Abstract
Introduction
When using free-water diffusion imaging or positron emission tomography (PET), it is established that substania nigra microstructure and presynaptic dopamine activity are impaired in early PD. It is not well understood if these two forms of degeneration are redundant, or if they each provide a unique contribution to the clinical motor and cognitive symptoms.
Methods
A total of 129 PD and 75 control individuals underwent motor and cognitive evaluations, and in vivo [11C]dihydrotetrabenazine (DTBZ) monoaminergic brain PET imaging and diffusion imaging. Correlations between free-water in the substantia nigra and striatal PET measures were analyzed. Unbiased multiple regression using a backward elimination method was performed between clinical severity and all imaging measures.
Results
Inverse correlations were found between free-water in posterior substantia nigra and DTBZ binding in putamen and caudate. Multiple regression revealed that increased free-water in the posterior substantia nigra, decreased DTBZ binding in putamen, and age were predictors of higher Hoehn and Yahr stage, MDS-UPDRS III scores, and posture and gait sub-scores. Increased posterior substantia nigra free-water alone was associated tremor scores. Free-water in caudate and putamen did not predict measures of motor performance. Decreased DTBZ binding in caudate, increased free-water in caudate and posterior substantia nigra were associated with higher dementia ratings.
Conclusions
These findings suggest that free-water in the posterior substantia nigra and presynaptic dopamine imaging in striatum are uniquely associated with the clinical symptoms of PD, indicating that each imaging modality may be measuring a unique mechanism relevant to nigrostriatal degeneration.
Keywords: Parkinson’s disease, basal ganglia, substantia nigra, Diffusion magnetic resonance imaging, PET, multimodal imaging
1. Introduction
Parkinson’s disease (PD) is the second most common age related neurodegenerative disease and its prevalence increases markedly with aging [1]. PD is characterized by progressive degeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNpc), typically observed in the ventrolateral tier [2]. The shortage of dopaminergic input to striatum along the nigrostriatal pathway is responsible for most of the classical motor manifestations in PD [2]. It is not well established if the neuroimaging modalities that measure the SN and striatum reflect redundant or unique mechanisms when related to clinical severity. If they are unique predictors then it would seem important that assaying the SN and striatum may benefit in tracking progression, and evaluating treatment interventions of PD.
Diffusion magnetic resonance imaging (dMRI) maps the diffusion properties of water and brings promise for identifying progression markers of the degeneration within the SN in PD [3]. Recently, a novel dMRI analysis technique using a bi-tensor model was developed to estimate the fractional volume of free-water within a voxel [4], which is expected to increase in neurodegenerative diseases [5]. Previous studies have found that free-water was elevated in the posterior SN (pSN) for PD when compared with healthy controls in single- and multi-site cohorts [6]. Furthermore, longitudinal studies across multiple sites and scanners revealed that free-water in pSN increased over a one year period of time in PD but not in controls, and free-water in pSN continued to increase over four years in PD, and these patterns were evident across sites [3, 7].
Since free-water provides an indirect measure of dopaminergic degeneration within the SN, we hypothesize the changes in nigral free-water relate to disease-related changes in the striatal dopaminergic nerve terminal density. Previous studies reported mixed results between nigral dMRI measures (focusing on fractional anisotropy, FA) and integrity of nigrostriatal dopaminergic nerve terminals evaluated with molecular imaging [8–10]. However, the reliability and utility of nigral FA as a biomarker of PD was questioned in recent meta-analyses [11, 12]. In addition, the radiotracers applied in previous studies include dopamine transporter ligands with single photon emission computed tomography (SPECT) [9, 10] and fluorodopa with positron emission tomography (PET) [8]. These methods may be affected by compensatory responses and dopaminergic treatments [13, 14]. Furthermore, fluorodopa uptake reflects not only the decarboxylation of fluorodopa to fluorodopamine, but also the egress of trapped fluorodopamine from synaptic vesicles, and this can be used to estimate dopamine turnover, which increases with disease severity [15]. The various methodologies may explain the different results. Another approach to determining the integrity of presynaptic dopaminergic terminal is to study vesicular monoamine transporter type 2 (VMAT2) binding, which correlates with striatal dopamine and fiber density, and as some studies suggest, it may be less susceptible to regulation by dopaminergic treatment[16]. To our knowledge, no study has explored the relation between striatal VMAT2 binding and nigral free-water imaging in PD.
In the current study, we assessed the relation of nigral free-water to striatal VMAT2 binding, and the association of these measures across the basal ganglia with motor symptoms and cognition function in PD. We hypothesized that nigral free-water correlates inversely with striatal VMAT2 binding, and that combination of these two imaging markers across the basal ganglia improves the prediction of motor and cognitive performance in PD. We expect that both measures are predictors because they represent different mechanisms in the nigrostriatal pathway, and because it has been suggested that the loss of melanized nigral neurons lags the loss of dopamine markers [17].
2. Methods
2.1. Participants
Participants in this cross-sectional study included 129 subjects with PD and 75 control subjects (Table 1). Subjects were recruited and tested at a single site, from October 2008 to June 2015. All PD subjects were assessed by board-certified neurologists who were experienced in movement disorders and fulfilled the UK Brain Bank Criteria for idiopathic PD [18]. The patients also underwent brain imaging to exclude atypical parkinsonian syndrome or vascular parkinsonism. Drug-induced parkinsonism was excluded based on the clinical history. None of the control subjects reported a history of neuropsychiatric or neurological diseases. Written informed consent was obtained from all subjects and approved by the local Institutional Review Board.
Table 1.
Demographic and Clinical data
| Clinical variables | PD | Controls | p |
|---|---|---|---|
| Sample size | 129 | 75 | - |
| Sex, Females/Males | 33/96 | 42/33 | <0.001* |
| Age, years | 65.38(7.84) | 65.97(11.55) | 0.277 |
| Age of onset, years | 59.35(8.58) | - | - |
| Disease duration, years | 6.03(4.21) | - | - |
| Education, years | 15.31(2.84) | 15.99(2.32) | 0.154 |
| Total LEDD | 699.35(541.17) | - | - |
| H&Y | 2.44(0.59) | - | - |
| MDS-UPDRS III | 32.60(14.27) | - | - |
| MDS-UPDRS Tremor | 6.89(5.19) | - | - |
| MDS-UPDRS posture and gait | 3.32(3.19) | - | - |
| MDS-UPDRS Bradykinesia | 16.81(8.45) | - | - |
| DRS total score | 139.41(4.63) | 140.92(2.99) | 0.042* |
| Attention | 36.28(1.33) | 36.57(0.72) | 0.181 |
| Initiation/Perseveration | 36.12(2.05) | 36.41(1.43) | 0.206 |
| Construction | 5.98(0.12) | 6.00(0.00) | 0.280 |
| Conceptualization | 37.26(1.78) | 37.68(1.21) | 0.252 |
| Memory | 23.77(1.89) | 24.25(1.48) | 0.023* |
Abbreviations: LEDD = levodopa equivalent daily dose; H&Y = Hoehn and Yahr stage; MDS-UPDRS III = the motor section of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale; MoCa = Montreal Cognitive Assessment; DRS = Dementia Rating Scale.
statistically significant difference.
Data were numbers or mean (SD).
2.2. Clinical assessment
Patients were assessed with the motor section of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III) in the off state, Hoehn and Yahr (H&Y) staging in the off state, Dementia Rating Scale (DRS) in the on state. MDS-UPDRS items for tremor dominant, bradykinesia, and posture and gait designations were rated to calculate tremor scores (sum of items 2.10, 3.15–18), bradykinesia scores (sum of items 3.2, 3.4–9, 3.14), and posture and gait scores (sum of items 2.12–13, 3.10–12) [19]. Daily dopaminergic medications were standardized into a levodopa equivalent daily dose (LEDD).
2.3. [11C]DTBZ PET acquisition and Preprocessing
All subjects underwent brain [11C]DTBZ VMAT2 PET imaging. Subjects receiving dopaminergic medication were imaged with [11C]DTBZ PET in the morning after dopaminergic medication had been withheld overnight. The radiosynthesis procedure, radiochemical purity, and scanning methods have been described in detail previously[20]. All PET imaging frames were spatially coregistered within subjects with a rigid body transformation to reduce the effects of subject motion during the imaging session. These motion corrected PET frames were spatially coregistered to the T1-weighted MRI scan using SPM8 software (Welcome Trust Center for Neuroimaging, London, UK). Interactive Data Language image analysis software (Research Systems, Inc., Boulder, CO) was used to manually trace regions of interest (ROIs) on the MRI scans to include the striatum of each hemisphere and neocortex. The striatal ROIs were defined bilaterally on the caudate nucleus and the putamen as previously described[20]. Neocortical ROI definition used semi-automated thresholding delineating the neocortical gray matter signal on the MRI images.
For each ROI, [11C]DTBZ distribution volume ratios (DVR), a measure of binding, was obtained using the Logan plot graphical analysis method with time activity curves for each ROI as the input function and the total neocortex ROI as the reference tissue (a reference region overall low in VMAT2 binding sites). The mean DVRs for putamen and caudate were derived by averaging the corresponding bilateral regions, respectively.
2.4. Diffusion MRI Acquisition and Preprocessing
Diffusion MRI images were collected on a 3T Philips scanner, using the following parameters: TR: 8044 ms, TE: 67 ms, flip angle: 90º, resolution: 1.75 mm isotropic, 15 non-collinear diffusion directions, b-value of 800 s/mm2 and one with a b-value of 0 s/mm2. FSL (fsl.fmrib.ox.ac.uk) was used for preprocessing all diffusion MRI data. The diffusion data were first corrected for eddy currents, then for head motion using a 3D affine registration, after which the brain was extracted. The motion and eddy current corrected volume was then used as input in two different procedures: (1) DTIFIT to calculate FA maps, and (2) custom written MATLAB (R2013a, The Mathworks, Natick, MA, USA) code [4] to calculate free-water and free-water corrected FA maps (FAT), which was consistent with prior work [4, 6]. To obtain a standardized space representation of the free-water and FAT maps, each map was registered to its respective template in standard space by a nonlinear warp using the Advanced Normalization Tools (ANTs) platform. FW and FAT templates were created from the average signal across a large cohort of aged control subjects available in our laboratory.
ROIs of the bilateral anterior SN (aSN), pSN, caudate nucleus, putamen, and globus pallidus were drawn in MNI space on one template image blind to all free-water data (Figure 1A), and the ROIs were used to extract free-water values from each subject, as previously reported [3]. The procedure was fully automated. The mean free-water and FAT for each region was calculated bilaterally. To be certain that the MNI space ROIs were accurately quantifying free-water and FAT, we compared free-water and FAT obtained in the MNI space with free-water and FAT obtained using hand drawn regions in patient space in 104 subjects using a previously published dataset [7, 21] from the University of Florida. Values were compared using intra-class correlation coefficients (ICC), and the ICC for the aSN, pSN, caudate nucleus, putamen and globus pallidus were 0.87, 0.92, 0.95, 0.92 and 0.95, respectively.
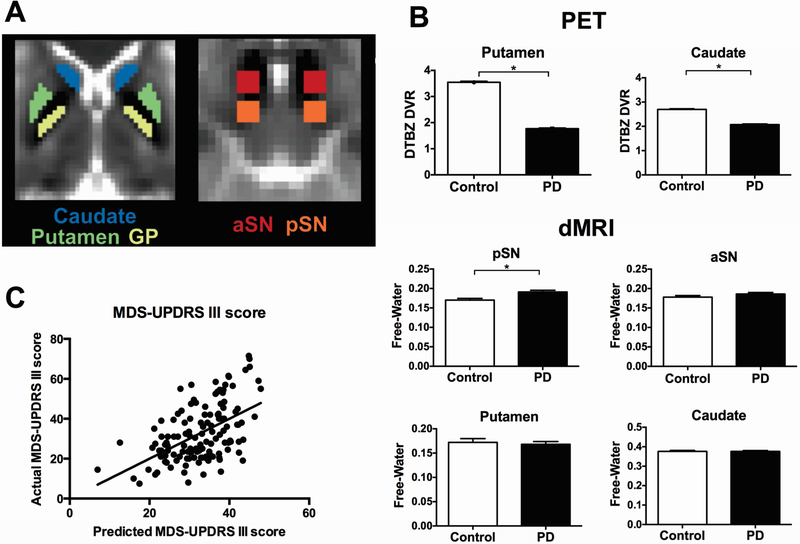
Figure 1.
A. Regions of interest for Diffusion MRI. The regions used in this study for diffusion MRI are shown for caudate, putamen, globus pallidus (GP), anterior substantia nigra (aSN), and posterior substantia nigra (pSN). Each region is shown in MNI space on a B0 image. B. Group comparison of imaging measures between Parkinson’s disease group and controls. Mean values for DTBZ DVR in putamen, DTBZ DVR in caudate, free-water in pSN, free-water in aSN, free-water in putamen, and free-water in caudate were shown for controls and PD patients. Each bar represented the group mean and errors bars represented ± standard error of the mean. *Statistically significant between-group differences. C. Multiple regression to associate imaging measures with clinical features in patients with Parkinson’s disease. The predicted versus actual plots for MDS-UPDRS III score is shown. The significant predictors for MDS-UPDRS III score in the regression model were listed on the right. DTBZ DVR = [11C]dihydrotetrabenazine distribution volume ratio; MDS-UPDRS III = the motor section of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale; DTBZ DVR = [11C]dihydrotetrabenazine distribution volume ratio.
2.5. Statistical analysis
Differences between groups in terms of demographic and clinical variables were performed by the Pearson Chi-square test, independent samples t-test or a Mann-Whitney U-test, where appropriate. Imaging data were compared between groups using analysis of covariance (ANCOVA) with sex as covariate. Correction via false discovery rate (FDR) was undertaken, and partial eta squared was reported as a measure of effect size. Pearson correlations were performed between striatal DTBZ DVRs and free-water values in aSN and pSN in all subjects and PD subjects respectively. To explore the relationship between motor symptoms, H&Y stage and striatal DTBZ DVRs and free-water in striatum and SN, we performed multiple regression analyses using the backward elimination method. The dependent variables were H&Y stage, MDS-UPDSR part III motor score, posture and gait score, tremor score, bradykinesia score, respectively, while the predictor variables were putamen DTBZ DVR, caudate DTBZ DVR, free-water in putamen, free-water in caudate, free-water in aSN, free-water in pSN, age and sex. Only free-water (and not FAT) was used in the regression analysis to simply the model, and because FAT has not proven different between PD and controls when corrected for free-water [21]. Measures including putamen DTBZ DVR, caudate DTBZ DVR, free-water in putamen, free-water in caudate, free-water in aSN and free-water in pSN, sex, age and education years were used also as independent variables in multiple regression analysis to determine which measures were best associated with DRS scores in PD subjects. Predictor variables were removed based on F probability>0.1. Multicollinearity in the resulting models was quantified using the variance inflation factor (VIF). Variables with VIF>5 were removed. Statistical significance was at p<0.05. Analyses were performed using IBM SPSS Statistics 22 (SPSS, Inc, Chicago, IL).
3. RESULTS
3.1. Demographic and clinical data
No significant group differences were found in age and education years. There were more men than women in the PD group compared with control group. As expected, the PD group had lower DRS scores compared with controls (Table 1).
3.2. Group differences in DTBZ DVR and free-water
Compared with controls, PD group had reduced DTBZ DVRs in putamen and caudate, and increased free-water in the pSN (Figure 1B). There were no significant group differences in bilateral mean free-water values in aSN, putamen, caudate and globus pallidus (Table 2 and Figure 1B). There were no differences in FAT between PD and control groups (Table 2).
Table 2.
Imaging data
| Imaging parameters | PD | Controls | PFDR corrected | Partial eta squared | |
|---|---|---|---|---|---|
| DTBZ DVR | Putamen | 1.768(0.261) | 3.545(0.327) | <0.001* | 0.890 |
| Caudate | 2.071(0.310) | 2.697(0.256) | <0.001* | 0.474 | |
| Free Water | pSN | 0.182(0.061) | 0.162(0.041) | 0.040* | 0.035 |
| aSN | 0.159(0.047) | 0.146(0.042) | 0.528 | 0.003 | |
| Putamen | 0.171(0.079) | 0.170(0.075) | 0.528 | 0.002 | |
| Caudate | 0.252(0.109) | 0.258(0.099) | 0.528 | 0.002 | |
| Globus pallidus | 0.204(0.111) | 0.182(0.075) | 0.477 | 0.008 | |
| FAT | pSN | 0.565(0.043) | 0.558(0.037) | 0.702 | 0.002 |
| aSN | 0.512(0.046) | 0.511(0.039) | 0.702 | 0.001 | |
| Putamen | 0.209(0.055) | 0.190(0.034) | 0.268 | 0.013 | |
| Caudate | 0.248(0.043) | 0.242(0.032) | 0.702 | 0.001 | |
| Globus pallidus | 0.333(0.061) | 0.313(0.032) | 0.268 | 0.017 | |
Abbreviation: DTBZ DVR = [11C]dihydrotetrabenazine distribution volume ratio; FAT = free-water corrected fractional anisotropy; pSN = posterior substantia nigra; aSN = anterior substantia nigra.
statistically significant difference.
Data were presented as mean (SD).
To estimate head motion during the diffusion imaging collection, we calculated the mean displacement between volumes in the diffusion MRI scan for each subject. We conducted a between-group t-test and found there were no significant differences in mean displacement [t(202)=1.631, p=0.104].
3.3. Correlation between free-water and DTBZ DVR
Significant inverse correlations were found between free-water in the pSN and DTBZ DVR in putamen (r =−0.230, p=0.001), and DTBZ DVR in caudate (r=−0.295, p<0.001) across all subjects. The correlation between free-water in aSN and DTBZ DVR in caudate was not significant (r=−0.128, p=0.068), and for putamen DTBZ DVR the correlation was not significant (r=−0.093, p=0.188). When testing these correlations in PD subjects, only an inverse correlation between caudate DTBZ DVR and pSN free-water was found (r=−0.213, p=0.016).
3.4. Associating clinical scores with DTBZ DVR and free-water using multiple regressions
Backward stepwise regression analysis produced a significant model, which associates MDS-UPDRS part III motor score with putamen DTBZ DVR, free-water in pSN, sex, and age (Table 3). Figure 1C shows the actual MDS-UPDRS part III motor scores versus predicted MDS-UPDRS part III motor scores. The model predicting posture and gait scores was significant with predictors DTBZ DVR in putamen, free-water in the pSN and age (Table 3). In a separate model testing imaging measures and MDS-UPDRS bradykinesia score, the model was significant, with predictors including DTBZ DVR in putamen, free-water in the aSN, and age (Table 3). In another model testing imaging measures and tremor scores was also significant, though the percent variance accounted for was low. Free-water in the pSN was the only significant tremor predictor (Table 3). The model predicting H&Y stage was significant with predictors including DTBZ DVR in putamen, free-water in the pSN, and age (Table 3).
Table 3.
Results of backward stepwise linear regression analyses for predictors in H&Y stage, MDS-UPDRS posture and gait score, MDS-UPDRS bradykinesia score, MDS-UPDRS tremor score and DRS score in PD subjects.
| Dependent Variable | Radj2 | F | p | Maximum VIF | Predictors selected by the model | standard. beta | t | P |
|---|---|---|---|---|---|---|---|---|
| H&Y | 23.9% | 14.371 | <0.001 | 1.118 | Putamen DTBZ DVR | −0.370 | −4.767 | <0.001 |
| pSN Free-Water | 0.134 | 1.655 | 0.095 | |||||
| Age | 0.304 | 3.749 | <0.001 | |||||
| Part III motor | 25.3% | 11.827 | <0.001 | 1.148 | Putamen DTBZ DVR | −0.395 | 5.127 | <0.001 |
| pSN Free-Water | 0.189 | 2.337 | 0.021 | |||||
| Age | 0.178 | 2.178 | 0.031 | |||||
| Sex | −0.135 | −1.731 | 0.086 | |||||
| Posture and gait | 24.6% | 14.903 | <0.001 | 1.118 | Putamen DTBZ DVR | −0.311 | −.023 | <0.001 |
| pSN Free-Water | 0.294 | 3.643 | <0.001 | |||||
| Age | 0.205 | 2.542 | 0.012 | |||||
| Bradykinesia | 22.5% | 13.419 | <0.001 | 1.008 | Putamen DTBZ DVR | −0.445 | −5.709 | <0.001 |
| aSN Free-water | 0.109 | 1.399 | 0.164 | |||||
| Age | 0.211 | 2.699 | 0.008 | |||||
| Tremor | 5.1% | 7.838 | 0.006 | pSN Free-water | 0.241 | 2.800 | 0.006 | |
| DRS | 23.1% | 10.622 | <0.001 | 1.118 | Caudate DTBZ DVR | 0.204 | 2.487 | 0.014 |
| Caudate Free-water | −0.256 | −3.139 | 0.002 | |||||
| pSN Free-water | −0.170 | −2.121 | 0.036 | |||||
| Education | 0.250 | 3.215 | 0.002 | |||||
Abbreviation: MDS-UPDRS = Movement Disorder Society Unified Parkinson’s Disease Rating Scale; H&Y = Hoehn and Yahr stage; DRS = Dementia Rating Scale; DTBZ DVR = [11C]dihydrotetrabenazine distribution volume ratio; pSN = posterior substantia nigra; aSN = anterior substantia nigra; VIF = variance inflation factor.
A multiple regression model explored the association between DRS total scores with DTBZ DVR and free-water. The model was significant. Included predictors were DTBZ DVR in caudate, free-water in caudate, free-water in the pSN, and education (Table 3).
4. Discussion
The current investigation confirmed our hypothesis and demonstrated an inverse correlation between nigral free-water and striatal VMAT2 binding. Multiple regression analyses found that increased free-water in the pSN, decreased VMAT2 binding in the putamen and age were associated with higher H&Y stage, MDS-UPDRS part III total motor scores, and posture and gait scores. This finding suggests that free-water imaging of the pSN and presynaptic dopamine imaging of the striatum are uniquely associated with PD clinical symptoms. Furthermore, VMAT2 binding in caudate, free water in caudate and pSN, and education associated with DRS total scores. These findings indicate that free-water in SN is correlated with striatal dopamine terminal degeneration, and both striatal dopaminergic denervation and free-water changes in specific structures within the basal ganglia are complimentary predictors of motor and cognitive deficits in PD patients.
Our results confirm previous single imaging modality studies showing increased pSN free-water and decreased striatal VMAT2 binding in PD compared with controls[3, 6, 20]. Increased pSN free-water was suggested to be a correlate of neurodegenerative alterations in the SN resulting in increased extracellular fluid space [6, 7]. Nigral dopaminergic neuronal degeneration in PD is focused initially and most pronounced in the ventrolateral tier of the SN, and this is the region where the pSN region is located. It is expected that the anterior region of the SN would be affected later in the disease [17]. This degeneration pattern may explain the detection of increased free-water in pSN but not in anterior SN in patients in relatively early stage. Decreased striatal VMAT2 in PD reflects diminished nigrostriatal dopaminergic nerve terminal integrity[20]. Prior studies examined relationships between imaging metrics obtained from the SN and imaging parameters pertaining to the integrity of the striatum [3, 6, 8]. In a small sample study of PD patients, it was found that increased mean diffusivity of the caudal section of SN was related to decreased putaminal [18F]dopa uptake [8]. Our analysis also complements previous findings demonstrating an inverse relation between pSN free-water levels and putaminal nigrostriatal terminal density evaluated with dopamine transporter SPECT imaging [6]. In a large cohort of subjects, we show an inverse correlation between SN free-water and striatal VMAT2 binding, indicating that nigral microstructural abnormalities evaluated by free-water were correlated with striatal dopaminergic denervation. The relatively low strength of the correlation between these two imaging modalities raises the possibility that the progression patterns of SN free-water and striatal VMAT2 binding changes may not be identical, and also may not be occurring in parallel. In a prior study, change of striatal VMAT2 binding was estimated to decline as early as 17 years before disease onset [22], which could indicate that the decline in nigrostriatal terminal integrity precedes increases in SN free-water resulting from SN dopaminergic perikaryal changes. This hypothesis is consistent with the model of Perlmutter and Norris which describes a non-linear relationship between measures of striatal dopamine terminal density and SN nigrostriatal perikaryal integrity [23]. Therefore, future longitudinal studies of early stage PD or pre-PD using both VMAT2 and free-water are needed to address this question.
In prior molecular imaging studies, diminished nigrostriatal dopaminergic terminals-as measured by 6-[18F]fluorolevodopa, 11C-DTBZ PET and SPECT DAT tracers-correlated with clinical severity particularly assessed by UPDRS motor scores[8, 24]. The current study found that aSN free-water and putaminal VMAT2 binding were associated with bradykinesia score. This is consistent with our prior study of PD that showed the baseline pSN free-water didn’t correlate with baseline bradykinesia score, but predicted the progression of bradykinesia over the next year [7]. Another multicenter longitudinal study of de novo PD showed that 1-year and 2-year increase in pSN free-water predicted subsequent progression on H&Y stage over 4 years [3]. Here, we found that pSN free-water and putaminal VMAT2 binding were both predictors for H&Y stage, MDS-UPDRS part III motor scores and posture and gait scores in PD patients. These results complement findings in the literature and validate MR-based free-water as a measure of PD pathology. Meanwhile, only pSN free-water but not striatal VMAT2 binding was included in the model related to tremor scores, although the percent of variance predicted was low. The different predictor patterns for tremor scores and other two motor symptoms scores are consistent with suggestions that tremor and bradykinesia have distinct pathophysiologic mechanisms [25]. Helmich suggested recently that parkinsonian tremor results from diminished dopaminergic innervation of the thalamus, not the striatum [26]. This hypothesis is also supported by recent studies testing the brain gray mater density and iron accumulation for different PD related motor symptoms [25, 27]. Our study demonstrates that posterior nigral free-water and putaminal VMAT2 bindings are both significant predictors of motor severity in PD, and thus they are not redundant measures, which is important if both are considered in future clinical trials.
We also found that pSN free-water, caudate free-water, and caudate VMAT2 binding were associated with diminished cognition as evaluated by DRS. These results are consistent with the established role of the caudate in cognition, as well as links between nigral dopaminergic input to caudate and impaired performance on tests of various functions including executive, verbal and visual memory functioning [28, 29]. Both caudate free-water and caudate VMAT2 binding, reflecting microstructural abnormalities and dopamine terminal dysfunction, respectively, were predictors of cognitive performance in PD. Caudate free-water and caudate VMAT2 binding were independent predictors of cognitive status in PD, suggesting that caudate free-water is measuring a caudate pathologic component separate from nigrostriatal terminal degeneration. Smith and colleagues argue that PD is marked by early, prominent loss of intralaminar thalamic neurons with substantial projections to the striatum. These thalamic neurons appear to play a particularly important role in cognition [30]. It is important to note that the model for predicting cognition in PD accounts for about 23.1% of the variation in cognitive performance. It suggests that the cognition changes in PD are unlikely to be related solely to nigrostriatal dysfunction or caudate microstructural changes. Extra-nigrostriatal and other neurotransmitter systems are also likely to be involved in the cognition of PD.
One limitation of the current study is the use of 15 directions and 1 b0 for the diffusion imaging protocol. While this is not an optimal protocol, the pattern of cross-sectional PD compared with control findings was consistent with the prior data in the literature using a free-water analysis approach [3, 6]. We are not recommending the use of a 15-direction sequence in the future, since additional directions and more b0 volumes provide better modeling and more stable estimates of the diffusion signal.
Our findings show that increased nigral free-water was negatively correlated with striatal dopaminergic denervation. In addition, free-water in structures along the nigrostriatal pathway correlates with both motor and cognition features of PD patients. Although further studies need to confirm these findings, our results suggest that SN free-water and striatal VMAT2 binding are possible markers for monitoring different aspects of nigrostriatal dopaminergic neuron degeneration and may be useful for assessing different features of PD.
Highlights.
A large cohort of PD and controls were assessed using PET and diffusion imaging
PD patients had increased nigral free-water than age-matched elderly controls
Nigral free-water inversely correlates with striatal dopaminergic dysfunction
Increased nigral free-water associates with motor and cognitive deficits in PD
Specific striatal dopaminergic patterns relate to motor and cognitive deficits in PD
Acknowledgements
The authors would like to thank all participants for their time and commitment to this research, and the National Institutes of Health for funding this study. This study was supported by the Supported by NIH grants (R01 NS052318; U01 NS102038 with additional support from P50 NS091856).
Disclosure statement:
Dr. Martijn L.T.M. Müller reports research support from the NIH, the Department of Veterans Affairs and the Michael J Fox Foundation. Dr. Nicolaas I. Bohnen reports research support from the NIH, Department of Veteran Affairs, the Michael J. Fox Foundation and Axovant Sciences. Dr. Roger L. Albin serves on the editorial boards of Neurology®, Annals of Neurology, Movement Disorders, and Neurobiology of Disease. He receives grant support from the NIH and the Michael J. Fox Foundation. Dr. Albin serves on the Data Safety and Monitoring Boards for the LEGATO-HD (Icon/Teva), IONISHTTRX (IONIS), and 251PP501 (Icon/Biogen) trials. Dr. David E. Vaillancourt reports grants from NIH, NSF, and Tyler’s Hope Foundation during the conduct of the study, and personal honoraria from NIH, National Parkinson’s Foundation, Sanofi, and Northwestern University unrelated to the submitted work. All other authors report no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Movement disorders : official journal of the Movement Disorder Society. 2014;29:1583–90. [DOI] [PubMed] [Google Scholar]
- [2].Kalia LV, Lang AE. Parkinson’s disease. Lancet (London, England). 2015;386:896–912. [DOI] [PubMed] [Google Scholar]
- [3].Burciu RG, Ofori E, Archer DB, Wu SS, Pasternak O, McFarland NR, et al. Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain : a journal of neurology. 2017;140:2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic resonance in medicine. 2009;62:717–30. [DOI] [PubMed] [Google Scholar]
- [5].Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, et al. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology. 2011;134:3590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ofori E, Pasternak O, Planetta PJ, Burciu R, Snyder A, Febo M, et al. Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiology of aging. 2015;36:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, et al. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain : a journal of neurology. 2015/May/20 ed2015. p. 2322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scherfler C, Esterhammer R, Nocker M, Mahlknecht P, Stockner H, Warwitz B, et al. Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain : a journal of neurology. 2013;136:3028–37. [DOI] [PubMed] [Google Scholar]
- [9].Schuff N, Wu IW, Buckley S, Foster ED, Coffey CS, Gitelman DR, et al. Diffusion imaging of nigral alterations in early Parkinson’s disease with dopaminergic deficits. Movement disorders : official journal of the Movement Disorder Society. 2015;30:1885–92. [DOI] [PubMed] [Google Scholar]
- [10].Lenfeldt N, Eriksson J, Astrom B, Forsgren L, Mo SJ. Fractional Anisotropy and Mean Diffusion as Measures of Dopaminergic Function in Parkinson’s Disease: Challenging Results. Journal of Parkinson’s disease. 2017;7:129–42. [DOI] [PubMed] [Google Scholar]
- [11].Hirata FCC, Sato JR, Vieira G, Lucato LT, Leite CC, Bor-Seng-Shu E, et al. Substantia nigra fractional anisotropy is not a diagnostic biomarker of Parkinson’s disease: A diagnostic performance study and meta-analysis. European radiology. 2017;27:2640–8. [DOI] [PubMed] [Google Scholar]
- [12].Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage Clinical. 2013;3:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Annals of neurology. 2000;47:493–503. [PubMed] [Google Scholar]
- [14].Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64:208–15. [DOI] [PubMed] [Google Scholar]
- [15].Sossi V, de la Fuente-Fernandez R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ. Dopamine transporter relation to dopamine turnover in Parkinson’s disease: a positron emission tomography study. Annals of neurology. 2007;62:468–74. [DOI] [PubMed] [Google Scholar]
- [16].Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. European journal of pharmacology. 1995;294:577–83. [DOI] [PubMed] [Google Scholar]
- [17].Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain : a journal of neurology. 2013;136:2419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42:1142–6. [DOI] [PubMed] [Google Scholar]
- [19].Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Movement disorders : official journal of the Movement Disorder Society. 2013;28:668–70. [DOI] [PubMed] [Google Scholar]
- [20].Miller NS, Kwak Y, Bohnen NI, Muller ML, Dayalu P, Seidler RD. The pattern of striatal dopaminergic denervation explains sensorimotor synchronization accuracy in Parkinson’s disease. Behavioural brain research. 2013;257:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Planetta PJ, Ofori E, Pasternak O, Burciu RG, Shukla P, DeSimone JC, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain : a journal of neurology. 2016;139:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].de la Fuente-Fernandez R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Annals of neurology. 2011;69:803–10. [DOI] [PubMed] [Google Scholar]
- [23].Perlmutter JS, Norris SA. Neuroimaging biomarkers for Parkinson disease: facts and fantasy. Annals of neurology. 2014;76:769–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Movement disorders : official journal of the Movement Disorder Society. 2000;15:692–8. [DOI] [PubMed] [Google Scholar]
- [25].Li X, Xing Y, Martin-Bastida A, Piccini P, Auer DP. Patterns of grey matter loss associated with motor subscores in early Parkinson’s disease. NeuroImage Clinical. 2018;17:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Helmich RC. The cerebral basis of Parkinsonian tremor: A network perspective. Movement disorders : official journal of the Movement Disorder Society. 2018;33:219–31. [DOI] [PubMed] [Google Scholar]
- [27].Martin-Bastida A, Lao-Kaim NP, Loane C, Politis M, Roussakis AA, Valle-Guzman N, et al. Motor associations of iron accumulation in deep grey matter nuclei in Parkinson’s disease: a cross-sectional study of iron-related magnetic resonance imaging susceptibility. European journal of neurology. 2017;24:357–65. [DOI] [PubMed] [Google Scholar]
- [28].Jokinen P, Bruck A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism & related disorders. 2009;15:88–93. [DOI] [PubMed] [Google Scholar]
- [29].Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, et al. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. NeuroImage. 2004;21:1497–507. [DOI] [PubMed] [Google Scholar]
- [30].Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, et al. The thalamostriatal system in normal and diseased states. Frontiers in systems neuroscience. 2014;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]