Abstract
Purpose
The purpose of this study was to characterize the composition of vaginal bacterial communities in a cohort of Black adolescent women and to determine how the species composition of these communities correlate with levels of estradiol, glycogen, and stress.
Methods
Twenty-one Black adolescent women were sampled longitudinally. The composition of their vaginal communities was determined by analyzing the sequences of the Vl-V3 region of l6S rRNA genes and they were grouped based on patterns in species abundances. The relationships between estradiol, glycogen, psychosocial stress, and the composition of these communities were assessed.
Results
Vaginal communities could be distinguished and classified into three groups that differed in the abundances of Lactobacillus. Eighty-one percent of study participants had communities dominated by species of Lactobacillus. Glycogen levels were higher in communities dominated by one or multiple species of Lactobacillus as compared to those having low proportions of Lactobacillus. Estradiol and psychosocial stress measurements did not differ among the three groups, while estradiol and glycogen exhibited a weak positive relationship that was not statistically significant.
Conclusions
The findings of this pilot study suggest that glycogen levels are associated with vaginal community composition in young Black women; however, estradiol and psychosocial stress are not. Additionally, the results suggest there is no simple relationship between levels of estradiol and the production of vaginal glycogen.
Keywords: vagina, microbiome, vaginal microbiome, microbial community, estrogen, glycogen, adolescent, stress
Sexually transmitted infections (STIs) are highly prevalent among adolescent women [1]. In 2016 alone, young women ages 10 – 19 y comprised nearly one fifth of chlamydia and gonorrhea cases [2], and these STIs increase HIV risk in adolescents [3]. Rates are especially high among young Black women [2]. While various physiological, behavioral, and psychosocial factors are thought to contribute [4–6], it is unclear why young Black women have such high risk. Poor sexual and reproductive health outcomes in women have been linked to the bacterial composition of the vaginal microbiome of older women, but we know very little about the vaginal microbiomes of Black adolescent women [7,8].
In pre-menopausal women older than 18 y, high proportions of Lactobacillus are associated with a lower prevalence of HIV and other STIs [9]. Lactobacillus species produce lactic acid, which is thought to inhibit colonization by pathogenic organisms [10–13], However, approximately 25% of reproductive age women have vaginal communities that are depleted of Lactobacillus [11], Moreover, the prevalence of communities dominated by Lactobacillus is greater in Asian and White women (80.2% and 89.7%, respectively) as compared to Black and Hispanic women (61.9% and 59.6%, respectively) [14]. In addition to these observed differences between women, point estimates of vaginal bacterial community composition can change rapidly [15–17], sometimes resulting in low proportions of lactobacilli that may expose women to windows of risk for acquiring infections. Currently, the factors accounting for the compositional differences observed between women and temporal changes within women are poorly understood. Thus, there is a need to understand drivers of changes in vaginal community composition, especially in young Black women.
The abundances of Lactobacillus species have been positively associated with circulating estrogen and vaginal glycogen content (reviewed in [11]). It has also been proposed that other factors such as psychosocial stress may influence vaginal community composition and account for the racial/ethnic differences in vaginal communities seen among adult women [18]. Thus, the main objective of this study was to determine whether vaginal bacterial community composition in Black adolescent women is associated with stress, estradiol, and glycogen. To explore this, we sampled the vaginas of twenty-one 14-year-old women at baseline and then monthly for six months. We determined the species composition of their vaginal bacterial communities, assessed levels of estrogen and glycogen, measured vaginal pH and Nugent scores, and assessed psychosocial stress. With these data, we addressed three questions: First, what kinds of vaginal bacterial communities do young Black women have? Second, do the levels of estradiol, glycogen, and stress differ among women with different kinds of communities? And third, what are the relationships between estradiol, glycogen, stress, and key species of the vaginal communities?
Materials and Methods
Study Design
Twenty-five self-identified Black women (ages 14.01 – 14.99 years) were recruited from neighborhood clinics in Indianapolis, where most participants received primary care, to participate in a longitudinal study to assess the relationships between stress, estrogen, and vaginal community composition. This study was approved by the Institutional Review Board at Indiana University. We focused on 14-year-olds because this age is developmentally meaningful interval in which hygiene practices (such as pad or tampon use, douching, and pubic hair removal) become common and the nature of sexual interpersonal relationships changes rapidly and studying this group fills gaps in existing research that cannot otherwise be addressed. Written, informed consent of both participants and a parent were obtained prior to enrollment. Exclusion criteria at enrollment included structural abnormalities of the vagina, chronic medical conditions that could alter the vaginal microbiome, pregnancy, immune deficiency conditions, and antibiotic use within the previous 90 days. Two participants were excluded due to antibiotic use, and two were excluded due to health complications or non-compliance. In total, 21 participants provided seven monthly, self-collected vaginal swab and saliva samples, and self-assessments of psychosocial stress, menses, and sexual behaviors. All study participants were post-menarcheal.
Estrogen and glycogen measurements
Saliva samples were used to measure estradiol levels because the method of sample collection is non-invasive, well accepted assays exist [19,20], and levels of salivary estradiol are well correlated with serum estradiol concentrations [20,21], For three days up to and including the scheduled day of vaginal swab sampling, once-per-day salivary samples were self-collected by passive drool into polypropylene cryotubes and frozen shortly after collection. Salivary estradiol concentrations were determined with a commercially available enzyme-linked immunosorbent assay kit (17β-Estradiol high sensitivity ELISA kit ADI-901–174, Enzo Life Sciences). The standard curve was prepared per manufacturer’s instructions but extended to eight standards with 7.8 pg/ml as the lowest value. The intra-assay variation was 4.2% and the inter-assay variation was 7.8%. During extraction, samples were concentrated 4× in assay buffer, and each was measured in duplicate using the protocol available at http://hdl.handle.net/2022/21883.
Glycogen in vaginal swab samples was quantified using the EnzyChrom Glycogen Assay Kit (BioAssay Systems) according to the manufacturer’s instructions. To measure vaginal pH, subjects inserted a gloved finger into the vagina for ten seconds then rolled the finger over a commercially available pH stick (pH-EcoCare™ Comfort; Merete Medical GmbH). The pH measurements were confirmed by a trained research associate and recorded in 0.5 increments. Self-obtained vaginal swabs (eSwab™ Copan Diagnostics Inc.) were used to assess Nugent score, and provide vaginal samples for STI testing and microbial community analyses. Nugent score is a Gram stain scoring system used to diagnose bacterial vaginosis (BV) [22], To assess Nugent scores, vaginal swabs were rolled onto glass microscope slides, air-dried in the field, and transported to the Infectious Diseases Laboratory at Indiana University. Slides were then stained according to standard procedures, and scored 0 – 3 (normal), 4 – 6 (intermediate), and 7 – 10 (abnormal/BV) [22], STI testing for chlamydia and gonorrhea was conducted using Abbott Realtime CT/NG assay on the Abbott m2000 platform (Abbott Molecular, DesPlaines, IL). Trichomonas vaginalis testing was performed using a validated real time PCR assay on the Abbott m2000 platform. The remainder of swab samples were stored at −80°C until DNA sequencing was done at the University of Idaho.
Measurement of psychosocial factors
Psychosocial factors were assessed using audio computer assisted self-interview [23], obtained at baseline, month three, and month six. The standardized scales (all previously validated in adolescent populations) addressed depression (the Patient Health Questionnaire [PHQ] [24]), stress (Perceived Stress Scale [PSS] [25]), and anxiety (the Brief Symptom Inventory [BSI][26]).
Determination of menstrual cycle phase
Throughout the study self-reported menses data were used to determine the start and end date of menstrual periods for subjects not on hormonal birth control. We assumed an average luteal phase of 14 days, one day for ovulation, and the remaining days to be follicular. Based on these criteria, we divided menstrual period into phases, and matched sample collection dates accordingly.
Microbial community analysis
Total genomic DNA was extracted from vaginal swab samples using chemical and mechanical lysis, and purified using QIAamp DNA mini kits (Qiagen) as described previously [27], The VIV3 region of l6S rRNA genes were amplified using a two-step PCR protocol, first amplifying the gene region using universal primers 27F and 534R, and then adding sample barcodes and sequence adapters. Amplicons were sequenced using an Illumina MiSeq platform in the Genomics Resources Core facility at the University of Idaho. High quality reads were obtained from all samples except one, resulting in 146 samples total. Forward and reverse reads were paired using FLASH [28], processed through DADA2 [29] to identify unique sequences, and these were classified to genus and species levels using SPINGO [30], A total of 422 taxa were identified. Of those, 44 taxa were present at a minimum of l% in at least two individuals or at least 5% in one individual. Using this filter, the remaining taxa (378) were considered uncommon and therefore grouped into an “other” category that was included in subsequent analyses. The relative abundances of the taxa found in these communities are reported in Table S1.
To group communities on the basis of similarities and differences in composition we performed complete-linkage hierarchical clustering on alt-Gower distances computed from taxon relative abundance data. Silhouette information was used to define nine clusters. Clusters were assigned to groups A, B, and C based on whether they were dominated by one species of Lactobacillus (group A), dominated by multiple species of Lactobacillus (group B), or had low proportions of lactobacilli (group C).
Statistics
Linear and linear mixed effects models were used for multiple analyses, including 1) modeling the means of the response variables estradiol, glycogen, vaginal pH, and psychosocial stress (BSI, PHQ, PSS) between groups; 2) modeling the means of estradiol between menstrual cycle phase; and 3) characterizing the linear relationship between estradiol and glycogen. Response variables were transformed where appropriate to avoid violating model assumptions. Statistical significance of these models was determined using analysis of variance (ANOVA). Statistical comparisons were performed by testing general linear hypotheses and multiple comparisons of the means using Tukey’s test. A Kruskal-Wallis rank sum test was used to evaluate group significant differences in Nugent score, with post-hoc analysis of multiple comparisons using a Dunn’s test with Bonferroni adjustment. Finally, Pearson correlation coefficients were used to explore relationships between metadata and key taxa. More details on these analyses can be found in the Supplemental Material.
Results
Study participants and metadata collection
We determined the relationships of stress, estrogen, and vaginal community composition in 21 Black women who averaged 14.6 ± 0.3 years of age at the time of enrollment (Table S2). The mean vaginal pH and Nugent score across all individuals were 5.2 ± 0.9 and 1.3 ± 2.5, respectively. This vaginal pH is higher than that reported for older reproductive age women. Vaginal glycogen levels across all samples showed substantial variability (mean glycogen 324.7 ± 399.1 μg/mL). Mean salivary estradiol across samples was 7.3 ± 4.5 pg/mL. Finally, across all measurements, the mean PHQ score was 5.9 ± 4.8 (range 0–25), the mean BSI score was 0.5 ±0.7 (range 0–3.7), and the mean PSS score was 18.5 ± 6.5 (range 7–38). These scores indicate that on average the women of this cohort experienced mild to moderate depression, low anxiety, and moderate to high stress.
Vaginal bacterial community composition
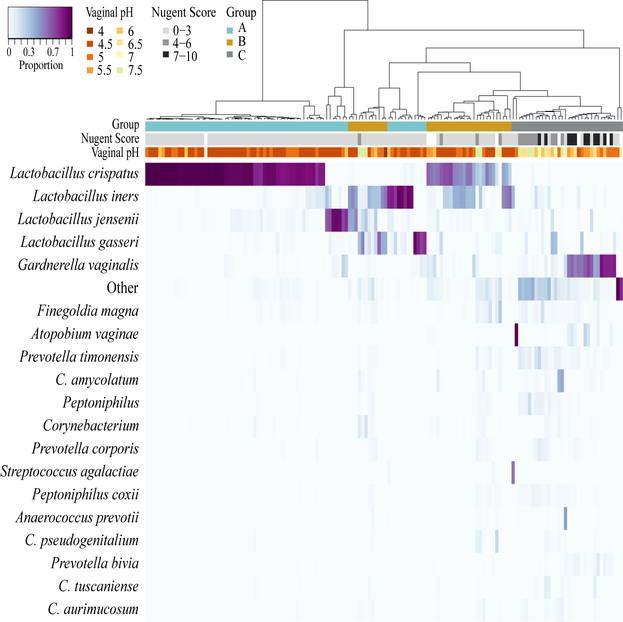
To characterize the composition of vaginal communities we sequenced the VI to V3 regions of 16S rRNA genes. Most participants (81%; 17 of 21) had communities that were dominated by Lactobacillus. Figure 1 shows a heat map of relative abundance data for the 20 most abundant taxa. The dendrogram in Figure 1 was used to identify nine clusters of communities that differed in composition. We observed that we could further group communities (i.e., combine clusters) based on the abundances of Lactobacillus species. Seven of the nine clusters had more than 50% total Lactobacillus, leaving two clusters in which the relative abundances of lactobacilli were less than 50%. The use of a 50% threshold to define “dominated” and “not dominated” was adopted from Klatt et al. [31] who used the same criterion in a study of how the abundance of lactobacilli was positively correlated with the efficacy of tenofovir. Of the seven clusters that were dominated by Lactobacillus, four were dominated by a single species of Lactobacillus and the remaining three had mixtures of Lactobacillus species. These became groups A and B, respectively. All clusters that had communities with low proportions of Lactobacillus were aggregated into group C. In addition to low proportions of lactobacilli, these communities had higher proportions of G. vaginalis and mixtures of other bacteria such as Atopobium vaginalis, Corynebacterium spp., Prevotella spp., Peptoniphilus spp., Streptococcus spp., and Anaerococcusprevotii (Figure 1). The mean relative abundances of key taxa in groups A-C are shown in Table S3.
Figure 1. Heatmap based on the relative proportions of the 20 most abundant taxa in vaginal communities of Black adolescent women.
The columns of the heatmap include 146 samples collected from 21 young women over a 6-month period. The corresponding dendrogram represents complete-linkage hierarchical clustering of samples based on alt-Gower distances. The colored bar immediately below the dendrogram indicates which clusters were combined to form three groups (A, B, and C).
Vaginal community composition is known to change over time in older, reproductive age women. To determine whether similar trends occur in young Black women, we created bar plots representing vaginal bacterial community composition over time for each subject (Fig. 2, Fig. S1–S2). Figure 2 shows plots for four subjects that illustrate the variability in community composition found within and between women of the cohort. Communities with high stability were seen in women of different groups. For example, Subject 35 (group A) maintained high proportions of L. crispatus over time, whereas Subject 11 (group C) maintained 50% or more G. vaginalis over time, hi contrast, Subject 31 had high proportions of G. vaginalis (group C) during the first three months, then transitioned once to a community dominated by L. gasseri (group A). Subject 24 started with a community dominated by L.jensenii (group A) and transitioned to different groups five times over the course of the study.
Figure 2. Examples of changes in community composition over time.
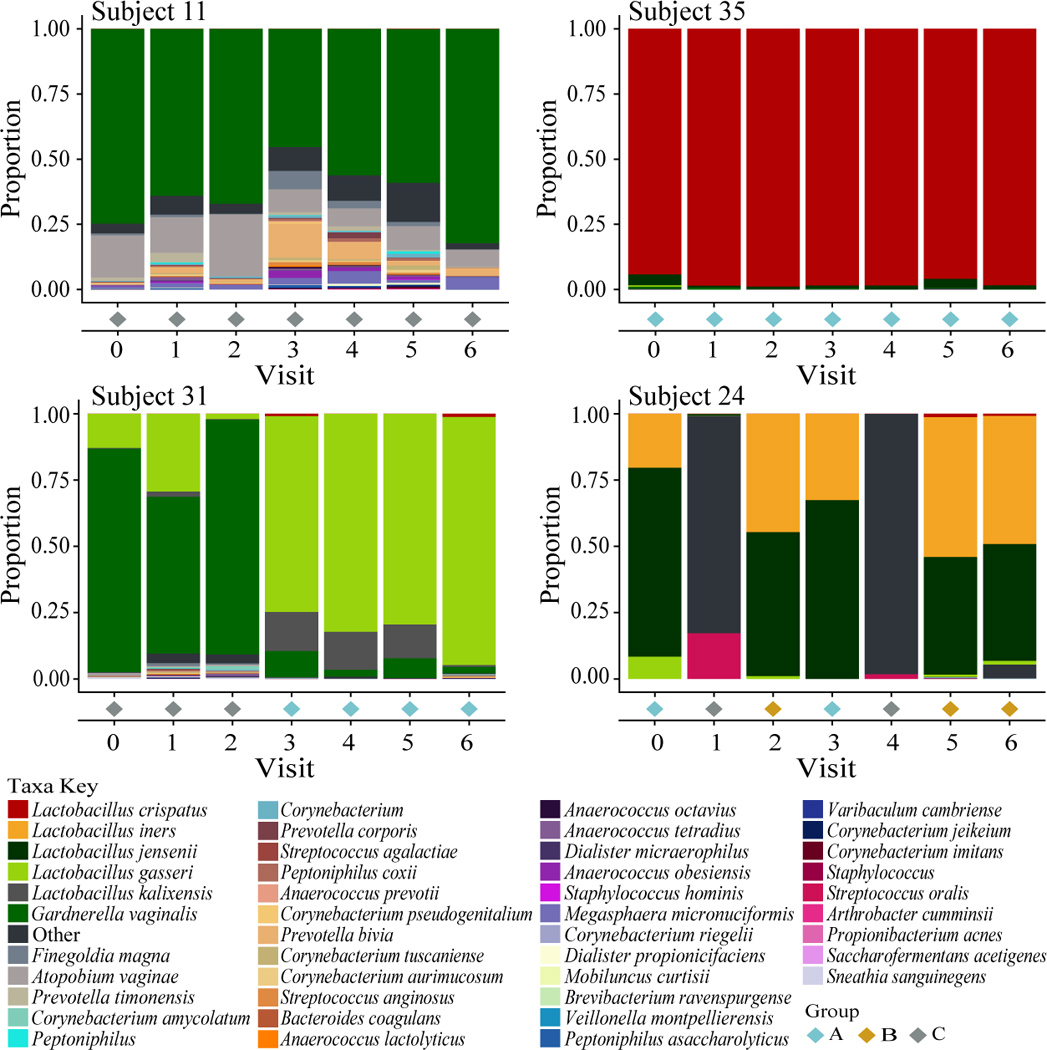
The stacked bar charts represent proportions of bacteria in each community over 7 monthly visits where zero was the baseline visit. Colors for each taxon are shown in the legend below the figure. The community group for each visit is highlighted by a colored diamond below each chart and the corresponding legend is listed in the bottom right. The profiles of these subjects were chosen to illustrate the temporal variability in community composition within subjects.
Differences between groups
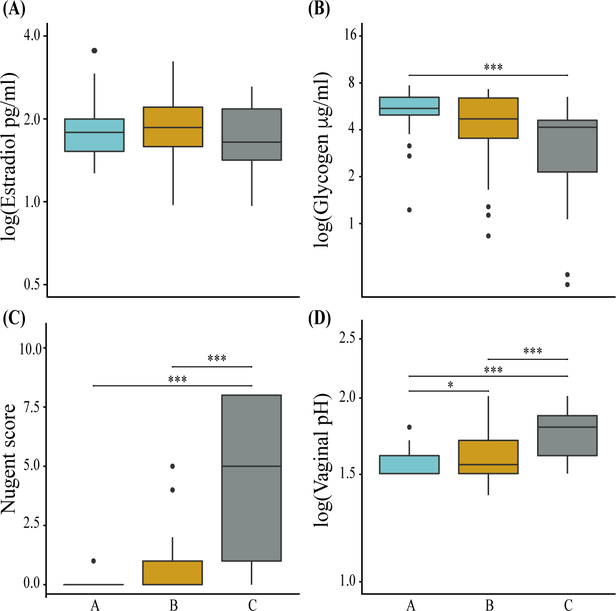
We determined if levels of estradiol, glycogen, vaginal pH, and Nugent score differed among groups A, B, and C. Vaginal pH and Nugent score were included in the analysis because they are well accepted correlates of vaginal community composition. We fit linear mixed effects models accounting for variation due to subject and performed non-parametric analyses where appropriate. The distributions of log-transformed estradiol and glycogen levels for each group are depicted by the boxplots in Figure 3a and 3b, respectively. There were no significant differences in estradiol levels among groups (χ2 = 4.4, p = 0.1). Estradiol levels are known to vary over the menstrual cycle; therefore, we compared estradiol measurements between menstrual cycle phase to test our ability to detect differences in estradiol levels (Figure S3). Mean estradiol concentrations were lowest in follicular samples and highest in peri-ovulatory samples; these differences were not significant (χ2= 5.1, p = 0.08). Although we were unable to discriminate between estradiol levels, we did find that vaginal glycogen differed significantly between groups A and C (z = −4.1, p < 0.001).
Figure 3. Differences in estradiol, glycogen, Nugent score, and vaginal pH measurements among groups.
The boxplots represent log-transformed estradiol (panel A), log-transformed glycogen (panel B), Nugent scores (panel C), and log-transformed vaginal pH (panel D) for samples in groups A, B, and C. Statistical significance (* p < 0.05, *** p < 0.001) is indicated above the bars.
Figure 3c and 3d show the distribution of Nugent scores and log-transformed vaginal pH for each group. As expected, group C had significantly higher Nugent scores and vaginal pH than did samples in groups A (Nugent: z = −7.6, pH: z = 6.5; p < 0.001 for both) and B (Nugent: z = −5.0, pH: z = 3.8, p < 0.001 for both). Moreover, groups A and B, both marked by high proportions of Lactobacillus, had similar Nugent scores yet differed significantly in vaginal pH (Nugent: z = −1.9, p = 0.2; pH: z = 2.9; p = 0.01).
To gain insight to whether measurements of psychosocial factors differed between groups, we used linear mixed effects models, incorporating variation due to subject when necessary, to test for differences in the mean values of perceived stress, anxiety, and depression. Summary statistics for the models are shown in Table 1. No significant differences in perceived stress (F =0.04, p = 1), anxiety (χ2 = 0.7, p = 0.7), or depression (χ2 = 3.2, p = 0.2) were identified.
Table 1.
Stress, anxiety, and depression in community groups A, B, and C.
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | |||||||
| Variable | Measure | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | Statisticd | p - valuee |
| PHQ scorea | Depression | 29 | 2.4 (0.2) | 19 | 2.0 (0.3) | 15 | 1.9 (0.3) | χ2 = 3.2 | 0.2 |
| BSI scoreb | Anxiety | 29 | 0.5 (0.1) | 19 | 0.5 (0.1) | 15 | 0.6 (0.2) | χ2 = 0.7 | 0.7 |
| PSS scorec | Stress | 29 | 4.3 (0.1) | 19 | 4.2 (0.2) | 15 | 4.2 (0.2) | F=0.04 | 1 |
PHQ (Personal Health Questionnaire - 9) is a self-report questionnaire consisting of 10 questions (9 asking about specific symptoms, and the final asking how impactful those symptoms are to assess the severity of depression).
BSI (Brief Symptom Inventory) – the anxiety subscale used here – is a self-report questionnaire consisting of 6 questions designed to clinically assess the level of anxiety in individuals.
PSS (Perceived Stress Scale) is a self-report questionnaire used to evaluate the degree to which particular situations in one’s life are deemed stressful.
The test statistic listed results from the linear mixed effects models conducted for PHQ and BSI, and the linear model conducted for PSS to test differences in their means between groups A, B, and C. The means and standard error (SE) that are reported above result from the modeled means (betas) and SE. Variation due to subject was incorporated where appropriate. Type II Wald chisquare tests were calculated for PHQ and BSI, and the F-test was calculated for PSS.
The p-value listed in this table is associated with the test statistic to the left.
Correlations between estradiol, glycogen, stress, and key taxa
We investigated the relationships between estradiol, glycogen, stress, and key taxa in the vaginal community by plotting Pearson correlation coefficients (Fig. S4). As expected, vaginal pH and Nugent score were positively correlated (r = 0.63), and both pH and Nugent score were negatively correlated with glycogen (r = −0.39 and −0.34, respectively) (Fig. S4 panel A). Vaginal glycogen was positively correlated with L. crispatus (r = 0.17) and L.jensenii (r = 0.26) (Fig. S4 panel B). Estradiol was positively correlated with L. iners (r = 0.37) but no other Lactobacillus species (Fig. S4 panel B). Furthermore, we did not observe a statistically significant correlation between levels of estradiol and glycogen.
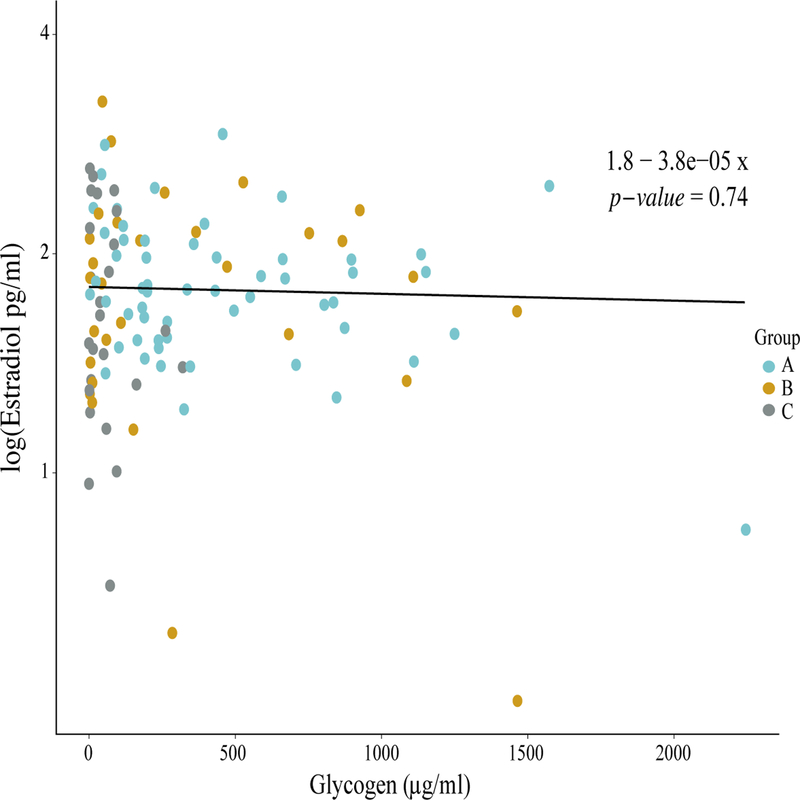
To further evaluate the relationship between estradiol and glycogen, we fit a linear mixed effects model incorporating variation due to subject. Figure 4 shows a scatterplot of log estradiol vs. glycogen content in all samples. Our model suggests a positive relationship between estradiol and glycogen (m = 1.4 e-4); however, in agreement with the correlation plots, this relationship was not statistically significant (p = 0.2) (Fig. 4).
Figure 4. The relationship between log-transformed estradiol and glycogen concentrations in Black adolescent women sampled longitudinally.
The amounts of salivary estradiol in each sample were log-transformed and modeled over corresponding glycogen measurements including subject as a random effect. The resulting linear model equation and p-value (shown in the upper right corner) were obtained by computing an analysis of variance (ANOVA) on the linear model. Each dot represents the log estrogen concentration and the corresponding glycogen value for a given sample. Dots are colored according to community group as shown in the legend to the right of the graph.
Discussion
We evaluated the relationships between mental health (perceived stress, depression, and anxiety), salivary estradiol, vaginal glycogen, and vaginal community composition in a small cohort of 14-year-old Black women. We showed that most of the cohort (81%) had communities that were dominated by species of Lactobacillus. The kind and abundances of Lactobacillus species served as a basis for the classification of communities into three groups: A, B, and C. Consistent with reports in older reproductive age women [11], some women had stable vaginal communities whereas others transitioned from one community group to another. Importantly, differences in groups were marked by differences in vaginal glycogen levels but not salivary estradiol or any of the mental health measures.
Few studies have used culture-independent methods to characterize vaginal community composition in adolescent women. A cross-sectional study found young women, ages 13–18, to have either vaginal communities that were dominated by L. iners or L. crispatus, or contained a mixture of L. crispatus, L.jensenii, and L. gasseri, or were heterogeneous in composition with low proportions of Lactobacillus [7]. Another study showed Lactobacillus species to be prominent members in the adolescent vaginal microbiome early in puberty, even prior to menarche [8]. The high prevalence of Lactobacillus in our cohort of young Black women (81%) is in agreement with previous studies, but is higher than that reported for older Black women (61.9%) [14]. As noted above, reproductive age Black women are less likely than White and Asian women to have Lactobacillus dominant communities [11,14], and more likely to have communities that have been associated with BV [32], The fact that more than three quarters of this cohort of young Black women had mostly Lactobacillus in their communities suggests there could be an age-associated transitional period in these vaginal communities. To understand this, we need more extensive longitudinal studies that evaluate the normal development of the vaginal microbiome in Black women over time. This will broaden our understanding of what is healthy and what leads to the health disparities observed later in the lives of Black women. In future studies the variability seen within ethnic groups might be lessened if host genetics were used to classify individuals instead of self-reported ethnicity.
Mental health such as chronic psychosocial stress has been associated with recurrent vulvovaginal candidiasis [33] and increased odds of vaginal conditions such as BV [34], The correlation between chronic stress and BV has been demonstrated to be even more prominent in pregnant women [18]. Culhane et al. found that pregnant women who experienced high stress were 2.2 times more likely to have BV than those who experienced low stress [18]. These associations between stress and BV likely result from stress-induced reduction in proteins involved in immune homeostasis that is associated with a decrease in the abundance of vaginal lactobacilli [35], Although we sampled vaginal microbial communities that varied in terms of the abundance of Lactobacillus and other species, we did not observe significant differences in psychosocial stress, depression, or anxiety among the women studied. The sample size of our pilot study could have contributed to our inability to detect differences in stress and this is the most likely explanation for the discrepancies between the findings of our study and others [18,34], However, it is also plausible that differences in the cohorts that were sampled in addition to variation in the perception of stress among cohorts of women could have played a role. Future research to evaluate the influence of psychosocial stress on vaginal community composition might include measuring a common biomarker of stress such as cortisol in addition to self-reported measures.
The positive association between estrogen, glycogen, and Lactobacillus over a woman’s lifespan [11] has led to an assumption that there is a simple linear relationship between estrogen and the levels of glycogen in the vagina. Our results suggest otherwise and are in general agreement with those of Mirmonsef et al. [36] who sampled older reproductive age women over time and found no relationship between estrogen levels and vaginal glycogen, In an effort to understand this, one might assume that the rate of glycogen production (and release from cells) is counterbalanced by the rate of glycogen metabolism by members of the vaginal community. The resulting pseudo-steady state could result in some relatively constant level of glycogen in vaginal secretions in instances where the rate of glycogen production exceeds the rate of consumption, In contrast, the steady-state concentration of glycogen would be near zero if the rate of glycogen consumption is equal to or greater than the rate of glycogen production. This reasoning could also be extended to explain how high levels of glycogen can persist in low estrogen environments [37], The relationship between the levels of estrogen and glycogen in the vagina might be further complicated by the fact that both the rates of glycogen production by the host and the rates of glycogen consumption by vaginal bacteria probably vary among women and over time within a woman. A second scenario emerges from the results of a study done by Pessina et al. [38] to evaluate the effects of steroidal hormones on the structure of the vaginal tissues of rats which showed that sub-physiological levels of estradiol thickened the vaginal epithelium more than physiological doses. Given this, it could well be that a positive linear relationship between estradiol and glycogen may only exist only at low concentrations of estradiol. As the estradiol level increases, its effect on glycogen production could be diminished or perhaps even saturated. The result would be a nonlinear relationship between estradiol levels and glycogen production in which glycogen is mainly influenced by the number of glycogen producing cells in the thickened vaginal epithelium.
In this study we sampled a relatively small number of individuals (21) infrequently over time and this limited our ability to resolve temporal changes in the vaginal microbiome and potential correlates of change (e.g., estradiol and stress), or to assess the effects of potentially confounding factors such as sexual activity or methods of birth control. Additionally, the sampling regimen precluded knowing the ovulatory status of subjects in a menstrual cycle. Since adolescents have a high frequency of anovulatory cycles [39] this might impact the vaginal environment in ways not commonly observed in adult women and confounded efforts to demonstrate an association between estradiol and microbiome composition. While these limitations exist, this is the first study done to evaluate estradiol, glycogen and stress as potential drivers of vaginal community composition in Black adolescent women. Our results support previous findings that vaginal glycogen content is positively correlated with Lactobacillus dominance in vaginal secretions from older reproductive age women [40] and confirm that there is no simple relationship between levels of estradiol and the production of vaginal glycogen.
Supplementary Material
Figure S1. Temporal changes in vaginal community composition. The occurrence of communities in groups A, B, and C are shown for each subject over time. The legend for the community groups is shown to the right of the plot. The red asterisk for Subject 20 at visit 2 indicates missing data. Subjects are vertically ordered according to the group they belonged to at their baseline visit and the number of transitions that were observed.
Figure S2. Vaginal community composition of study participants sampled longitudinally. The stacked bar charts represent proportions of bacteria in each community over 7 visits at monthly intervals, where zero represents the baseline visit. Colors for each taxon are shown in the legend at the lower right comer. The graphs are ordered according to group they belonged to at their baseline visit and the number of transitions that were observed. There was no data for Subject 20 visit 2, thus this subject only has 6 visits. The stacked bar charts for Subjects 11, 24, 31, and 35 can be found in Figure 2.
Figure S3. Changes in estradiol over the menstrual cycle. Samples were categorized by menstrual cycle phase based on self-reported menses data. Log-transformed estradiol concentrations from women in follicular (black), peri-ovulatory (white), and luteal (grey) phases are shown in box plots. The number of samples for each phase is indicated above the corresponding box.
Figure S4. Correlations between psychosocial factors, metadata, and key taxa. Panel A shows correlations between psychosocial stress factors and sample metadata. Panel B shows correlations between psychosocial stress factors and sample metadata with key taxa in the vaginal microbiome. Pearson correlation coefficients (labeled within each ellipse) were used to build these correlation plots. The sign and magnitude of the correlation is reflected in the color bar to the right of each plot. Shades of blue represent positive correlations, while shades of red represent negative correlations. Solid, black circles represent correlations that were not significant (p > 0.05).
Implications and Contribution.
The positive association between Lactobacillus-dommance and glycogen levels suggest that factors affecting vaginal glycogen levels may influence clinical conditions such as bacterial vaginosis and alter susceptibility to other urogenital infections. Future studies might elucidate these factors and explore whether glycogen levels could serve as a surrogate that predicts community composition.
Acknowledgements:
Financial support to complete the research was provided by grant funding through the National Institute of Allergy & Infectious Diseases (1R56AI108775) for which J.D.F and L.J.F were co-principal investigators. K.L.N is supported by a Presidential Scholar Fellowship from the University of Idaho. The funding sources did not play a role in writing the manuscript or the decision to submit it for publication. L.J.F., as the corresponding author, had full access to all of the data in the study and had final responsibility for the decision to submit the manuscript for publication.
We would like to thank Maria Schneider for her help in preparing genomic DNA extractions and amplicons for sequencing. We are also grateful to the Institute for Bioinformatics and Evolutionary Studies (IBEST) Genomics Resources Core staff and Computational Resources Core staff for technical support; and Celeste Brown for discussions and feedback regarding data analysis.
Abbreviations:
- STI
Sexually transmitted infection
- HIV
Human Immunodeficiency Virus
- BV
bacterial vaginosis
- PHQ
Patient Health Questionnaire
- PSS
Perceived Stress Scale
- BHI
Brief Symptom Inventory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors report no conflict of interest.
References
- [1].Forhan SE, Gottlieb SL, Sternberg MR, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics 2009;124(6):1505–12. DOI: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. Atlanta: U.S. Department of Health and Human Services; 2017. [Google Scholar]
- [3].Newbern EC, Anschuetz GL, Eberhart MG, et al. Adolescent Sexually Transmitted Infections and Risk for Subsequent HIV. Am J Public Health 2013;103(10): 1874—81. DOI: 10.2105/AJPH.2013.301463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015;18(2 Suppl 1):19408 DOI: 10.7448/LA.S.18.2.19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hulland EN, Brown JL, Swartzendruber AL, et al. The association between stress, coping, and sexual risk behaviors over 24 months among African-American female adolescents. Psychol Health Med 2014;20(4):443–56. DOI: 10.1080/13548506.2014.951369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: Conceptual issues and research evidence. Psychol Sex Orient Gender Divers 2013;1(S):3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamamoto T, Zhou X, Williams CJ, et al. Bacterial populations in the vaginas of healthy adolescent women. J Pediatr Adolesc Gynecol 2009;22(1): 11–8. DOI: 10.1016/j.jpag.2008.01.073. [DOI] [PubMed] [Google Scholar]
- [8].Hickey RJ, Zhou X, Settles ML, et al. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015;6(2):e00097–15–14. DOI: 10.1128/mBio.00097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Borgdorff H, Tsivtsivadze E, Verhelst R, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 2014;8(9):1781–93. DOI: 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boskey ER, Cone RA, Whaley KJ, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod 2001; 16(9): 1809—13. [DOI] [PubMed] [Google Scholar]
- [11].Hickey RJ, Zhou X, Pierson JD, et al. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 2012;160(4):267–82. DOI: 10.1016/j.trsl.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ma B, Fomey LJ, Ravel J. Vaginal Microbiome: Rethinking Health and Disease. Annu Rev Microbiol 2012;66:371–89. DOI: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van de Wijgert JHHM, Borgdorff H, Verhelst R, et al. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE 2014;9(8):el05998–10. DOI: 10.1371/joumal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 2011;108 Suppl 1:4680–7. DOI: 10.1073/pnas.l002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gajer P, Brotman RM, Bai G, et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci Transl Med 2012;4(132):132ra52–2. DOI: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect 2010;86(4):297–302. DOI: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS 1997,8(8):489–94. [DOI] [PubMed] [Google Scholar]
- [18].Culhane J, Rauh J, McCollum KF, et al. Maternal Stress is Associated With Bacterial Vaginosis in Human Pregnancy. Matem Child Health J 2001;5(2):127–134. [DOI] [PubMed] [Google Scholar]
- [19].Gray SH, Ebe LK, Feldman HA, et al. Salivary Progesterone Levels Before Menarche: A Prospective Study of Adolescent Girls. J Clin Endocrinol Metab 2010;95:3507–11.DOI: 10.1210/jc.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clin Chem 1990;36:1769–73. [PubMed] [Google Scholar]
- [21].Dielen C, Fiers T, Somers S, et al. Correlation between saliva and serum concentrations of estradiol in women undergoing ovarian hyperstimulation with gonadotropins for IVF/ICSI. Facts Views Vis Obgyn 2017;9(2):85–91. DOI: 10.1002/14651858.CD005289.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gutiérrez JP, Torres-Pereda P. Acceptability and reliability of an adolescent risk behavior questionnaire administered with audio and computer support. Rev Panam Salud Publica 2009;25(5):418–22. [DOI] [PubMed] [Google Scholar]
- [24].Kroenke K and Spitzer RL. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psych Annals 2002; 32(9), 509–515. [Google Scholar]
- [25].Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav 1983;24(4):385–396. [PubMed] [Google Scholar]
- [26].Derogatis L and Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13(3):595–605. [PubMed] [Google Scholar]
- [27].Yuan S, Cohen DB, Ravel J, et al. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 2012;7(3):e33865 DOI: 10.1371/joumal.pone.0033865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011;27(21):2957–63. DOI: 10.1093/bioinforniatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: High resolution sample inference from amplicon data. Nat Meth 2016;13(7):581–583. DOI: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Allard G, Ryan FJ, Jeffery IB, et al. SPINGO: a rapid species-classifier for microbial amplicon sequences. BMC Bioinformatics 2015;16:324 DOI: 10.1186/sl2859-015-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].KIatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017;356(6341):938–45. DOI: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- [32].Fettweis JM, Brooks JP, Serrano MG, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiol 2014;160(Pt_10):2272–82. DOI: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Akimoto-Gunther L, Bonfim-Mendonça P de S, Takahachi G, et al. Highlights Regarding Host Predisposing Factors to Recurrent Vulvovaginal Candidiasis: Chronic Stress and Reduced Antioxidant Capacity. PLoS ONE 2016;11(7):e0158870 DOI: 10.1371/joumal.pone.0158870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nansel TR, Riggs MA, Yu K-F, Andrews WW, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol 2006;194(2):381–6. DOI: 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology 2015;156(9):3265–76. DOI: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mirmonsef P, Hotton AL, Gilbert D, et al. Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal pH, Estrogen, and Progesterone. PLoS ONE 2016;11(4):e0153553 DOI: 10.1371/joumal.pone.0153553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ayre WB. The glycogen-estrogen relationship in the vaginal tract. J Clin Endocrinol 1951;11:103–110. DOI: 10.1210/jcem-ll-l-103. [DOI] [PubMed] [Google Scholar]
- [38].Pessina MA, Hoyt RF, Goldstein I, Traish AM. Differential effects of estradiol, progesterone, and testosterone on vaginal structural integrity. Endocrinology 2006;147(1):61–9. DOI: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- [39].Rosenfield RL. Clinical review: Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab 2013;98(9):3572–83. DOI: 10.1210/jc.2013-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mirmonsef P, Hotton AL, Gilbert D, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS ONE 2014;9(7):el02467 DOI: 10.1371/joumal.pone.0102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Temporal changes in vaginal community composition. The occurrence of communities in groups A, B, and C are shown for each subject over time. The legend for the community groups is shown to the right of the plot. The red asterisk for Subject 20 at visit 2 indicates missing data. Subjects are vertically ordered according to the group they belonged to at their baseline visit and the number of transitions that were observed.
Figure S2. Vaginal community composition of study participants sampled longitudinally. The stacked bar charts represent proportions of bacteria in each community over 7 visits at monthly intervals, where zero represents the baseline visit. Colors for each taxon are shown in the legend at the lower right comer. The graphs are ordered according to group they belonged to at their baseline visit and the number of transitions that were observed. There was no data for Subject 20 visit 2, thus this subject only has 6 visits. The stacked bar charts for Subjects 11, 24, 31, and 35 can be found in Figure 2.
Figure S3. Changes in estradiol over the menstrual cycle. Samples were categorized by menstrual cycle phase based on self-reported menses data. Log-transformed estradiol concentrations from women in follicular (black), peri-ovulatory (white), and luteal (grey) phases are shown in box plots. The number of samples for each phase is indicated above the corresponding box.
Figure S4. Correlations between psychosocial factors, metadata, and key taxa. Panel A shows correlations between psychosocial stress factors and sample metadata. Panel B shows correlations between psychosocial stress factors and sample metadata with key taxa in the vaginal microbiome. Pearson correlation coefficients (labeled within each ellipse) were used to build these correlation plots. The sign and magnitude of the correlation is reflected in the color bar to the right of each plot. Shades of blue represent positive correlations, while shades of red represent negative correlations. Solid, black circles represent correlations that were not significant (p > 0.05).