Abstract
Objective:
To determine whether serum etonogestrel concentrations in contraceptive implant users are associated with certain individual patient characteristics.
Study Design:
We enrolled reproductive-age women using etonogestrel contraceptive implants between 12–36 months duration and measured a single serum etonogestrel concentration. Participants also completed a questionnaire about demographics.
Results:
We enrolled 350 participants; median age was 22.5 years (range 18.0–39.1), median months of implant use was 26.0 (range 12.0–36.0), and median body mass index was 25.7kg/m2 (range 18.5–52.0). Our study population was primarily White/Caucasian (46.6% [163/350]) and Hispanic/Latina ethnicity (51.4% [180/350]). The median serum etonogestrel concentration was 137.4pg/mL and etonogestrel concentrations varied 12.4 fold in the population (range 55.8–695.1 pg/mL). Using forward stepwise linear regression, months of implant use (β= −1.74, p<0.001) and body mass index (β= −3.10, p<0.001) were both significantly associated with decreased serum etonogestrel concentration with Black/African American race as a positive effect modifier (β= 18.24, p=0.099); R-squared for the model = 0.13.
Conclusions:
Individuals demonstrated a wide variability in serum etonogestrel concentrations, which can potentially affect side-effect profiles and efficacy. Increasing body mass index and longer duration of implant use were associated with small decreases in serum etonogestrel concentrations, while self-reported Black/African American race was associated with a non-significant increase. Despite these findings, most of etonogestrel variability was unaccounted for, suggesting that other clinical, pharmacologic, and genetic factors contributing to variability in etonogestrel concentrations remain to be determined.
Keywords: body mass index, contraceptive implant, etonogestrel, pharmacokinetics
1.0. Introduction
The etonogestrel (ENG) contraceptive implant (Nexplanon®, formerly Implanon®, Merck & Co., Whitehouse Station NJ) remains the most efficacious long acting reversible contraceptive method available in the United States [1]. Use of this contraceptive method is increasing, especially among young adults and adolescents where reported use of the ENG contraceptive implant has more than tripled from 2006 to 2015 (0.6% versus 3.0%, respectively) [2, 3]. The ENG implant has a well-described pharmacokinetic profile given its steady-state drug release and relative plateau of serum values in an individual after the first year of use [4, 5]. However, wide variability exists in inter-individual serum ENG concentrations among women using the ENG implant for identical periods of time. McNicholas et al measured serum ENG levels in 157 women in the third year of contraceptive implant use and demonstrated a range of values from 63.8–802.6pg/mL, a greater than 12-fold difference between the highest and lowest values [6]. Pharmacokinetic studies of other contraceptive methods, including combined hormonal contraceptive pills, have demonstrated similar variability in serum drug levels [7, 8]. Although prior research has not demonstrated an association between lower ENG levels and contraceptive efficacy in a general population of implant users, serum drug level variability may impact the efficacy of less effective hormonal contraceptive methods (e.g. combined and progestin-only pills), which do not provide continuous hormone release [6, 9].
Prior studies have largely focused on the association of body mass index (BMI) with variability in ENG pharmacokinetics, but often with non-significant or even conflicting findings [6, 10, 11]. A cross-sectional, descriptive study of 52 ENG implant users with at least one year of use found no statistically significant difference between normal weight, overweight, and obese participants [11]. In fact, both the overweight and obese participants had higher median ENG serum levels when compared to the normal weight women (33.3% and 4.2% higher, respectively) [11]. A larger prospective cohort study of 291 implant users that followed women past 3 years of use found a significant difference in serum ENG concentrations based on BMI [6]. This finding, however, did not follow a linear trend as overweight participants had higher serum ENG concentrations than both the normal weight and obese participants at 4 years of use and there were no statistically significant differences between normal weight, overweight, and obese participants at 3 and 5 years of implant use [6]. Median serum ENG concentration was, however, consistently lowest in the obese group [6]. This unclear relationship between BMI and serum ENG concentrations is consistent with the currently limited understanding of exactly how BMI affects steroid hormone metabolism [12].
Given that medication efficacy may be associated with serum drug levels, there is an urgent need to better understand the factors that contribute to variability in serum hormone levels for women using hormonal contraception. The ENG contraceptive implant is an ideal candidate for studies on variability in serum concentrations given its steady-state drug release and independence from issues of protocol adherence [1]. We aimed to better characterize serum ENG concentrations accounting for certain individual patient characteristics within a large racially/ethnically diverse group of women. We hypothesized that age, duration of implant use, BMI, race, and ethnicity would be associated with serum ENG concentrations in contraceptive implant users and account for some of the known pharmacokinetic variability of this contraceptive method.
2.0. Materials and Methods
In this pharmacokinetic study, we recruited English or Spanish speaking reproductive age women (18–45 years old) with an ENG contraceptive implant in place for at least 12 and no more than 36 months. We chose this duration of implant use for our inclusion criteria because the ENG implant has a pharmacokinetic burst early in the first year of use that then resolves to a relative steady-state for the remaining two years of use [4]. We chose 36 months as our upper enrollment limit for duration of implant use as this is the current Food and Drug Administration approved duration of use [1]. We excluded women using any medications or supplements that could impact serum ENG levels through inhibition or induction of cytochrome P-450 (CYP) enzymes (specifically CYP-3A4) [13]. We also excluded women with any reported medical conditions that could impact baseline liver function (e.g. hepatitis, cirrhosis) or a measured BMI less than 18.5kg/m2, as low BMI has been associated with abnormal metabolism. The protocol was approved by the Colorado Multiple Institutional Review Board and all participants gave written informed consent before study initiation.
For eligible participants, we determined the length of implant use by participant report and confirmed presence of the implant by physical exam. We excluded women who could not remember when they had the implant placed, unless they had documentation of the date of insertion. We then collected a single blood sample from each participant for serum ENG concentration analysis. Participants then completed a questionnaire to obtain self-reported demographics.
For ENG analysis, the blood was allowed to clot for at least 10 minutes at room temperature, was centrifuged, and then the serum stored in aliquots at −80°C. We shipped all de-identified frozen serum samples to the Biomarkers Core Laboratory of the Irving Institute of Clinical and Translational Research at Columbia University Medical Center (New York City, NY). The samples underwent analysis using a previously validated ultra performance liquid chromatography tandem mass-spectrometry (LC-MS) assay protocol [14]. We extracted ENG from human serum samples using liquid-liquid extraction by mixing 500μL of serum samples spiked with deuterated internal standard (D8-progesterone) with 7mL of hexane/dichloromethane (3/2) [14]. We vortexed the mixture for 10 minutes, centrifuged it, and then evaporated the organic layer under nitrogen stream and resuspended it in 25% methanol. We performed LC-MS analysis on an analytical platform comprising a triple quadrupole Waters Xevo TQ-S (Waters, Milford, MA) mass spectrometer equipped with an electrospray ionization source and integreated with a Waters Acquity UPLC controlled by Mass Lynx Software 4.1 [14]. We performed chromatographic separation on a Waters C18 BEH column (BEH C18 1.7μm, 2.1×50mm) maintained 50°C with mobile phases of water (solvent A) and acetonitrile (solvent B) containing 0.1% formic acid maintained at 600μL/min [14]. We operated the mass spectrometer under multiple reaction monitoring (MRM) mode with positive electrospray ionization. For MRM, we utilized the following transitions for quantitation: etonogestrel 325.2>108.9, and D8-Progesterone 323.2>100.0. We performed peak integration and data analysis with TargetLynx 4.1. We quantitated ENG by comparing the integrated peak areas of unknown against those of known amounts of purified standards [14].
Lower limit of quantification, defined as the level at which the residual of the calibration line is <20% of the expected concentration combined with a signal to noise ratio >10, was determined to be 25 pg/ml. We performed serum ENG concentration quantification in three batches at enrollment targets of 150, 250, and 350 participants. We included three levels (low, medium and high) of quality controls along with a study pool sample to assess the batch effect. The mean intra-assay precision for the assay was 3.2%. The inter-assay variability across the batches assessed using the pool sample was 1.8%.
We used IBM SPSS™ version 24 statistical software for all statistical analyses. We performed descriptive frequencies and conducted both simple linear regression and multivariable linear regression to identify predictors of serum ENG concentrations. We chose the pertinent participant characteristics/demographics of age, months of implant use, BMI, race, and ethnicity as variables to create our linear regression model based on the prior literature and plausible pharmacologic associations [6, 7, 12]. We utilized information criterion to identify the best linear regression model using a forward step-wise approach. With a sample size of 350, we had greater than 90% power to find an R2 ≥ 0.05 assuming an alpha level of 0.05 and all five predictors in the multivariable linear regression model [15].
3.0. Results
We recruited 350 participants over the course of 15 months (March 2016 to May 2017). Table 1 shows pertinent participant characteristics. The median age of the participants was 22.5 years (range 18.0 – 39.1) with a median duration of ENG implant use of 25.7 months (range 12 – 36). The participants had a median BMI of 25.7kg/m2 (range 18.5–52.0). Using the National Institutes of Health categorization, less than half of participants (43.8% [152/350]) were normal weight, whereas 101 participants (29.1%) met criteria for overweight, 83 participants (23.9%) for obesity, and 11 participants (3.2%) for extreme obesity [16]. The most frequent self-reported race was White/Caucasian (46.6% [163/350]) and 51.4% (180/350) of participants reported Hispanic/Latina ethnicity. Amongst the 77 participants who responded with “No response/Unknown” in relation to self-identified race, all identified their ethnicity as “Hispanic/Latina.”
Table 1:
Summary characteristics of age, duration of implant use, and body-mass index for all 350 etonogestrel contraceptive implant users with self-reported race and ethnicity
| Median (Range) | |
|---|---|
| Age (years) | 22.5 (18.0 – 39.1) |
| Months of implant use | 26.0 (12.0 – 36.0) |
| BMI (kg/m2) | 25.7 (18.5 – 52.0) |
| n (%) | |
| Race | |
| White/Caucasian | 163 (46.6) |
| Black/African American | 40 (11.4) |
| Asian/Pacific Islander | 19 (5.4) |
| Native American/Alaskan | 7 (2.0) |
| More than one | 44 (12.6) |
| No response/Unknown | 77 (22.0) |
| Ethnicity | |
| Hispanic/Latina | 180 (51.4) |
| Non-Hispanic | 170 (48.6) |
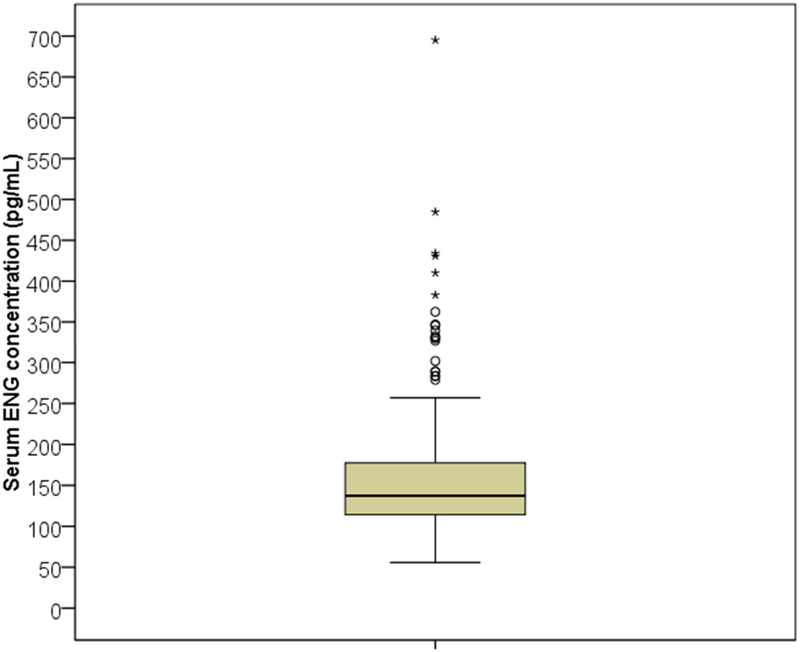
The median ENG serum concentration was 137.4pg/mL (range 55.8–695.1); Figure 1 demonstrates the distribution of serum ENG concentrations with an interquartile range of 63.5pg/mL. There were 36 participants (10.3%) with ENG levels less than 90pg/mL, which is the manufacturer’s reported minimum serum concentration for consistent prevention of ovulation [1]. Table 2 shows the pertinent characteristics of participants with serum ENG concentrations less than 90pg/mL compared to the rest of the cohort. Participants with ENG concentrations less than 90pg/mL had a longer median duration of implant use and higher median BMI compared to all other participants (p < 0.001 and p = 0.022, respectively). Both groups were similar in self-reported race and ethnicity and no participants had contraceptive implant failures at the time of enrollment.
Figure 1:
Box-plot of serum etonogestrel (ENG) concentrations for all 350 participants. The box represents the first and third quartiles (interquartile range = 63.5pg/mL) with the band inside the box representing the median (137.4pg/mL). Whiskers represent the data within 1.5 interquartile range of the upper and lower quartile.
Table 2:
Characteristics and demographics of participants with serum ENG concentrations less than 90pg/mL (N=36) and participants with serum ENG concentrations greater than or equal to 90pg/mL (N=314).
| ENG < 90pg/mL (n = 36) |
ENG ≥ 90pg/mL (n = 314) |
||
|---|---|---|---|
| Median (Range) | p-value | ||
| Age (years) | 22.4 (19.1 – 30.8) | 22.5 (18.0 – 39.1) | 0.86* |
| Months of implant use | 35.0 (15.0 – 36.0) | 24.0 (12.0 – 36.0) | <0.001* |
| BMI (kg/m2) | 29.2 (21.9 – 52.0) | 25.3 (18.5 – 48.1) | 0.022* |
| n (%) | |||
| BMI category± | 0.01† | ||
| Normal weight | 7 (19.4) | 145 (46.6) | |
| Overweight | 13 (36.1) | 88 (28.3) | |
| Obesity | 15 (41.7) | 68 (21.9) | |
| Extreme obesity | 1 (2.8) | 10 (3.2) | |
| Race | 0.50† | ||
| White/Caucasian | 16 (44.4) | 147 (46.8) | |
| Black/African American | 6 (16.7) | 34 (10.8) | |
| Asian/Pacific Islander | 1 (2.8) | 18 (5.7) | |
| Native American/Alaskan | 0 (0.0) | 7 (2.2) | |
| More than one | 7 (19.4) | 37 (11.8) | |
| No response/Unknown | 6 (16.7) | 71 (22.6) | |
| Ethnicity | 0.86† | ||
| Hispanic/Latina | 19 (52.8) | 161 (51.3) | |
| Non-Hispanic | 17 (47.2) | 153 (48.7) |
Independent samples medians test
Pearson’s Chi-squared test
BMI categories defined using the National Institute of Health classifications [16]
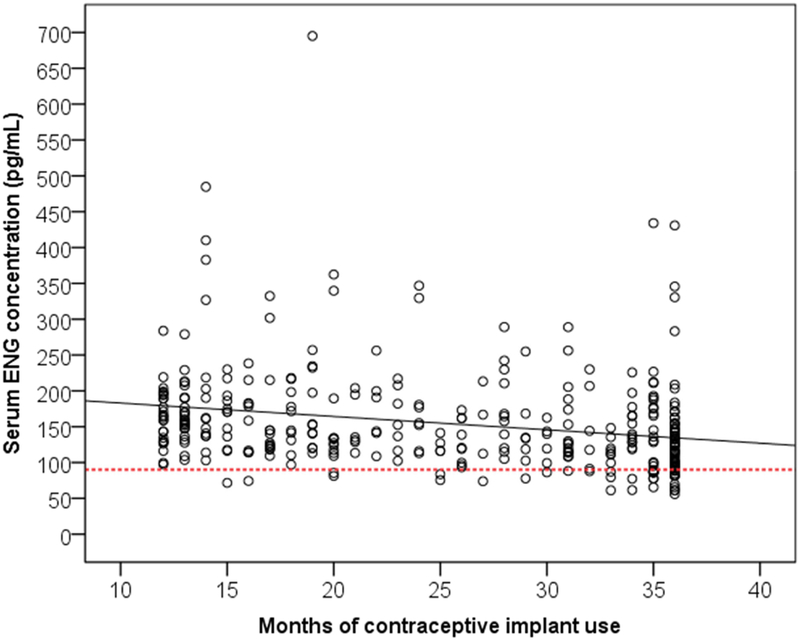
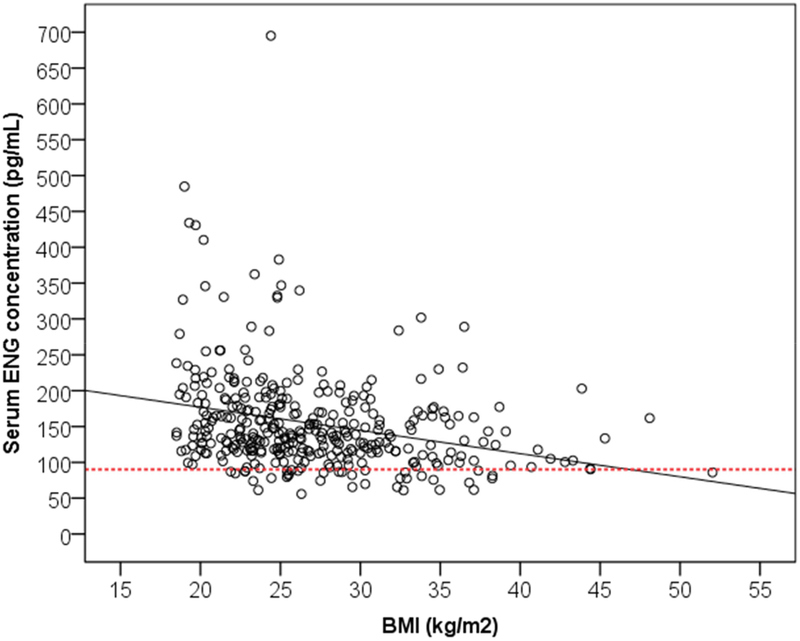
Three variables of interest had statistically significant associations with serum ENG concentrations when we performed simple linear regression: months of implant use (β = −1.87, p < 0.001), BMI (β = −3.24, p < 0.001), and Black/African American race (β = 23.84, p = 0.043). Table 3 provides the results from the forward stepwise linear regression; the final model had an R-squared of 0.13. Months of implant use (β = −1.74, p <0.001) and BMI (β = −3.10, p < 0.001) demonstrated slight negative correlations with serum ENG concentrations (see Figure 2 and 3 respectively). For every one month of implant use past the first 12 months the serum ENG concentrations declined on average by 1.74pg/mL, and for every 1kg/m2 increase in BMI the serum ENG concentrations decreased on average by 3.1pg/mL. Self-reported Black/African American race still demonstrated a large positive correlation with serum ENG concentrations (β = 18.24, p = 0.099), but this association was not statistically significant.
Table 3:
Beta-coefficients and 95% confidence intervals for variables in the final linear regression model
| Variable | β-coefficient | 95% confidence interval | p-value |
|---|---|---|---|
| Months of implant use | −1.74 | −2.51, −0.97 | <0.001 |
| BMI (kg/m2) | −3.10 | −4.27, −1.93 | <0.001 |
| Black/African American race | 18.24 | −3.48, 39.96 | 0.099 |
Figure 2:
Scatterplot of etonogestrel (ENG) serum concentrations based on months of contraceptive implant use. Line of best fit denoted by solid black line. The dotted line marks serum ENG concentration of 90pg/mL.
Figure 3:
Scatterplot of etonogestrel (ENG) serum concentrations based on body mass index (BMI) (kg/m2). Line of best fit denoted by solid black line. The dotted line marks serum ENG concentration of 90pg/mL.
4.0. Discussion
In this large and diverse group of subdermal ENG implant users, we found a 12.4-fold difference between the minimum and maximum serum ENG concentrations, which is consistent with variability seen in prior investigations [6, 11, 12]. We found two participant characteristics associated with decreases in ENG serum concentrations: duration of implant use and BMI. Prior smaller studies have demonstrated that serum ENG concentrations reach a relative steady state after the first year of use in ENG contraceptive implant users with only a slight downward trend thereafter [4]. Our large study of 350 participants confirms this finding and reaffirms that the decline between 12 and 36 months, while statistically significant, is small and not clinically concerning given that the vast majority of contraceptive implant users would not fall below the level for consistent ovulatory suppression (90pg/mL) solely based on duration of implant use.
Much attention has focused on whether BMI affects the efficacy of the ENG contraceptive implant. We found that for every 1kg/m2 increase in BMI, serum ENG concentrations decreased by 3.10pg/mL. These data differ from the findings of smaller cohorts by Morrell et al and McNicholas et al, who found no significant association between BMI and serum ENG concentrations and only a positive association between overweight women and serum ENG concentrations [6, 11]. Though statistically significant, this association between increasing BMI and lower serum ENG concentrations also likely has limited clinical significance. Given the median serum ENG concentration in our population and those reported previously, the effect of BMI was not significant enough in the majority of patients to cause the ENG concentration to drop below 90pg/mL. Furthermore, the clinical cut-off of 90pg/mL is associated with lack of consistent ovulatory suppression with the contraceptive implant, but the minimum serum ENG concentration needed to maintain contraceptive efficacy by other progestin effects has not yet been determined [1].
Though self-reported Black/African American race initially appeared to be significantly associated with serum ENG concentrations, this association was ultimately not statistically significant in the final model. This finding may have been in-part due to the inaccuracy of self-reported race when compared to actual genetic ancestry [17]. Self-reported race becomes even less useful in populations of increased admixture including Hispanic/Latina populations. Mexican Americans have been found to have up to 3% African ancestry as compared to Puerto Ricans who have up to 16% African ancestry [17]. Given the prevalence of Hispanic/Latina ethnicity in our study population, self-reported race may not have accurately reflected the true genetic ancestry of our participants. Thus, the imprecision of self-reported race/ethnicity may have contributed to the overall lack of significant associations with serum ENG concentrations in our final model.
The major strength of this study was the size and diversity of our cohort of ENG implant users. With this large sample size, we were able to detect significant associations between patient characteristics and serum ENG concentrations. By analyzing a medication with a controlled-release rate and steady-state pharmacokinetics, we were able to efficiently capture each individual’s pharmacokinetic profile with only a single ENG measurement during the relative steady-state period between 12 and 36 months [1, 4]. Our study population was also relatively young with a median age of 22.5 years and only 18 participants over the age of 30 years. This age group represents a time of relatively normal physiological metabolism as compared to the developing metabolism in childhood/adolescence and the altered metabolism associated with advanced age [18]. Thus, the findings of this study were less likely to be confounded by age-related changes in drug metabolism. This age group is also representative of the general population of ENG implant users who are typically younger women [3].
The primary limitation of our study was that we did not measure all possible patient characteristics that may impact serum ENG concentrations, but rather focused on variables identified in the previous studies and self-reported race/ethnicity. We also used self-reported duration of implant use, which may not have been entirely accurate for all participants. We attempted to control for these potential recall errors by measuring duration in months, which allowed for a larger margin of error as compared to measuring in weeks or days. Another limitation is that we only obtained a single serum ENG concentration for each participant. However, previously published pharmacokinetic studies of the contraceptive implant have shown that it reaches a relative steady-state of serum ENG concentration in an individual roughly after the first 12 months of use, making a single measurement appropriate in this setting [1, 4].
Individual participant characteristics that were captured in this study explained only a small portion of the variability seen in the metabolism of the ENG steroid hormone. Investigating novel areas of interindividual differences (e.g. genetics, depth/location of implant, microbiomes) may help to account for some of the pharmacokinetic variability in both serum ENG levels and other sex steroid hormones used in hormonal contraception. By identifying such novel interindividual differences associated with altered metabolism of steroid hormones, precision medicine clinical tools may lead to improved patient counseling regarding steroid hormone efficacy and side-effects. Given how frequently steroid hormones are used by women throughout their lifetimes, it is imperative that we gain a better understanding of how individual patient characteristics, including genetic variants, can impact the pharmacology and functionality of these medications.
Implications.
Although increases in body mass index are associated with lower etonogestrel levels in contraceptive implant users, the majority of women will maintain serum concentrations that consistently suppress ovulation. Furthermore, certain patient characteristics can only explain a small portion (13%) of the variability in serum etonogestrel levels among contraceptive implant users.
5.0. Acknowledgments
The authors would like to thank Dr. Serge Cremers at the Biomarkers Core Laboratory at Columbia University for assisting with the etonogestrel analysis.
6.0 Funding
This work was primarily supported by the Society of Family Planning Research Fund [grant number SFPRF17–3]. This work was also supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Teal has served on scientific advisory boards of Allergan and Bayer Healthcare, and serves on a Data Monitoring Board for a study funded by Merck and Co. Dr. Teal and Dr. Lazorwitz receive research funding from Merck and Co. for an Investigator Initiated Study on drug-drug interactions with the etonogestrel contraceptive implant. The University of Colorado Department of Obstetrics and Gynecology has received research funding from Bayer, Agile Therapeutics, Merck and Co, and Medicines360. Dr. Guiahi’s time was supported by the Society of Family Planning Junior Investigator Career Grant SFPRF10-JI1. The authors have no other conflicts of interest to disclose.
Declaration of interests
☐ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
☒The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Clinical Trial Registration:
References
- [1].Hatcher RA, Trussell J, Stewart F, et al. Contraceptive technology. New York: Ardent Media: Inc; 2011. [Google Scholar]
- [2].Abma JC, Martinez GM. Sexual Activity and Contraceptive Use Among Teenagers in the United States, 2011–2015. National health statistics reports. 2017:1–23. [PubMed] [Google Scholar]
- [3].Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS data brief. 2014:1–8. [PubMed] [Google Scholar]
- [4].Le J, Tsourounis C. Implanon: a critical review. The Annals of pharmacotherapy. 2001;35:329–36. [DOI] [PubMed] [Google Scholar]
- [5].Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of Etonogestrel Released From the Contraceptive Implant Implanon. Contraception. 1998;58:283–8. [DOI] [PubMed] [Google Scholar]
- [6].McNicholas C, Swor E, Wan L, Peipert JF. Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration. American journal of obstetrics and gynecology. 2017;216:586.e1–.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fotherby K Variability of pharmacokinetic parameters for contraceptive steroids. Journal of steroid biochemistry. 1983;19:817–20. [DOI] [PubMed] [Google Scholar]
- [8].Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bennink HJ. The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5 Suppl 2:12–20. [PubMed] [Google Scholar]
- [10].Mornar S, Chan LN, Mistretta S, Neustadt A, Martins S, Gilliam M. Pharmacokinetics of the etonogestrel contraceptive implant in obese women. American journal of obstetrics andgynecology. 2012;207:110.e1–6. [DOI] [PubMed] [Google Scholar]
- [11].Morrell KM, Cremers S, Westhoff CL, Davis AR. Relationship between etonogestrel level and BMI in women using the contraceptive implant for more than 1 year. Contraception. 2016;93:263–5. [DOI] [PubMed] [Google Scholar]
- [12].Simmons KB, Edelman AB. Hormonal contraception and obesity. Fertil Steril. 2016;106:1282–8. [DOI] [PubMed] [Google Scholar]
- [13].U.S. Food and Drug Administration. Drug Development and Drug Interactions: Table of Substrates, Inhibitors, and Inducers.
- [14].Thomas T, Petrie K, Shim J, Abildskov KM, Westhoff CL, Cremers S. A UPLC-MS/MS method for therapeutic drug monitoring of etonogestrel. Ther Drug Monit. 2013;35:844–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Soper D A-priori Sample Size Calculator for Multiple Regression [Software]. 2018.
- [16].Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. 1998. [PubMed]
- [17].Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Human genomics. 2015;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wilson K, Hanson J. The effects of extremes of age on drug action. Methods Find Exp Clin Pharmacol. 1980;2:303–12. [PubMed] [Google Scholar]