Abstract
Objective:
To assess fitness and outcomes in older women undergoing cytoreductive surgery for advanced ovarian cancer (OC).
Methods:
A prospective study of OC patients referred to Geriatrics Clinic for preoperative evaluation. All completed the electronic Rapid Fitness Assessment (eRFA) and were followed by Geriatrics Service during inpatient postoperative course, co-managed by Surgical Service. Outcomes were 30-day Intensive Care Unit (ICU) admission, emergency room (ER) visit, readmission, mortality, adverse surgical events. Descriptive statistics were used.
Results:
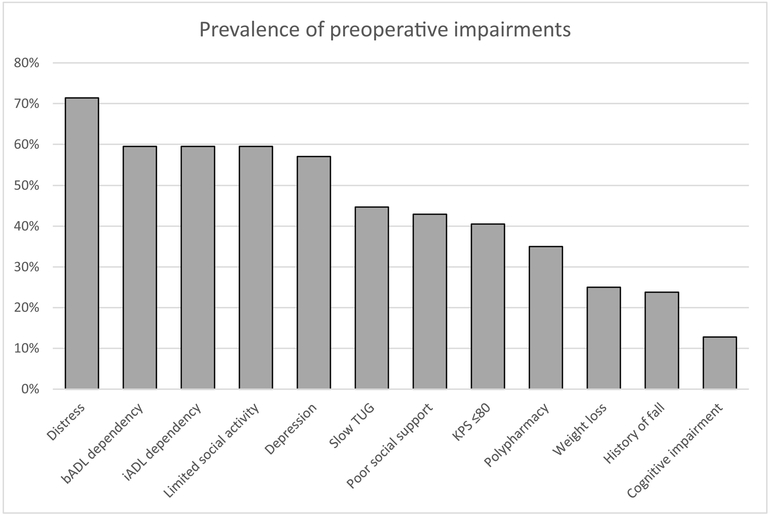
Forty-two women (median age 79, range 74–88), 38 with newly diagnosed advanced OC, 4 with recurrent OC, underwent cytoreductive surgery between 5/2015 and 1/2018. Preoperative age-related impairments per eRFA: high level of distress (71%), functional dependency (59%), limited social activity (59%), depression (57%), slow Time Up and Go (54%), Karnofsky Performance Score (KPS)≤80 (41%), poor social support (43%), polypharmacy (35%), weight loss>10 lbs (25%), fall history (244%), cognitive impairment (13%). Median number of comorbid conditions=3.
Among 38 newly diagnosed women, 26 (68%) had stage IIIC, 11 (29%) stage IV. Sixteen (42%) underwent primary debulking surgery, 22 (58%) neoadjuvant chemotherapy followed by interval debulking surgery. Median duration of surgery=245.5 minutes (range 95–621); median hospital length of stay=6 days (range 0–22). Optimal debulking rate=97%, complete gross resection rate=63%. One patient was admitted to ICU, 26% had 30-day ER visit, 10% were readmitted. Any complication, minor complication, major complication occurred in 58%, 55%, 8%, respectively. Median time from surgery to postoperative chemotherapy=34.5 days (range 19–66). Median follow-up=15.7 months (range 3.7–38.0), 12-month survival=93.3%. There was no 180- day mortality.
Conclusion:
Cytoreductive surgery among older women with advanced OC and frailty can be performed safely in a tertiary care center with preoperative/postoperative geriatric and surgical co-management.
INTRODUCTION
Three-quarters of women with ovarian cancer will present with advanced stage disease and require treatment with a combination of extensive surgery and chemotherapy. Over half of patients with newly diagnosed ovarian cancer are 65 years of age or older [1, 2], and the number of older women with ovarian cancer will grow as the population of older adults continues to increase steadily [3, 4]. Older women gain the same cancer-related survival benefit from aggressive surgery for advanced stage ovarian cancer, but are at higher risk of surgical morbidity and mortality [5]. Older women are also at higher risk of toxicity from chemotherapy [6], with some studies reporting rates of 50% grade 3 or higher toxicity [7]. The optimal sequence of chemotherapy and surgery are unknown for this cohort. Older women are commonly excluded from clinical trials [2, 3, 6] and are less likely to be offered surgical management, despite evidence demonstrating the feasibility of surgical treatment in this age group [4].
It has been suggested that the fitness (or frailty, i.e. decreased level of fitness) of the older cancer patient, rather than chronological age, should be considered in treatment decision-making. The geriatric assessment (GA) is considered the gold standard for assessing frailty. It evaluates multiple key domains which have been shown to be associated with increased surgical risk and chemotherapy-related toxicities: functional and nutritional status, comorbidity and medications, cognition, psychological status, and social support [1, 7]. Frailty, a decrease in physiologic reserve beyond the normal aging process [8], has been linked to a higher risk of surgical complications, as well as longer hospital stays and 30-day readmission rates [9]. There is a growing body of literature linking frailty to chemotherapy toxicity and completion [10–12]. Data is still limited on the fitness of older women who undergo cytoreductive surgery. However, a recent collaborative study was able to demonstrate an association between a GA and postoperative complications in women with advanced stage gynecologic malignancy [13]. There is minimal data available to show which interventions may improve surgical outcomes in older, frail women with ovarian cancer. In the non-oncologic setting, collaboration between surgeons and geriatricians has led to improved outcomes in older patients. The benefits of co-management on length of stay, complication rates, and mortality are most prominently demonstrated in the care of older patients with hip fractures [14, 15]. In one institution, after implementation of a quality improvement program including co-management of hip fracture patients, rates of adverse events were cut in half: number of days in the hospital postoperatively (4.6 vs. 8 days expected), the rate of readmission (9.7% vs. 19.4%), and mortality (1.5% vs. 3.2%) [14]. Similar benefits of co-management have already been demonstrated for other cancer types. International societies call for treatment of older cancer patients based on an individualized assessment of fitness rather than age alone, using standardized geriatric tools [16, 17].
In this study, we aimed to provide a more comprehensive picture of fitness and outcomes in older women with ovarian cancer who underwent cytoreductive surgery at a single institution, using a shared care model based on collaboration between gynecologic oncology surgeons and geriatricians.
METHODS
Patients
After obtaining approval from the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC), we conducted a prospective study of women with newly diagnosed ovarian cancer who were referred to the Geriatrics Clinic for evaluation before cytoreductive surgery. As per standard of care, the majority of patients aged 75 or older were referred to the Geriatrics Clinic for preoperative evaluation, unless the surgical team agreed that the patient had already received preoperative clearance from either a primary care provider or other subspecialist such as a cardiologist. This unique and interconnected relationship with the Geriatrics Service at our institution has only increased since its inception in 2009 [1, 18]. During preoperative clearance, patients completed a geriatric assessment (GA). At MSKCC, the GA is conducted via electronic Rapid Fitness Assessment (eRFA) [19] (Table 1). This tool allows for simultaneous patient-directed completion of the components of the GA via a computer interface, as well as data capture. Following geriatric preoperative evaluation, ovarian cancer care was determined by the Surgical Service utilizing institutional and national guidelines. During the inpatient postoperative period, patients were followed by the Geriatrics Service in a consulting role, with the surgical team in a primary role.
Table 1.
Domains of the geriatric assessments evaluated by the eRFA
| Domain | Tool used for evaluation |
|---|---|
| Functional Status | Patient-rated Karnofsky Performance Status (KPS) |
| Basic Activities of Daily Living (bADL) | |
| Instrumental Activities of Daily Living (iADL) | |
| Time up and Go (TUG) | |
| History of fall within the past 12 months | |
| Use of assistive device | |
| Social Support | Four-item Medical Outcomes Study Social Support Survey |
| Social Activity | Medical Outcomes Study Social Activity Survey |
| Nutritional status | Weight change within the last 6 months |
| Emotional status | Distress Thermometer |
| Geriatrics Depression Scale 4-item questionnaire | |
| Cognition | Mini-Cog test |
| Comorbidity and Polypharmacy | Comorbidity and number of prescribed medications, herbals, supplements, vitamins |
Outcomes
The following outcomes were considered: intensive care unit (ICU) admission, emergency room (ER) visit after discharge, readmission, and adverse surgical events (all within 30 days from surgery), as well as overall mortality. All postoperative surgical complications were captured using an institutional Surgical Secondary Events (SSE) reporting system, which is completed by the surgical team until 30 days postoperatively. Major complications were defined as grade 3 or higher. ICU admission, ER visits, and readmission within 30 days, as well as overall mortality, were obtained from the medical record.
Surgical and Cancer Characteristics
Disease and treatment characteristics were obtained from a prospectively maintained ovarian cancer patient database, and from medical record review. Disease factors included newly diagnosed versus recurrent disease, age at diagnosis, stage, and histology. Advanced stage was defined as stage III or IV disease, based on the 2014 International Federation of Gynecology and Obstetrics (FIGO) staging criteria. Treatment factors included treatment approach: primary debulking surgery (PDS) versus neoadjuvant chemotherapy (NACT) with or without interval debulking surgery (IDS); route of IDS (laparoscopic versus open), operative (OR) time, estimated blood loss (EBL), and surgical outcome: complete gross resection (CGR), optimal resection (residual disease >0 cm but ≤1 cm), or suboptimal resection (residual disease >1 cm). Data on the number of NACT cycles, and reason for NACT, were also collected. Other variables included length of postoperative hospital stay, time to the start of postoperative chemotherapy, and overall survival (OS) (time between disease diagnosis and last follow-up, or death). For patients with recurrent disease, progression-free survival (PFS) was also calculated (time between disease diagnosis and recurrence).
Statistical Analysis
Descriptive statistics were used to describe the patient cohort, preoperative impairments, and postoperative outcomes of interest. The Chi-squared test was used for nominal data, and the Man-Whitney U test for continuous variables. Survival was evaluated using the Kaplan-Meier estimator.
RESULTS
Between May 2015 and January 2018, 123 women 74 years of age and older presented to our institution for treatment of newly diagnosed advanced stage ovarian cancer. Of these, 30 (24%) were treated with upfront surgery; the remaining 93 (75%) were treated with NACT. Of the 93 women treated with NACT, 48 (52%) eventually underwent IDS. In total, 78 women has surgery, either PDS or IDS, during the interval. At the time, institutional guidelines recommended that women 75 and older should be referred to geriatrics for preoperative evaluation, with younger women referred on a case-by-case basis. Of the 78 women treated surgically, 67 (86%) were 75 years of age or older, and 38 of these women (57%) underwent such an evaluation (Table 2). In the same time frame, 4 additional women were treated surgically for recurrent ovarian cancer. The median age at presentation was 79 years (range 74 – 88) for the entire cohort.
Table 2.
Patient demographics
| N | |
|---|---|
| Median age, years (range) | 79 (74 – 88) |
| Median number of comorbidities (range) | 3 (0–8) |
| Recurrent ovarian cancer / SDS | 4 (10) |
| IV | 11 (29) |
| Median number of NACT cycles (range) | 4 (3–6) |
| Mixed | 1 (3) |
Among the 38 patients with newly diagnosed ovarian cancer, 26 (68%) had stage IIIC disease, 11 (29%) had stage IV disease, and 1 (3%) presented with stage IIIB disease. Thirty-four patients (89%) had tumors of high-grade serous histology; the remaining 4 presented with rarer histologies. Sixteen patients (42%) were treated with PDS, and 22 (58%) received NACT followed by IDS. Nine women (41%) were treated with NACT because they met the Aletti criteria [20], 8 (36%) due to extent of disease, 3 (14%) based on recommendations by outside physicians, and 2 (9%) for unique patient-specific reasons. All women completed the GA prior to surgical intervention, either before PDS or IDS, after NACT was completed. Of the women who underwent IDS, 3 (14%) had a robotic-assisted laparoscopic procedure. The median number of administered NACT cycles was 4 (range 3 – 6).
The median duration of surgery was 245.5 minutes (range 95 – 621), with primary surgeries lasting longer (median 278 minutes, range 136 – 621) than interval surgeries (median 222.5 minutes, range 95 – 481) (p=0.046) (Table 3). Yet there was no difference in EBL between PDS and IDS, with a median EBL of 275 mL (range 50 – 1985, p=0.077). Overall, the rate of optimal debulking (residual disease ≤1 cm) during PDS and IDS was 97% (n=37), with a 63% rate of CGR (n=24). Rates of optimal debulking and CGR did not differ between the PDS and IDS groups (p=0.739). The median hospital length of stay was 6 days (range 0 – 22), with no difference between the PDS and IDS groups (p=0.097). The median time to the start of postoperative chemotherapy was 34.5 days (range 19 – 66) and did not differ between the PDS and IDS groups (p=0.099). The median follow-up was 15.7 months (range 3.7 – 38.0). At 12 months postoperatively, 93% of patients with newly diagnosed ovarian cancer were alive.
Table 3.
Surgical and treatment characteristics of women with newly diagnosed ovarian cancer (*patients with >1cm residual disease were excluded from analysis)
| All new diagnoses (n=38) | PDS (n=16) | IDS (n=22) | P value | |
|---|---|---|---|---|
| Median operative time, minutes (range) | 245.5 (95–621) | 278 (136–621) | 222.5 (96–481) | 0.046 |
| Median estimated blood loss, mL (range) | 275(50–1985) | 300 (100–1985) | 225 (50–900) | 0.077 |
| ➢ 1 cm | 1 (3) | 0 (0)* | 1 (5)* | |
| Median hospital stay, days (range) | 6 (0–22) | 7 (4–22) | 6 (0–18) | 0.097 |
| Median time to postoperative chemotherapy, days (range) | 34.5 (19–66) | 41 (22–53) | 30 (19–41) | 0.099 |
The 4 women treated with secondary debulking surgery (SDS) for recurrent ovarian cancer were all originally diagnosed with stage IIIC high-grade serous disease. Two were originally treated with PDS followed by chemotherapy; the 2 others received NACT followed by IDS. One patient underwent NACT due to a concurrently diagnosed pulmonary embolism, and 1 was treated with NACT based on extent of disease. All 4 patients met our institutional criteria for SDS [21]. CGR was achieved in 2 women (1 PDS, 1 IDS); the other 2 had residual disease ≤1 cm. Three patients were treated with SDS for a first recurrence, diagnosed at 20.2, 25.3, and 30.9 months, respectively, after the original diagnosis. The fourth woman was treated with SDS for a second recurrence (66.6 months later).
The median time to completion of the eRFA was 10 minutes (range 2 – 35). The most prevalent preoperative age-related impairments, identified via the eRFA, were high level of distress (71%), followed by functional dependency for basic and instrumental activities of daily living, limited social activity (60%), and depression (57%) (Figure 1). Other impairments included a TUG >10 seconds (46%), KPS≤80 (41%), poor social support (43%), and polypharmacy (35%). Less prevalent impairments were weight loss of >10 lbs within the past six months (25%), a history of fall within the past year (24%), and cognitive impairment (13%). The median number of comorbid conditions was three.
Figure 1.
Prevalence of pre-operative impairments bADL – basic activities of daily living, iADL – instrumental activities of daily living, TUG – Time Up and Go, KPS – Karnofsky Performance Score
Based on the results of the eRFA, patients were referred to various support services. Thirty-eight (90%) women were referred to physical therapy and 13 (31%) to occupational therapy. Only 1 (2%) required rehabilitation in a facility postoperatively. Thirty-seven (88%) women underwent nutritional counseling; 35 (83%) and 11 (26%) spoke to a case manager or social worker, respectively. Two (5%) patients were referred to psychiatry.
Only 1 patient (2%) had an unplanned admission to the ICU within 30 days after surgery for cardiopulmonary support brought on by atrial fibrillation. Eleven (26%) presented to the ER within 30 days, and 4 (10%) were readmitted. Two patients were readmitted for infection management, 1 for work-up of chest pain, and 1 for acute neurologic changes. Surgical complications occurred in 24 (58%) patients; major complications (grade 3 or 4) occurred in 3 (8%) patients. There was no 30-, 90-, or 180-day mortality.
In the same time period 40 additional women aged 74 and older were treated with surgery for newly diagnosed advanced stage ovarian cancer but did not undergo preoperative geriatric evaluation; 14 (35%) underwent PDS and 26 (65%) IDS. The reasons for omitting this evaluation are hard to ascertain on review of the records; some included surgeon preference, or the fact that the patient already had established care with a primary care provider or specialist. In this cohort, 6 patients (15%) had an unplanned admission to the ICU within 30 days of surgery. Seven (18%) presented to the ER for evaluation within 30 days, and 6 (15%) were readmitted. Four patients were readmitted for treatment of infection, 1 for electrolyte imbalance secondary to an ileus, and 1 for dyspnea evaluation. Grade 3/4 surgical complications were present in 4 patients, and there were 2 mortalities:1 within 90 days and 1 within 180 days of surgery. Comparing the five main outcomes between women who did and did not have a preoperative geriatrics evaluation yielded no statistical difference.
DISCUSSION
Care of the older ovarian cancer patient is multimodal and complex, with disease and comorbidity closely interrelated. A plethora of research provides options for ovarian cancer treatment, rooted in extensive surgical resection and combination chemotherapy. Geriatrics and the study of frailty is a rapidly growing field. However, the application of geriatrics principles to the care of women with ovarian cancer and other gynecologic malignancies is in its infancy. Our study examined the collaboration between surgeons and geriatricians at our institution, including those caring for older women with ovarian cancer. We were able to demonstrate that this cohort had a high rate of preoperative impairments but a low rate of postoperative events, and no 180- day mortality. This demonstrates the strength of co-management in this population.
The treatment for advanced stage ovarian cancer includes a combination of extensive surgery and chemotherapy, either of which could be given as upfront treatment. The results of three randomized trials comparing PDS versus NACT/IDS have failed to show a benefit to either approach [22–24]. However, the latest randomized non-inferiority trial [25], and many retrospective studies [26], show a survival benefit for PDS. Such data promotes individualized decision-making in the treatment of every woman diagnosed with advanced stage ovarian cancer, based equally on patient and disease characteristics. However, a bias regarding older women exists. Older women are commonly seen as unable to tolerate extensive surgery and are thus treated with NACT. Unfortunately, the effect of this bias is even more pronounced in clinical trials, as two of the most recently reported randomized trials excluded women aged 75 years and older [22,25]. Data published from the SEER database demonstrates an increase in NACT use in women 65 and older: from 16% in 2000 to 35.4% in 2013 [27]. Since this population comprises half of women diagnosed with ovarian cancer, as well as the largest “data deficient” group, objective measures of patient fitness in this population are needed to help physicians make informed choices for appropriate surgical candidates.
Frailty has been shown to be associated with poorer outcomes. A systematic review combining 20 studies, including approximately 3,000 older patients with solid or hematologic malignancies, demonstrated that frail patients undergoing surgical treatment for cancer had a 3.19 times higher chance of a 30-day postoperative complication and a 2.67 times higher risk of 30-day postoperative mortality [28]. As many as 86% of the patients included in these studies were classified as frail. However, only one of the 20 studies included women with gynecologic cancers [29]; of the 37 patients evaluated in that study, 10 were diagnosed with ovarian cancer, highlighting a need for more research focused on frailty in this population.
Many different tools for assessing frailty have been created, but the GA is considered the gold standard. At our institution, the GA is delivered to patients via the eRFA. The electronic format of this GA allows for more accurate data capture. Using the eRFA, we identified high rates of preoperative impairments in our cohort of women with advanced stage ovarian cancer. Five impairments (distress, bADL and iADL dependency, limited social activity, and depression) were present in more than 50% of women. These high rates of impairment resulted in a high percentage of patients being referred to physical therapy, nutritional counseling, and case management. Simultaneously, we observed low rates of 30-day postoperative events. Such a mismatch between observed deficiencies and complications highlights the importance of evaluation and co-management by physicians who are well-versed in frailty.
Our study is not without limitations. As it is a single institution study, the results may not be generalizable. We did not report on patients seen by the Geriatrics Service who were not cleared for surgery; in the past, however, we have shown that this constitutes approximately 5% of all patients evaluated by the Geriatrics Service for preoperative risk stratification [30]. Our study could not test whether patients’ overall excellent outcomes were due to geriatric co-management or other factors. A control group, preferably in a randomized study, is needed to address this question. As the sample size of our study was small, no relationship between age-related impairments and outcomes could be explored. Larger and possibly multi-institutional studies are needed to test such relationship. Additionally, only 50% of eligible women were referred for preoperative geriatrics evaluation. The reasons for this are multifactorial. However, we have recently made institutional changes to increase this referral rate, basing referrals on fitness and not just on age alone; and we are referring patients earlier, even before they begin NACT.
Nonetheless, our study has significant strengths. It focused on very old women (aged 74 or older). We were able to show the degree of aging-related impairments in this population using the gold standard for frailty, the GA. Using geriatrics and surgical datasets, we were able to capture significant details about the treatment these patients received before and after surgery, and treatment-related outcomes. Although highly selected, this cohort of very old women were safely and successfully treated with surgery for advanced stage ovarian cancer. Although there were no statistical differences, in terms of outcomes studied, between women treated with surgery who did and did not have a preoperative geriatrics evaluation, there were 2 mortalities in the group that did not undergo evaluation, and many more unplanned ICU admissions (6 vs 1). We believe that, with a larger sample size, these clinically significant differences would be even more pronounced. In conclusion, our study demonstrates that cytoreductive surgery for advanced ovarian cancer can be safely performed in women aged 74 or older, even in patients presenting with at least some degree of frailty. Appropriate preoperative evaluation and postoperative co-management by a geriatrics team is feasible and may play a role in outcomes. In the future, additional studies should assess the impact of geriatric co-management on postoperative outcomes in older women with ovarian cancer.
Highlights.
Older women with advanced ovarian cancer have a high rate of preoperative impairment.
We simultaneously demonstrated a low rate of postoperative events.
Geriatric co-management plays a key role in outcomes for these women.
ACKNOWLEDGEMENTS
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None of the authors declare any conflicts of interest.
Disclosures:
Dr. Chi reports personal fees from Bovie Medical Co., personal fees from Verthermia Inc., personal fees from C Surgeries, from Intuitive Surgical Inc., outside the submitted work.
Dr. Long Roche reports other* from Intuitive Surgical Inc., outside the submitted work (*airfare to a survivorship conference, where she spoke).
Dr. O’Cearbhill reports personal fees from Clovis, personal fees from Tesaro, outside the submitted work.
This study was presented as a poster at the International Society of Geriatric Oncology 2018 Annual Conference in Amsterdam, Netherlands, November 16–18, 2018.
REFERENCES
- 1.Tew WP. Ovarian cancer in the older woman. J Geriatr Oncol 2016;7:354–61. [DOI] [PubMed] [Google Scholar]
- 2.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen MLG, van der Burg MEL, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011:29:3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korc-Grodzicki B, Downey RJ, Shahrokni A, Kingham TP, Patel SG, Audisio RA. Surgical considerations in older adults with cancer. J Clin Oncol 2014;32:2647–53. [DOI] [PubMed] [Google Scholar]
- 5.Langstraat C, Aletti GD, Cliby WA. Morbidity, mortality and overall survival in elderly women undergoing primary surgical debulking for ovarian cancer: a delicate balance requiring individualization. Gynecol Oncol 2011;123:187–91. [DOI] [PubMed] [Google Scholar]
- 6.Ferrero A, Fuso L, Tripodi E, Tana R, Daniele A, Zanfagnin V, et al. Ovarian Cancer in Elderly Patients: Patterns of Care and Treatment Outcomes According to Age and Modified Frailty Index. Int J Gynecol Cancer 2017;27:1863–71. [DOI] [PubMed] [Google Scholar]
- 7.Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 2016;34:2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uppal S, Igwe E, Rice LW, Spencer RJ, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol 2015;137:98–101. [DOI] [PubMed] [Google Scholar]
- 9.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013:206:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falandry C, Weber B, Savoye AM, Tinquaut F, Tredan O, Sevin E, et al. Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: a GINECO prospective trial. Ann Oncol 2013;24:2808–13. [DOI] [PubMed] [Google Scholar]
- 11.Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol 2005;16:1795–800. [DOI] [PubMed] [Google Scholar]
- 12.von Gruenigen VE, Huang HQ, Beumer JH, Lankes HA, Tew W, Herzog T, et al. Chemotherapy completion in elderly women with ovarian, primary peritoneal or fallopian tube cancer - An NRG oncology/Gynecologic Oncology Group study. Gynecol Oncol 2017;144:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed A, Deng W, Tew W, Bender D, Manner RS, Littell RD, et al. Pre-operative assessment and post-operative outcomes of elderly women with gynecologic cancers, primary analysis of NRG CC-002: An NRG oncology group/gynecologic oncology group study. Gynecol Oncol 2018;150:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc 2008;56:1349–56. [DOI] [PubMed] [Google Scholar]
- 15.Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014;28:e49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Droz JP, Aapro M, Balducci L, Boyle H, Van den Broeck T, Cathcart P, et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol 2014;15:e404–14. [DOI] [PubMed] [Google Scholar]
- 17.Shuman AG, Patel SG, Shah JP, Korc-Grodzicki B. Optimizing perioperative management of geriatric patients with head and neck cancer. Head Neck 2014;36:743–9. [DOI] [PubMed] [Google Scholar]
- 18.Korc-Grodzicki B, Tew W, Hurria A, Yulico H, Lichtman S, Hamlin P, Bosl G. Development of a Geriatric Service in a Cancer Center: Lessons Learned. J Oncol Pract 2017;13:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahrokni A, Tin A, Downey RJ, Strong V, Mahmoudzadeh S, Boparai MK, et al. Electronic Rapid Fitness Assessment: A Novel Tool for Preoperative Evaluation of the Geriatric Oncology Patient. J Natl Compr Canc Netw 2017;15:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol 2007;197:676.e1–7. [DOI] [PubMed] [Google Scholar]
- 21.Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006;106:1933–9. [DOI] [PubMed] [Google Scholar]
- 22.Fagotti A, Vizzielli G, Ferrandina G, Fanfani F, Gallotta V, Chiantera V, et al. Survival analyses from a randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer with high tumor load (SCORPION trial). J Clin Oncol 2018;36(15 Suppl):5516 Available: http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.5516. [Google Scholar]
- 23.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249–57. [DOI] [PubMed] [Google Scholar]
- 24.Vergote I, Trope CG, Amant F, Kristense GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- 25.Onda T, Satoh T, Kasamatsu T, Nakanishi T, Takehara K, Miyamoto K, et al. Comparison of survival between upfront primary debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomized trial: JCOG0602. J Clin Oncol 2018;36(15 Suppl):5500 Available: http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.5500. [Google Scholar]
- 26.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM Jr, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol 2012;124:10–4. [DOI] [PubMed] [Google Scholar]
- 27.Meyer LA, He W, Sun CC, Zhao H, Wright AA, Suidan RS, et al. Neoadjuvant chemotherapy in elderly women with ovarian cancer: Rates of use and effectiveness. Gynecol Oncol 2018;150:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091–101. [DOI] [PubMed] [Google Scholar]
- 29.Courtney-Brooks M, Tellawi AR, Scalici J, Duska LR, Jazaeri AA, Modesitt SC, et al. Frailty: an outcome predictor for elderly gynecologic oncology patients. Gynecol Oncol 2012;126:20–4. [DOI] [PubMed] [Google Scholar]
- 30.Williams TR, Alexander K, Korc-Grodzicki B, Sarraf S, Shahrokni A. Preoperative evaluation of older cancer patients: Why some patients did not proceed with planned surgery. J Clin Oncol 2017;35(31 Suppl):38 Available: http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.31_suppl.38. [Google Scholar]