Abstract
Some organisms, such as the Mexican axolotl, have the capacity to regenerate complicated biological structures throughout their lives. Which molecular pathways are sufficient to induce a complete endogenous regenerative response in injured tissue is an important question that remains unanswered. Using a gain-of-function regeneration assay, known as the Accessory Limb Model (ALM), we and others have begun to identify the molecular underpinnings of the three essential requirements for limb regeneration; wounding, neurotrophic signaling, and the induction of pattern from cells that retain positional memory. We have previously shown that treatment of Mexican axolotls with exogenous retinoic acid (RA) is sufficient to induce the formation of complete limb structures from blastemas that were generated by deviating a nerve bundle into an anterior-located wound site on the limb. Here we show that these ectopic structures are capable of regenerating and inducing new pattern to form when grafted into new anterior-located wounds. We additionally found that the expression of Alx4 decreases, and Shh expression increases in these anterior located blastemas, but not in the mature anterior tissues, supporting the hypothesis that RA treatment posteriorizes blastema tissue. Based on these and previous observations, we used the ALM assay to test the hypothesis that a complete regenerative response can be generated by treating anterior-located superficial limb wounds with a specific combination of growth factors at defined developmental stages. Our data shows that limb wounds that are first treated with a combination of FGF-2, FGF-8, and BMP-2, followed by RA treatment of the resultant mid-bud stage blastema, will result in the generation of limbs with complete proximal/distal and anterior/posterior limb axes. Thus, the minimal signaling requirements from the nerve and a positional disparity are achieved with the application of this specific combination of signaling molecules.
Introduction:
One of the long-term goals of regenerative medicine is to elicit an endogenous regenerative response in humans through the application of defined signaling molecules. This may be particularly important in the context of regenerating complicated multi-cell-type organs that need to have proper integration with the remaining tissues to restore function. To work towards this goal, we used a gain-of-function limb regeneration assay in the Mexican axolotl, a model organism that is capable of regenerating complete limbs, to test the signaling requirements for the induction of a regenerative response in non-regenerative lateral limb wounds.
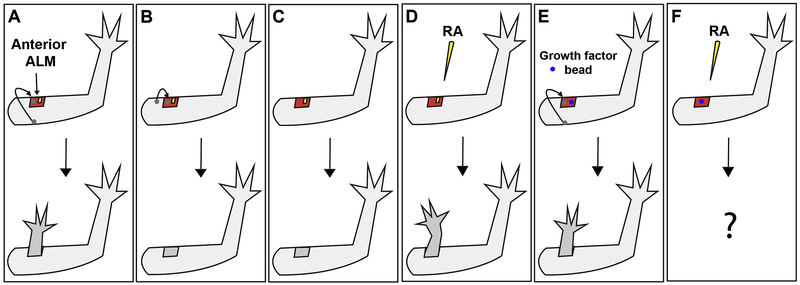
In the axolotl, superficial lateral limb wounds do not elicit a regenerative response unless a nerve and a graft of tissue from the opposing limb axis are present in the wound site (Figure 1A-C) (Endo et al., 2004; Lheureux, 1977; Maden and Holder, 1984). This surgical manipulation is known as the Accessory Limb Model (ALM) and is a powerful gain of function regeneration assay that can be used to test the basic steps of regeneration (wounding, innervation, and positional discontinuity) (McCusker and Gardiner, 2013; McCusker et al., 2016; 2014; Satoh et al., 2008a; 2008b; 2007). Using this assay, it was observed that simple wounding and innervation are sufficient to induce the formation of the regenerative organ known as the blastema (Endo et al., 2004). In an eloquent combinatorial study, Makanae et al. discovered that limb wounds lacking nerves but treated with different combinations of FGF’s and BMPs or TGFb are able to undergo blastema formation in the ALM (Makanae et al., 2014). However, only certain combinations of these factors in conjunction with a tissue graft from the opposing axis on the limb were capable of generating limb structures (Makanae et al., 2014). For example, both combinations of FGF-2 and FGF-8 with either BMP-2 or BMP-4 are capable of generating limb blastemas in the ALM. However, only the latter cocktail will result in the formation of ectopic limb structures when skin from the opposite side of the limb is grafted into the wound (Makanae et al., 2014). These findings indicate that these factors can take the place of the endogenous neurotrophic signals during blastema formation; however, only specific combinations of these factors make the cells capable of generating the new pattern of the regenerate (Figure 1E) (Makanae et al., 2014; 2013).
Figure 1: Growth factor replacement of tissue-specific signaling during limb regeneration.
(A) Ectopic limb structures are generated from an anterior-located wound site with a nerve (yellow) and a graft of full-thickness skin (grey circle) from the posterior side of the limb (Endo et al., 2004). (B) A regenerative response is not induced by the same manipulation as in “A” when the grafted tissue is obtained from the anterior side of the limb. (Phan et al., 2015) (C) A wound site with only a nerve (no graft) does not result in regeneration (Endo et al., 2004). (D) Treatment of a wound site with only a nerve, with retinoic acid (RA) results in the formation of ectopic limbs. (McCusker et al., 2014) (E) Implantation of a gelatin bead soaked in FGF-2, FGF-8, and BMP-2 into an anterior-located wound with a tissue graft (no nerve) results in the formation of limb structures (Makanae et al., 2014). (F) In the current study, we are combining the treatments described in “D” and “E” to determine whether ectopic limbs can be induced in superficial wounds treated consecutively with growth factors.
Once the blastema cells receive the appropriate trophic signals, either from nerves or a combination of specific signaling molecules (BMP-4, FGF-2 and FGF-8) to become capable of generating new pattern; the blastema must contain cells with positional identities from different axes of the limb to generate new limb structures (Endo et al., 2004; Makanae et al., 2014; Niazi et al., 1985). In the ALM, this is most commonly achieved by grafting tissue from the opposing side of the limb into the wound site. We have additionally shown that treatment with exogenous retinoic acid (RA) can take the place of tissue grafts in innervated ALM wounds (Figure 1D) (McCusker et al., 2014). RA is an important patterning molecule during embryogenesis and is hypothesized to reprogram the positional information of blastema cells to the most posterior, ventral, proximal (PVPr) limb identity (Maden, 1983; Crawford and Stocum, 1988; Kim and Stocum, 1986; Ludolph et al., 1990; Bryant and Gardiner, 1992; Scadding, 1999; McCusker et al., 2014). Consistent with this hypothesis, we observed that RA treatment of ALM blastemas located in the anterior or dorsal sides of the limb, as opposed to the posterior and ventral locations, results in a complete regenerative response (McCusker et al., 2014). This result indicates that the mature tissue is resistant to RA-mediated reprogramming while the blastema tissue is reprogrammed so to generate sufficient positional disparity with the surrounding mature tissue such that a regenerative response is elicited (McCusker et al., 2014). To further test this hypothesis, in the current study we analyzed the expression of an anterior positional limb marker, Alx4, and a posterior patterning signaling molecule, Sonic Hedgehog (Shh), in anterior and posterior located blastemas. We observed that anterior blastemas lose the expression of Alx4 and gain the expression of Shh when treated with RA, while no changes in expression are observed in the mature limb tissues.
In addition to RA, other biological molecules can induce the formation of limb structures in ALM blastema in the absence of a tissue graft. However, the ability for ALM blastemas located at different positions around the limb circumference to form growths, as well as the overall pattern complexity of these growths, differ according to the molecules that are applied. For example, decellularized extracellular matrix (ECM) from the posterior side of the limb induces hypomorphic limb structures that look like a digit when grafted into an anterior located ALM site (Phan et al., 2015). However the reverse manipulation of grafting anterior ECM into a posterior ALM does not result in an ectopic growth (Phan et al., 2015), indicating that position specific cues are present in the ECM of the limb. Specific signaling molecules have also been applied to ALM wounds to determine whether they can induce pattern formation without the presence of a skin graft. For example, similar to what we have observed with RA treatments, overexpression of Shh results in the formation of ectopic limb structures in anterior, but not posterior, located ALMs and this ability is dependent on FGF signaling (Nacu et al., 2016). Interestingly, the overexpression of FGF-8 can induce the formation of ectopic limbs in both anterior and posterior ALMs, and this ability is dependent on Shh activity (Nacu et al., 2016). It should be noted that the structures that are induced by either Shh or FGF-8 lack normal limb pattern. The limbs are incomplete in terms of both their proximal-distal and anterior-posterior patterns; they lack a stylopod, they appear to be missing one of the elements in the zeugopod, and their autopod exhibits symmetry along the anterior-posterior axis (Nacu et al., 2016). Thus, it appears that although these signaling molecules confer positional cues to the blastemas cells, additional positional signals are required to generate limbs with complete anterior-posterior and proximal-distal axes. Because of this, in the current study we have decided to use RA treatment to induce pattern formation because it is the only molecule thus far that can induce limbs with complete anterior-posterior and proximal-distal axes to form in the ALM (McCusker et al., 2014).
Another important aspect that should be noted is that Shh and FGF-8 overexpression do not appear to permanently alter the positional information in the blastema cells. This is exhibited by the observation that the growths that are induced by increased Shh or FGF-8 activity do not regenerate upon amputation (Nacu et al., 2016). Additionally, if tissues from these anterior-located ectopic structures are grafted into either an anterior or posterior located ALM, they are only able to induce structures in the latter, indicating that the ectopic growths have only anterior positional information (Nacu et al., 2016). This also reveals that, although they provide positional cues in the blastema and the blastema cells are responding to these cues, neither Shh nor FGF-8 are permanently changing the positional information in the blastema cells. In the current study, we perform similar assays on RA-induced structures in the ALM and found that they are able to both regenerate, and induce ectopic structures to form in anterior-located ALMs, suggesting that posterior positional information has been stabilized in the RA-treated blastemas. This indicates that the roles of Shh and FGF-8 in patterning are likely to be downstream of the interactions between cells with positional information, which can be reprogramed by RA.
Last, we show that the generation of anatomically complete forelimbs can be induced in superficial “non-regenerative” anterior-located wounds through the application of specific signaling molecules at different time points. We first treated the wound with FGF-2, FGF-8, and BMP-2 to induce the formation of an ectopic blastema that is capable of pattern formation, as Makanae et al. had previously shown (Makanae et al., 2014). These blastemas were then treated with RA to induce a positional disparity in the wound site (McCusker et al., 2014). The resultant limbs contained all three limb segments, the zeugopod, stylopod, and autopod, with complete anterior/posterior asymmetry. Thus, FGF-2, FGF-8, BMP-2, and RA treatments alone provided the signaling requirements that are sufficient for complete limb regeneration at a site of injury.
Results and discussion:
Retinoic acid treatment reprograms the positional memory of blastema cells
It has been well-established that retinoic acid treatment alters the pattern in regenerating and developing limbs. Exogenous RA results in the proximilization (Maden, 1983; Niazi et al., 1985) and posteriorization of blastema cells (McCusker et al., 2014). Recently, it was shown that ectopic activation of Shh signaling also results in the generation of ectopic limb structure in anterior, but not posterior, located blastemas, indicating that Shh also posteriorizes the anterior blastema (Nacu et al., 2016). As we have explained above, these Shh-induced structures do not regenerate when amputated (Nacu et al., 2016), revealing that these structures do not have enough positional diversity to generate new pattern. Moreover, grafts of tissue from the Shh induced structures into an anterior located ALM do not result in the generation of ectopic structures; therefore, the positional identity of the outgrowths appear to be only anterior in nature (Nacu et al., 2016). Together, these data suggests that while Shh signaling is providing posterior pattern information in anterior-located blastemas, this signal is not permanently altering the positional memory in these cells. Thus, we queried whether exogenous RA acted in a similar manner.
To test this hypothesis, we induced the formation of ectopic limbs by treating anterior located blastemas with RA, at a dosage of 150μg per gram of body weight, on transgenic animals expressing GFP. Once the ectopic structures had finished growing, we analyzed the positional information in the tissues by 1) amputating the ectopic structures to determine whether they regenerated and thus had enough positional diversity to make a new structure (Figure 2, Table 1), and 2) grafting skin from the GFP+ amputated structures into a new anterior-located ALM on a white animal to test whether the tissue has posterior positional information (Figure 3, Table 2).
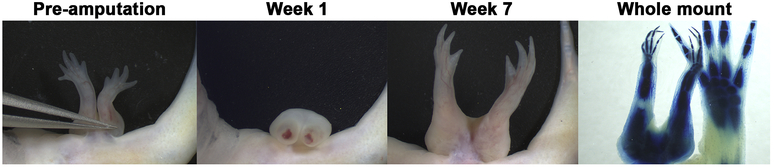
Figure 2: RA induced ectopic limbs exhibit regenerative capabilities in response to amputation.
Representative images of an RA-induced ectopic structure, a paired limb phenotype, which regenerated over 7 weeks in response to amputation. Each limb within the pair was restored in its entirety, with complete limb pattern. After 7 weeks the limbs where collected and prepared for whole mount staining to allow for visualization of the skeletal pattern of the regenerated structure.
Table 1:
Regenerative capacities of RA induced ectopic limbs
| Phenomenological category | Number of ectopic structures amputated |
Percentage displaying regenerative capacity |
|---|---|---|
| Bulbous mass | 3 | 2 (66.7%) |
| Single or paired multiple symmetrical elements | 2 | 2 (100%) |
| Single multiple asymmetrical elements or single complete limbs | 7 | 2 (100%) |
| Paired limbs or paired multiple asymmetrical elements* | 5** | 4.5 (90%***) |
includes one paired structure composed of a complete limb and another axis with multiple symmetrical elements.
6 ectopic paired structures were amputated; however, one animal died 3 weeks after amputation and was not included in the subsequent analysis.
Only 1 of the 10 individual limbs of the patterned paired structures failed to regenerate.
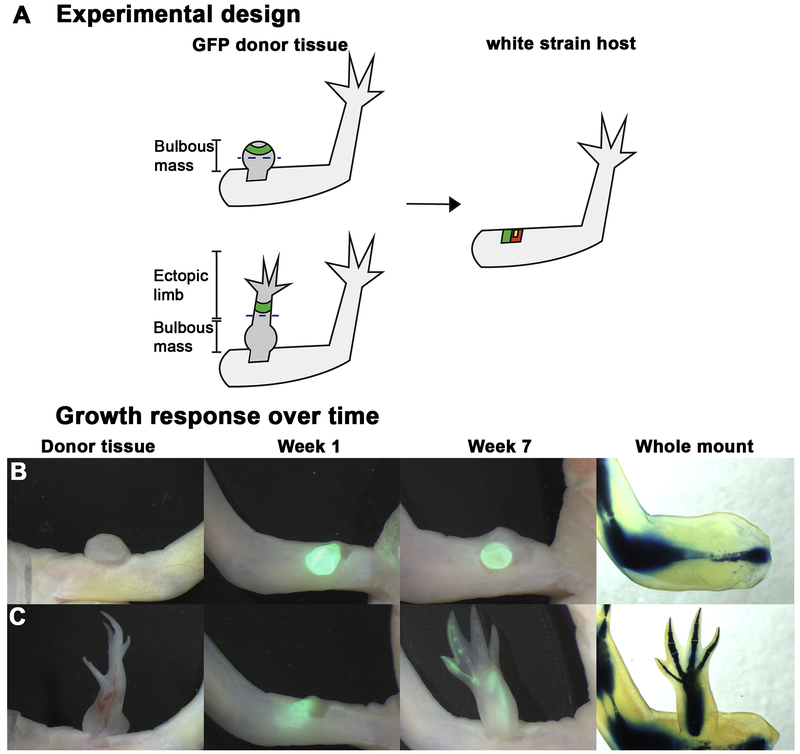
Figure 3: Full thickness skin derived from anteriorly located, RA-induced ectopic limbs is sufficient to elicit pattern when grafted into anterior, innervated wound sites.
A) Diagrammatic representation of tissue harvest sites and amputation planes in RA-induced bulbous masses and patterned ectopic outgrowths on GFP donor animals. Dashed line represents the plane of amputation. The amputated structures were monitored for a regenerative response. The green tissue represents the region of full thickness skin grafted into innervated, anterior wound sites on white animals. Yellow square represents the deviated nerve, red square represents the wound site. B-C) Growth response over time. Representative live images of the regenerative response elicited when skin was grafted from anteriorly located RA-induced ectopic structures into anterior innervated wound sites. Skin grafts (green fluorescent tissue) were obtained either from a bulbous mass (B), or from a patterned, RA-induced outgrowth (C) (see table 2). After 7 weeks the limbs where collected and prepared for whole mount skeletal staining to visualize the pattern of the regenerated structure
Table 2:
Induction of ectopic growths in anterior ALMs from RA-induced tissue grafts
| Donor tissue | Total ALMs performed |
ALMs Counted* |
No Element |
Single Nodule |
Multiple symmetrical Elements |
Multiple asymmetrical Elements |
Complete Limb |
|---|---|---|---|---|---|---|---|
| Bulbous mass graft | 3 | 3 | 3 (100%) | 0 | 0 | 0 | 0 |
| Multiple symmetrical elements | 4 | 4 | 0 | 2 (50%) | 2 (50%) | 0 | 0 |
| Multiple asymmetrical elements OR complete limb | 17 | 16 | 5 (31.25%) | 0 | 1 (6.25%) | 6 (37.5%) | 4 (25%) |
Only wounds that formed a blastema were further analyzed for growth phenotypes
As we have seen previously, RA treatment of anterior located ALMs results in the generation of complete limb structures with high frequency; however, sometimes we observe the generation of structures with less complicated patterns, such as single masses of cartilage (bulbous mass) or multiple symmetrical elements (Supplemental Figure 1)(McCusker et al., 2014). Regardless of the complexity of pattern, these ectopic growths were amputated as described in the materials and methods, were followed for 7 weeks, and the limbs were harvested and whole mount stained for cartilage to assess the regenerative phenotypes. We observed that the RA-induced ectopic structures were able to regenerate a pattern similar to the original, regardless of the complexity of the original ectopic structure (Table 1, Figure 2). This result indicates that RA treatment stably alters the positional memory in the regenerating cells, which have enough positional diversity to generate new structure when amputated.
To determine whether the positional memory in the anterior-located blastemas had been stably reprogramed to a posterior identity by RA treatment, we grafted a complete circumference of skin from the ectopic limbs into a new anterior located ALM (Figure 3, Table 2). We used a complete circumference of skin because the anterior/posterior sides of the ectopic growths were not always evident, and we wanted to ensure that all the cells that could cause a positional discontinuity in the ALM were present in the graft. Interestingly, while we observed that most of the grafts from the most completely patterned ectopic limbs resulted in induction of ectopic growths in anterior ALMs, the grafts from the bulbous masses do not (Figure 3, Table 2). These observations indicate that the more complicated structures have stabilized posterior information, while the bulbous masses are either composed of cells with anterior limb information only, or have an identity outside of the limb field as previously hypothesized (Thoms & Stocum, 1984).
In summary, exogenous RA and Shh both provide posterior information that results in the formation of ectopic limb structures in anterior-located ALMs (Figure 2) (Nacu et al., 2016). However, Shh induced structures only have anterior positional memory (Nacu et al., 2016), while RA induced structures have posterior positional memory as well (Figure 3). This indicates that RA is stably reprogramming the positional memory in the regenerating cells, while Shh is providing a transient signal that the blastema cells are responding to. Our hypothesis is that RA reprograms blastema cells to have a posterior positional memory, and they use this memory to induce the expression of signaling molecules, such as Shh, to communicate this posterior information to the surrounding cells. Thus, we next tested whether exogenous RA promoted the expression of Shh in limb blastemas.
RA signaling differentially alters Shh and Alx4 expression in anterior and posterior located ALM blastemas
We have previously shown that RA treatment induces the formation of ectopic limbs in anterior but not posterior located ALM blastemas (McCusker et al., 2014). This result indicates that 1) RA treatment results in the generation of cells with posterior identities in the anterior-located blastema and 2) that the mature limb tissue must be resistant to RA-mediated positional reprogramming. Additionally, as we have explained above, since RA stably reprograms blastema cells to have a posterior identity, while Shh appears to provide transient posterior information, we hypothesized that RA signaling is upstream of Shh in the regenerate. To test these hypotheses we performed a marker analysis using Q-RTPCR on anterior and posterior located mid-bud staged blastemas (induced by innervating a lateral limb wound) and mature limb tissue at two or seven days after treatment with either DMSO or RA, (Figure 4). We analyzed the expression of Alx4, which is a marker for anterior limb tissue, and Shh, which is strongly expressed in the posterior side of the developing limb (Panman et al., 2005; Tickle and Towers, 2017).
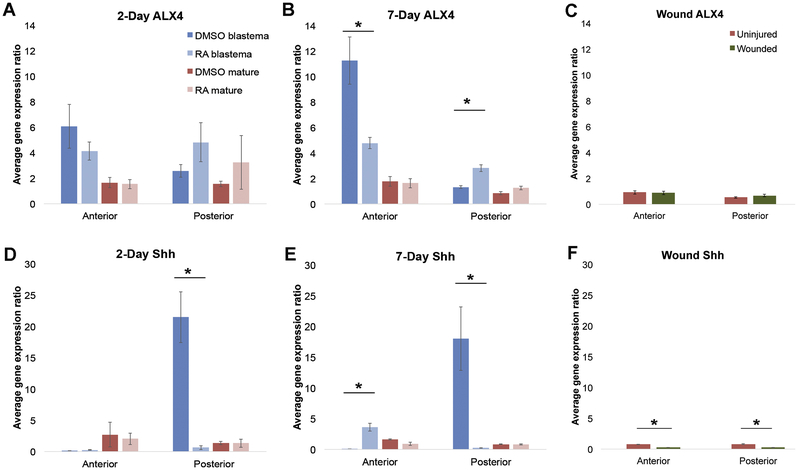
Figure 4: Expression analysis of Shh and Alx4 in anterior and posterior located blastemas and mature limb tissue treated with RA.
(A-F) Histograms of the relative expression of Shh and Alx4 in anterior and posterior located tissues using the Pfaffl method (Pfaffl, 2001). Dark blue bars are DMSO treated blastemas, light blue bars are RA treated blastemas, dark red bars are DMSO treated mature tissue, and pink bars are RA treated mature limb tissue, and green bars are lateral wound sites. (A-B) Alx4 expression was quantified in anterior and posterior located mid-bud staged blastemas or mature tissue from animals that have been treated with DMSO or RA at 2 (A) and 7 (B) days post treatment. (C) Alx4 expression was quantified in anterior and posterior located mature skin or 9 day wounds. (D-E) Shh expression was quantified in anterior and posterior located mid-bud staged blastemas or mature tissue from animals that have been treated with DMSO or RA, at 2 (D) and 7 (E) days post treatment. (F) Shh expression was quantified in anterior and posterior located mature skin or 9 day wounds. Error bars are SEM. We performed statistical analyses by T-tests, whereby the RA- and DMSO-treated versions of each sample type were compared (* p < 0.05).
Exogenous RA resulted in a significant decrease in the expression of Alx4 by seven days post treatment in anterior located blastemas (Figure 4B) (compare dark blue bars (control) to light blue bars (RA treatment)). This change was not observed in the anterior located mature tissues, which expresses Alx4 in a relatively low abundance (compare red (control) and pink (RA treated) bars) (Figure 4B). RA treatment also altered the expression of Shh in anterior blastemas (Figure 4D and E). The abundance of Shh transcripts is relatively low in the DMSO treated anterior blastemas. However, by seven days post RA treatment the expression of Shh is significantly increased in this but not in the mature treated tissue. This data indicates that RA treatment decreases the expression of an anterior gene (Alx4) and increases the expression of a posterior gene (Shh) in anterior located blastemas. These observations are consistent with the idea that these anterior blastemas have been posteriorized.
Surprisingly, RA treatment also altered Alx4 and Shh expression in posterior-located blastemas (Figure 4), despite these blastemas failing to produce a regenerate (McCusker et al., 2014). Although the overall abundance of Alx4 expression is relatively low in posterior located blastemas, RA treatment results in a modest but significant increase in expression of this gene by seven days post treatment (Figure 4B). Shh expression is relatively high in the DMSO treated posterior blastemas. This expression pattern is consistent with that reported by (Nacu et al., 2016) and appears to be nerve dependent because its expression in lateral (non-innervated) limb wounds is low (Figure 4F). RA almost completely abolished Shh expression in posterior blastemas by two days post treatment, and this pattern was maintained at the seven-day time point (Figure 4D and E). This result was surprising because RA has been shown to be required for Shh expression in the developing limb bud (Stratford et al., 1996). Regardless, our observation that RA treatment resulted in differential expression patterns of Shh in anterior and posterior blastemas indicates that there is position-specific regulation at play. This molecular data is also consistent with previous findings from Kim and Stocum, who observed that double-anterior, but not double posterior, limb grafts could be induced to regenerate limbs with a complete anterior posterior axis with RA treatment (Kim and Stocum, 1986).
Our data demonstrates that Shh expression is regulated by exogenous RA differentially in anterior and posterior blastema cells. Interestingly, we also observed a temporal effect on Shh expression in the blastema in response to RA. Posterior blastema tissue reacted quickly to RA by significantly decreasing Shh expression, while the anterior counterpart did not show a significant upregulation of Shh until 7 days post treatment. This fast and slow response, respectively, might be due to direct and indirect regulation of the Shh gene by RA signaling. We have identified a number of putative Retinoic Acid Responsive Elements (RAREs) in and around Shh (Supplemental Table 1), thus it is possible that activated RA receptors bind to and inhibit the transcription of Shh. The slow response that we observe in the anterior wounds could be due to the upstream effects that exogenous RA signaling has on the positional memory in the regenerating cells, such that Shh expression is driven by the generation of a population of regenerating cells with a disparity in positional information.
Together these data are also consistent with the idea that RA treatment posteriorizes the anterior located blastema cells, while mature tissue appears to be resistant to this effect. However, our finding that the expression of anterior and posterior markers is also effected in the RA-treated posterior blastema cells is unexpected because these cells already have a posterior identity. RA treatment has been hypothesized to reprogram limb blastema cells to a posteriorventral identity rather than a purely posterior identity (Bryant and Gardiner, 1992; McCusker et al., 2014; Scadding and Maden , 1994; Ludolph et al., 1990); and we therefore speculate that this could explain the change in Alx4 and Shh expression in the posterior blastemas documented here. Alternatively, it is also possible that RA treatment is reprogramming the blastema to an anterior identity as has been postulated previously (Kim and Stocum 1986). Additionally, our data does not reveal the localization of Shh transcripts in the blastemas, which is likely to be different in the RA treated anterior blastema that will grow into a limb structure, and the DMSO treated posterior blastema that will not form a limb. Further experimentation is required to understand the differential responses to exogenous RA documented in blastema cells that arise from different locations on the limb circumference.
Induction of limb regeneration in lateral wounds treated with FGF-2, FGF-8, BMP-2 and Retinoic acid
The above-described experiments indicate that exogenous RA signaling induces the formation of ectopic limb structures in anterior ALMs by proximalizing the positional memory in these cells. This reprograming capacity appears to be dependent on the limb cells being in a regeneration permissive environment because the expression of Alx4 and Shh is unaltered in mature limb tissues (Figure 4C, F). Nerve signaling plays an essential role in the establishment and maintenance of the blastema (Singer, 1952). Since regeneration in limb wounds without nerves can be rescued by treatment with different combinations of FGFs and BMPs (Makanae et al., 2014), we hypothesized that these molecules are sufficient to render the limb cells capable of being positionally reprogramed by RA treatment.
To test this idea we first generated blastemas by implanting gelatin beads (ranging in 0.5 to 1 mm in diameter) that have been soaked in a cocktail of 300 ng/ul of FGF-2, FGF-8, and BMP-2 each, as performed previously (Makanae et al., 2014), into anterior-located wounds two days after wounding, so that a wound epithelium had formed. This combination of growth factors was previously shown to most robustly induce the formation of ectopic blastemas that are capable of generating new pattern when tissue from the opposite limb axis is grafted into the wound as well (Makanae et al., 2014), and thus is the reason why we chose this combination for the current study. Of the 38 wounds that were implanted with growth factor soaked beads (GF-beads), 25 of them formed blastemas. Once these blastemas had reached the mid-bud blastema stage (approximately 7-10 days), the animals were intraperitoneally injected in the flank region with RA at a concentration of 150μg per gram of body weight, as previously described (McCusker et al., 2014 and Niazi et al., 1985). Of these RA treated blastemas, we observed that 52% of them resulted in the generation of ectopic structures, some of which result in the generation of up to three complete limb axes (Figure 5, Table 3, Supplemental Figure 2). These limbs contain all three limb segments (zeugopod, stylopod, and autopod), as well as having complete anterior/posterior axis (radius and ulna, and digits 1 to 4). None of the 20 control ALMs, which were implanted with a bead soaked in PBS resulted in the formation of ectopic limb structures (Figure 5, Table 3).
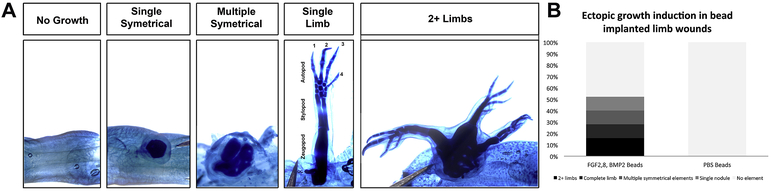
Figure 5: Induction of limb regeneration after consecutive treatment of lateral wounds with FGF-2, FGF-8 and BMP-2 beads and Retinoic Acid.
(A) Representative images of the growth phenotypes elicited by the consecutive treatment of an anterior-located wound site with growth factors. Images are of the wound sites of whole mount Victoria blue stained limbs. A spectrum of growth phenotypes was observed, ranging from no ectopic growths; a single nodule of cartilage; multiple symmetrical cartilage elements (often with a joint); a single complete limb (having all three segments; the zeugopod, stylopod, and autopod with digits 1-4), to 2 or more limb axes. (B) The histogram represents the percentage of the total number of GF-bead implanted and RA-treated blastemas (N = 25), or control bead implanted (N = 20) that generated each growth phenotype observed (all data is in table 3).
Table 3:
Ectopic limb induction in bead implanted limb wounds
| Bead Content | Total bead implanted wounds |
Blastema formation* |
No Element |
Single Nodule |
Multiple symmetrical Elements |
Complete Limb** |
2+ limbs*** |
|---|---|---|---|---|---|---|---|
| FGF-2,-8, BMP-2 Beads | 38 | 25 | 12 (48%) | 3 (12%) | 3 (12%) | 3 (12%) | 4 (16%) |
| PBS | 20 | 2 | 2 (100%) | 0 | 0 | 0 | 0 |
Only wounds that formed a blastema were further analyzed for growth phenotypes
includes phenotypes exhibiting bifurcation in the autopod and/or zeugopod
Includes phenotypes with 3 limb axes with one or more axes being distally hypomorphic
Although our observation that 52% of the treated blastemas result in ectopic limb structures appears low; it is higher than what we expected. Previous studies using either beads soaked in growth factors (Makanae et al., 2014) or retinoic acid (McCusker et al., 2014) in the ALM do not exhibit 100% penetrant phenotypes. In both instances, the induction of a phenotype occurred in roughly 70% of the ALMs. Additionally, these previous studies only had one treatment, as opposed to two in the current study, and thus the amount of variation that could negatively effect the induction of an ectopic response, such as dosage of the factors or staging of the blastema at the time of treatment, is greater. Considering we rarely see blastemas in the control ALMs that were implanted with a 1 mm bead soaked in PBS (10% of control ALMs) (Table 3), it is not surprising that these ALMs fail to form ectopic structures (Figure 5B). Importantly, this observation also indicates that the wounding surgery and bead implantation do not cause enough of an injury to induce an ectopic growth response on their own in the ALM.
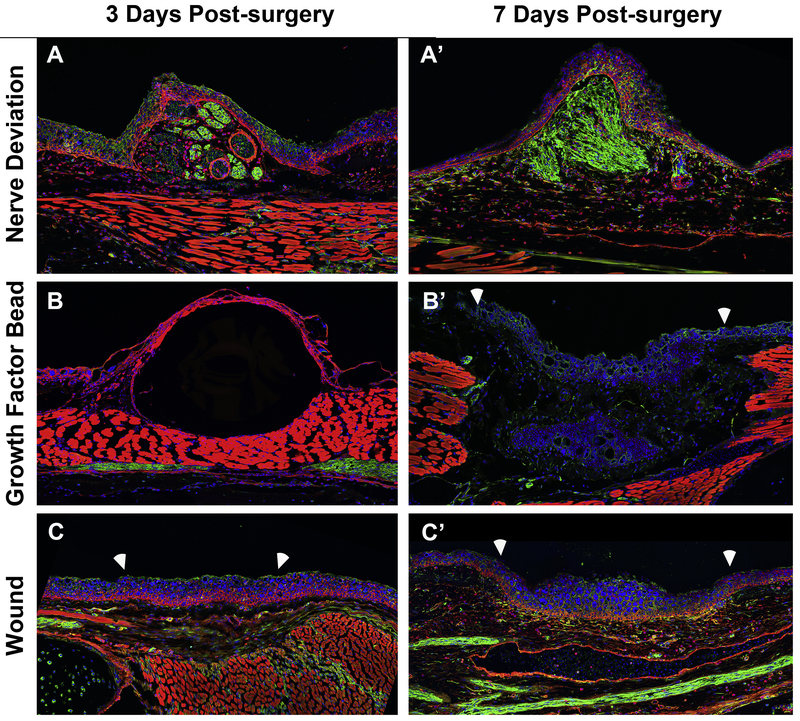
Because FGFs are known to have chemoattractant properties it is possible that the GF-beads attract nearby nerves to innervate the wound site, thus supplementing/replacing the exogenous neurotrophic signals (Li and Muneoka, 1999). To test this possibility, we analyzed the innervation in wound sites with a deviated nerve, an implanted GF-bead, or untreated, 3 and 7-days post surgery, by immunofluorescence for acetylated tubulin, a nerve marker, (Figure 6). Although the innervated wound site has robust nerve staining; the fluorescent signal in the GF-bead and untreated wounds is low at 7-days, the time point by which an early blastema has formed (Figure 6). This observation indicates that the growth factor cocktail sufficiently replaces endogenous neurotrophic signaling, as opposed to attracting near-by nerves, in the wound sites during the time of RA treatment. It is possible, however, that these ectopic limbs become innervated at later stages of development.
Figure 6: Innervation of lateral wound sites with and without the implantation of gelatin beads with growth factors.
Fluorescent images were obtained of anteriorly-located wound sites on longitudinally sectioned limbs that were immunostained with acetylated-tubulin to visualize nerves (green), rhodamine conjugated phalloidin to stain the actin cytoskeleton in the cells of the different tissues (red), and DAPI to stain the nuclear DNA (blue). Images were obtained of wound sites with a deviated nerve, 3 (A) and 7-days (A’) post surgery; (B, B’) with a gelatin bead soaked in FGF-2, FGF-8, and BMP-2, and (C, C’) a wound site with no other manipulation. White arrows delineated the boundary between the wound site and the surrounding mature tissue.
In summary, previous studies using the ALM regeneration assay have been able to induce the formation of ectopic limb structures when, 1) a nerve and a graft with opposing positional information, or 2) a bead soaked in FGFs and BMPs and a graft with opposing positional information, or 3) a nerve and the application of RA or overexpression of either Shh or FGF are present in wound site. In all of these scenarios, at least two factors in the ALM are providing endogenous signals – a wound with either a nerve, or a tissue graft. However, so far, no one has induced a complete limb with only one endogenous singling source – the wound. Here we show complete ectopic limbs could be formed in limb wounds that were treated with exogenous signals that replace the need for signals from both the nerve and the tissue graft with opposing positional information. This observation also indicates that treatment of limb wounds with a combination of FGF-2, FGF-8, and BMP-2 are sufficient to make the wounded limb cells capable of being positionally reprogrammed by RA.
Growth factor bead size impacts the migration of dermally-derived cells in the wound site
We had previously observed that anterior and dorsal located blastemas that were treated with exogenous RA sometimes resulted in the formation of up to two complete limb axes, which we have named the paired-limb phenotype (McCusker et al., 2014). We postulated that this paired limb phenotype is caused by the interaction of the RA-reprogrammed blastema cells and the cells at the dorsal edge of the ALM wound (McCusker et al., 2014). Based on the Polar Coordinate Model of regeneration (French et al., 1976), we predicted that if the dorsal edge of the anterior located wound has an anterior/dorsal positional identity, then two complete mirror-image limbs with double-ventral handedness would form (McCusker et al., 2014). This response is exactly what was observed in the anterior-located paired-limb phenotype (McCusker et al., 2014). Thus, we were surprised when we observed that up to three complete limb axes form in the current study as a result of GF-bead implantation and RA treatment. Since we are not introducing an additional positional disparity compared to this previous study, we speculated that either the dosage of the exogenous growth factors or the bead itself could impact the maximum number of limb fields generated.
Although the precise molecular mechanisms by which positional disparities in cells drive the formation of new positional information during regeneration are unknown; local cell-cell interactions are thought to be essential for this to occur (Bryant et al., 1981). Thus, we speculated that because of the large size of the beads relative to the wound site (0.5 to 1mm bead in a 2mm wound), the gelatin beads might restrict the migration and/or interaction between cells in the blastema and this could result in the formation of an additional limb axis. A similar phenomenon has been observed during embryogenesis, where by the implantation of a physical barrier, such as a bead alone or in combination with growth factors, into the embryonic limb field is sufficient to generate supernumerary limbs in amphibians (Sessions and Ruth, 1990) and chickens (Cohn et al., 1995).
To test this possibility we treated blastemas generated by innervated wounds alone or implanted with 0.5 mm or 1.0 mm gelatin beads soaked in PBS, with RA (Supplemental Figure 3). These bead sizes represent the two polar ends of the size scale employed in the previously described growth factor study that generated up to 3 complete fields (Figure 5). We observed that RA-treated innervated wounds without the beads resulted in the formation of up to 2 complete limb axes, as previously described in McCusker et al., 2014; while, the bead-implanted wounds resulted in up to 3 complete axes (Table 4). We also implanted crystals of DiI into the mature dermis, close to the wound periphery in the above described wound sites, to observe how the dermally-derived cells migrate into ALM blastemas with differently sized gelatin beads (no exogenous growth factors) (Figure 7). Because the basement membrane separates the dermis from the epidermis in the mature tissue, the cells in the dermis took up the dye as they migrated into the blastema, whereas very few epidermal cells took in the label. At 2 and 3 weeks post-surgery, the DiI positive cells were evenly distributed in the no bead-implanted blastemas. In contrast, the presence of the DiI positive cells in both 0.5 and 1.0 mm bead-implanted blastemas were often restricted to a portion of the blastema, and sometimes formed clusters of cells at the apical tip of the blastema (Figure 7A and B).
Table 4:
Effect of beads, implanted into the site of injury, on ectopic limb patterning using the ALM treated with Retinoic Acid
| Bead size | Total ALMs performed |
ALMs Counted* |
No Element |
Single Nodule |
Multiple symmetrical Elements |
Complete Limb** |
2 limbs | More than 2 limbs*** |
|---|---|---|---|---|---|---|---|---|
| no bead | 16 | 13 | 0 | 2 (15.4%) | 0 (0%) | 1 (7.7%) | 10 (77%) | 0 |
| 0.5mm beads | 16 | 16 | 0 | 3 (18.8%) | 1 (6.3%) | 9 (56.3%) | 1 (6.3%) | 2 (12.5%) |
| 1 mm beads | 16 | 13 | 0 | 0 | 3 (21.4%) | 7 (58%) | 2 (16.6%) | 0 |
Two animals did not survive RA treatment. Three limbs did not have blastemas at time of RA treatment. Percentage is the % of counted ALMs with phenotype.
includes phenotypes exhibiting bifurcation in the autopod and/or zeugopod
Includes phenotypes with 3 limb axes with one or more axes being distally hypomorphic
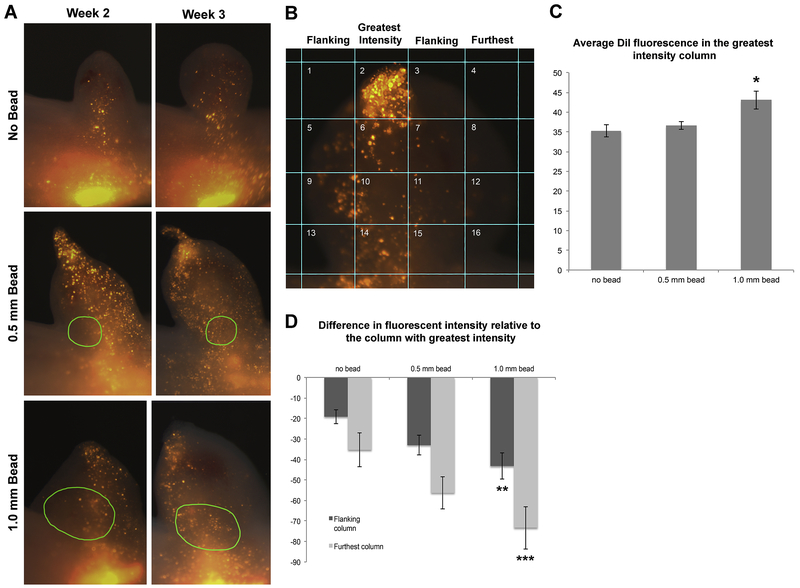
Figure 7: Size of implanted bead alters migration trajectory of dermal-derived blastemal cells.
(A) Representative images of DiI-labeled dermal cells that contribute to ectopic blastemas with no implanted bead (top panel), a 0.5 mm bead (middle), or a 1.0 mm bead (lower) at 2 and 3 weeks post-surgery. Green circles in middle and lower panels indicate the perimeter of the implanted beads that were visible via autofluorescence in the green channel. (B) The images of the blastemas were divided into 16 squares and the average fluorescence intensity of the blastema tissue in each square was measured. The column with the highest average intensity (second column in this example) (C) was compared with the average intensity of neighboring columns (flanking) and the columns farther away (furthest), and the average difference in intensity is presented in the histogram in panel (D). The y-axis in C and D corresponds to arbitrary fluorescent units. N = 13 blastemas measured for the no bead samples; N = 16 for 0.5 mm samples, and N = 12 for 1.0 mm bead samples. Error bars are SEM, and one-way ANOVA and post-hoc Turkey tests for significance were performed (* p = 0.04 comparing no bead to 1.0 mm bead; ** p = 0.01 comparing flanking columns between no bead and 1.0 mm bead, and *** p=0.003 comparing furthest column in the no bead compared to the 1.0 mm bead samples).
To quantify these observations we divided each blastema image into 16 squares (Figure 7B), and measured the average DiI fluorescence in the blastema tissue in each square. We used these values to calculate the average fluorescence in the four columns; identify the columns with the highest average intensity, and then compared this highest average fluorescence with the average fluorescence in surrounding columns. The closest columns were designated as “flanking”, and more distant columns called “furthest” (Figure 7B). We found that the average fluorescence of the greatest intensity column increased in the blastemas with beads (Figure 7C). We also found that the difference in the fluorescent intensity between the highest intensity column and the neighboring columns increased in blastemas with implanted beads, the 1.0mm bead implanted blastemas having the greatest (and a significant) difference compared to the blastemas with no implanted beads (Figure 7D).
Together these results indicate that the migration of the DiI labeled cells is more restricted in the bead-implanted blastemas. Since dermal-derived cells play an important role in patterning the regenerate (Endo et al., 2004; Muneoka et al., 1986), we suspect that this restricted migration may prevent interactions between cells that migrate to the distal tip of the blastema, and those in the basal region of the blastema (below the bead), potentially leading to the generation of additional limb axes. In the future, we should be able to modulate the pattern of the regenerate by refining the growth factor delivery such that it does not restrict cell migration.
Conclusion:
In the current study we identified the combination of signals that are sufficient to induce a complete regenerative response in superficial, lateral (non-regenerative) limb wounds. To generate blastemas that were capable of pattern formation, we treated the anterior-located wound site with a combination of FGF-2, FGF-8, and BMP-2 to substitute for the required nerve signals, as was previously performed (Makanae et al., 2014). The resulting blastemas were then treated with RA, which reprograms the positional memory in the cells to a posterior identity (Figure 2 and 3), and thus generates a positional disparity in the anterior blastema cells. Our expression analysis of Alx4 and Shh further supports the hypothesis that RA treatment of the anterior-located blastemas results in the reprograming of the blastema cells to a posterior identity (Crawford and Stocum 1988; Bryant and Gardiner 1992; McCusker et al., 2014). This sequential method of treatment resulted in the generation of limb structures with complete proximal/distal and anterior/posterior axes from the wound sites, and indicates that FGF-2, FGF-8, BMP-2, and RA accomplish the signaling requirements that normally come from the nerves and the positional disparity. However, future studies will be required to identify the essential wound-specific signals.
This combination of signals, which results in the generation of complete limbs, is an improvement over Shh or FGF-8 treatments that result in the generation of incomplete limb structures (Nacu et al., 2016). However, the use of gelatin beads to deliver growth factors to the injury site results in the generation of supernumerary limbs and is likely the result of altered cell migration and/or interactions in the ectopic blastema. In the future, novel methods of growth factor delivery that have minimal impact on cell migration and interactions will lead to the improved outcome of a single, complete, limb structure.
One interesting outcome of this study was the location-specific transcriptional responses of the blastema tissues to RA treatment (Figure 4). This is a logical outcome, however, since RA treatment induces regeneration in anterior, but not posterior blastemas (Kim and Stocum, 1986, McCusker et al., 2014). We previously hypothesized that this differential regenerative response is due to the interaction of the RA-reprogrammed blastema cells with the surrounding mature wound tissue, which has differing positional information depending on where the wound is located (McCusker et al., 2014). While this might be the case, our data here indicate that the blastema cells themselves are responding differently to the same molecular signal.
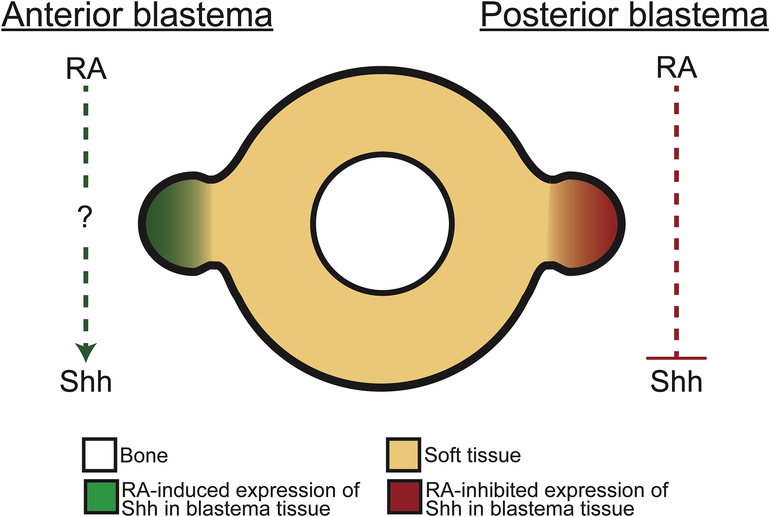
For example, RA treatment increases Shh expression in anterior-located blastemas, while the same treatment results in a loss of Shh expression in the posterior-located blastemas, which normally express abundant levels of Shh (Figure 4). We predict multiple potential RAREs are present in and around the Shh gene (Supplemental Table 1), which brings forth the possibility that Shh expression is directly regulated by RARs. Since the expression of Shh in posterior blastemas is effected so quickly after RA treatment, we hypothesize that this is occurring by direct regulation via RARs (Figure 8). RA treatment has the opposite effect on Shh expression in the anterior-located blastemas. In this instance Shh expression slowly increases in this tissue after treatment, indicating that there is some intermediate downstream of RA signaling that regulates Shh expression in anterior blastema cells (Figure 8). While a number of genes downstream of RA signaling could regulate Shh expression, members of the Hox family, which are positively regulated by RA signaling (Reviewed in Cunningham and Deuster, 2015), have been shown to play an important role in the positive regulation of Shh expression in developing limb buds (Lettice et al., 2017). Differential expression of other transcriptional factors and co-factors around the circumference of the limb may also contribute to our observations. Why and how RA signaling would directly regulate Shh in posterior blastema cells, while simultaneously indirectly regulating Shh in anterior cells are important outstanding questions.
Figure 8: Model for RA-mediated regulation of Shh expression in anterior and posterior blastema cells.
An anterior innervated wound site fails to generate an ectopic limb unless treated with exogenous RA. This treatment induces the expression of Shh, a posterior specific signaling factor, by 7 days post-treatment. As this change in Shh expression demonstrates a slow response to RA treatment, it can be hypothesized that this effect is indirect. A posterior innervated wound site fails to generate an ectopic limb, even if treated with exogenous RA. This tissue naturally expresses Shh and exogenous RA treatment rapidly inhibits the expression of this gene. We speculate that, due to the rapid response to RA treatment, that Shh is directly regulated by this pathway in posterior blastema cells.
Last, future studies that compare the differential activation of the FGF, BMP, and RA pathways in regenerative and non-regenerative wounds and animals will likely provide key insights into why some injuries regenerate while other do not. For example, Monaghan et al. observed that the expression of FGFR1 and BMP2R is significantly greater in regenerating limb wounds compared to flank wounds (non-regenerative) in the axolotl (Monaghan et al., 2012). It was additionally observed that crabp1, a protein that plays an essential role in regulating RA nuclear signaling, expression was significantly greater in limb wounds and this increased expression was dependent on nerve signaling (Monaghan et al., 2012). Thus, regenerative competency could, in part, depend the ability of a tissue to respond to FGF, BMP, and RA signaling.
Materials and Methods:
Animal husbandry
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental work was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts, Boston. The experiment described in Figure 5 in this study was performed on wild type (RRID:AGSC_100L), and the remaining experiments were performed on white-strain (RRID:AGSC_101J) and GFP-strain (RRID:AGSC_110J) Mexican axolotls (Ambystoma mexicanum) measuring approximately 5-8 cm snout to tail tip (3-4 cm snout to vent). Experimental animals were either spawned at UMass Boston or obtained from the Ambystoma Genetic Stock Center at the University of Kentucky. Animals were anesthetized using a 0.1% MS222 solution (Ethyl 3-aminobenzoate methanesulfonate salt, Sigma), pH 7.0.
Surgical procedures
Two types of surgeries were performed in this study: 1) lateral wounding, and 2) nerve deviations. In order to ensure the generation of wounded tissue of only a single circumferential positional identity, all wounds were restricted to a single quadrant of the limb. The lateral wound surgery was performed by making a 2 mm by 2 mm excision of full-thickness skin, ensuring no damage to the underlying muscle, along the anterior or posterior of the forelimb stylopod. Nerve deviations were performed as described in (McCusker et al., 2014) and (Endo et al., 2004) on 2 mm by 2 mm anterior or posterior-located wound sites. Beads were synthesized as described below, and were implanted below the 2-day old wound epithelium that had formed over the wound sites. 38 limbs were used for the implantation of growth factor soaked beads, of which 25 generated blastemas, and 20 control limbs were implanted with PBS soaked beads, of which 2 formed small blastemas (See table 3). 48 limbs were used to determine the effect of bead size on cell migration within the blastema, 16 per experimental group (see table 4).
RNA isolation and cDNA synthesis
For the expressional analysis portion of this study 32 limbs were used per injury type per limb axis. Anterior and posterior located nerve deviations were performed as in McCusker et al. 2014, and treated with DMSO or RA 7 days after surgery (mid-bud staged blastemas). Anterior and posterior stump and blastema tissues were then collected 2 or 7 days post DMSO or RA injection. Each of the biological replicates were generated from pooled tissues (4 blastemas per sample). RNA was extracted in Tripure Isolation Reagent (Sigma) and isolated with the Nucleospin RNA XS kit (Macherey-Nagel) according to the manufacturers’ specifications. Subsequently, cDNA libraries were generated with the Transcription first strand cDNA synthesis kit (Roche), using the anchored oligo(dT)18 primer as specified by the manufacturer. Three technical replicates of each of the three or four biological replicates per sample were performed.
QRT-PCR
Using Q-RTPCR (Azuraquant™ Green Fast qPCR Mix Lo-Rox, Azura Genomics Inc.) the relative transcription levels of anterior and posterior positional genes were determined between the different experimental groups and tissue types. Forward and reverse primer sequences for ef1α, Alx4, and Shh are presented in Table 5. Primer sequences were generated using contig data derived from the Ambystoma EST (http://www.ambystoma.org/) and Axolotlomics EST Databases (https://www.axolotl-omics.org/home).
Table 5:
Primer sequences used for the transcriptional analysis of anterior and posterior tissue identity in the axolotl.
| Name | Sequence |
|---|---|
| ef1 α Forward | 5’ – AACATCGTGGTCATCGGCCAT |
| ef1 α Reverse | 5’ – GGAGGTGCCAGTGATCATGTT |
| Alx4 Forward | 5’ – GGGTAACCTCTTTGGCACTG |
| Alx4 Reverse | 5’ – TTAAGTGCCCTGTCATGTGG |
| Shh Forward | 5’ – AGTGACCAGACCGGTGAAAG |
| Shh Reverse | 5’ – CGCTCCGTCTCTATCACGTA |
Synthesis and implantation of Growth Factor Beads to induce ectopic blastemas
Gelatin Microsphere Beads were prepared as described in (Makanae et al., 2014). Dried beads were rehydrated in the growth factor cocktail, composed of 300 ng/μl each of FGF-basic (FGF-2), FGF-8, and BMP-2 (R&D Systems) for 3 hours at 4°C prior to implanting below the anterior-located 48-hour wound epidermis. When beads were implanted into a wound site with a deviated nerve, the bead was placed in such a way to ensure the nerve retained contact with the wound epithelium. Control beads were rehydrated in 1× PBS. Beads of approximately 1 mm or 0.5 mm in diameter were selected for implantation (Supplemental Figure 3). As the wounds are not sutured closed after implantation, the animals were incubated on ice for 2 hours to allow for natural wound healing to close the wound and secure the bead in place, before returning to Hotfreters solution.
Retinoic acid treatment
Anteriorly located ectopic blastemas were allowed to develop until midblastema (MB) stage (approximately 7-10 days), at which point animals were injected intraperitoneally in the flank with RA (150 μg/gram of body weight) as described in (McCusker et al., 2014 and Niazi et al 1985). Animals were kept in the dark for 2 days following the injection to minimize the photo-inactivation of RA. Live images of blastemas were taken on a weekly basis starting on the day of RA injection and continuing until fully-regenerated skin had formed over the regenerate or wound site, at which time the limbs were collected for further analysis.
Positional information regenerative assay
The same method described above was employed to generate RA-induced ectopic structures from anterior located blastemas on 30 GFP-strain animals. At 7 weeks, 18 had generated patterned structures. Full thickness skin was harvested and the structures were amputated as depicted in Figure 3. As the anterior-posterior axis of the ectopic structure was not visually discernable, a complete circumference of skin was collected from the outgrowths. Structures constituted by bulbous masses alone were amputated just proximal to this harvest site (Figure 3A). For outgrowths exhibiting patterned elements distal to the bulbous mass, a ring of skin was collected from each independent structure in the region proximal to the autopod or tapered digit-like structure but distal to the bulbous mass (Figure 3A). Each collected tissue sample was grafted into an innervated anterior-located wound site on the forelimb (as described above) of white strain animals (32 sites generated). The amputated outgrowths and tissue grafts were imaged on a weekly basis until regeneration had been completed at which time the regenerates were collected for whole mount cartilage staining.
DiI labeling of dermal-derived cells and quantification of migration
DiI (Invitrogen) was placed in the dermal layer at the dorsal edge of the ALM wound site immediately after beads were implanted as performed previously in Endo et al., 2004. Live images were obtained weekly using a Zeiss Discovery V8 Stereomicroscope with an Axiocam 503 color camera and Zen software. We quantified the distribution of the DiI labeled cells in the blastemas at 3 weeks following the bead implantation because this was the time when we observed the greatest qualitative difference between the samples. To quantify the distribution of DiI cells we divided each blastema image into 16 squares (Figure 7B), and measured the average DiI fluorescence in the blastema tissue in each square. We used these values to calculate the average fluorescence in the four columns; identify the columns with the highest average intensity, and then compared this highest average fluorescence with the average fluorescence in surrounding columns. The closest columns were designated as “flanking”, and more distant columns called “furthest” (Figure 7B).
Whole mount cartilage staining and phenotype scoring
Whole mount Victoria blue staining was performed as described in (Bryant and Iten, 1974) on limbs 8-weeks after the growth factor saturated gelatin beads were implanted into the anterior wound sites (7-weeks after RA treatment). The presence of Victoria blue stained ectopic cartilage structures in the wound site was observed, and were scored according to the number of skeletal elements present in the wound site (Figure 5, Supplemental Figure 2). Wound sites that did not have any cartilage elements were scored as “no growth”. Wound sites that had a single (often spherical shaped) cartilage nodule were scored as “single symmetrical” growths. Wound sites that had multiple cartilage elements, with or without an obvious joint and without an obvious anterior-posterior axis were scored as “multiple symmetrical” growths. Single complete limbs with all limb segments (zeugopod, stylopod, and autopod) were scored as “single limb” growths. Wounds that formed three limb structures that were either completely separated or one limb formed independently and the other two limbs were fused at the stylopod, were scored as “2+ limbs”.
Histology and immunostaining
Harvested limb tissues were fixed overnight at 4°C in a 4% Paraformaldehyde solution in 1X PBS, softened in 10% EDTA at room temperature for 2 days, soaked in 30% sucrose overnight at 4°C, and equilibrated and flash-frozen in OCT compound (Tissue-Tek) and sectioned into 10 uM sections for immunodetection. Immunofluorescence was performed as described in (McCusker et al., 2015). The nerves were detected using an acetylated-tubulin primary antibody (Sigma-Aldrich) diluted to 1:100 in 1× PBS-Tween (incubated overnight at 4°C), and Alexa Fluor® 488 conjugated goat antimouse IgG (Thermo Scientific) secondary antibody diluted to 1:200 in 1× PBST, washed 1× 20 minutes with PBST with DAPI and rhodamine-conjugated phalloidin, washed 2× with 1×PBS, and mounted in Vectasheild. Fluorescent tilescanned images were obtained using a 63× objective with a Zeiss Axio observer Z1 inverted fluorescent microscope with an Apotome 2, and a Hamamatsu Orca 4 Flash LTE cooled monochrome camera.
Putative RARE identification
Putative retinoic acid response elements (RAREs) were identified with the aid of JASPAR (Waki et al., 2011). Genomic libraries (Keinath et al., 2015; Nowoshilow et al., 2018) encoding the axolotl Alx4 and Shh genes were identified, and a region of 3000 nucleotides around and including each of these genes was then analyzed for putative RAREs with a threshold of 80 and 79% respectively.
Supplementary Material
Supplemental Figure 1: Representative images of RA-induced ectopic structures which exhibited regenerative capabilities in response to amputation (see table 2). 94% of these ectopic structures were restored to their original pattern, the outstanding 6% patterned more complex structures. After 7 weeks the limbs where collected and prepared for whole mount staining to allow for visualization of the skeletal pattern of the regenerated structure.
Supplemental Figure 2: Development of different growth phenotypes over time. Left panels are representative live images of the GF-bead induced blastemas and their development over time (Day of RA injection, 2, 3, 5, and 7 weeks post-injection). Right panels are images of whole mount Victoria blue stained growths that formed in anterior-located limb wounds.
Supplemental Figure 3: Implantation of differently sized gelatin beads. Gelatin beads were sorted into 0.5 (A) and 1 mm (B) groups. (C-D) Nerve deviations (outlined in yellow) into wound sites (white square) were performed and these sites were then allowed to form a wound epithelium for 2 days. The dorsal edge of the wound epithelium (WE) was opened, the WE was separated from the underlying mesenchyme to make a pocket, and beads of either 0.5 mm (C) or 1.0 mm (D) were inserted below the WE (beads outlined in blue). A crystal of DiI was placed in the mature dermis on the dorsal edge of the wound site (outlined in pink).
Highlights:
Sequential treatment of anterior located wounds with FGF2, FGF8, BMP2, and retinoic acid are sufficient to induce complete limb structures.
Retinoic acid induced ectopic limbs can regenerate and induce new pattern to form when grafted into anterior-located ALMs.
Anterior and posterior blastemas respond differentially to retinoic acid treatment in the expression of anterior and posterior positional markers, Alx4 and Shh.
The induction of extra limb axes in the treated wounds is a result of the treatment procedure, which restricts cell migration in the blastema.
Acknowledgements:
The authors wish to thank Dr. David Gardiner, Dr. Susan Bryant, Dr. Anne Phan, and Dr. Julian Sosnik for their insightful discussions. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R15HD092180.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bryant SV, French V, and Bryant PJ (1981). Distal regeneration and symmetry. Science 212, 993–1002. [DOI] [PubMed] [Google Scholar]
- Bryant SV, and Gardiner DM (1992). Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev. Biol. 152, 1–25. [DOI] [PubMed] [Google Scholar]
- Bryant SV, and Iten LE (1974). The regulative ability of the limb regeneration blastema of Notophlhalmus viridescens: Experiments in situ. W. Roux' Archiv F. Entwicklungsmechanik 174, 90–101. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK and Tickle C (1995). Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739–746. [DOI] [PubMed] [Google Scholar]
- Crawford K, and Stocum DL (1988). Retinoic acid coordinately proximalizes regenerate pattern and blastema differential affinity in axolotl limbs. Development 102, 687–698. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, and Deuster G, (2015). Mechanisms of retinoic acid signaling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 16(2), 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Bryant SV, and Gardiner DM (2004). A stepwise model system for limb regeneration. Dev. Biol. 270, 135–145. [DOI] [PubMed] [Google Scholar]
- French V, Bryant PJ, Bryant SV, (1976) Pattern regulation in epimorphic fields. Science. 193, 969–981. [DOI] [PubMed] [Google Scholar]
- Keinath MC, Timoshevskiy VA, Timoshevskaya NY, Tsonis PA, Voss SR, and Smith JJ (2015). Initial characterization of the large genome of the salamander Ambystoma mexicanum using shotgun and laser capture chromosome sequencing. Sci Rep 5, 16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, and Stocum DL (1986). Retinoic acid modifies positional memory in the anteroposterior axis of regenerating axolotl limbs. Dev. Biol. 114, 170–179. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Devenney P, De Angelis C, and Hill RE (2017). Conserved sonic hedgehog limb enhancer consists of discrete functional elements that regulate precise spatial expression. Cell Reports 20, 1396–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lheureux PE (1977). Importance des associations de tissus du membre dans le developpement des membres surnumeraires induits par deviation de nerf chez le Triton Pleurodeles waltlii Michah. Development 38, 151–173. [PubMed] [Google Scholar]
- Li S, and Muneoka K (1999). Cell migration and chick limb development: chemotactic action of FGF-4 and the AER. Dev. Biol. 211, 335–347. [DOI] [PubMed] [Google Scholar]
- Ludolph D, Cameron JA, and Stocum DL (1990) The effect of retinoic acid on positional memory in the dorsoventral axis of regenerating axolotl limbs. Dev. Biol 140, 41–52. [DOI] [PubMed] [Google Scholar]
- Maden M (1983). The effect of vitamin A on the regenerating axolotl limb. J. Embryol. Exp. Morph 77, 273–295. [PubMed] [Google Scholar]
- Maden M, and Holder N (1984). Axial characteristics of nerve induced supernumerary limbs in the axolotl. W. Roux' Archiv F. Entwicklungsmechanik 193, 394–401. [DOI] [PubMed] [Google Scholar]
- Makanae A, Hirata A, Honjo Y, Mitogawa K, and Satoh A (2013). Nerve independent limb induction in axolotls. Dev. Biol. 381, 213–226. [DOI] [PubMed] [Google Scholar]
- Makanae A, Mitogawa K, and Satoh A (2014). Co-operative Bmp- and Fgf-signaling inputs convert skin wound healing to limb formation in urodele amphibians. Dev. Biol. 396, 57–66. [DOI] [PubMed] [Google Scholar]
- McCusker CD, and Gardiner DM (2013). Positional Information Is Reprogrammed in Blastema Cells of the Regenerating Limb of the Axolotl (Ambystoma mexicanum). PLoS ONE 8, e77064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CD, and Gardiner DM (2014). Understanding positional cues in salamander limb regeneration: implications for optimizing cell-based regenerative therapies. Dis Model Mech 7, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CD, Athippozhy A, Diaz-Castillo C, Fowlkes C, Gardiner DM, and Voss SR (2015). Positional plasticity in regenerating Amybstoma mexicanum limbs is associated with cell proliferation and pathways of cellular differentiation. BMC Dev. Biol. 15, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker CD, Diaz-Castillo C, Sosnik J, Phan A, and Gardiner DM (2016). Cartilage and bone cells do not participate in skeletal regeneration in Ambystoma mexicanum limbs. Dev. Biol. 416, 26–33. [DOI] [PubMed] [Google Scholar]
- McCusker C, Lehrberg J, and Gardiner D (2014). Position-specific induction of ectopic limbs in non-regenerating blastemas on axolotl forelimbs. Regeneration 1, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan JR, and Maden M (2012). Visualization, of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev. Biol. 368, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan JR, Athippozhy A, Seifert AW, Putta S, Stromberg AJ, Maden M, Gardiner DM, and Voss SR (2012). Gene expression patterns specific to the regenerating limb of the Mexican axolotl. Biology Open 1, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Fox WF, and Bryant SV (1986). Cellular contribution from dermis and cartilage to the regenerating limb blastema in axolotls. Dev. Biol. 116, 256–260. [DOI] [PubMed] [Google Scholar]
- Nacu E, Gromberg E, Oliveira CR, Drechsel D, and Tanaka EM (2016). FGF8 and SHH substitute for anterior-posterior tissue interactions to induce limb regeneration. Nature 533, 407–410. [DOI] [PubMed] [Google Scholar]
- Niazi IA, Pescitelli MJ, and Stocum DL (1985). Stage-dependent effects of retinoic acid on regenerating urodele limbs. W. Roux' Archiv F. Entwicklungsmechanik 194, 355–363. [Google Scholar]
- Nowoshilow S, Schloissnig S, Fei J-F, Dahl A, Pang AWC, Pippel M, Winkler S, Hastie AR, Young G, Roscito JG, et al. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature 554, 50–55. [DOI] [PubMed] [Google Scholar]
- Panman L, Drenth T, Tewelscher P, Zuniga A, and Zeller R (2005). Genetic interaction of Gli3 and Alx4 during limb development. Int. J. Dev. Biol. 49, 443–448. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001). A new mathematical model for relative quantification in realtime RT-PCR. CORD Conference Proceedings 29, e45–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AQ, Lee J, Oei M, Flath C, Hwe C, Mariano R, Vu T, Shu C, Dinh A, Simkin J, et al. (2015). Position information in axolotl and mouse limb ECM is mediated via heparan sulfates and FGF during limb regeneration in the Axolotl (Ambystoma mexicanum). Regeneration 2, 182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Graham GM, Bryant SV, and Gardiner DM (2008a). Neurotrophic regulation of epidermal dedifferentiation during wound healing and limb regeneration in the axolotl (Ambystoma mexicanum). Dev. Biol. 319, 321–335. [DOI] [PubMed] [Google Scholar]
- Satoh A, Bryant SV, and Gardiner DM (2008b). Regulation of dermal fibroblast dedifferentiation and redifferentiation during wound healing and limb regeneration in the Axolotl. Dev. Growth Differ. 50, 743–754. [DOI] [PubMed] [Google Scholar]
- Satoh A, Gardiner DM, Bryant SV, and Endo T (2007). Nerve-induced ectopic limb blastemas in the Axolotl are equivalent to amputation-induced blastemas. Dev. Biol. 312, 231–244. [DOI] [PubMed] [Google Scholar]
- Scadding SR, and Maden M (1994). Retinoic acid gradients during limb regeneration. Dev. Biol. 162, 608–617. [DOI] [PubMed] [Google Scholar]
- Scadding SR (1999). Citral, an inhibitor of retinoic acid synthesis, modifies pattern formation during limb regeneration in the axolotl Ambystoma mexicanum. Can. J. Zool. 77, 1835–1837. [Google Scholar]
- Sessions SK, and Ruth SB (1990). Explanation for Naturally Occurring Supernumerary Limbs in Amphibians. J. Exp. Zool. 254, 38–47. [DOI] [PubMed] [Google Scholar]
- Singer M (1592). The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 27, 169–200. [DOI] [PubMed] [Google Scholar]
- Stratford T, Horton C, and Maden M (1996). Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr. Biol. 6, 1124–1133. [DOI] [PubMed] [Google Scholar]
- Thoms S and Stocum DL (1984) Retinoic acid-induced pattern duplication in regenerating urodele limbs. Dev. Biol. 103, 319–328. [DOI] [PubMed] [Google Scholar]
- Tickle C, and Towers M (2017). Sonic Hedgehog Signaling in Limb Development. Front Cell Dev. Biol. 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Nakamura M, Yamauchi T, Wakabayashi K-I, Yu J, Hirose-Yotsuya L, Take K, Sun W, Iwabu M, Okada-Iwabu M, et al. (2011). Global mapping of cell type-specific open chromatin by FAIRE-seq reveals the regulatory role of the NFI family in adipocyte differentiation. PLoS Genet 7, e1002311–e1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Representative images of RA-induced ectopic structures which exhibited regenerative capabilities in response to amputation (see table 2). 94% of these ectopic structures were restored to their original pattern, the outstanding 6% patterned more complex structures. After 7 weeks the limbs where collected and prepared for whole mount staining to allow for visualization of the skeletal pattern of the regenerated structure.
Supplemental Figure 2: Development of different growth phenotypes over time. Left panels are representative live images of the GF-bead induced blastemas and their development over time (Day of RA injection, 2, 3, 5, and 7 weeks post-injection). Right panels are images of whole mount Victoria blue stained growths that formed in anterior-located limb wounds.
Supplemental Figure 3: Implantation of differently sized gelatin beads. Gelatin beads were sorted into 0.5 (A) and 1 mm (B) groups. (C-D) Nerve deviations (outlined in yellow) into wound sites (white square) were performed and these sites were then allowed to form a wound epithelium for 2 days. The dorsal edge of the wound epithelium (WE) was opened, the WE was separated from the underlying mesenchyme to make a pocket, and beads of either 0.5 mm (C) or 1.0 mm (D) were inserted below the WE (beads outlined in blue). A crystal of DiI was placed in the mature dermis on the dorsal edge of the wound site (outlined in pink).