Infants are particularly susceptible to a variety of breathing disorders during sleep, including obstructive sleep apnea. Polysomnography, a diagnostic modality that is the gold standard for the assessment of sleep-disordered breathing in children, is well-suited to evaluate these young patients.
Breathing is irregular in neonates, whose respiratory rate is faster and more variable than older children 1. Distinguishing between normal and abnormal breathing during sleep or wake can be difficult, especially in infants born prematurely or with congenital conditions. The traditional definition of “prolonged” apnea in infants includes cessation of breathing for 20 seconds or longer, or briefer if associated with bradycardia or cyanosis 2. Neonatologists generally have not distinguished between central or obstructive pauses in breathing. However, polysomnography is increasingly being used in infants to evaluate for obstructive sleep apnea, particularly in high-risk infants and has several advantages over other modes of assessment. However, there are limited data available for infant polysomnography, which presents challenges for those performing, interpreting, and treating based on the results of these studies.
While most research on the evaluation of pediatric obstructive sleep apnea (OSA) using polysomnography has focused on older children, infants are uniquely suited to develop OSA. Up to 50% of total upper airway resistance occurs in the nasopharynx, and narrowing of the nasopharynx or other parts of the craniofacial anatomy due to congenital abnormalities like mandibular hypoplasia, laryngomalacia, or mid-face hypoplasia can further contribute to upper airway obstruction during the neonatal period. The highly compliant airway of the infant and relative ventilatory instability can further contribute to a propensity for upper airway obstruction during sleep in infants. Infants with OSA carry a significant healthcare burden. A high percentage of infants diagnosed with OSA have a history of prematurity or underlying congenital condition and require the coordination of care by multiple subspecialties 3.
INDICATIONS FOR POLYSOMNOGRAPHY IN INFANTS/INFANTS AT HIGH-RISK FOR OBSTRUCTIVE SLEEP APNEA
As with older children, the primary indication for polysomnography in infants is for the evaluation of sleep related breathing disorders. Especially in infants, the clinical history and physical examination alone are poor predictors of objectively measured upper airway obstruction. Many otherwise healthy infants without obstructive sleep apnea frequently snore 4, but snoring has not been found to be predictive of OSA presence or severity in infants with cleft palate and micrognathia 5,6. Polysomnography is indicated when clinical evaluation is suggestive of sleep related breathing disorders and remains the gold standard diagnostic modality for the evaluation of OSA in all children, including infants 7. There are a number of factors that may predispose infants to obstructive sleep apnea, including craniofacial conditions like mandibular or mid-face hypoplasia 8,9, laryngomalacia10, nasal obstruction11, or conditions that cause hypotonia12, among other factors.
History and physical exam can be unreliable for the assessment of OSA so polysomnogram should be repeated following treatment of infants with OSA 13. While there have been limited studies of the long-term efficacy of positive airway pressure (PAP) for OSA in infants, continuous positive airway pressure (CPAP) has been shown to be effective in eliminating obstructive events even in very young infants 14,15. As with older children, infants requiring treatment of OSA with CPAP should have a titration polysomnogram to ensure adequate pressure.
In addition to OSA, polysomnography can be useful in the evaluation of hypoventilation in infants; continuous monitoring of end-tidal and/or transcutaneous CO2 is recommended for children undergoing testing. For infants with central disorders of hypoventilation, polysomnography can be used both for the initial diagnostic testing and then to assess treatment with positive pressure ventilation. For children with congenital central hypoventilation syndrome, the recommendation is for evaluation of ventilation during sleep and then routine reassessment of ventilatory needs 16.
Current evidence suggests that polysomnography has a limited role in the evaluation of acute, resolved respiratory events in infants. Polysomnography has been recommended as part of the evaluation for apparent life-threatening event (ALTE) when the history and physical examination are consistent with obstructive sleep apnea 13. However, polysomnography is not part of the standard evaluation for infants with low risk brief resolved unexplained events (BRUE) that are considered for OSA by history and physical examination 17.
While more limited pneumograms, or limited channel studies, have traditionally been used in the assessment of apnea of prematurity, polysomnography has the advantage of having a standardized scoring system for both central and obstructive pauses. Few studies have thus far attempted to use polysomnography to evaluate central apnea in infants. Brockman and colleagues found a tremendous amount of variability in central apneas using home polygraphs, especially at one month of age 18. Full-night attended polysomnography is the gold standard for the evaluation of OSA in children, and abbreviated testing is not considered sensitive or specific enough to be relied on for the clinical diagnosis of OSA 19. The utility of briefer “nap studies” in the evaluation of obstructive sleep apnea have not been compared to full-night polysomnography in infants. As neonates do not have robust circadian rhythms, some centers use daytime testing in young infants, who will sleep and wake to feed both during the daytime and night.
CHALLENGES IN CONDUCTING POLYSOMNOGRAPHY IN INFANTS
Polysomnography in infants comes with unique challenges and requires personnel that are comfortable both with administering and interpreting the test (Table 1). Because infant sleep is less consolidated than older children, sleep efficiency may be reduced. Infants wake periodically to feed, and may require equipment to be adjusted frequently, making the study more labor-intensive for technologists.
Table 1.
Challenges in infant polysomnography
| Challenge | Potential solution |
|---|---|
| Sleep less consolidated than in older children | Adjust study time if needed to ensure adequate sleep time |
| Significant age-related EEG variability | Staff scoring and interpreting studies should be familiar with current AASM guidelines |
| Respiratory signal quality less reliable, irregular breathing | Ensure adequate staff to replace or relocate equipment as needed |
| Lack of end-tidal CO2 waveform plateau | Use calibrated transcutaneous CO2 monitoring as a secondary measure to evaluate hypoventilation |
| Limited nasal masks available for PAP titrations | Have appropriate masks available in sleep lab prior to titration studies |
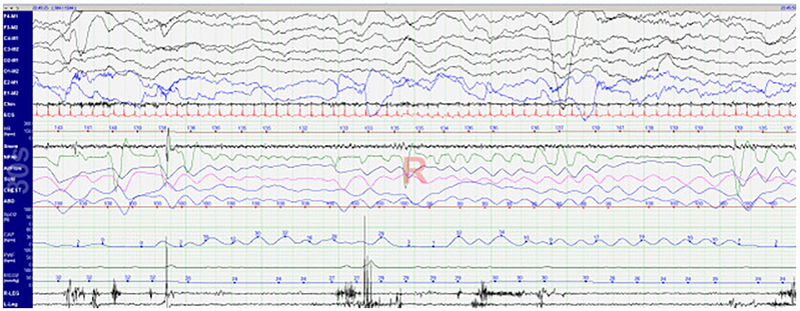
Breathing is less regular in infants during sleep, particularly during REM sleep. Airway closure has been shown to occur even in central apneas in neonates 20, and distinguishing between obstructive and central apneas is more difficult in infants than older children (Figure 1). Tidal volumes are smaller, so relatively small changes can result in greater aberrations to both thermistor and nasal pressure waveforms. The proper positioning of respiratory flow sensors is critical in acquiring quality data from the study. Paradoxical breathing is common even in healthy infants, so this is often not a useful parameter to use in the assessment of obstructive events. Because of their rapid respiratory rate, the end-tidal CO2 waveform may not have a reliable plateau in infants, making the value less reliable. Transcutaneous CO2 monitoring may be used as a secondary measure 21.
Figure 1. 30-second epoch of REM sleep in a healthy 2-week old infant.
Of note are intermittent variability in airflow seen in the thermistor (AirFlow) and nasal pressure (NPAF) signals as well as thoraco-abdominal asynchrony (CHEST and ABD) and lack of end-tidal plateau (CAP).
There is significant age-related variability in normal EEG and respiratory patterns in infants, making scoring sleep challenging, particularly in preterm infants. There are now standardized guidelines for assessing sleep in infant polysomnogaphy21,22, but even among experts in the field there can be variability in scoring 23. Technologists and physicians scoring and interpreting studies on infants 37-48 weeks post-conceptional age should be familiar with and comfortable using infant sleep staging 21.
Limited nasal masks are available for non-invasive positive pressure in infants, so polysomnograms to titrate PAP can be more challenging in infants than older children. Care should be taken to ensure a proper mask seal before attempting these studies. Accurately interpreting studies with infants being treated with high-flow cannula systems can be problematic as this airflow may distort thermistor and nasal pressure signals, making them uninterpretable.
CONCLUSIONS
With increased awareness of sleep-disordered breathing in infants, there is a need for standardized diagnostic tools for these patients. Polysomnography has several benefits for the evaluation of OSA in infants, including standardized setup and scoring of events. In addition, polysomnography can be used to assess for appropriate CPAP pressure in infants with obstructive sleep apnea and may be beneficial in the evaluation of ALTE with symptoms of sleep-disordered breathing. However, polysomnography has several important limitations. Data regarding normative values for polysomnography in infants is limited to studies using older or non-standardized methodology or small studies. There have not been studies comparing polysomnography to more limited polygraphy, daytime testing, or briefer nap studies in infants at different ages.
There is a need for more robust normative sleep respiratory data in infants at different ages to be able to distinguish between normal and pathologic pauses in breathing. An assessment of the appropriateness of applying scoring rules for apnea and hypopneas to infants with a rapid respiratory rate and irregular breathing during REM sleep would also be beneficial. As the evaluation and management of infants with sleep-disordered breathing involves a multidisciplinary team, collaboration between neonatologists, primary care pediatricians, and other specialists is critical to ensure that the appropriate workup and treatment plan is initiated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hooker EA, Danzl DF, Brueggmeyer M, Harper E. Respiratory rates in pediatric emergency patients. J Emerg Med. 1992;10(4):407–410. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. Task Force on Prolonged Apnea. Prolonged apnea. Pediatrics. 1978;61(4):651–652. [PubMed] [Google Scholar]
- 3.Qubty WF, Mrelashvili A, Kotagal S, Lloyd RM. Comorbidities in infants with obstructive sleep apnea. J Clin Sleep Med. 2014;10(11):1213–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn A, Groswasser J, Sottiaux M, et al. Clinical symptoms associated with brief obstructive sleep apnea in normal infants. Sleep. 1993;16(5):409–413. [DOI] [PubMed] [Google Scholar]
- 5.Maclean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012;97(12):1058–1063. [DOI] [PubMed] [Google Scholar]
- 6.Anderson IC, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48(5):614–618. [DOI] [PubMed] [Google Scholar]
- 7.Katz ES, Mitchell RB, D'Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185(8):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inverso G, Brustowicz KA, Katz E, Padwa BL. The prevalence of obstructive sleep apnea in symptomatic patients with syndromic craniosynostosis. Int J Oral Maxillofac Surg. 2016;45(2):167–169. [DOI] [PubMed] [Google Scholar]
- 9.MacLean JE, Hayward P, Fitzgerald DA, Waters K. Cleft lip and/or palate and breathing during sleep. Sleep Med Rev. 2009;13(5):345–354. [DOI] [PubMed] [Google Scholar]
- 10.Camacho M, Dunn B, Torre C, et al. Supraglottoplasty for laryngomalacia with obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope. 2016;126(5):1246–1255. [DOI] [PubMed] [Google Scholar]
- 11.Samadi DS, Shah UK, Handler SD. Choanal atresia: a twenty-year review of medical comorbidities and surgical outcomes. Laryngoscope. 2003;113(2):254–258. [DOI] [PubMed] [Google Scholar]
- 12.Testa MB, Pavone M, Bertini E, Petrone A, Pagani M, Cutrera R. Sleep-disordered breathing in spinal muscular atrophy types 1 and 2. Am J Phys Med Rehabil. 2005;84(9):666–670. [DOI] [PubMed] [Google Scholar]
- 13.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNamara F, Sullivan CE. Effects of nasal CPAP therapy on respiratory and spontaneous arousals in infants with OSA. Journal of applied physiology. 1999;87(3):889–896. [DOI] [PubMed] [Google Scholar]
- 15.Bedi PK, Castro-Codesal ML, Featherstone R, et al. Long-term Non-Invasive Ventilation in Infants: A Systematic Review and Meta-Analysis. Front Pediatr. 2018;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med. 2010;181(6):626–644. [DOI] [PubMed] [Google Scholar]
- 17.Tieder JS, Bonkowsky JL, Etzel RA, et al. Brief Resolved Unexplained Events (Formerly Apparent Life-Threatening Events) and Evaluation of Lower-Risk Infants. Pediatrics. 2016;137(5). [DOI] [PubMed] [Google Scholar]
- 18.Brockmann PE, Poets A, Poets CF. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3months. Sleep Med. 2013;14(12):1323–1327. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–755. [DOI] [PubMed] [Google Scholar]
- 20.Upton CJ, Milner AD, Stokes GM. Upper airway patency during apnoea of prematurity. Arch Dis Child. 1992;67(4 Spec No):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry RB, Albertario CL, Harding SM, Medicine ftAAoS The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.5. Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 22.Grigg-Damberger MM. The Visual Scoring of Sleep in Infants 0 to 2 Months of Age. J Clin Sleep Med. 2016;12(3):429–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowell DH, Brooks LJ, Colton T, et al. Infant polysomnography: reliability. Collaborative Home Infant Monitoring Evaluation (CHIME) Steering Committee. Sleep. 1997;20(7):553–560. [PubMed] [Google Scholar]