlap exists between the two syndromes, some investigators have posited that they are variants of the same illness. I have viewed this as an empirically testable hypothesis and have summoned considerable amounts of data suggesting the two illnesses differ. Were differences to exist, that would suggest different pathophysiological processes for each - leading to different treatments.
Myalgic encephalomyelitis/Chronic fatigue syndrome [for succinctness, CFS] and fibromyalgia [FM] are medically unexplained illnesses, predominantly in women, characterized by disabling fatigue and by widespread pain with tenderness, respectively. Because there are currently no known biomarkers that can be used to diagnose each of these illness processes, diagnosis is based on clinical criteria. One major difference between the two diagnoses is that the existence of any medical cause of severe fatigue excludes patients from receiving the diagnosis of CFS; in contrast, there are no medical exclusions in making the diagnosis of FM. Instead, patients with no other cause for body-wide pain are diagnosed as having primary FM while those with coexisting rheumatological diagnoses receive the diagnosis of secondary FM. This difference in diagnosis is responsible for a ten-fold difference in prevalence with CFS occurring approximately 0.3%1 of the population and FM in about 4%2.
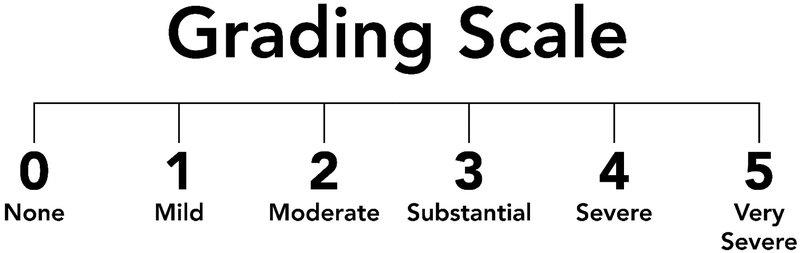
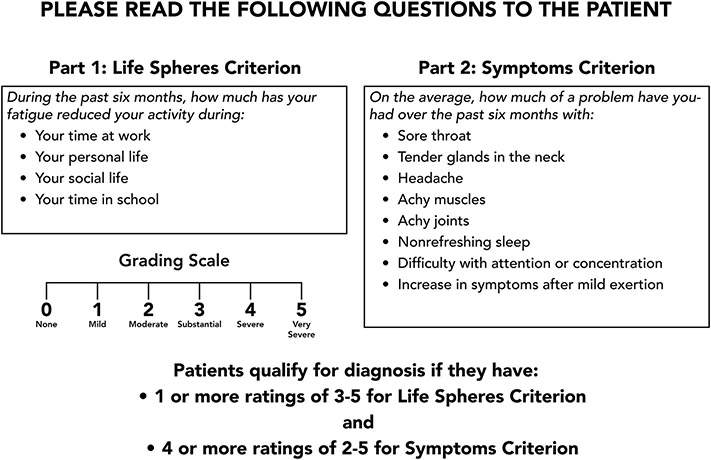
I was a part of the committees that arrived at the 1994 and 2015 case definitions for CFS, and so those are the ones described here. The 1994 CDC-based case definition defined the illness as new onset of fatigue, lasting at least 6 months and severe enough to produce a substantial decrease of activity at work, school or while doing personal or social activities. We have operationalized the way we assess severity of fatigue by asking patients to score their reduction in activity using a 5 point visual analog scale ranging from 0 for none, 3 for substantial and 5 for very severe. We then ask patients to provide duration of as well as burden from [using the same 0 to 5 visual analog scale] the following symptoms: feverishness, sore throat, tender lymph glands, headache; myalgia, arthralgia, unrefreshing sleep, difficulty with concentration/attention, and the report that even minimal physical or mental/emotional efforts produce a flare up of all these other symptoms. Figures 1 and 2 show how to facilitate making this diagnosis. Figure 1 depicts the visual analog scale going from none [0] to very severe [rating of 5]. The doctor holds the scale shown in the in front of the patient and then reads the text in Figure 2 to the patient. Figure 2 allows the examiner to first assess the effects of the patient’s fatigue on his or her life sphere. We define a “substantial” reduction in activity as endorsement of at least one of the life spheres at a 3 or greater. Patients who do not report having fatigue sufficient to produce such a reduction in activity would receive the diagnosis of idiopathic chronic fatigue rather than the more severe diagnosis of CFS. But if the patient fulfills the life sphere criterion, the examiner than goes onto the list of 8 symptoms which accompany severe fatigue and are used to make the diagnosis of CFS; these are sore throat; tender lymph glands; headache; myalgia; arthralgia; unrefreshing sleep; and postexertional malaise. If the patient rates four of these 8 symptoms on the list as a moderate or greater problem, that patient fulfills the symptom severity criterion and then receives the diagnosis of CFS [in our research we require patients to rate symptoms as substantial or worse to be included in studies].
Figure 1.
Visual analog scale shown to patients during their evaluation for the possible diagnosis of CFS. To use this most efficiently, put this on one side and Figure 2 on the back side of a piece of paper.
Figure 2:
Read this script to the patient. If the patient fulfills the criteria noted herein, that patient will fulfill the 1994 CDC case definition for CFS. If the patient reports problems with unrefreshing sleep, cognitive problems and post-exertional malaise, the patient would also fulfill criteria for the 2015 clinical case definition developed by an expert committee at the IOM.
Next, blood tests are done to eliminate many of the myriad medical causes of fatigue including anemia, occult liver dysfunction, hypothyroidism, an underlying inflammatory disorder via sedimentation rate and C reactive protein, Lyme Disease via C6 Lyme Elisa, and rheumatological disease via rheumatoid factor and ANA. Because some severe psychiatric illnesses could present with fatigue, those with a history of psychotic or bipolar disorders, recent eating disorder or problems with alcohol or drug abuse would be excluded from receiving the diagnosis.
In contrast to this research case definition, a committee of experts met at the Institute of Medicine in 2015 and came up with an easier way to make the clinical diagnosis of CFS. The case definition was similar in defining the illness as requiring new onset of 6 months of fatigue producing a substantial decrease in activity. In addition, patients must have moderate, substantial or severe problems [2, 3, or 4 respectively on our visual analog scale] occurring at least half the time with unrefreshing sleep, post-exertional malaise, and either cognitive problems or objective evidence of orthostatic intolerance. To determine if a patient has physiological evidence for the complaint of orthostatic intolerance, we record physiological data while supine and then every minute during a 10 minute lean test where the patient is asked to keep feet together leaning against a wall without talking; a video of this procedure can be found at https://www.facebook.com/painandfatigue/videos/1318728218235453/. The two most common examples of physiological manifestations of orthostatic intolerance are orthostatic tachycardia with increases in heart rate exceeding 30 beats per minute or orthostatic hyperventilation with drops in exhaled CO2 to 31 mmHg or lower. Earlier work suggested that delayed hypotension was also common, but we were unable to replicate this finding in a controlled study3.
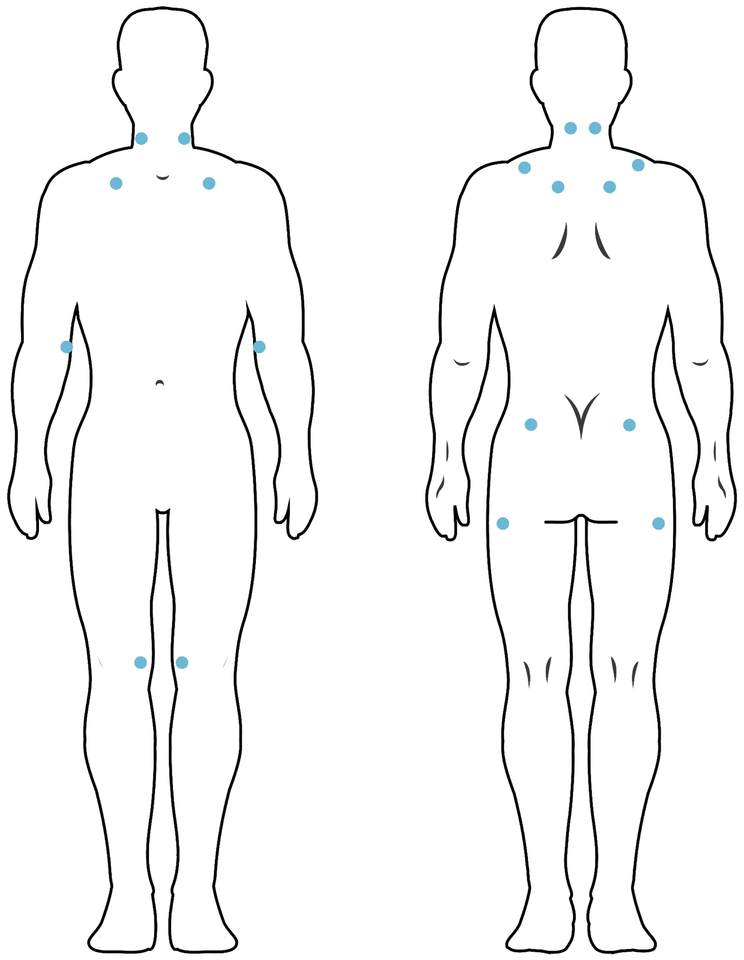
The diagnosis of FM requires widespread pain lasting at least 3 months and accompanied by tenderness on palpation with pressure of 4 kg in at least 11 of 18 locations [see figure 2 for their location]; however, patients usually report pain wherever they are pressed – thus the notion of widespread pain. However, the American College of Rheumatology came up with an operational definition of “widespread:” Pain on left and right sides of the body, above and below the waist, and accompanied by pain in cervical spine, anterior chest, thoracic spine or low back. Patients fulfilling these criteria often report symptoms also consistent with the diagnosis of temporo-mandibular joint/muscle disorder4.
Despite the difference in prevalence between the two syndromes, the core symptoms of fatigue, sleep problems and cognitive difficulties exist across both syndromes and lead to significant comorbidity between them; we have found that 34% of 313 patients diagnosed with CFS had comorbid FM5. The fact that these two syndromes co-exist so often has led some to question whether they are, in fact, distinct diagnostic entities. Wessely et al., for example, have suggested that the “similarities between them outweigh the differences” – a position similar to that taken by other researchers6. We have termed this the “unitary” hypothesis – a position our own data and those of others do not support, as we will show. A revision of the original 1990 case definition for FM published in 20107 has blurred the diagnostic differences between the two illnesses with the result of nearly doubling the rate at which CFS patients also receive the diagnosis of FM compared to when the 1990 case definition is used8. Doing this leaves open the research question of whether the two illnesses are variants of one another or are due to different pathophysiological processes. If the former hypothesis were true, discrete case definitions corresponding to distinct illness syndromes would be unnecessary since the pathophysiological underpinnings of the two syndromes would be similar; plans to treat one syndrome would apply to the other as well. Therefore, a critical question at this juncture is to answer the question as to whether CFS and FM are the same or different illnesses.
Although overlap between the occurrence of CFS and FM exists, there are differences between the illnesses. For example, substance P is increased in the spinal fluid of FM patients but not in the spinal fluid of CFS patients9. This increase in FM has recently been replicated and extended to also show an increase in corticotrophin releasing hormone with both of these leading to further increases in IL-6 and TNF via mast cell activation10. Of potential importance is the recent suggestion that this inflammatory process can be limited with treatment with IL-3711. A critical next step will be to move these studies to a side by side comparison of FM with CFS to determine if differences in this immune regulatory process differs between the illnesses.
We have reviewed the work of others in which biochemical, physiological and genetic differences have been reported between the two syndromes12; moreover, many of our own studies also show differences between the two illnesses – supporting the position that the illnesses are different, produced by different pathophysiological processes.
Because our studies were subsumed under the auspices of an NIH-funded CFS Cooperative Research Center, we studied patients with CFS only or with CFS+FM and did not recruit those with FM only for study. The research revealed: 1) Those in the CFS only but not the CFS+FM group showed neuropsychological dysfunction and an elevated brain serotonergic response to tryptophan infusion relative to controls13,14. 2) Those with CFS+FM did not but CFS only showed an altered physiological response to a standardized sub-maximal exercise test through reduced blood pressure and an increased stroke index. 3) Concerning post-traumatic stress disorder, CFS only patients had community rates of having this diagnosis on diagnostic psychiatric interview – i.e., 1.5%, while those with CFS+FM were substantially and significantly higher – 8.5% [Natelson, unpublished data]. Another group also reported this15. 4) Approximately twice as many CFS only patients developed their illness following a sudden, influenza-like onset compared to those with CFS+FM5. 5) Finally, our group has identified a series of patients diagnosed with obstructive sleep apnea based on data recorded during overnight polysomnography16. Fourteen percent of these patients had CFS only, four percent fulfilled case criteria for the diagnosis of CFS+FM [4%]; in contrast, none of these patients had FM only. These findings indicate discordance in rates of CFS and FM; compared to substantially higher rates for CFS [compare 14% to 0.3% in community samples], rates of FM only were not different from those found in community samples [~4% in both17]. This review strongly suggests that CFS only and CFS+FM are categorically different and not just the same disorder that differs in severity.
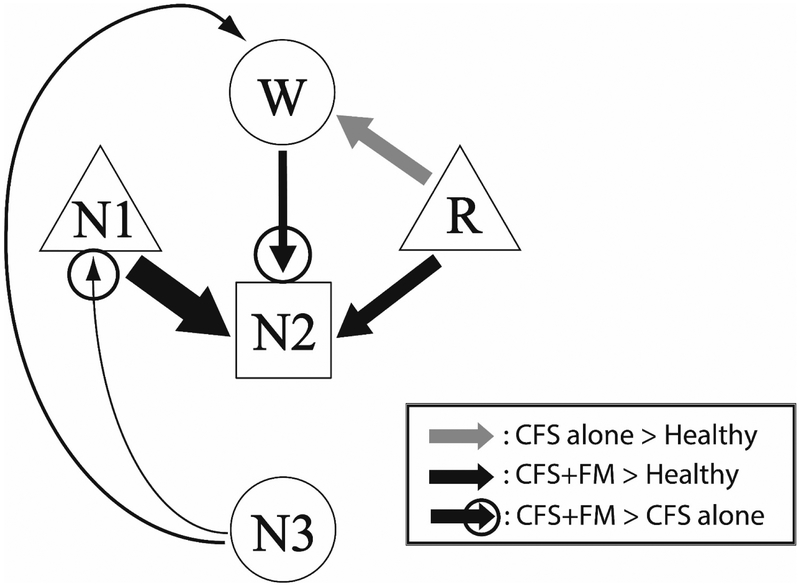
Most recently, we have turned our focus on the sleep architecture of patients with CFS only and those with CFS+FM and have found these to differ18. These differences were not seen using standard Rechtschaffen and Kales (R-K) criteria but were seen using our newer approach that determined the probability for transitioning among the various sleep phases19. The original RK method of assessing sleep rates the sleep stage status of every sequential 30 sec epoch following sleep onset. In general people fall into light sleep early on with sleep getting deeper later in the night. This allows sleep researchers to quantify the duration of light and deeper sleep over the entire night as well as periods when the sleeper wakes after sleep onset. However, this method only provides a rather coarse representation of sleep architecture. In contrast, we count every transition from one sleep stage to another. So for example, as one falls asleep, a transition might occur between wake and light [N1] sleep. Then as the patient falls deeper asleep, transitions may occur from N1 to N2 or even N3 sleep both of which capture the deeper stages of sleep.
The data from this study are shown in Figure 3: CFS only patients had a very different abnormality from CFS+FM patients in the form of increased transitions from REM to wake. In contrast, CFS+FM patients, but not CFS only or healthy controls, had data indicative of sleep disruption – namely, higher probabilities of transitioning from slow wave sleep to N1. As a compensation, these patients also showed evidence for greater sleep pressure than CFS only by having increased transitions from Wake to N2.
Figure 3:
This figure depicts the location of the 18 tender points that should be probed for tenderness at a force of 4 kg. Usually the patient will report pain at substantially less pressure than 4 kg. If the patient reports tenderness at more than 10 of the 18 locations, that fulfills the tender point requirement for the diagnosis of FM.
These differences in sleep architectures indicate that patients with CFS+FM exhibit an additional sleep-disrupting component to their disease not found in CFS only, supporting the idea that pathophysiological differences exist between CFS only and CFS+FM; such differences may mean that CFS+FM is a different illness from CFS without FM. We have recently received NIH funding to allow us to use a novel method to make this determination by non-pharma-cologically improving some elements of sleep. A report from Swiss researchers studied the sleep architecture of 10 young men during two 45 min naps – one on a normal bed and the other on a bed that moved back and forth like a cradle20 (while this is an oscillating movement, the authors called it “rocking” – a term I will continue to use here also). Sleep in the rocking bed condition was better than in the stable bed condition with shorter periods of light sleep, increases in N2 sleep accompanied by a higher density of sleep spindles, and evidence for improved deep sleep (significantly higher delta power throughout the nap). Because in our data, only the CFS+FM patients had disrupted sleep specifically in the pattern improved by the rocking bed, the hypothesis we will test with the newly available funding is that the rocking bed will improve the sleep disruption of the CFS+FM patients significantly more than it will for the sleep of the CFS only patients.
Going forward, we have also recently learned that the NIH will fund the next step of our proteomics effort using spinal fluid collected from patients who were not using brain-active medications to determine if CFS alone is a different illness from CFS+FM. Our initial study compared spinal fluid from a mixed sample of CFS patients in comparison to spinal fluid from patients whose fatigue followed well treated Lyme Disease21. Importantly, the proteomic analysis identified over 600 proteins that seemed unique to CFS based on limits of detection – thus differentiating it both from the Lyme patients and from healthy controls. However inferences about these results were limited by the need to pool over 20 CFS subjects’ spinal fluids to attain a volume sufficient for analysis. Advances in methodology have now obviated the need for so much pooling. With the new NIH support, we can limit pooling to three subjects per group and are able to assay five such groups; thus, we will be able to aliquot spinal fluids from patients with either CFS only or with CFS+FM in discrete pools. Being able to do this will allow us to eliminate outliers and determine whether the proteome of those with CFS only is similar to or quite different from those with CFS+FM -- a major step forward.
Figure 4:
This figure shows a summary of all the transitions among sleep stages for CFS only, CFS+FM and healthy controls. Note that the sleep architecture of the CFS only patients differs considerably from those with CFS+FM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. ArchInternMed 1999;159:2129–37. [DOI] [PubMed] [Google Scholar]
- 2.White KP, Speechley M, Harth M, Ostbye T. The London fibromyalgia epidemiology study: The prevalence of fibromyalgia syndrome in London, Ontario. Journal of Rheumatology 1999;26:1570–6. [PubMed] [Google Scholar]
- 3.LaManca JJ, Peckerman A, Walker J, et al. Cardiovascular response during head-up tilt in chronic fatigue syndrome. Clinical Physiology 1999;19:111–20. [DOI] [PubMed] [Google Scholar]
- 4.Fraga BP, Santos EB, Farias Neto JP, et al. Signs and symptoms of temporomandibular dysfunction in fibromyalgic patients. The Journal of craniofacial surgery 2012;23:615–8. [DOI] [PubMed] [Google Scholar]
- 5.Ciccone DS, Natelson BH. Comorbid illness in the chronic fatigue syndrome: A test of the single syndrome hypothesis. PsychosomMed 2003;62:268–75. [DOI] [PubMed] [Google Scholar]
- 6.Barsky AJ, Borus JF. Functional somatic syndromes. Annals of Internal Medicine 1999;130:910–21. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res(Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 8.Kaseeska K, Brown M, Jason LA. Comparing two fibromyalgia diagnostic criteria in a cohort of chronic fatigue syndrome patients. BullIACFS/ME 2011;19:47–57. [Google Scholar]
- 9.Evengård B, Nilsson CG, Lindh G, et al. Chronic fatigue syndrome differs from fibromyalgia. No evidence for elevated substance P levels in cerebrospinal fluid of patients with chronic fatigue syndrome. Pain 1998;78:153–5. [DOI] [PubMed] [Google Scholar]
- 10.Tsilioni I, Russell IJ, Stewart JM, Gleason RM, Theoharides TC. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. The Journal of pharmacology and experimental therapeutics 2016;356:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastrangelo F, Frydas I, Ronconi G, et al. Low-grade chronic inflammation mediated by mast cells in fibromyalgia: role of IL-37. Journal of biological regulators and homeostatic agents 2018;32:195–8. [PubMed] [Google Scholar]
- 12.Abbi B, Natelson BH. Is Chronic Fatigue Syndrome the same illness as Fibromyalgia: Evaluating the 'single syndrome' hypothesis. QJMed 2013;106:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook DB, Nagelkirk PR, Peckerman A, Poluri A, Mores J, Natelson BH. Exercise and cognitive performance in chronic fatigue syndrome. Med Sci Sports Exerc 2005;37:1460–7. [DOI] [PubMed] [Google Scholar]
- 14.Weaver SA, Janal MN, Aktan N, Ottenweller JE, Natelson BH. Sex differences in plasma prolactin response to tryptophan in Chronic Fatigue Syndrome patients with and without comorbid fibromyalgia. JWomens Health (Larchmt) 2010;3:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy-Byrne P, Smith WR, Goldberg J, Afari N, Buchwald D. Post-traumatic stress disorder among patients with chronic pain and chronic fatigue. Psychological Medicine 2004;34:363–8. [DOI] [PubMed] [Google Scholar]
- 16.Pejovic S, Natelson BH, Basta M, Fernandez-Mendoza J, Mahr F, Vgontzas AN. Chronic fatigue syndrome and fibromyalgia in diagnosed sleep disorders: a further test of the 'unitary' hypothesis. BMC Neurol 2015;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 18.Kishi A, Natelson BH, Togo F, Struzik ZR, Rapoport DM, Yamamoto Y. Sleep-Stage Dynamics in Patients with Chronic Fatigue Syndrome with or without Fibromyalgia. Sleep 2011;34:1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishi A, Struzik ZR, Natelson BH, Togo F, Yamamoto Y. Dynamics of sleep stage transitions in healthy humans and patients with chronic fatigue syndrome. AmJPhysiol RegulIntegrComp Physiol 2008;294:R1980–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayer L, Constantinescu I, Perrig S, et al. Rocking synchronizes brain waves during a short nap. Curr Biol 2011;21:R461–2. [DOI] [PubMed] [Google Scholar]
- 21.Schutzer SE, Angel TE, Liu T, et al. Distinct cerebrospinal fluid proteomes differentiate post-treatment lyme disease from chronic fatigue syndrome. PLoSOne 2011;6:e17287. [DOI] [PMC free article] [PubMed] [Google Scholar]