1. Background
Effective treatment of obstructive sleep apnea (OSA) is an important goal due to its impact on quality of life, and the known cardiovascular and neurocognitive consequences of this common chronic disease. OSA is also associated with an increasing economic and social burden, estimated to be over $12 billion in the US in 2015 [1]. Continuous positive airway pressure (CPAP) has excellent efficacy for controlling OSA, but its effectiveness is limited by variable adherence to therapy. The fact that CPAP devices objectively record usage data provides a unique opportunity to monitor adherence. A recent systematic review demonstrated that CPAP adherence did not increase across clinical trials over the last 20 years despite continuous improvement in technical innovation and support [2]. Studies included in the systematic review were generally small and often performed under close monitoring conditions that are not reflective of real world usage. Therefore, such approaches may not be generalizable or sustainable. Moreover, randomized controlled clinical trials typically exclude severe patients, including those with profound sleepiness or at high risk of complications, making participants less representative of typical clinical practice. Advances in technology, intensive support, and patient/provider education are all thought to improve adherence to CPAP therapy. Recent digital health innovation using patient engagement tools has also been associated with improved CPAP use compared with usual care [3]. There is a need for physicians, payers and policy makers to have access to real world data on CPAP adherence in order to opmitize models of care and patient outcomes. Hence, the aim of this study was to define CPAP adherence over the first 90 days using data from a large cloud database, as reflective of current technology and real-world clinical care.
2. Methods
We assessed de-identified data from a large cloud database of positive airway pressure (PAP) users to examine adherence to therapy during the period 1-Oct-2014 to 31-Oct-2017. AirView (ResMed Inc., San Diego, USA) is a HIPAA-compliant web-based solution for healthcare specialists intended to transfer and display device and therapeutic information that has been transmitted remotely from the patient’s therapy device. All Air10 platform PAP devices (ResMed Inc., San Diego, USA) have an on-board mobile communications chip which connects to the secure cloud infrastructure automatically. Patient compliance and therapy data are uploaded daily, typically within one hour after each therapy session. All AirView communication and storage is encrypted to meet required international privacy and security standards. The AirView database is hosted in a secure facility in the USA. Patients on the AirView platform in the USA are managed by private and academic sleep centres, home medical equipment providers (HMEs) and primary care offices, with a wide geographical distribution. Compliance data are automatically provided to the HME to facilitate patient care, and the patient can also nominate their caring physician to access the data. The information provided includes usage data and therapy issues (eg, high mask leak, high residual AHI or central apnea index). Approximately 98% of Air10 devices setup with patients have data in AirView. The HME gives permission to ResMed to de-identify protected health information in accordance with 45 C.F.R. § 164.514(b) and use such de-identified data for analytics by ResMed. The HME is responsible consenting each patient and approximately 96% of records are thereby included. The Air10 platform became available in August 2014, and was rapidly adopted such that older devices currently represent <0.2% of devices setup in the USA. Mobile communications costs are not borne by the patient but are purchased by the HME as part of their population management process. Furthermore, a patient engagement tool, My Air (ResMed Inc, San Diego, USA), became available in October 21 2014 and the iOS app became available August 2016. Patients do not pay for access to MyAir irrespective of HME signup.
Patients were included if they were adults >18 years, started treatment on the AirSense/AirCurve 10 (ResMed Corp) PAP platform (Air10) within the US, were registered on the cloud database by their HME, and if they used a single PAP modality for ≥1 hour during the first 90 days of therapy. The one hour threshold was chosen to minimise selection bias while ensuring that machine calculated parameters such as AHI and respiratory rate are averaged over a sensible minimum timeframe. The primary outcome was adherence, which was defined using the US Center for Medicare and Medicaid Services (CMS) definition (≥4 hours’ PAP use on 70% of nights in a consecutive 30-day period in the first 90 days of therapy). The time to achieve CMS compliance was taken as the number of days taken from first day of use to the day at which the compliance threshold was met. The study was reviewed by an Institutional Review Board (IRB) and deemed exempt from IRB oversight.
All data were analysed in a secure, anonymised database physically separate from the main production server. Statistical analyses were performed using the R language (R development core team). Missing compliance data are imputed as zero. The first day of therapy is taken as the patient AirView setup date. For other data fields, including clinical metrics and respiratory events, missing data are not imputed and not included in calculations of mean values, standard deviations and proportions.
3. Results
During the period of analysis, 2.62 million patients met the inclusion criteria; 23.4% of patients were excluded for the following reasons: >1 HME (1.9%), invalid data entry (10.1%), more than one PAP modality used or data from SD card (9.2%), age ≤18 years (0.7%), <1 hour usage in first 90 days (1.4%). The mean (±SD) age was 55.7 ± 13.8 years. The following single PAP modalities were used: APAP (50%), CPAP (41%), bilevel (5.6%), bilevel Auto (2.4%), ASV (0.7%), ASV Auto (0.5%).
The observed 90-day PAP adherence rate using the CMS definition was 75%. Participants used PAP on a median 93% of nights for a mean of 5.1 ± 2.5 (SD) hours/night across all nights and 6.0 ± 2.0 h/night on nights used (see Table 1). The median proportion of days with non-zero usage was 93.3%. A histogram of CPAP usage is shown in Fig. 1. The mean residual AHI was 3.2 ± 4.5 events/hour. The mean 95th percentile pressure (CPAP and APAP) was 10.9 ± 2.8 cmH2O. The mean 95th percentile leak of 22.8 ± 19.6 L/min.
Table 1.
Adherence data from the first 90 days of therapy.
| Adherence measures | Values (n = 2,621,182) Median (IQR)a |
|---|---|
| CMS compliance in first 90 days, n (%) | 1,955,961 (74.6) |
| Time to achieve CMS compliance, days | 23.00 (21.00, 27.00) |
| Device usage, h/session | 6.18 (4.79, 7.35) |
| Daily usage (all days), h/night | 5.54 (3.42, 7.04) |
| Proportion of days with non-zero usage, % | 93.3 (72.2, 98.9) |
| Proportion of days compliant (usage ≥ 4 h/night), % | 80.0 (46.7, 95.6) |
CMS, Center for Medicare and Medicaid Services; IQR, interquartile range; SD, standard deviation. CMS Compliance definition: ≥4 hours’ PAP use on 70% of nights in a consecutive 30-day period in the first 90 days of therapy.
Data not normally distributed.
Fig. 1.
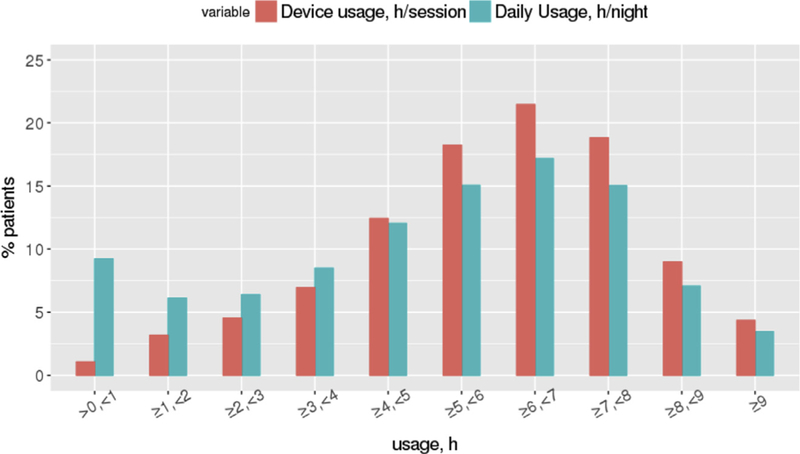
Histogram of PAP usage.
4. Discussion
To our knowledge this is the largest analysis of objective CPAP usage ever undertaken using real world data. The short term adherence rate of 75% in this analysis appears higher than is generally acknowledged in clinical practice and reported in the literature. The observed treatment characteristics in our study demonstrate excellent efficacy during therapy, with a mean residual AHI of 3.2/hour and acceptable leaks. The mean usage of 5.1 hours is also higher than that found in a meta-analysis of 82 clinical trials (weighted pooled mean of 4.6 h/night) [2]. Among larger CPAP trials, a study of a social cognitive theory intervention involving 206 patients found that 55.2% of patients achieved >4hours use per night with standard care at three months [4]. The APPLES study (n = 443 active CPAP) found that 42% achieved the MCS adherence threshold at six months [5]. More recently, a randomized controlled trial of telehealth strategies in 1455 patients found that 54% in the usual care group achieved MCS adherence at 90 days, rising to 73% in the telemedicine group, who received education and automated feedback [6].
Given that OSA is a chronic disorder associated with considerable morbidity and mortality, there is an imperative to optimize adherence to therapy. The observed adherence rate in this study compares favorably to that reported for pharmacotherapy in other chronic diseases. For example, high non-adherence rates have been reported for the use of inhalers in asthma (>50%) [7] and COPD (77%) [8], and medication use in hypertension (45%) [9], diabetes (7—64%) [10], and epilepsy (38%) [11]. Poor adherence to prescribed medications accounts for substantial worsening of disease, death, and increased health care costs.
The long-term effects of suboptimal PAP adherence bring to light the health related impact of untreated OSA. It is not sufficient simply to prescribe a PAP machine and consider the patient to be treated. There are many factors influencing PAP adherence, both medical and non-medical (eg, psychosocial), and that these need to be considered when both prescribing CPAP as well as designing cost-effective interventions to improve adherence. We believe that big data analysis such as the one reported here can offer new and important insights to enhance PAP adherence.
There is a need for health care systems to embrace digital information that can enhance patient engagement and outcomes of therapy. Although there are many challenges related to ethics, data ownership, storage, usage, and reimbursement that require clarification [12], sleep medicine is well placed to develop new chronic disease models of care, enabled by digital health data such as CPAP adherence. It is critical that progress is based on ethical patient-centered principles, to safe-guard against the misuse of “big data.” It has been proposed that academic-industry partnerships offer a path to maximise the benefits that are achievable from appropriate use of such data, provided there are rigorous controls on who can access the data and for what purpose it is used [13].
Although our study has a large sample size there are important limitations that need to be considered. The observational nature of the study raises potential for important biases. First, we do not have data about patients who may have been prescribed treatment but did not proceed with it. Prior studies suggest that up to 24% of patients may not fulfill their PAP prescription [14] and a proportion of patients may not even turn on their PAP machine. Exclusions related to invalid data entry may have further biased the sample. In addition, we lack detailed knowledge about participants’ demographic and clinical characteristics because these data are not available in the database and cannot be linked to existing data due to privacy issues.
Notwithstanding these notable limitations, using a big data approach we observed excellent adherence among patients initiating PAP therapy, with 75% of patients achieving the CMS compliance threshold. This adherence rate compares favorably to those reported in the OSA literature, as well as with other chronic medical therapies. The large sample size in this study suggests that the findings are likely to reflect real world clinical care in the US. Future work is needed to examine longer term adherence to PAP therapy and outcomes.
Acknowledgements and disclosures
The authors wish to thank the following for statistical analysis: Haixiang Shi, PhD and Yang Yan, M. Sc. Stat. Authors JA and AB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. ResMed Corp, USA funded the study and their employees (JA, AB, CMN) contributed to the design and conduct of the study, analysis, and interpretation of the data and preparation of the manuscript for publication. PAC has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding. He has received research support from ResMed, SomnoMed, Australia, and Zephyr Sleep Technologies, Canada. He is a consultant/adviser to Zephyr Sleep Technologies, and Narval, France. He has a pecuniary interest in SomnoMed related to a previous role in R&D (2004). JLP is supported by the French National Research Agency, France in the framework of the “Investissements d’avenir” program (ANR-15–1DEX-02). His department has received research support from Philips Respironics, USA, Fisher and Paykel, New Zealand, and ResMed. HW has received consulting/speaking fees from ResMed and Inspire Medical. AM relinquished all outside personal income as an Officer of the ATS in 2012. ResMed gave a philanthropic donation to UC San Diego, but AM receives no personal income from ResMed.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2019.01.004.
References
- [1].Watson NF. Health care savings: the economic value of diagnostic and therapeutic care for obstructive sleep apnea. J Clin Sleep Med 2016;12(8):1075–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg 2016;45(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Malhotra A, Crocker ME, Willes LE, et al. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018;153(4):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bartlett D, Wong K, Richards D, et al. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. Sleep 2013;36:1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstruction sleep apnea patients: the apnea positive pressure long-term efficacy study (APPLES). Sleep 2012;25:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hwang D, Chang JW, Benjafield AV, et al. Effect of telemedicine education and telemonitoring on continous positive airway pressure adherence. Am J Respir Crit Care Med 2018;197:117–26. [DOI] [PubMed] [Google Scholar]
- [7].Mes MA, Katzer CB, Chan AHY, et al. Pharmacists and medication adherence in asthma: a systematic review and met-analysis. Eur Respir J 2018;52: 1–10. [DOI] [PubMed] [Google Scholar]
- [8].Sulaiman I, Cushen B, Green G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructice pulmonary disease. Am J Respir Crit Care Med 2017;195:1333–43. [DOI] [PubMed] [Google Scholar]
- [9].Abegaz TM, Shehab A, Gebreyohannes A, et al. Nonadherence to antihypertensive drugs: systematic review and meta-analysis. Med Open 2017;96(4) (e5641). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cramer JA. A systematic review of adherence with medication for diabetes. Diabetes Care 2004;27:1218–24. [DOI] [PubMed] [Google Scholar]
- [11].Getnet A, Woldeyohannes SM, Bekana L, et al. Antiepileptic drug nonadherence and its predictors among people with epilepsy. Behav Neurol 2016. 10.1155/2016/3189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Randerath W, Bassetti CL, Bonsignore MR, et al. Challenges and perspective in obstructive sleep apnoea. ERJ; 2018. https://doi/org/10.10.1183/13993003.02616-2017. [Google Scholar]
- [13].Jain SH, Rosenblatt M. Is big data the new frontier for academic-industry collaboration. JAMA 2014:2171–2. [DOI] [PubMed] [Google Scholar]
- [14].Lin HS, Zuliani G, Amjad EH, et al. Treatment compliance in patients lost to follow-up after polysomnography. Otolaryngol Head Neck Surg 2007. February;136(2):236–40. [DOI] [PubMed] [Google Scholar]


