Abstract
Introduction:
Rapid Eye Movement (REM) sleep behavior disorder (RBD) is characterized by dream enactment and is associated with incidence of neurodegenerative disorders, especially Parkinson’s disease (PD). Whether PD with RBD constitutes a distinct subtype with unique progression is unknown. Here, we investigated motor and cognitive symptom progression in patients with self-reported RBD features in adult life.
Methods:
We screened for RBD in a cohort of 776 PD patients whom we ascertained using a population-based strategy. Among participants with at least one follow-up (60%), we compared those with and without probable RBD (pRBD) estimating hazard rate ratios for progression events UPDRS-III≥ 35 and MMSE≤ 24.
Results:
Prevalence of pRBD at baseline was 21%. In adjusted Cox regression models among patients with a Postural Instability and Gait Dysfunction (PIGD) phenotype, those with pRBD progressed faster to a UPDRS-III≥ 35 (HR= 1.92, 95% CI= 1.12; 3.27). Also, all patients with pRBD progressed twice as fast to a MMSE score≤ 24 (HR= 2.04, 95% CI= 1.13; 3.69). In sensitivity analyses, using alternative definition of pRBD and accounting for bias due to loss to follow-up results remained similar.
Discussion:
Employing data from one of the largest population-based studies of PD, in which movement disorder specialists assessed patients, we confirm evidence that pRBD features are a clinical marker for faster cognitive decline and possibly also motor progression in PD patients, the latter for patients with a PIGD subtype early in disease.
Keywords: Parkinson’s disease, REM sleep behavior disorder, motor progression, cognitive decline, sleep
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD) is characterized by dream enactment, usually associated with dreams of violent content, and classified according to the International Classification of Sleep Disorders (ICSD-2) as a parasomnia, an event accompanying sleep, instead of a sleep disorder [1]. RBD occurs due to motor activity during REM sleep resulting from transient loss of muscle atonia normally present during this sleep stage, sometimes resulting in injuries to the patient and/or bed partners. The disorder is considered rare, with a prevalence of less than 1% in general population [2], but with much higher prevalence in those afflicted by neurodegenerative diseases known as synucleinopathies, including Parkinson’s disease (PD), Dementia with Lewy Bodies (DLB), and Multiple Systems Atrophy (MSA) [3].
Population-based studies estimated the prevalence of RBD symptoms in PD as 15% [4], while a meta-analysis including different study types estimated a 24% prevalence [5]. Characteristics associated with RBD in previous PD studies include male sex, older age, longer disease duration, and greater motor severity [6]. Attention to RBD has grown as it has become known for its link to neurodegenerative pathology [7] and as a prodromal marker of Parkinsonism. About 75% of those suffering from RBD develop PD or a Parkinsonism within about 10 years [8,9]. Furthermore, it has been suggested that PD presenting with RBD symptoms may constitute a distinct PD subtype, with features such as autonomic dysfunction, hallucinations, more axial symptoms, and faster cognitive decline [3,10].
Elucidating whether PD with RBD indeed constitutes a distinct phenotype with a unique etiology and disease course or is indistinguishable from idiopathic PD without RBD is crucial for upcoming neuroprotective trials and clinical care. To date, most studies on PD with RBD enrolled few subjects, selected participants from tertiary clinical settings, and/or relied on cross-sectional designs. Since prospective and population-based epidemiological studies may help us gain better insights into the role of RBD in PD, we investigated how self-reported RBD-like features manifesting in adult life are related to motor and cognitive symptom progression in a large population-based PD patient cohort.
Methods
Research Ethics
The UCLA Institutional Review Board approved all phases of the study protocol, and participants were informed of all procedures and their rights, and provided written informed consent.
Study design
PD patients enrolled in the Parkinson’s Environment and Genes Study (PEG), were identified in two independent waves (PEG1 & PEG2), from the population of three California counties. In the first wave, new onset PD cases (≤3 years from diagnosis) in the region were identified by contacting health professionals, and in the second wave, PD cases (≤5 years from diagnosis) were identified through a population-based PD registry. Eligible cases had lived in California five years at minimum and agreed to participate [11]. Baseline neurologic exams occurred between 2001 and 2007 (PEG1), and 2011–2017 (PEG2). PEG1 participants were seen up to four times during follow-up thus far, on average 3.2 years apart. For PEG2, there has only been one follow-up thus far, on average 3.3 years after baseline. Figure 1 shows flowchart for baseline recruitment and follow-ups.
Figure 1.
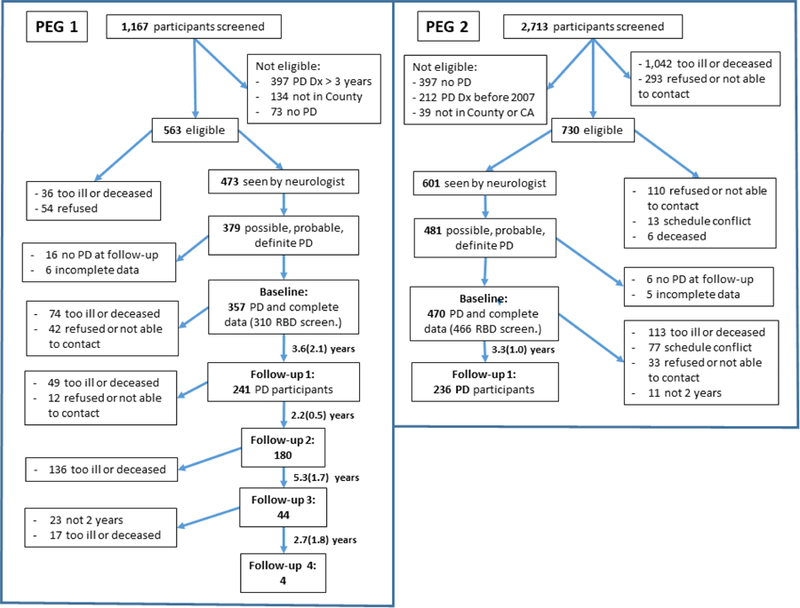
Flowchart of Parkinson’s Environment and Genes (PEG) Study, first and second cohorts: baseline and follow-ups
Data collected
At baseline and each follow-up, UCLA movement disorder specialists confirmed a diagnosis of idiopathic PD and evaluated motor features using the Unified Parkinson’s Disease Rating Scale (UPDRS parts I, III and IV) and Hoehn and Yahr staging (HY). At each time point, over 80% of the participants were evaluated in an ‘off’ (≥12 hours) medication state. For those ‘on’, we added a correction factor to their UPDRS-III total score, equal to the mean difference of ‘off’ and ‘on’ scores in all patients. We also used the average of the whole sample to impute missing items (mainly due to disability impeding evaluation of specific items such as ‘arise from chair’). We adopted the MDS version of the UPDRS-III in 2016, thus, scores derived from this scale were corrected by subtracting six points.
At baseline, participants were screened for RBD (Figure S1) answering four questions about nighttime sleep as an adult: 1- acting out dreams, 2- talking/yelling/screaming, 3- walking, 4- aggressive behaviors (1- definitely happened, 2- may have happened but not sure, 3- unlikely to have happened, 4- I don’t know if happened). We defined probable presence of RBD features (pRBD) based on questions # 1 and 4 only, as an answer of definitely happened to at least one with the other being at least may have happened, i.e. they were certain that they had acted out dreams or shown aggressive behaviors during sleep, and did not negate the possibility of the other action completely. Trained researcher assistants also collected data on demographics, lifestyle and environmental exposures, medical history, and applied standardized instruments: UPDRS patient questionnaire (parts IB+II), Mini-Mental State Examination (MMSE), and Geriatric Depression Scale (GDS) [11]. UPDRS-I and II were only administered at follow-up. From these interview data, we calculated Levodopa Equivalent Dose (LED), as previously described [12].
PD clinical progression was defined in terms of time to a motor and a non-motor outcome. A UPDRS-III score≥ 35 (higher score represents worse motor function) was chosen as a meaningful threshold for motor progression because it represents, on average [13], motor progression to a stage where patients start presenting some dependency for functional activities, equivalent to a HY stage 3 and to 60% in Schwab and England scale. For cognitive decline, a MMSE score≤ 24 (lower scores represent worse cognition) was chosen as the threshold, as previously done [12]. Time to event was defined as the interval in years from baseline (time=0) to the first time the event was recorded at a follow-up visit; those with the event at baseline were excluded from progression analyses.
Using items scores from UPDRS-III at baseline, we classified participants into motor subtypes of Postural Instability and Gait Dysfunction (PIGD), Tremor Dominant (TD), or Indeterminate (IND), as previously described [14]. Summing up specific items from UPDRS-III, we calculated subscores of bradykinesia, rigidity, tremor, axial [15], and PIGD features.
Statistical analysis
Analyses were conducted in statistical software package SAS (SAS Institute) version 9.4, and forest plot figure was generated in R (package forestplot). Cross-sectional comparisons of clinical and lifestyle characteristics between groups with and without pRBD were tested using t-tests or linear regressions for continuous characteristics, and chi-square or logistic regressions (ordinal logistic regression for more than two categories) for categorical.
We used Cox proportional hazards regression models to obtain hazard rate ratios and 95% confidence intervals (CI) comparing clinical progression between groups with and without pRBD. We assessed the proportional hazards assumption plotting product-limit survival curves for each outcome and time variables, stratified by pRBD, confirming that hazard rates were proportional between groups. All regression models were fitted by maximum likelihood methods.
We selected covariates for adjustment in regression models based on assumptions derived from previous knowledge and encoded using DAG (Directed Acyclic Graph) (Figure S2) methodology [16]. Baseline characteristics assumed to be confounders were: sex, age at PD diagnosis, PD duration, ethnicity (minority yes/no), baseline wave (PEG1/2), smoking in pack-years, and years of education (for cognition) and comorbidities (note: comorbidities (high blood pressure, diabetes type 2, anxiety, and depression) did not change estimates under these scenarios, and we did not include these to avoid generating spare data strata). The models were also stratified by motor subtypes.
To account for lack of information about outcomes on the 44% of participants not seen for follow-up, we used Inverse Probability of Censoring Weighting (IPCW) [16], generating weights conditional on presumed determinants of loss to follow-up (Supplemental Methods, and Table S4). In sensitivity analyses, we defined pRBD only by an answer ‘definitely happened’ to Question #1-acting out dreams (Table S4).
Results
Overall prevalence of pRBD in adult life was 21% (15% in PEG1 and 25% in PEG2), shown in Table 1. Fewer pRBD participants were females (24 vs. 40%) and more self-reported diagnoses of myocardial infarction, anxiety, and depression before baseline. Patients reporting pRBD had slightly lower mean MMSE scores, longer average disease duration, and a trend for a higher LED (p=0.06). Average time from baseline to first follow-up was 3.4 (SD= 1.6, min-max=0.7–15) years overall and by pRBD status.
Table 1.
Baseline distribution of PD patients’ characteristics: overall and by pRBD status.
| Characteristics | Total1 | With pRBD | Without pRBD | p-value: With vs. Without | |||
|---|---|---|---|---|---|---|---|
| N or Mean | (% or SD) | N or Mean | (% or SD) | N or Mean | (% or SD) | ||
| Study-related factors | |||||||
| Total number | 776 | (100) | 160 | (21) | 616 | (79) | |
| Study wave, PEG1 | 310 | (40) | 45 | (15) | 265 | (85) | 0.0013 |
| PEG2 | 466 | (60) | 115 | (25) | 351 | (75) | |
| Total with follow-up | 477 | (61) | 90 | (56) | 387 | (63) | |
| Average time, baseline to first follow-up, years2 | 3.4 | (1.6) | 3.4 | (1.5) | 3.4 | (1.6) | |
| Min – Max | 0.7 – 15.1 | 0.9 – 7.1 | 0.7 – 15.1 | ||||
| Demographics | |||||||
| Age at interview, years | 70.5 | (10.2) | 70.0 | (9.6) | 70.6 | (10.4) | 0.504 |
| Min – Max | 34 – 92 | 37 – 92 | 34 – 92 | ||||
| Sex, females | 283 | (37) | 38 | (23) | 245 | (40) | 0.00015 |
| Ethnicity, White | 588 | (76) | 123 | (76) | 467 | (76) | 0.695 |
| Latino | 134 | (17) | 30 | (19) | 105 | (17) | |
| Other | 54 | (7) | 9 | (6) | 45 | (7) | |
| Years of education | 13.7 | (4.5) | 14.3 | (3.7) | 13.6 | (4.7) | 0.084 |
| PD Clinical factors | |||||||
| Age at PD diagnosis, years | 67.4 | (10.7) | 66.4 | (9.8) | 67.7 | (9.8) | 0.046 |
| Min – Max | 23 – 89 | 35 – 88 | 23 – 89 | ||||
| PD duration, years | 3 | (2.5) | 3.5 | (3.1) | 3 | (2.5) | 0.046 |
| Min - Max | 0 – 16 | 0 – 16 | 0 – 15 | ||||
| Motor subtype, Tremor Dominant | 199 | (26) | 38 | (24) | 164 | (27) | 0.207 |
| PIGD | 471 | (61) | 97 | (60) | 376 | (61) | |
| Indeterminate | 106 | (14) | 27 | (17) | 79 | (13) | |
| PD Treatment-related factors | |||||||
| PD medication, any | 692 | (89) | 151 | (93) | 544 | (88) | 0.127 |
| LED, mg | 404 | (336) | 459 | (349) | 388 | (332) | 0.067 |
| Dyskinesia (n=424) | 78 | (19) | 20 | (19) | 58 | (18) | 0.987 |
| Medical factors (self-reported) | |||||||
| High Blood Pressure | 418 | (54) | 83 | (51) | 337 | (54) | 0.607 |
| Diabetes, type 2 | 150 | (19) | 35 | (22) | 116 | (19) | 0.507 |
| Cancer, any | 213 | (28) | 40 | (25) | 175 | (28) | 0.507 |
| Stroke | 74 | (10) | 15 | (9) | 60 | (10) | 0.807 |
| Heart attack | 73 | (9) | 20 | (12) | 53 | (9) | 0.307 |
| Traumatic Brain Injury | 85 | (11) | 20 | (12) | 66 | (11) | 0.707 |
| Anxiety | 194 | (25) | 57 | (35) | 138 | (22) | 0.0037 |
| Depression | 233 | (30) | 62 | (38) | 172 | (28) | 0.017 |
| Anxiety medication use, any | 141 | (18) | 37 | (23) | 104 | (17) | 0.037 |
| Depression medication use, any | 219 | (28) | 58 | (36) | 162 | (26) | 0.017 |
| Lifestyle factors | |||||||
| Smoker, never | 429 | (55) | 84 | (52) | 346 | (56) | 0.607 |
| Quit | 320 | (41) | 73 | (45) | 249 | (40) | |
| Current | 26 | (3) | 5 | (3) | 22 | (4) | |
| Smoking, pack-years | 9 | (19) | 9.1 | (17) | 9.1 | (17) | 0.996 |
| Physical activity levels, current | |||||||
| Very low | 493 | (65) | 108 | (67) | 388 | (64) | 0.207 |
| Low | 146 | (19) | 27 | (17) | 119 | (20) | |
| Moderate | 75 | (10) | 19 | (12) | 57 | (9) | |
| High | 48 | (6) | 7 | (4) | 41 | (7) | |
| BMI (n=557) | 27.5 | (5.4) | 27.6 | (5.5) | 27.6 | (5.5) | 0.826 |
| underweight (<18.5) | 174 | (31) | 37 | (31) | 137 | (31) | 0.507 |
| normal (18.5–24) | 17 | (3) | 3 | (3) | 14 | (3) | |
| overweight (25–29) | 205 | (37) | 44 | (38) | 161 | (37) | |
| obese (>29) | 161 | (29) | 32 | (28) | 129 | (29) | |
| Average sleep duration current, hours | 7.6 | (1.8) | 7.8 | (1.9) | 7.5 | (1.8) | 0.056 |
| Lifetime coffee consumption | |||||||
| Low | 179 | (26) | 28 | (19) | 152 | (28) | 0.097 |
| Medium | 367 | (53) | 87 | (59) | 283 | (51) | |
| High | 150 | (22) | 33 | (22) | 117 | (21) | |
| Alcohol use, never (n= 578) | 66 | (13) | 7 | (11) | 59 | (14) | 0.307 |
| Alcohol use, high lifetime consumption (n= 539) | 279 | (57) | 58 | (65) | 223 | (56) | 0.407 |
Total with RBD screening at baseline interview.
Average follow-up time in years from baseline to first follow-up point. The total average follow-up time for all 776 subjects, i.e. from baseline to last follow-up, was 4.8(1.6) (note: only the PEG 1 cohort had more than 1 follow-up exam).
p-values obtained from chi-square, testing equality of pRBD prevalence in PEG 1 vs. PEG 2.
p-values obtained from t-tests, testing equality of characteristic comparing patients with vs. without pRBD.
p-values obtained from chi-square, testing equality of characteristic comparing patients with vs. without pRBD.
p-value obtained from linear regression of characteristic on pRBD status, adjusted for sex and age at baseline interview.
p-value obtained from logistic regression of characteristic on pRBD status, adjusted for sex and age at baseline interview. Ordinal logistic regression was used for characteristics with more than two categories (motor subtype, physical activity, BMI, coffee consumption).
Of the 44% (362 out of 832) missing follow-up information, most had died or were severely debilitated at our last attempt of contact. Those without follow-up information had a similar prevalence of pRBD at baseline, but were older, had longer PD duration, exhibited a PIGD subtype, and had more comorbidities (Table S1).
Table 2 shows cross-sectional associations of pRBD with motor and non-motor outcomes. At baseline, motor signs (UPDRS-III total score and subscores, and HY≥3) were similar in both groups, while at first follow-up (average PD duration of 6.1±2.8 years), pRBD was associated with slightly higher bradykinesia and axial UPDRS-III subscores. MMSE scores were lower at both times for those with pRBD, while GDS scores were similar. At first follow-up, non-motor symptoms measured by UPDRS-I/II, were worse in pRBD, specifically, patients reported more hallucinations.
Table 2:
Baseline and follow-up motor and non-motor outcomes by baseline pRBD status.
| With pRBD | Without pRBD | Adjusted p-value2 | |||
|---|---|---|---|---|---|
| N or Mean1 | (% or 95% CI1) | N or Mean1 | (% or 95% CI1) | ||
| Baseline | |||||
| PD duration, years | 3.5 | (3.1) | 3.0 | (2.5) | |
| Motor (n=776) | |||||
| UPDRS-III, total | 22.7 | (19.8, 25.6) | 23.1 | (20.5, 25.7) | 0.90 |
| Tremor | 2.8 | (2.1, 3.4) | 3.0 | (2.4,3.6) | 0.23 |
| Rigidity | 4.9 | (4.2, 5.5) | 4.9 | (4.3, 5.5) | 0.90 |
| Bradykinesia | 1.3 | (1.0, 1.5) | 1.2 | (1.0, 1.4) | 0.25 |
| Axial | 5.3 | (4.4, 6.2) | 5.2 | (4.4, 6.1) | 0.80 |
| PIGD | 1.1 | (0.7, 1.5) | 1.2 | (0.8, 1.5) | 0.96 |
| HY≥ 3, yes | 25 | (16) | 99 | (16) | 0.92 |
| Non-motor (n=775) | |||||
| MMSE | 26.9 | (26.2, 27.6) | 27.2 | (26.6, 27.9) | 0.04 |
| GDS | 3.4 | (2.5, 4.2) | 3.2 | (2.4, 4.0) | 0.57 |
| First follow-up | |||||
| PD duration, years | 6.3 | (3.0) | 6.1 | (2.7) | |
| Motor (n=463) | |||||
| UPDRS-III, total | 23.4 | (18.6, 28.2) | 22.6 | (18.2, 27.0) | 0.49 |
| Tremor | 2.3 | (1.1, 3.4) | 3.0 | (2.0, 4.1) | 0.03 |
| Rigidity | 5.2 | (4.2, 6.3) | 5.1 | (4.1, 6.0) | 0.53 |
| Bradykinesia | 1.6 | (1.2, 2.0) | 1.4 | (1.1, 1.8) | 0.04 |
| Axial | 5.7 | (4.1, 7.3) | 5.2 | (3.8, 6.7) | 0.19 |
| PIGD | 1.1 | (0.4, 1.8) | 1.1 | (0.5, 1.8) | 0.73 |
| HY≥ 3, yes | 22 | (27) | 94 | (26) | 0.50 |
| Non-motor (n=477) | |||||
| MMSE | 27.1 | (26.0, 28.2) | 27.7 | (26.7, 28.7) | 0.02 |
| GDS | 3.6 | (2.3, 4.9) | 3.3 | (2.2, 4.5) | 0.44 |
| UPDRS-I3 | 9.3 | (6.9, 11.7) | 7.2 | (5.0, 7.2) | 0.005 |
| UPDRS-II4 | 10.1 | (6.9, 13.3) | 8.6 | (5.6, 11.5) | 0.11 |
| Autonomic symptoms score5 | 3.9 | (2.6, 5.2) | 3.2 | (2.0, 4.4) | 0.06 |
| Orthostatic hypotension symptoms, yes6 | 53 | (60) | 213 | (54) | 0.24 |
| Hallucinations, yes | 21 | (24) | 43 | (12) | 0.001 |
| UPDRS patient questionnaire | 16.4 | (11.9, 20.8) | 14.1 | (10.0, 18.2) | 0.11 |
Means and CI’s adjusted for sex and PD duration at baseline or at first follow-up. Numbers and percentages (for HY, orthostatic hypotension symptoms and hallucinations) are crude.
p-values obtained from linear (continuous) or logistic (binary) regressions of outcome on pRBD status, adjusted for: age at diagnosis, sex, PD duration at baseline or first follow-up, race, baseline wave (PEG1/2), and years of education for MMSE. For outcomes at first follow-up, baseline value was also included (except for UPDRS-I and II, because not available at baseline). Estimates and 95% CI’s are shown in online supplement, Table S3.
UPDRS-I items: cognitive impairment, hallucinations, depressed mood, anxious mood, apathy, features of dopamine dysregulation syndrome, sleep problems, daytime sleepiness, pain, urinary problems, constipation, lightheadedness, fatigue.
UPDRS-II items: speech, saliva/drooling, chewing/swallowing, eating tasks, dressing, hygiene, handwriting, hobbies, turning in bed, tremor, getting off car/chair/bed, walking/balance, freezing.
Autonomic symptoms items: urinary problems, constipation, lightheadedness, saliva/drooling, chewing/swallowing.
Answer yes to item: lightheadedness.
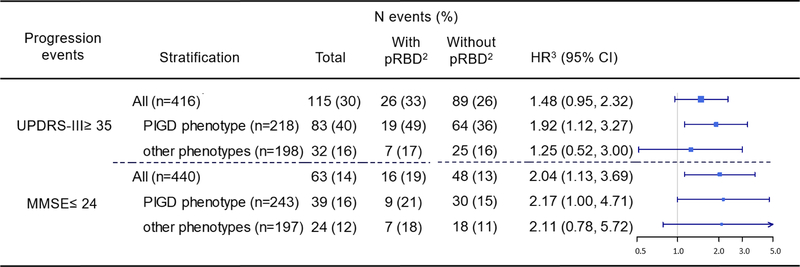
Of participants with at least one follow-up motor evaluation (n=416), a total of 115 (30%) developed the event UPDRS-III≥ 35 (Figure 2) and the incidence was higher in those with pRBD (33%). In Cox models adjusted for potential confounders, pRBD PD patients progressed faster to a UPDRS-III≥ 35 than those without pRBD (HR= 1.48), but the HR estimate was not formally statistically significant at alpha=0.05 (p=0.08, 95% CI= 0.95; 2.32). When stratifying by motor phenotypes, only among PIGD patients pRBD was a risk factor for faster progression to a UPDRS-III≥ 35 (HR= 1.92, 95% CI= 1.12; 3.27).
Figure 2.
Hazard rate ratios (HR) estimated for PD clinical progression events and pRBD.
1Total corresponds to those without the events (UPDRS-III ≥ 35 and MMSE ≤ 24) at baseline.
2Percentages refer to the total participants in each group (with and without pRBD).
3Adjusted for age at diagnosis, sex, PD duration at baseline, race (minority yes/no), pack-years of smoking, baseline cohort, and years of education for MMSE.
The group with pRBD also had a greater incidence for a MMSE≤ 24 during follow-up (19% compared to 13% in without pRBD). The hazard ratio for progression to this cognitive event for those with pRBD was twice that of those without (HR= 2.04, 95% CI= 1.13; 3.69); models stratified by motor phenotypes yielded similar size, but less precise estimates (Figure 2), that were not formally statistically significant for the non-PIGD phenotype stratum.
Using the alternative definition, prevalence of pRBD increased from 21 to 25%, and results remained similar (Table S4). Finally, accounting for bias due to loss to follow-up using IPCW, effect estimates were also similar to the ones obtained without (using conventional naïve CI’s).
Discussion
In this large community-based Parkinson’s disease study that followed new onset patients, RBD features in adult life were associated with faster cognitive decline, while there was only a trend observed towards a potentially faster motor symptoms progression among those with pRBD. Progression of motor dysfunction associated with pRBD was only faster among those who exhibited a PIGD motor subtype at baseline, while associations of pRBD and cognitive decline did not differ between subtypes. The average motor progression rate during follow-up in our cohort (1.9 points/year in UPDRS-III, Table S2) was similar to what has been reported (2.2 points/year) by a UK population-based study [17] of 132 patients with incident PD, followed for a similar average period (five years from PD diagnosis).
Prevalence of pRBD in our cohort is in the lower range of all estimates used in a recent meta-analysis (19 to 69%) [18] based on studies that recruited participants in select clinical settings rather than from communities. The higher prevalence of pRBD in our second (PEG2) compared to first patient enrolment wave (PEG1) might reflect the higher proportion of male participants enrolled in PEG2 (68% vs. 57%). Apart from being a chance finding, this may also reflect increased RBD awareness in more recent years, or other study participants’ characteristics that differed at baseline.
Only one previous longitudinal population-based study [4] has examined pRBD in PD, reporting on 231 Norwegian patients. Although that cohort had a much longer disease duration at baseline (on average 8.6 years for patients without pRBD and 11.1 for those with pRBD), compared with our population, its baseline prevalence of pRBD (15%) was equal to our first enrolment wave. Characteristics of participants with pRBD were also similar (i.e., more males, higher LED, longer PD duration, and similar frequency of dyskinesia). That study also found less tremor and lower overall UPDRS-III scores in participants with pRBD, but did not evaluate motor subtypes or UPDRS subscores, and it might also have been affected by selection for milder PD cases, due to the long average disease duration at baseline.
A faster progression of motor symptoms in PD with RBD has been noted previously in four smaller studies selecting participants from tertiary clinics [3,10,19,20]. In Canada, 36 PD patients underwent sleep laboratory evaluation [21]; those with RBD had less tremor, but disease severity or other motor manifestations were not different over time. A longitudinal French study followed 100 PD patients from a University Hospital for two years [20], and reported slightly higher UPDRS-III scores and on-medication axial subscores in pRBD affected patients at baseline and follow-up. In 61 newly diagnosed PD patients from a Neurology clinic in Portugal [19] followed for two years, pRBD was associated with PIGD subtype at baseline, and with worse motor symptoms over time. In our study, pRBD was not associated with UPDRS-III scores or motor subtypes at baseline, but our cohort had a much shorter PD duration. Thus, while pRBD may not be an indicator of worse motor symptoms early in the disease, among those with a PIGD subtype it may be a predictor of much faster motor decline, as suggested a decade ago [21].
Another study evaluating rate of motor symptom progression in PD in relation to RBD, recruited 113 participants from two movement disorders clinics in Canada, and followed 76 for an average of 4.5 years, performing exams in sleep laboratories both times [10]. Using cluster analysis, investigators identified three PD clinical subtypes; the one dominated by slowly progressing motor symptoms had the lowest prevalence of RBD (19%); another cluster featured a high (60%) pRBD prevalence and orthostatic hypotension at baseline, with intermediate motor progression. The third cluster exhibited the highest RBD prevalence (93%) combined with mild cognitive impairment in neuropsychiatric evaluations, orthostatic hypotension, axial motor subtype and the fastest motor progression. The clustering together of RBD features, faster motor progression, preponderance of axial (PIGD) subtypes, and/or cognitive progression, corroborate our findings. However, our results suggest that while presence of pRBD is associated with accelerated cognitive decline in all patients, its impact on motor progression seems to be restricted to PD with PIGD motor features.
In accordance with some other previous studies [10,19,22], we found pRBD patients had generally worse non-motor symptoms at follow-up, with higher scores in UPDRS-I and in autonomic dysfunction symptoms items, especially higher frequencies of orthostatic hypotension symptoms and hallucinations. Implications of RBD for depressive symptoms in PD have not yet been investigated, but antidepressants may cause RBD-like symptoms. In our cohort, GDS scores did not differ significantly between groups at both times, but the pRBD group reported more depression diagnoses and antidepressant medication use. When we adjusted models for these factors, however, estimates remained unchanged.
Our findings that pRBD accelerates time to reach MMSE≤ 24 corroborate those of several previous studies that found increased risk of dementia or cognitive decline with RBD [3,23–25]. We report this finding for the first time in a cohort of PD patients sampled from an identifiable source population. In this cohort, we also obtained a similar annual rate difference (Table S2: Adjusted MD, with vs. without pRBD) of MMSE points decline as that reported from a multi-site international cohort of 423 PD patients [26], where pRBD patients declined on average 0.3 points in MOCA scores more per year than no-pRBD.
No experimental models of RBD in PD are available thus far [27], but the neurodegenerative nature of RBD is established. In RBD, the brain stem circuitry of the subcoeruleus nucleus and the ventromedial medulla, which promote normal motor activity suppression during REM sleep, are damaged [2,7]. While multiple neurotransmitter systems innervate these structures, cholinergic neurons play a central role. These are essential for maintenance of cognition, as is REM sleep in general, linking RBD with cognitive impairment and dementia. Additionally, damage to brain stem structures with diverse innervation, manifesting clinically as PIGD symptoms may link PIGD and RBD (since axial symptoms result mainly from non-dopaminergic impairment). In our cohort, pRBD was not associated with motor subtype at baseline, but pRBD was an important marker for faster clinical motor progression in those with PIGD symptoms at baseline. Future studies expanding our understanding of this phenomenon are needed.
Using questionnaires to screen for RBD provides less specificity and sensitivity than objectively confirming a lack of atonia in polysomnography exams [28]. While questionnaires may introduce bias due to measurement error, they are the only feasible way to assess RBD in large populations. Longer 13-question screening questionnaires than ours, such as the RBD Screening Questionnaire (RBDSQ) and RBD-Hong Kong (RBD-HK) are available, but 94% sensitivity and 87% specificity were reached for a single question, that asks about ‘acting out your dreams while asleep’ [28]. Our pRBD definition aimed to increase specificity, but in sensitivity analyses with an alternative definition, results for motor and cognitive progression were similar. Furthermore, even unspecific motor behaviors or vocalizations during REM sleep have been found to be early indicators of PD [29].
Like most longitudinal studies, we lost patients during follow-up due to death or disabilities, but we used Cox models to account for censoring, in addition to IPCW to account for potential selection bias due to such censoring, resulting in estimates mostly unchanged. It is not clear whether the rate of progression of motor and cognitive symptoms in PD is indeed linear [30] as assumed in most epidemiological studies of progression. Thus, to avoid this assumption, our main results are obtained from Cox models, which only assume that differences in hazard rates are multiplicative.
Ours is a large population-based study with movement disorder specialist confirmed PD diagnoses and motor assessments. We present evidence that pRBD features may be an early clinical marker of faster cognitive decline and progression of motor symptoms in PD, the latter particularly for patients with marked PIGD symptoms early in the disease. RBD-features may be a simple and useful screening for treatment trials and in clinical practice to identify those at risk for faster progression, who may benefit from pharmacological (changes in drug schemes) and non-pharmacological (including physical activity and prevention of falls) interventions.
Supplementary Material
Highlights.
This is one of the largest population-based cohort studies in Parkinson’s disease (PD).
Of 776 patients screened for RBD at baseline, 477 were followed on average 3 years later.
Probable REM Sleep Behavior Disorder (pRBD) prevalence at baseline was 21%.
PD patients with pRBD progressed almost twice as fast as those without to a MMSE score≤ 24.
In those with PIGD motor subtype, pRBD was associated an 80% faster progression to a UPDRS-III≥ 35.
Acknowledgements
We acknowledge the contributions of Dr Yvette Bordelon for her work as a Movement Disorders specialist conducting clinical assessments for the study. We would also like to thank all patients with Parkinson’s disease and their caregivers, and all the PEG Study staff, for their time and their efforts that made this research possible.
Funding Sources
This research study has been funded by the National Institute of Environmental Health Sciences of the National Institutes of Health (grants numbers: R01 ES010544, U54-ES012078, P01-ES016732, P50-NS038367, and initial pilot funding P30-ES07048), and by the American Parkinson’s disease Association (grant number 20161386).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None.
References
- [1].Thorpy MJ, Classification of Sleep Disorders, Neurotherapeutics 9 (2012) 687–701. doi: 10.1007/s13311-012-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fraigne JJ, Torontali ZA, Snow MB, Peever JH, REM sleep at its core - Circuits, neurotransmitters, and pathophysiology, Front. Neurol 6 (2015) 1–9. doi: 10.3389/fneur.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Postuma RB, Bertrand J-A, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, Panisset M, Gagnon J-F, Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study., Mov. Disord 27 (2012) 720–6. doi: 10.1002/mds.24939. [DOI] [PubMed] [Google Scholar]
- [4].Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP, Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson’s disease over time., J. Neurol. Neurosurg. Psychiatry 79 (2008) 387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- [5].Zhang J, Xu C-Y, Liu J, Meta-analysis on the prevalence of REM sleep behavior disorder symptoms in Parkinson’s disease, BMC Neurol 17 (2017) 23. doi: 10.1186/s12883-017-0795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhu R, Xie C, Hu P, Wang K, Clinical variations in Parkinson’s disease patients with or without REM sleep behaviour disorder: a meta-analysis, Sci. Rep 7 (2017) 40779. doi: 10.1038/srep40779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKenna D, Peever J, Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder, Mov. Disord 00 (2017) 1–9. doi: 10.1002/mds.27003. [DOI] [PubMed] [Google Scholar]
- [8].Jozwiak N, Postuma RB, Montplaisir J, Latreille V, Panisset M, Chouinard S, Gagnon J, REM sleep behavior disorder and cognitive impairment in Parkinson’s disease, (2017). [DOI] [PMC free article] [PubMed]
- [9].Iranzo A, Fernández-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, Gelpi E, Vilaseca I, Sánchez-Valle R, Lladó A, Gaig C, Santamaría J, Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: Study in 174 patients, PLoS One 9 (2014). doi: 10.1371/journal.pone.0089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fereshtehnejad S-M, Romenets SR, Anang JBM, Latreille V, Gagnon J-F, Postuma RB, New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression, JAMA Neurol 72 (2015) 863. doi: 10.1001/jamaneurol.2015.0703. [DOI] [PubMed] [Google Scholar]
- [11].Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D, Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease., Neurology 73 (2009) 1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Keener AM, Paul KC, Folle A, Bronstein JM, Ritz B, Cognitive impairment and mortality in a population-based Parkinson’s disease cohort, J. Parkinsons. Dis 8 (2018) 353–362. doi: 10.3233/JPD-171257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shulman LM, The clinically important difference on the unified Parkinson’s disease rating scale, Arch. Neurol 67 (2010) 64–70. 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- [14].Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC, How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale, Mov. Disord 28 (2013) 668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- [15].Gigante AF, Bruno G, Iliceto G, Guido M, Liuzzi D, Mancino PV, De Caro MF, Livrea P, Defazio G, Action tremor in Parkinson’s disease: Frequency and relationship to motor and non-motor signs, Eur. J. Neurol 22 (2015) 223–228. doi: 10.1111/ene.12583. [DOI] [PubMed] [Google Scholar]
- [16].Hernán MA, Hernández-Díaz S, Robins JM, A structural approach to selection bias, Epidemiology 15 (2004) 615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- [17].Evans JR, Mason SL, Williams-Gray CH, Foltynie T, Brayne C, Robbins TW, a Barker R, The natural history of treated Parkinson’s disease in an incident, community based cohort., J. Neurol. Neurosurg. Psychiatry 82 (2011) 1112–1118. doi: 10.1136/jnnp.2011.240366. [DOI] [PubMed] [Google Scholar]
- [18].Zhang X, Sun X, Wang J, Tang L, Xie A, Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: a meta and meta-regression analysis, Neurol. Sci (2016) 1–8. doi: 10.1007/s10072-016-2744-1. [DOI] [PubMed] [Google Scholar]
- [19].Bugalho P, Viana-Baptista M, REM sleep behavior disorder and motor dysfunction in Parkinson’s disease - A longitudinal study, Park. Relat. Disord 19 (2013) 1084–1087. doi: 10.1016/j.parkreldis.2013.07.017. [DOI] [PubMed] [Google Scholar]
- [20].Lavault S, Leu-Semenescu S, Tezenas Du Montcel S, Cochen De Cock V, Vidailhet M, Arnulf I, Does clinical rapid eye movement behavior disorder predict worse outcomes in Parkinson’s disease?, J. Neurol 257 (2010) 1154–1159. doi: 10.1007/s00415-010-5482-y. [DOI] [PubMed] [Google Scholar]
- [21].Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J, REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features., J. Neurol. Neurosurg. Psychiatry 79 (2008) 1117–21. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- [22].Postuma RB, Gagnon JF, Vendette M, Montplaisir JY, Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease, Brain 132 (2009) 3298–3307. doi: 10.1093/brain/awp244. [DOI] [PubMed] [Google Scholar]
- [23].Romenets SR, Gagnon J-F, Latreille V, Panniset M, Chouinard S, Montplaisir J, Postuma RB, Rapid eye movement sleep behavior disorder and subtypes of Parkinson’s disease, Mov. Disord 27 (2012) 996–1003. doi: 10.1002/mds.25086. [DOI] [PubMed] [Google Scholar]
- [24].Nomura T, Inoue Y, Kagimura T, Nakashima K, Clinical significance of REM sleep behavior disorder in Parkinson’s disease, Sleep Med 14 (2013) 131–135. doi: 10.1016/j.sleep.2012.10.011. [DOI] [PubMed] [Google Scholar]
- [25].Sinforiani E, Pacchetti C, Zangaglia R, Pasotti C, Manni R, Nappi G, REM behavior disorder, hallucinations and cognitive impairment in Parkinson’s disease: A two-year follow up, Mov. Disord 23 (2008) 1441–1445. doi: 10.1002/mds.22126. [DOI] [PubMed] [Google Scholar]
- [26].Chahine LM, Xie SX, Simuni T, Tran B, Postuma R, Amara A, Oertel WH, Iranzo A, Scordia C, Fullard M, Linder C, Purri R, Darin A, Rennert L, Videnovic A, Del Riva P, Weintraub D, Longitudinal changes in cognition in early Parkinson’s disease patients with REM sleep behavior disorder, Parkinsonism Relat. Disord 27 (2016) 6–10. doi: 10.1016/j.parkreldis.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fifel K, Piggins H, Deboer T, Modeling sleep alterations in Parkinson’s disease: How close are we to valid translational animal models?, Sleep Med. Rev 25 (2016) 95–111. doi: 10.1016/j.smrv.2015.02.005. [DOI] [PubMed] [Google Scholar]
- [28].Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, Oertel W, yo-el Ju A Single-Question Screen for REM Sleep Behavior Disorder: A Multicenter Validation Study, 27 (2012) 913–916. doi: 10.1002/mds.25037.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sixel-Döring F, Zimmermann J, Wegener A, Mollenhauer B, Trenkwalder C, The Evolution of REM Sleep Behavior Disorder in Early Parkinson Disease., Sleep (2016) 1737–1742. doi: 10.5665/sleep.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kuramoto L, Cragg J, Nandhagopal R, Mak E, Sossi V, de la Fuente-Fernandez R, Stoessl AJ, Schulzer M, The Nature of Progression in Parkinson’s Disease: An Application of Non-Linear, Multivariate, Longitudinal Random Effects Modelling, PLoS One 8 (2013). doi: 10.1371/journal.pone.0076595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.