Abstract
Objective
To evaluate clinical outcomes of patients with BRCA-associated ovarian cancer who developed brain metastases (BM).
Methods
Patients with epithelial ovarian, fallopian tube, and primary peritoneal cancer (EOC) and BM, treated at a single institution from 1/1/2008–7/1/2018, were identified from two institutional databases. Charts and medical records were retrospectively reviewed for clinical characteristics and germline BRCA mutation status. Appropriate statistics were used.
Results
Of 3649 patients with EOC, 91 had BM (2.5%). Germline mutation status was available for 63 (69%) cases; 21 (35%) of these harbored a BRCA1/2 mutation (15 BRCA1, 6 BRCA2). Clinical characteristics were similar between groups.
BM were diagnosed at a median of 31 months (95% CI, 22.6–39.4) in BRCA-mutated (mBRCA) and 32 months (95% CI, 23.7–40.3) in wild-type BRCA (wtBRCA) (p=0.78) patients. Brain metastases were the only evidence of disease at time of BM diagnoses in 48% (n=10) mBRCA and 19% (n=8) wtBRCA (p=0.02) patients. There was no difference in treatment of BM by mutation status (p=0.84). Survival from time of BM diagnosis was 29 months (95%CI, 15.5–42.5) in mBRCA and 9 months (95% CI, 5.5–12.5) in wtBRCA patients, with an adjusted hazard ratio (HR) of 0.53, p=0.09; 95% CI, 0.25–1.11. HR was adjusted for presence of systemic disease at time of BM diagnosis.
Conclusion
This is the largest study to date comparing outcomes in patients with EOC and BM by mutation status. mBRCA patients were more likely to have isolated BM, which may be a factor in their long survival. This supports the pursuit of aggressive treatment for mBRCA EOC patients with BM. Additional studies examining the correlation of BRCA mutational status with BM are warranted.
Keywords: Ovarian cancer, Brain metastasis, BRCA mutation
INTRODUCTION
Epithelial ovarian, fallopian tube, and primary peritoneal cancer (EOC) is a rare disease, accounting for approximately 22,000 new cancer cases and over 14,000 deaths in the U.S.annually. It is the fifth leading cause of cancer-related death among women [1]. Although over 75% of patients respond to initial treatment, including surgical resection and chemotherapy, most will recur [2]. The predominant site of recurrence is within the peritoneal cavity [3]. Brain metastases (BM) are rare, with an incidence ranging from 1 to 2% [4], and are often a late complication of EOC. Patients who develop BM have a poor prognosis, with reported overall survival (OS) of only 6–12 months [5–7]. The mainstay of treatment in this setting includes radiation therapy (RT) and surgery, when feasible [8]. Previous studies have shown that performance status, the number of brain metastases, monotherapy for brain recurrence, and poorly controlled extracranial tumor are associated with worse outcomes [6, 7].
BRCA1 and BRCA2 proteins are components of the homologous recombination repair pathway for double strand DNA breaks. When the homologous recombination repair pathway is disrupted, as it is when either the BRCA1 or BRCA2 (BRCA1/2) gene is mutated, a patient is predisposed to hereditary breast and ovarian cancer [9]. Previous studies have shown that EOC patients with a BRCA1/2 mutation are more likely to develop visceral metastases than patients without this mutation [10, 11]. Specifically, these patients may be more likely to develop BM [11, 12]. Despite this, EOC patients with BRCA1/2 gene mutations have a significantly longer OS than patients without a BRCA mutation; this is likely attributable to improved response to DNA-damaging agents such as platinum [13]. The effects of BRCA1/2 mutation on EOC-associated BM has not been explored.
Knowledge of a patient’s BRCA1/2 status has become imperative in the management of EOC, as patients with deleterious mutations are likely to benefit from a new class of therapy: Poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors. Three PARP inhibitors are currently FDA-approved for the management of EOC: olaparib, rucaparib, and niraparib. Although treatment with PARP inhibitors has demonstrated clinical benefit in all patients with EOC, patients with BRCA1/2 mutation (in particular, germline mutations) treated with PARP inhibitors have a longer median progression-free survival (PFS) than patients without the mutation [14–16]. Furthermore, olaparib was recently shown to dramatically improve PFS in patients with BRCA-associated EOC when used as frontline therapy, and has been FDA-approved for use in the first-line maintenance setting for this subgroup of patients [17].
Despite the relatively favorable outcome of EOC patients with BRCA1/2 mutations, there is a dearth of literature exploring the effects of this mutation in patients with EOC-associated BM, and the potentially promising benefits these women may derive from PARP inhibitors. We therefore sought to evaluate the impact of BRCA1/2 mutation status on clinical presentation and survival in patients with EOC who developed BM.
METHODS
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC). The MSKCC Institutional Database (IDB) and the Gynecology Disease Management Team Database were queried for patients with EOC who developed BM between January 1, 2008 and December 31, 2018. It is worth noting that a portion of our cohort (those diagnosed with BM between 2008 and 2010) has been previously described, although not in relation to BRCA mutation status [6].
A retrospective review of medical records was performed to identify demographic and clinical characteristics. Patient charts were queried for germline BRCA1/2 mutation status. Data were collected from reports of either commercially available ovarian cancer gene panels—Myriad Genetics (Myriad Genetics, Inc., Salt Lake City, UT) and GeneDx (Gene Dx, Inc., Gaithersburg, MD)—or the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Mutations (MSK-IMPACT). MSK-IMPACT is a multi-gene targeted capture panel with return of results for somatic and germline mutations [18–20]. MSK-IMPACT initially included germline analysis of 76 genes, and more recently 88 genes, which have been associated with hereditary cancer predisposition per the American College of Medical Genetics (ACMG) guidelines [21]22]. Given the time period of patient ascertainment in this cohort, most patients did not have somatic tumor gene analysis. Therefore, the current study is based solely on germline tumor mutations.
Time to BM diagnosis was calculated from the date of pathologic EOC diagnosis until the date of magnetic resonance (MR) image-confirmed BM diagnosis. OS was calculated from the date of initial pathologic EOC diagnosis until death or last follow-up. Survival from time of BM diagnosis was calculated from the date of image-confirmed BM diagnosis until death or last follow-up. Platinum sensitivity at the time of first relapse was defined as radiologically demonstrated progression, at or greater than 6 months from completion of the last dose of upfront platinum therapy. Presence or absence of extracranial disease at time of BM diagnosis was determined by a computed tomography (CT) scan.
Statistical analyses were performed using SPSS software, version 25.0 (IBM, Armonk, NY). Dichotomous outcomes were compared with the Chi-squared test, except when cell sizes were less than 10, in which case Fisher’s exact test was utilized. Survival was calculated using the Kaplan-Meier method, and compared using the Logrank test. Survival was adjusted for presence of systemic disease at the time of BM diagnosis using Cox proportional hazards model. Results were considered statistically significant if P<0.05 and/or if 95% confidence intervals (CIs) did not cross 1.0.
RESULTS
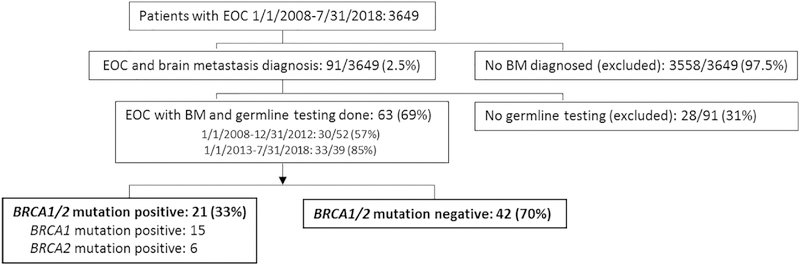
During the 10-year study period, 3649 patients with EOC were seen at our institution for at least 1 cancer treatment related visit. Of these, 91 were noted to have BM (2.5%) (Figure 1). Germline BRCA1/2 mutation status was available for 63 of these 91 (69%) cases: 21 (33%) patients had a BRCA mutation (15 BRCA1 mutation, 6 BRCA2 mutation).
Figure 1.
Patients selected for inclusion
Of 3649 patients with EOC seen between 1/1/08 and 7/31/18, 91 (2.5%) were diagnosed with BM. Of these, 63 had germline testing performed. When grouped by 5-year increments, 30 of 52 (57%) patients diagnosed 1/1/2008–12/31/2012 and 33 of 39 (85%) patients diagnosed 1/1/13–7/31/18 underwent genetic testing. 21 patients had a BRCA mutation: 15, BRCA1; 6, BRCA2. 42 patients were germline BRCA mutation negative.
The majority (n=58, 92%) of these patients were treated at our institution for most of their disease course (including pre- and post-BM diagnosis). Among the patients with BM there were no significant differences in baseline characteristics between those with mBRCA and those with wtBRCA (Table 1). Although not included in this report, it is noteworthy that the clinical characteristics of patients with EOC and BM who did not have germline testing were not significantly different from those who did.
Table 1.
Clinical characteristics of patients with EOC diagnosed with BM, by mutation category
| Clinical characteristic |
BRCA1/2 positive n=21 n (%) |
BRCA1/2 negative n= 42 n (%) | p-value |
|---|---|---|---|
| Median age at diagnosis (range) | 54.0 (36–80) | 59.5 (41–86) | 0.28 |
| Histology | |||
| High-grade serous | 21 (100) | 35 (83) | 0.56 |
| Carcinoma, NOS | 0 (0) | 1 (2) | |
| Mixed histology | 0 (0) | 2 (5) | |
| Endometrioid | 0 (0) | 1 (2) | |
| Clear cell | 0 (0) | 1 (2) | |
| Carcinosarcoma | 0 (0) | 2 (5) | |
| Low-grade serous | 0 (0) | 0 (0) | |
| Stage at diagnosis | |||
| I/II | 0 (0) | 1 (2) | 0.48 |
| III/IV | 21 (100) | 41 (98) | |
| Primary treatment | |||
| PDS | 11 (52) | 21 (50) | 0.86 |
| NACT | 10 (48) | 21 (50) | |
| Debulking surgery (primary or interval) | |||
| Optimal | 18 (86) | 33 (79) | 0.76 |
| Sub-optimal | 0 (0) | 0 (0) | |
| Unknown/did not have surgery | 3 (14) | 9 (21) | |
| Intraperitoneal chemotherapy | |||
| Received | 10 (48) | 11 (26) | 0.09 |
| Did not receive | 11 (52) | 31 (74) | |
| Platinum sensitivity at first recurrence | |||
| Sensitive | 17 (81) | 26 (62) | 0.13 |
| Resistant | 4 (19) | 16 (38) | |
NOS, not otherwise specified; PDS, primary debulking surgery; NACT, neoadjuvant chemotherapy
Median age at diagnosis was 59 years (range, 36–86). The most common histologic subtype was high-grade serous (HGS) carcinoma (n=56; 89%). Most patients had stage III/IV disease at initial diagnosis (n=62; 98%) (Table 1). Primary treatment included frontline cytoreductive surgery in 51% (n=32), and neoadjuvant chemotherapy in 49% (n=31). Cytoreductive surgery was considered optimal in 81% (n=51) of patients. As previous research has suggested that patients treated with intraperitoneal (IP) chemotherapy had extra-abdominal disease recurrence more frequently than those treated with IV chemotherapy [22], we noted that, of the entire cohort, 67% (n=42) received IP chemotherapy. Sixty-eight percent (n=43) were considered to have platinum-sensitive disease at the time of first recurrence.
The majority of these patients (n=59; 94%) were diagnosed with and treated for their BM at our institution: 20 (95%) mBRCA, 39 (93%) wtBRCA. Forty percent (n=25) of BM cases were confirmed by pathology, with an additional 5% (n=3) showing adenocarcinoma in cerebrospinal fluid cytology. Of the 25 tumors submitted for pathology review, 24 (96%) showed HGS carcinoma consistent with a primary, and 1 (4%) tumor sample showed necrotic tissue that was insufficient for further analysis.
BM were diagnosed at a median of 31 months (95% CI, 22.6–39.4) from the time of initial diagnosis in the mBRCA group, and 32 months (95% CI, 23.7–40.3) in the wtBRCA group (p=0.78) (Table 2). BM were diagnosed at the time of first recurrence in 38% (n=8) of mBRCA and 20% (n=8) of wtBRCA patients (p=0.10). There was no extracranial disease at the time of BM diagnoses in 48% (n=10) of mBRCA patients and 19% (n=8) wtBRCA patients (p=0.02). The number of BM lesion at diagnosis was 1 in 24% (n=5) of mBRCA and 36% (n=15) of wtBRCA patients (p=0.34), while the remaining patients were noted to have 2 or more lesions. Two patients (3%) had leptomeningeal disease at the time of BM diagnosis; both were wtBRCA patients.
Table 2.
Characteristics of BM diagnosis in women with EOC, by mutation category
| Brain Metastasis characteristics | BRCA1/2 positive n=21 n (%) | BRCA1/2 negative n= 42 n (%) | p-value |
|---|---|---|---|
| Time to BM diagnosis (m, 95% CI) | 31 (22.6–39.4) | 32 (23.7–40.3) | 0.78 |
| BM as first recurrence | |||
| First recurrence | 8 (38) | 8 (20) | 0.10 |
| Not first recurrence | 13 (62) | 34 (80) | |
| Extracranial disease | |||
| Present | 11 (52) | 34 (81) | 0.02 |
| Absent | 10 (48) | 8 (19) | |
| Number of BM lesions | |||
| 1 lesion | 5 (24) | 15 (36) | 0.34 |
| More than 1 lesion | 16 (76) | 27 (64) | |
BM, brain metastasis
There was no difference in treatment of BM between patients with or without BRCA mutation (p=0.84). Best supportive care was provided to 5% (n=3) of the cohort; all 3 of these patients had widely metastatic disease, and declined treatment. Ninety percent (n=57) underwent RT; of these, 49% (n=28) also had surgical resection of their disease. Of the 57 patients who underwent any RT, 17 (30%) had whole brain RT and 40 (70%) had lesion-targeted RT. In those patients who underwent both surgery and RT, the surgical resection of the lesion(s) was done first, followed by RT to the surgical bed. Thirty-nine patients (68%) were noted to have radiologic response to RT (including complete response, partial response, and stable disease). There was no difference in response to RT by BRCA status (p=0.90). Two patients (3%) did not have treatment specifically for BM, but instead underwent close monitoring of their BM while they continued the systemic chemotherapy they had received prior to their BM diagnosis. Treatment information was not available for 1% (n=1); this patient was lost to follow-up after her BM diagnosis.
None of the patients received a PARP inhibitor as part of treatment specifically for BM. However, PARP inhibitor therapy was used by 19% of patients (n=12) at some point for treatment of systemic disease; 7 of these patients were BRCA mutation positive and 5 were not. Of the 5 wtBRCA patients who received a PARP inhibitor, 4 received it as part of a clinical trial (during which a PARP inhibitor was given in combination with chemotherapy) prior to their diagnosis with BM: 2 of these patients were prescribed rucaparib, 1 niraparib, and 1 veliparib. One patient received niraparib as treatment for late-stage disease, after her tumor was noted to have a somatic BRCA1 mutation. Of the 7 BRCA mutation positive patients, 3 were prescribed a PARP inhibitor (olaparib) before their BM diagnosis; 4 were prescribed a PARP inhibitor after their BM diagnosis (2 olaparib, 1 rucaparib and 1 niraparib).
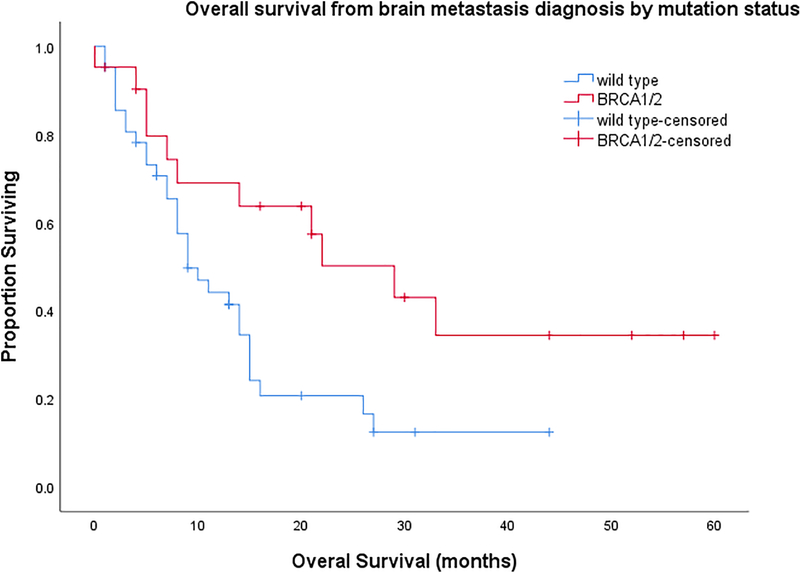
After a median follow-up of 48 months (range, 13–105), there were 42 deaths (67% of included patients). Median OS was 70 months (95%CI, 52.1–87.8) for patients with a BRCA mutation and 52 months (95%CI, 41.9–62.1) for patients with no mutation. Survival from the time of BM diagnosis was 29 months (95%CI, 15.5–42.5) for patients with a BRCA mutation and 9 months (95% CI, 5.5–12.5) for those without a mutation (p=0.015), adjusted HR 0.53, p=0.09; 95% CI, 0.25–1.11). HR was adjusted for presence of systemic disease at the time of BM diagnosis (Figure 2).
Figure 2.
Overall survival from time of brain metastasis in patients with EOC
Kaplan-Meier curve for overall survival by mutation category and adjusted HR (aHR) for death. HR adjusted for presence of extracranial disease.
DISCUSSION
EOC-associated BM has historically carried a poor prognosis, with median survival of less than 12 months following diagnosis of BM. It has been demonstrated that risk factors, including a greater number of metastases and poorly controlled extracranial disease, confer worse outcomes [6, 7]. Previous research suggests that extraperitoneal disease, including BM, may be more common in patients who carry a BRCA1/2 gene mutation [11, 12]. However, there is little data on the clinical outcomes of patients with EOC-associated BM, with respect to BRCA mutation status.
In a study exploring the relationship between BRCA1/2 mutations and EOC metastatic to the central nervous system (CNS), Jernigan et al. used a family history of hereditary breast and ovarian cancer (HBOC) as a surrogate for germline mutation [12]. The study had limited data: of 32 cases with a family history of HBOC, only 7 underwent germline testing. However—although it did not establish a correlation between HBOC status and survival—this hypothesis-generating report showed that the presence of BRCA mutation/history of HBOC was more common in women with EOC metastatic to the CNS.
More recently, Ratner et al. conducted a time-to-event analysis of a cohort of patients with EOC, to determine whether patients with a germline BRCA mutation were more likely to develop BM compared to wtBRCA patients [11]. This analysis identified 46 of 4515 EOC patients with BM (overall rate, 1%) and noted a higher rate of BM among mBRCA (3%), compared to wtBRCA (0.6%) patients. Importantly, there was no survival difference between the two groups following diagnosis of BM.
The present study builds on these reports in describing outcomes of patients with EOC and BM by BRCA mutation status. We found that, although time to diagnosis of BM does not differ by mutation status, patients with a BRCA mutation are more likely to have BM diagnosed in the absence of systemic disease and have a non-statistically significant longer survival time following BM diagnosis, than patients without a BRCA mutation. Importantly, there were no differences in treatment of BM between the two groups; only 3 patients in the entire cohort received best supportive care, and the remainder received RT, surgery, chemotherapy, or a combination of these. Our findings support prior research noting lack of extracranial disease as a predictor for longer survival following diagnosis of BM [6]. Similar to previous findings, when controlling for presence of extracranial disease, a statistically significant difference in survival between the mutated and wild-type groups disappears [11]. This may be because OS is primarily driven by disease burden and extent of disease systemically; or it may be due to the relatively small cohort of patients in this study.
We show that patients with BRCA1/2 mutation are more likely than those without a mutation to have BM without extracranial disease, which may contribute to their long survival. This is probably related to the exquisite chemosensitivity of BRCA-associated EOC to platinum-based therapy, facilitating systemic disease control. This is also the case in HER2-positive breast cancer, where isolated CNS metastases in the setting of systemic disease control is seen with the extremely effective anti-cancer therapy trastuzumab. [23]. Platinum-based chemotherapy provides excellent systemic control but has limited penetrance of the blood/brain barrier, and therefore plays a limited role in preventing CNS tumor spread.
The mutation status of patients with EOC-related BM is critically important, as these patients may be candidates for PARP inhibitor therapy. Although none of the women in our cohort received PARP inhibitors specifically for BM, these novel therapies have potential implications for the treatment of BM.
Preclinical studies suggest that neither rucaparib nor olaparib cross the blood/brain barrier [24–26]. However, there is a case report of olaparib used successfully as treatment for leptomeningeal disease in 1 patient with mBRCA EOC [27]. After a 2-year disease-free interval following completion of primary treatment, the patient was noted to have disease recurrence with isolated BM. She underwent resection and RT to the lesion beds but had another BM recurrence 1 year later and was treated with RT alone. Over the next several months she was noted to have leptomeningeal disease, at which time single-agent carboplatin was attempted; however, she was intolerant of the drug. Given her worsening neurologic symptoms, she was started on single-agent olaparib and derived 12 months of clinical benefit from the therapy before progression of disease was noted, and the drug discontinued [27].
Niraparib has been shown to cross the blood/brain barrier, and its efficacy in BM treatment has been confirmed in animal models [26, 27]. Veliparib, a PARP inhibitor that is not currently FDA-approved for EOC, also crosses the blood/brain barrier and has demonstrated promising results when used with whole brain RT, in treating BM associated with solid tumors [28]. As more patients—especially in the maintenance setting—are prescribed PARP inhibitors that can penetrate the blood/brain barrier, it is plausible that the rate of CNS disease will decrease as these highly active drugs infiltrate this disease sanctuary.
The current study is limited by its retrospective nature and small sample size. Of note, 31% (n=28/93) of patients with BM in our database were excluded from analysis due to their unknown BRCA status. The Society of Gynecologic Oncology did not endorse genetic testing for all EOC patients until 2014, and our study period began in 2008. When grouped by 5-year increments, 30 of 52 (57%) patients diagnosed between January 1, 2008 and December 31, 2012, and 33 of 39 (85%) patients diagnosed between January 1, 2013 and July 31, 2018 underwent genetic testing. Although most of the patients included in our study received their care (pre- and post- BM diagnosis) at our institution, we do not have the longitudinal data on the treatment of all patients with EOC seen at our institution; therefore, the stated 2.5% rate of BM may be an under- or an over-estimate.
Recent studies have shown that patients with somatic BRCA1/2 gene mutations also have an improved prognosis independent of germline mutation status, and up to 6% of HGS tumors harbor a somatic BRCA mutation [13, 29, 30]. Furthermore, somatic BRCA mutations are biologically similar to germline BRCA mutations, and patients with somatic BRCA mutations also show improved responses to PARP inhibitors [15, 17]. Unfortunately, due to the time period of this study, only 33% (n=21/63) of patients had somatic tumor profiling. Therefore, we were unable to include data on somatic BRCA mutation status and BM outcomes.
BM in EOC is a rare occurrence. Although this was a relatively small cohort, it is the largest analysis to date comparing outcomes of patients with EOC and BM by BRCA1/2 mutation status. We have shown that women with a BRCA mutation are significantly more likely to have BM without extracranial disease; this may contribute to their long survival. The long survival we observed supports the pursuit of aggressive, disease-targeted treatment for BM in BRCA1/2 mutated EOC patients. Additional studies examining the correlation of both somatic and germline BRCA mutation with brain metastases, and the evolving use of PARP inhibitor therapy, are warranted.
HIGHLIGHTS.
Approximately 2.5% of epithelial ovarian cancer patients develop brain metastases
There was no difference in time from EOC diagnosis to brain mets in mBRCA vs wtBRCA
mBRCA patients were more likely to have brain mets without extracranial disease
Median survival from brain mets diagnosis to death was longer in mBRCA patients
ACKNOWLEDGEMENTS
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Disclosures:
Dr. Beal reports other*, outside the submitted work (*MMT shareholder)
Dr. Chi reports personal fees from Bovie Medical Co., personal fees from Verthermia Inc., personal fees from C Surgeries, outside the submitted work.
Dr. Cadoo reports other* from Astra Zeneca, other** from Syndax Pharmaceuticals, outside the submitted work (*travel, accommodation, meal; institutional support for therapeutic trial; **institutional support for therapeutic trial)
Dr. Konner reports personal fees from AstraZeneca, personal fees from Immunogen, personal fees from Clovis Oncology, outside the submitted work.
Dr. Long Roche reports other* from Intuitive Surgical Inc., outside the submitted work (*airfare to a survivorship conference, where she spoke).
Dr. Makker reports grant and personal fees from Eisai for service on Advisory Board, National PI E7080-A001-111; grant and personal fees from Merck for service on Scientific Advisory Board; International PI 775-03/E7080-G000-309; grant from Takeda (MSKCC PI C31004); personal fees from ArQule for service on Advisory Board, honoraria; grant and personal fees from Karyopharm (SAC and PI of 14-110); grant from AstraZeneca (PI-16-1491); grant from Lilly (PI 15-079 and 18-107); personal fees from IBM Watson, outside the submitted work.
Dr. O’Cearbhill reports personal fees from Clovis for service on Advisory Board, personal fees from Tesaro for service on Advisory Board, outside the submitted work.
Footnotes
Conflict of Interest Statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CS, Maxwell GL, Darcy KM, Hamilton CA, McGuire WP. Current approaches and challenges in managing and monitoring treatment response in ovarian cancer. J Cancer 2014;5:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amate P, Huchon C, Dessapt AL, Bensaid C, Medioni J, Le Frère Belda MA, et al. Ovarian cancer: sites of recurrence. Int J Gynecol Cancer 2013;23:1590–6. [DOI] [PubMed] [Google Scholar]
- 4.Pietzner K, Oskay-Oezcelik G, El Khalfaoui K, Beohmer D, Lichtenegger W, Sehouli J. Brain metastases from epithelial ovarian cancer: overview and optimal management. Anticancer Res 2009;29:2793–8. [PubMed] [Google Scholar]
- 5.Cohen ZR, Suki D, Weinberg JS, Marmor E, Lang FF, Gershenson DM, et al. Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neurooncol 2004;66:313–25. [DOI] [PubMed] [Google Scholar]
- 6.Teckie S, Makker V, Tabar V, Alektiar K, Aghajanian C, Hensley M, et al. Radiation therapy for epithelial ovarian cancer brain metastases: clinical outcomes and predictors of survival. Radiat Oncol 2013;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchetti C, Ferrandina G, Cormio G, Gambino A, Cecere S, Lorusso D, et al. Brain metastases in patients with EOC: Clinico-pathological and prognostic factors. A multicentric retrospective analysis from the MITO group (MITO 19). Gynecol Oncol 2016;143:532–8. [DOI] [PubMed] [Google Scholar]
- 8.Pothuri B, Chi DS, Reid T, Aghajanian C, Venkatraman E, Alektiar K, et al. Craniotomy for central nervous system metastases in epithelial ovarian carcinoma. Gynecol Oncol 2002;87:133–7. [DOI] [PubMed] [Google Scholar]
- 9.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 2011;108:18032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourley C, Michie CO, Roxburgh P, Yap TA, Harden S, Paul J, et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol 2010;28:2505–11. [DOI] [PubMed] [Google Scholar]
- 11.Ratner E, Bala M, Louie-Gao M, Hazard S, Brastianos P. Brain metastases in primary ovarian cancer: real-world data. Ann Oncol 2018;29(Suppl 8):viii332–358. [Google Scholar]
- 12.Jernigan AM, Mahdi H, Rose PG. Epithelial Ovarian Cancer Metastatic to the Central Nervous System and a Family History Concerning for Hereditary Breast and Ovarian Cancer--A Potential Relationship. Int J Gynecol Cancer 2015;25:1232–8. [DOI] [PubMed] [Google Scholar]
- 13.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 2014;20:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154–64. [DOI] [PubMed] [Google Scholar]
- 16.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852–61. [DOI] [PubMed] [Google Scholar]
- 17.Moore K, Columbo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495–505. [DOI] [PubMed] [Google Scholar]
- 18.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyman DM, Solit DB, Arcila ME, Cheng DT, Sabbatini P, Baselga J, et al. Precision medicine at Memorial Sloan Kettering Cancer Center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today 2015;20:1422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 2017;19:249–55. [DOI] [PubMed] [Google Scholar]
- 22.Tanner EJ, Black DR, Zivanovic O, Kehoe SM, Dao F, Konner JA, et al. Patterns of first recurrence following adjuvant intraperitoneal chemotherapy for stage IIIC ovarian cancer. Gynecol Oncol 2012;124:59–62. [DOI] [PubMed] [Google Scholar]
- 23.Lim YJ, Lee SW, Choi N, Kwon J, Eom KY, Kang E, et al. Failure patterns according to molecular subtype in patients with invasive breast cancer following postoperative adjuvant radiotherapy: long-term outcomes in contemporary clinical practice. Breast Cancer Res Treat 2017;163:555–63. [DOI] [PubMed] [Google Scholar]
- 24.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 2008;105:17079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray J, Thomas H, Berry P, Kyle S, Patterson M, Jones C, et al. Tumour cell retention of rucaparib, sustained PARP inhibition and efficacy of weekly as well as daily schedules. Br J Cancer 2014;110:1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikule K, Wilcoxen K. The PARP inhibitor, niraparib, crosses the blood brain barrier in rodents and is efficacious in a BRCA2-mutant intracranial tumor model [abstract]. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2015 Nov 5–9; Boston, MA. Philadelphia (PA): AACR; Mol Cancer Ther 2015;14(12 Suppl 2):Abstract nr B168. [Google Scholar]
- 27.ZEJULA® (niraparib) [package insert]. Waltham, MA: Tesaro, Inc; 2017. [Google Scholar]
- 28.Mehta MP, Wang D, Wang F, Kleinberg L, Brade A, Robins HI, et al. Veliparib in combination with whole brain radiation therapy in patients with brain metastases: results of a phase 1 study. J Neurooncol 2015;122:409–17. [DOI] [PubMed] [Google Scholar]
- 29.Norquist BM, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner S, et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 2018;24:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]