Abstract
Background:
Stepping is critical for responding to perturbations, whether externally induced or self-initiated. Falls post-stroke is equally likely to happen from either mechanism. The objective of the study was, to examine lateral stepping performance during waist-pull induced reactive steps and voluntary choice reaction time steps in chronic stroke and controls.
Methods:
In this cross-sectional study participants with chronic stroke (N=10) and ageand gender-matched controls (N=10) performed reactive and voluntary lateral steps. Step initiation time, global step length, step clearance, and step velocity were calculated. Other measures for reactive step included, Balance tolerance limit (perturbation magnitude when recovery transitioned from single to multiple steps), and step type. The Community Balance & Mobility Scale, and hip abductor and adductor isokinetic asymmetry torque ratio were assessed.
Results:
The paretic and non-paretic leg were combined since step characteristics did not differ. Step (voluntary vs. reactive) by group (stroke vs. controls) was significant for step initiation time. The stroke group took longer initiating a voluntary step (P=0.004). Reactive and voluntary steps were executed slower (P=0.041), with a reduced step length (P=0.028) by the stroke group. The stroke group had a lower balance tolerance limit (P=0.01) and took reactive medial steps more frequently (P=0.001). The Community Balance & Mobility Scale (P>0.001), and hip abductor and adductor asymmetry torque ratio (P>0.001; P=0.015) was reduced in the stroke group.
Significance:
Our findings indicate individuals post-stroke are slower initiating and executing reactive and voluntary steps. Though the reactive step timing is less impaired, this may be a method for enhancing faster voluntary movements and training reactive balance.
Keywords: Stroke, Lateral, Balance, Perturbation, Voluntary, Reactive, Stepping
Stroke is one of the leading causes of long-term disability in the United States.1 Falls, in particular, are a significant secondary complication with 40% of individuals experiencing a serious fall within the first year.2 The risk of a hip fracture on the paretic side increases by four-fold with poor balance and a tendency to fall toward the paretic side.3 Preventing a fall often requires increasing the body’s base of support with a quick and fast step to slow down the momentum of the body’s center of mass. Balance recovery is often not effective, as indicated by the high fall rate.4 Asymmetrical weakness, altered sensation, impaired coordination,5 and neuromuscular control contribute to the relative difficulty of stepping to recover balance. Thus, understanding balance recovery through stepping is essential for reducing falls in this population.
In order to maintain balance equilibrium, control can occur proactively in advance of a predictable and known forthcoming disturbance or reactively in response to an external force acting on the body. The mechanisms by which the central nervous system anticipates or quickly senses instability and reacts is different in proactive or voluntarily initiated actions than in reactive balance control. Proactive control that accompanies voluntary movements are estimates of the perceived threat of instability and involve cortical and subcortical networks.6 The capacity to move is influenced by cognitive and psychological factors that can affect the willingness to move the center of mass outside the base of support. Reactive reflex-like responses are more prominently brainstem and spinally mediated, initiated after an external disturbance to balance. Both forms of motor control are important after stroke since the incidence of falls caused by external forces acting on the body, such as slips, trips or pushes, equals those from predictable conditions of instability.7
In healthy older adults, reactive steps are initiated faster with a larger step length and height compared to voluntary steps,8 which may indicate a conservative response to reduce balance instability. In people after stroke, voluntary steps in the sagittal plane are characterized by slow reaction times, poorly coordinated muscles responses and smaller movement amplitudes, limiting the response to challenges to stability.9,10 Similarly, perturbation-induced reactive steps are initiated more slowly,7,11 with a reduced center of mass velocity, and reduced first step length.11 When comparing reactive and voluntary balance recovery, reactive steps are typically initiated faster, with a shorter duration than voluntary steps.12 However, in contrast with sagittally oriented imbalance, falls are more challenging to recover in the lateral direction than the forward direction among healthy older adults.13 In this regard, there is less information on balance recovery in the frontal plane after stroke.14,15 One would expect balance control in the lateral direction to be more challenging after a stroke because of the asymmetrical distribution of sensory and motor deficits.5,16 To address these issues, this study examined the influence of a stroke on the spatial-temporal characteristics of stepping in the frontal plane by comparing visually cued voluntary stepping and waist-pull induced reactive stepping, between community-dwelling individuals with chronic stroke and age- and gender-matched healthy controls. We hypothesized that the spatial-temporal step characteristics would differ between the voluntary and reactive steps, and between stroke compared to controls. We expected individuals after stroke would have a reduced step length and clearance and move slower during the voluntary steps because of their unwillingness to risk balance instability by moving outside of their base of support. The reactive steps are more reflexive and do not rely on similar cortical processes as voluntary steps. Therefore, we expected the stroke group to initiate and execute faster reactive steps, with a greater step length and height compared to voluntary steps. Furthermore, we anticipated reactive and voluntary steps would have reduced spatial-temporal step characteristics in stroke compared to the control group.
Methods
Ten community-dwelling adults with hemiparesis and ten age- and gender-matched controls participated in the study. Participants included in the study were >6 months post-stroke, 50 years of age or older, able to walk 10 meters with or without an assistive device, could stand unsupported for 5 minutes and did not have a medical condition that significantly impacted their ability to walk beyond the effects of the stroke. Participants gave informed consent to participate, and the University of Maryland, Baltimore Institutional Review Board approved the study protocol.
Participants performed reactive steps induced by a lateral waist-pull perturbation controlled by a motor-driven system and visually cued voluntary choice reaction time steps in a laboratory setting. Participants received 24 lateral waist-pull perturbations, three trials in each direction, paretic/non-paretic and left/right at four magnitudes for stroke and control subjects, respectively. The perturbation magnitudes had a fixed acceleration of 720 cm/s2, and the velocity (v) and displacement (d) were as follows, Level 1 v=18.0 cm/s; d=8.6 cm, Level 2 v=27.0 cm/s d=12.1 cm, Level 3 v=36.0 cm/s d=15.7 cm, and Level 4 v=45cm/s d=19.3 cm. The perturbation magnitudes are known to induced steps in older adults17,18 and individuals after stroke.12 Verbal instructions were to, “respond naturally and if necessary prevent yourself from falling.” The Balance tolerance limit (BTL) defined as the perturbation magnitude whereby reactive balance recovery transitioned from single step recovery to multiple step recovery was determined for each person. At BTL the first step was categorized into three step types, 1) a lateral step where the passively loaded leg moves sideways in the waist-pull direction, 2) a crossover step whereby the passively unloaded leg moves toward and past the loaded leg in front (cross front) or behind the body (cross back), or 3) a medial step where the unloaded leg moves toward but not beyond the loaded leg and was followed by a lateral step.18 The voluntary reaction time steps consisted of 10 lateral voluntary reaction time steps and ten crossover voluntary reaction time steps (five paretic/non-paretic, five left/right). The direction of the cross over step (cross front or cross back) taken during induced waist-pull perturbation determined the step type performed for the voluntary reaction time steps (cross front or cross back). A light cue was positioned in front of the participant at eye level, which indicated the time and direction of the step. Participants were asked to step as fast as possible, with no instructions given on the number of steps to be used. The direction of each trial of the reactive and voluntary steps was randomized to reduce the anticipation and learning effects.
Participants were fitted with a safety harness, which did not provide support and stood in their comfortable stance width on two adjacent force platforms (Advanced Mechanical Technology Inc., Watertown, MA, USA). Before the start of the trial, ground reaction forces were monitored visually to ensure symmetrical weight bearing before the trial started. The ground reaction forces were sampled at 600 Hz. A reflective marker was affixed on the lateral malleoli and recorded for 7 seconds per trial at a sampling rate of 120Hz, using a 10-camera motion analysis system (Vicon, Oxford, UK)
Participants underwent a physical function assessment that included, Community Balance & Mobility,19 Timed Up and Go20 and Activities-specific Balance Confidence.21 The Chedoke-McMaster Stroke Assessment Impairment Inventory, assessed leg and foot motor recovery in the stroke group.22 The stages of motor recovery are graded from 1–7, with seven classified as normal and one as flaccid. The Biodex System Pro4 (Biodex Medical Systems, NY, USA) assessed peak isokinetic joint torques bilaterally over five trials at 30°/s for hip abduction and adduction in side lying. The cutaneous sensation assessed on the plantar surface of the foot with a series of Semmes-Weinstein monofilaments, ranging from 1.65–6.65, with the lowest value representing normal cutaneous sensation.23
Data analysis
Customized Matlab programs were used to calculate step initiation time, first global step length, first step clearance and step velocity. Step initiation time was calculated relative to the onset of the light cue (voluntary reaction time step) or the waist-pull perturbation (reactive step) and first step liftoff from the ground reaction forces. First step characteristics of global step length, step clearance, and step velocity were identified from the ankle marker. Global step length calculated as the square root of the sum of squares of displacement in the anteroposterior and mediolateral direction, step clearance defined as the maximum vertical displacement and step velocity calculated by global step length divided by step duration. Global step length and step clearance were normalized to the person’s height and expressed as a percent. Peak isokinetic torque was defined as a torque symmetry ratio (paretic/non-paretic or non-dominant/dominant). Statistics
Statistical analyses were performed using SPSS for Windows version 22.0 (Chicago, IL, USA). Between-group differences were determined for BTL with a Chi-square test, step type with Mann-Whitney U test, and demographics and clinical measures with parametric and non-parametric t-tests. Between-group comparisons between the paretic and non-paretic voluntary and reactive step parameters were made with multivariate analyses (Table 1) using the trials. Twelve trials (7 paretic; 5 non-paretic) for the voluntary steps are excluded because the participants stepped with the wrong leg and 8 trials (7 paretic side pulls; 1 non-paretic side pulls) are excluded from the induced perturbation due to markers falling off during stepping. Adjustments were made for the reactive steps to account for differences in step type and perturbation magnitude. Adjustments were made for the voluntary steps to account for differences in step type. No significant differences were found between the paretic and non-paretic sides, for the following analyses the data were combined. A repeated measures analysis of variance with simple contrasts were used to compare step (voluntary, reactive) as the within-subject factor and between-group differences between stroke and control group for the first step initiation time, global step length, step clearance, and step velocity. Analyses included the reactive steps at BTL and the voluntary steps. Data included are average across trials with one data value for reactive and voluntary steps. A sub-analysis of the reactive step data was performed using a multivariate analysis of covariance with the BTL and step type as covariates and the variables of first step initiation time, global step length, step clearance, and step velocity. The step type was coded from the smallest to the largest, for example the medial step would have the smallest spatiotemporal characteristics due to the constraints of the stance limb and was coded 1. Thus, based on this principle, lateral steps were coded 2 and crossover steps were coded 3. The reactive step type characteristics among stroke, paretic, non-paretic and controls were examined with a Kruskal Wallis Test and when an effect occurred the Mann-Whitney U test to determine the difference. Bonferroni corrections were performed to correct for multiple comparisons with an adjusted P value (P ≤ .025). Data are presented at median and as a range (25–75 percentile). A Pearson correlation coefficient was used to determine the relationship between hip abductor and adductor torque of the leg controlling lateral stability and the step initiation time of the voluntary cross over and lateral reaction time and individual trials for reactive steps. The significance level was set at P <0.05.
Table 1.
Paretic and non-paretic leg step parameters for voluntary RT and reactive steps, expressed as mean (95% CI).
| Voluntary steps | Reactive Steps | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Paretic N=93 |
Non-paretic N=95 |
P value |
Adj. P value | Paretic N=26 |
Non-paretic N=26 |
P value |
Adj. P value |
| Step Initiation time (ms) | 1064.0 (979.6–1148.4) |
1010.5 (927.0–1094.0) |
0.38 | 0.24 | 486.9 (437.8–535.9) |
534.6 (487.4–581.8) |
0.17 | 0.11 |
| Step length (% of body height) | 18.0 (16.0–20.0) |
19.6 (17.6–21.6) |
0.25 | 0.88 | 17.8 (11.6–23.9) |
18.5 (12.6–24.4) |
0.86 | 0.83 |
| Step clearance (% of body height) | 4.4 (3.2–5.7) |
5.1 (3.9–6.3) |
0.43 | 0.85 | 4.1 (2.5–5.7) |
3.7 (2.1–5.2) |
0.69 | 0.62 |
| Step Velocity (m/s) | 0.21 (0.18–0.23) |
0.29 (0.26–0.31) |
0.21 | 0.11 | 0.29 (0.21–0.38) |
0.27 (0.19–0.36) |
0.71 | 0.67 |
Results
The mean age of the stroke group was 62.8±9.4 years, with five males and five females (Table 2). Six participants had left hemiparesis and four right sided. The cutaneous sensation was not significantly different between the stroke and control group (P=0.31). The stroke group scored significantly lower on Community Balance & Mobility (t(19)=−6.3, P<0.001) and Activities-Specific Balance Confidence (t(19)=−3.3, P=0.006) and took longer to complete the Timed Up and Go (t(19)= 2.6, P=0.017) compared to controls. The torque symmetry ratio for the hip abductor (t(19)=−5.6, P<0.001) and adductor (t(19)=−2.7, P=0.015) was significantly reduced in stroke compared to controls.
Table 2.
Characteristics of stroke and control group, expressed as mean (standard deviation)
| Stroke | Controls | P value | |
|---|---|---|---|
| Age (years) | 62.8 (9.4) | 64.8 (8.5) | |
| Gender | 5 Female / 5 Male | 5 Female / 5 Male | |
| Post-stroke (years) | 13.7 (range 2 – 48) | ---- | |
| Paretic side | 4 Right / 6 Left | ---- | |
| Body mass index (kg/m2) | 29.2 (8.4) | 26.8 (5.0) | |
| Clinical Measures | |||
| CMSA Score (leg+foot, /14) | 7.9 (2.0) | ---- | ---- |
| Cutaneous Sensation | 3.61–4.37 (4.31) | 3.78–4.17 (3.84) | 0.31 |
| TUG (seconds) | 11.1 (2.1) | 6.9 (1.2) | 0.017 |
| CB&M Scale (/96) | 36.4 (13.5) | 74.9 (13.0) | >0.001 |
| ABC (/100) | 81.5 (12.7) | 96.1 (5.5) | 0.006 |
| Hip Abductor torque (%) | 0.64 (0.11) | 1.1 (0.20) | >0.001 |
| Hip Adductor torque (%) | 0.80 (0.18) | 1.2 (0.36) | 0.015 |
Cutaneous sensation presented as 25th-75th quartile (median), stroke data is presented for paretic side
Reactive versus Voluntary Stepping
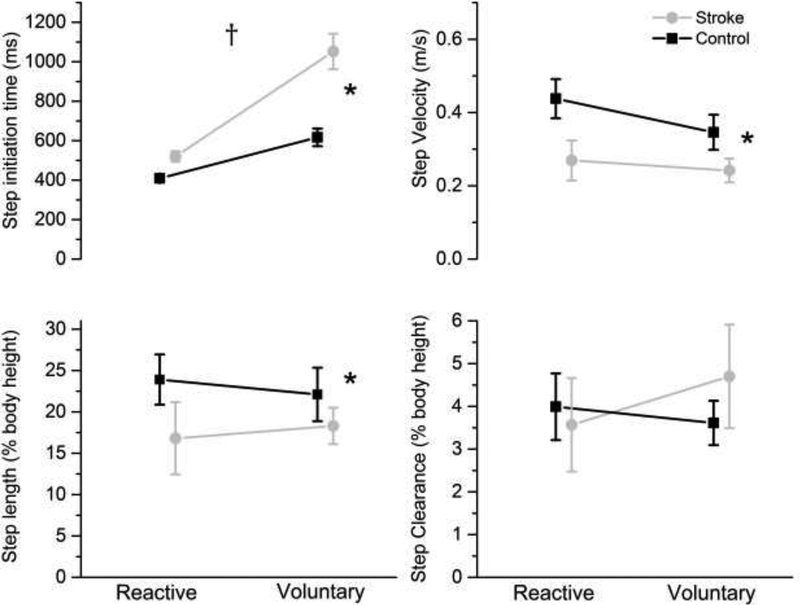
Step initiation time was significant for step (voluntary, reactive) by group interaction (F1,19=10.8, P=0.004) (Figure 1; Table 3). The stroke group took longer to initiate the voluntary reaction time step (F1,19=60.8, P=0.001) compared to controls with no between-group differences in reactive steps. Overall, step initiation time was significantly longer for voluntary steps than reactive steps (F1,19=12.6, P<0.001). There were significant between-group differences, with the stroke group having a slower step velocity (F1,19=4.9, P=0.045) and reduced global step length (F1,19=5.7, P=0.02) for voluntary reaction time and reactive steps. There were no significant differences in first step clearance between step or group.
Figure 1.
First step characteristics of reactive and voluntary steps for the stroke (gray circles) and control (black squares) group represented by means and standard error. † P<0.05 step x group interaction; * P<0.05 stroke significantly different from controls
Table 3:
Voluntary and reactive step characteristics in stroke and controls, mean (95% CI).
| Voluntary steps | Reactive Steps | P value | |||||
|---|---|---|---|---|---|---|---|
| Step | Group |
Step X group |
|||||
| Measure | Stroke N=10 |
Control N=10 |
Stroke N=10 |
Control N=10 |
|||
| Step Initiation time (ms) | 1052.4 (905.0–1199.8) |
520.0 (475.1–565.0) |
645.4 (490.1–800.8) |
409.0 (361.6–456.4) |
>0.001 | 0.001 | 0.001 |
| Step length (% of body height) | 18.3 (12.5–24.1) |
23.0 (16.9–29.1) |
16.8 (8.9–24.7) |
23.9 (15.6–32.2) |
0.91 | 0.02 | 0.63 |
| Step clearance (% of body height) | 4.7 (2.7–6.7) |
3.8 (1.7–5.9) |
3.6 (1.6–5.6) |
4.0 (1.9–6.1) |
0.59 | 0.83 | 0.45 |
| Step Velocity (m/s) | 0.24 (0.16–0.33) |
0.33 (0.24–0.42) |
0.27 (0.16–0.38) |
0.44 (0.32–0.55) |
0.06 | 0.045 | 0.25 |
Reactive Steps
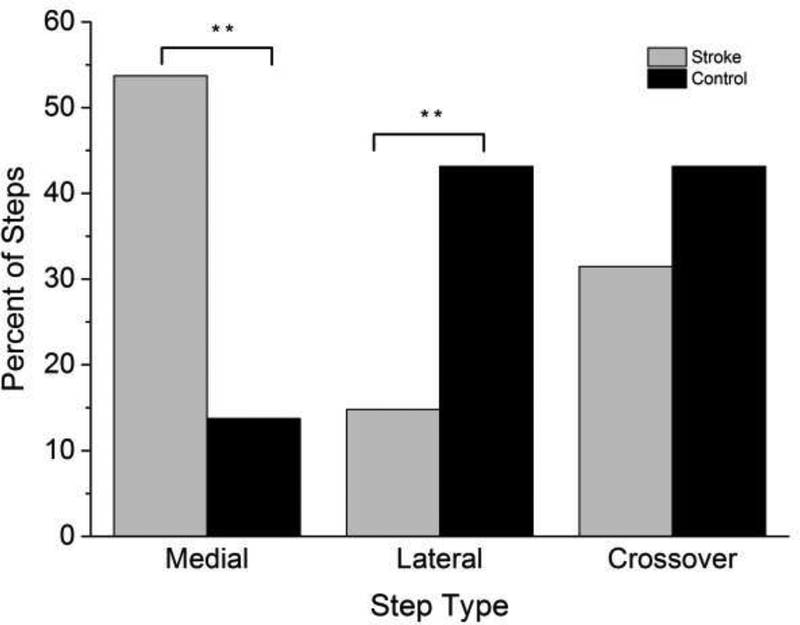
The median BTL of the stroke group was significantly less than controls (stroke 3.0 (IQR 0), control 4.0 (IQR 2.0), U=109.0 (Z=−2.9), P=0.01). Significant differences in percent of each step type indicated that the stroke group took medial steps more often (χ2=13.8, P=0.001) and used lateral steps less frequently (χ2=7.1, P=0.001) than controls (Figure 2). The percent of crossover steps was not significantly different between groups (χ2= 2.83, P=0.065).
Figure 2.
Percent of total trials for reactive first step types for all trials in the stroke group (gray bars) and control (black bars) group for a medial step, lateral step and crossover step. ** P=0.001 stroke significantly different from control group
Based on the group differences in BTL and step type, a subanalysis was performed with the velocity of the waist-pull perturbation and step type used as covariates. After adjusting for perturbation magnitude and step type, there were significant between-group differences in step initiation time (F1,91=21.9, P<0.001), step velocity (F1,91=3.1, P =0.05), and step clearance (F1,91=10.8, P<0.001), but not global step length (F1,91=2.3, P =0.099) for the reactive steps.
In comparing step types by paretic, non-paretic and controls (Table 4) a medial step initiated with the paretic was slower H(2)=16.8, P>0.001, compared to the non-paretic leg (Mann–Whitney U=32.0, P=0.004) and control group (Mann–Whitney U=16.0, P<0.006). The step clearance was also significantly different H(2)=10.3, P=0.006 for the crossover steps, with a larger step clearance for the paretic compared to the control group (Mann- Whitney U=12.0, P=0.002). Analyses for the lateral step could not be performed due to the low incident of lateral steps in stroke (paretic=3; non-paretic=4).
Table 4:
Reactive step characteristics of the medial and crossover step for paretic, nonparetic and control group, median (range: 25–75 percentile)
| Step type | Paretic | Non-paretic | Controls | P value |
|---|---|---|---|---|
| Medial | N=17 | N=11 | N=7 | |
| Step Initiation time (ms) | 506.7 (458.3– 535.0)*† | 606.7 (520.0–698.3) † | 366.7 (365.0–413.3) | >0.001 |
| Step length (% of body height) | 5.6 (3.4–8.4) | 7.3 (3.5–8.7) | 7.1 (4.4–9.0) | 0.65 |
| Step clearance (% of body height) |
0.6 (0.3–2.0) | 1.3 (0.5–1.7) | 0.9 (0.2–2.3) | 0.76 |
| Step Velocity (m/s) | 0.09 (0.06–0.16) | 0.17 (0.08–0.21) | 0.16 (0.13–0.22) | 0.15 |
| Lateral | N=3 | N=4 | N=22 | |
| Step Initiation time (ms) | 533.3 (511.7–533.3) | 447.5 (417.1–756.7) | 414.1 (385.0–450.0) | |
| Step length (% of body height) | 28.7 (28.6–28.6) | 16.6 (5.9–18.3) | 21.4 (14.9–23.1) | |
| Step clearance (% of body height) |
3.3 (1.5–3.3) | 1.6 (1.3–2.4) | 2.9 (1.9–3.3) | |
| Step Velocity (m/s) | 0.56 (0.56–0.56) | 0.53 (0.16–0.57) | 0.59 (0.44–0.70) | |
| Crossover | N=6 | N=11 | N=22 | |
| Step Initiation time (ms) | 393.3 (372.1–412.5) | 396.7 (353.3–520.0) | 403.3 (362.9–436.3) | 0.78 |
| Step length (% of body height) | 38.8 (27.2–42.4) | 37.9 (36.6–40.5) | 36.3 (31.1–37.0) | 0.30 |
| Step clearance (% of body height) |
10.1 (8.2–11.6)* † | 8.7 (6.1–0.9.8) | 6.4 (3.9–7.6) | 0.006 |
| Step Velocity (m/s) | 0.41 (0.31–0.48) | 0.42 (0.37–0.49) | 0.46 (0.40–0.49) | 0.49 |
P value, group differences with Kruskal Wallis Test
significantly difference from non-paretic side with Mann-Whitney U test
significantly different from control group
Association between initiation time and hip torque
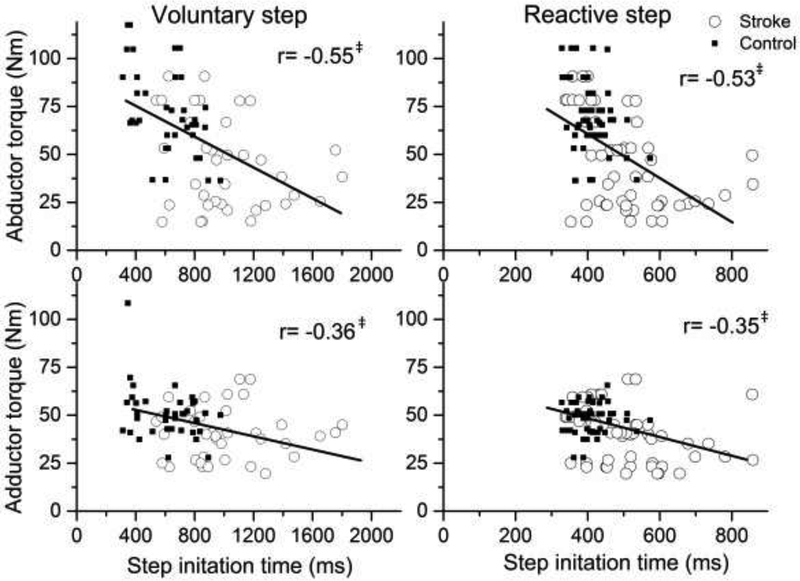
Reactive step initiation time was negatively associated with hip abductor (r(91)=−0.53; P<0.001) and adductor torque (r(91)=−0.35; P=0.01). Similarly, a negative association was observed between the hip abductor (r(91)=−0.55; P<0.001) and adductor torque (r= −0.36; P<0.001) and voluntary reaction time step initiation time (Figure 3).
Figure 3.
The relationship between voluntary and reactive step initiation time and hip abductor and adductor torque for stroke (open circles) and controls (filled squares) ‡P<0.001
Discussion
The findings of this study highlight differences initiating and executing voluntary reaction time steps versus perturbation-induced reactive steps in the frontal plane after stroke compared to healthy controls. Overall, reactive steps were initiated earlier than the visually cued rapid voluntary step in both groups. The stroke group took longer to initiate the voluntary reaction time step compared to healthy controls. Furthermore, the stroke group executed reactive steps more slowly, with a reduced first global step length compared to controls. The stroke group transitioned to a multiple step recovery pattern at a lower perturbation, and predominantly initiated the first step with the passively unloaded leg (medial step) and used the passively loaded leg (lateral step) less frequently compared to controls.
Among the different mediolateral reactive steps, lateral steps are biomechanically more stable, and less risky than other steps, such as crossover and medial steps, which lead to limb collisions and multiple steps, and are characteristic of older adult fallers.17,24 Initiating a lateral step widens the base of support and increases stability, and adequate hip abductor muscle torque is necessary for this adjustment.25 The stroke group took fewer lateral reactive steps, which are biomechanically more stabilizing. Instead, they initiated medial steps more frequently. Similar findings are reported in older adults at risk of falls, with more medial steps taken,26 which also was related to reduced hip abductor torque.27 Thus, reduced capacity to quickly generate hip abductor-adductor or other leg muscle torques may limit the ability for taking a lateral step.28 Delayed and absent hip abductor muscle responses are reported in the paretic muscles after stroke. Individuals with stroke are unable to recruit their paretic hip abductors quickly and compensate with their non-paretic legs to maintain balance.29 Greater impairments are found during step initiation than walking in the paretic hip abductor muscles,30 indicating the initial activation of the muscles is more challenging in stroke. We observed significant group differences in hip abductor-adductor torque asymmetry ratio. There was a significant relationship in the hip torque and the time taken to initiate a reactive and voluntary reaction time step. In our previous study, we showed that the hip aductors disciminated the step type that was taken.15 Similarly, de Kam et al. found that a single lateral step recovery required quick and large muscle burst from the hip abductors.14 This may indicate delays in step initiation may be related to the changes that occur in the hip muscles after a stroke, such as reduced motor units and type II fibers that allow for the motor units to be recruited quickly to initiate movement.5
Reduced somatosensation is associated with fall risk and is another reason for not taking a lateral step as the first reactive.31 Though, both groups in our study had diminished light touch of the plantar aspect of the foot. Previously, it has been shown after a stroke that people with impaired sensation are more likely to step with their passively unloaded leg (crossover or medial) but not specifically medial steps.15 Medial steps may be biomechanically less stabilizing than other step types but may represent a compensatory strategy that limits the base of support adjustment with a shorter first step length, while not requiring comparatively large torques from the hip muscles to recover balance. The findings appear to indicate that people after stroke have a limited capacity to execute a lateral step.
Lower perturbation magnitudes are observed in stroke, transitioning from a single step recovery to multiple steps.14 Similar to our study the balance limit threshold was a transition from single to multiple steps, the stroke group had a lower balance limit threshold than controls, which may place them at risk of falling with larger perturbation magnitudes. Previous research also found the older adult faller transition to multiple steps at a lower balance tolerance limit compared to younger adults.32,33 This could indicate reactive stepping may be a stored motor plan based on prior experiences and triggered by other factors. Some factors may include reduced hip torque or cognitive aspects that influence prior experiences of poor balance, and a fear of falling that may influence the behaviors associated with balance. Schinkel-Ivy et al34 did not find a relationship between balance confidence and reactive stepping in stroke. However, the two-stroke groups had Activities-Specific Balance Confidence values below the cutoff score of 81.1 use to identify fallers,35 indicating both groups are impaired in reactive stepping. In our study, we observed group differences in balance confidence and reactive and voluntary reaction time step behaviors. Thus, relative to older adults with more balance confidence people after stroke with impaired reactive and voluntary reaction time stepping score lower on balance confidence.
Similar to studies in stroke12 and aging,8 the time to initiate reactive steps was faster than for voluntary RT steps. Our study found that the stroke group was slower to initiate voluntary reaction time steps, whereas the initiation time for reactive steps was not significantly different from controls. The faster reactive step initiation time compared with voluntary reaction time steps in the stroke group, indicated a capacity to move faster. Though, the percent of lateral reactive steps in stroke is low (~10% in stroke, compared to ~45% in healthy), most of the steps are medial which allow individuals in the stroke group to take advantage of the passive momentum from the pull, leading to a faster step initiation. The mechanism of the reactive balance recovery likely engages less cortical processing that is slow and thus may be a means of training faster responses. For example, training faster reaction time voluntary stepping with reactive stepping has been demonstrated in older adults,36 although, this has not been applied to people after stroke.
The study was a first effort in determining the responses to challenging balance perturbations, and it is limited by the sample size. The limited step types, especially for the lateral steps restricted analysis of the induced step trials with each leg. Although, the study was focusing on natural self-selected responses to induced perturbations the results reflect a preferred performance.
Conclusions
Effective stepping strategies are important for preventing falls. Reactive step initiation time was faster than the voluntary reaction time step for both groups. People after stroke executed reactive and voluntary reaction time steps more slowly with a reduced step length compared with people of similar age. Though, reactive steps taken by the stroke group were largely medial steps which may be a compensatory strategy used to reduce the amount of hip torque necessary to generate a quick lateral step. Further studies investigating the effectiveness of reactive stepping strategies would be needed to understand whether the capacity to generate hip muscle torque is related to step types.
Highlights.
Implication for rehabilitation
Voluntary and reactive protective step reactions are important for preventing falls.
Self-initiated steps after stroke are initiated and executive slower.
Individuals after stroke initiate reactive steps almost as fast as controls.
People with weaker hip abductor/adductor torque initiate protective steps slower.
Acknowledgments
Declaration of interest: The contents of this publication were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (H133P100014, H133F140027). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this publication do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the Federal Government. This study was also supported by the American Heart Association (14CRP19880025) and The National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center P30-AG028747.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persson CU, Hansson PO, Sunnerhagen KS. Clinical tests performed in acute stroke identify the risk of falling during the first year: postural stroke study in Gothenburg (POSTGOT). J Rehabil Med. 2011;43(4):348–353. doi: 10.2340/16501977-0677 [DOI] [PubMed] [Google Scholar]
- 3.Mulley G, Espley AJ. Hip fracture after hemiplegia. Postgr Med J. 1979;55(642):264–265. https://www.ncbi.nlm.nih.gov/pubmed/471862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–1213. http://www.ncbi.nlm.nih.gov/pubmed/19235120. [PubMed] [Google Scholar]
- 5.Garland SJ, Gray VL, Knorr S. Muscle activation patterns and postural control following stroke. Motor Control. 2009;13(4). doi: 10.1123/mcj.13.4.387 [DOI] [PubMed] [Google Scholar]
- 6.Movement Massion J., posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. https://www.ncbi.nlm.nih.gov/pubmed/1736324. [DOI] [PubMed] [Google Scholar]
- 7.Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27(6):526–533. doi: 10.1177/1545968313478486 [DOI] [PubMed] [Google Scholar]
- 8.Luchies CW, Wallace D, Pazdur R, Young S, DeYoung AJ. Effects of age on balance assessment using voluntary and involuntary step tasks. J Gerontol A Biol Sci Med Sci. 1999;54(3):M140–4. https://www.ncbi.nlm.nih.gov/pubmed/10191842. [DOI] [PubMed] [Google Scholar]
- 9.Gray VL, Ivanova TD, Garland SJ. Effects of fast functional exercise on muscle activity after stroke. Neurorehabil Neural Repair. 2012;26(8). doi: 10.1177/1545968312437944 [DOI] [PubMed] [Google Scholar]
- 10.Melzer I, Goldring M, Melzer Y, Green E, Tzedek I. Voluntary stepping behavior under single- and dual-task conditions in chronic stroke survivors: A comparison between the involved and uninvolved legs. J Electromyogr Kinesiol. 2010;20(6):1082–1087. doi: 10.1016/j.jelekin.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Salot P, Patel P, Bhatt T. Reactive Balance in Individuals With Chronic Stroke: Biomechanical Factors Related to Perturbation-Induced Backward Falling. Phys Ther. 2016;96(3):338–347. doi: 10.2522/ptj.20150197 [DOI] [PubMed] [Google Scholar]
- 12.Martinez KM, Mille ML, Zhang Y, Rogers MW. Stepping in persons poststroke: comparison of voluntary and perturbation-induced responses. Arch Phys Med Rehabil. 2013;94(12):2425–2432. doi: 10.1016/j.apmr.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 13.Mille ML, Johnson-Hilliard M, Martinez KM, Zhang Y, Edwards BJ, Rogers MW. One step, two steps, three steps more ... Directional vulnerability to falls in community-dwelling older people. J Gerontol A Biol Sci Med Sci. 2013;68(12):1540–1548. doi: 10.1093/gerona/glt062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Kam D, Roelofs JMB, Bruijnes AKBD, Geurts ACH, Weerdesteyn V. The Next Step in Understanding Impaired Reactive Balance Control in People with Stroke: The Role of Defective Early Automatic Postural Responses. Neurorehabil Neural Repair. 2017;31(8):708–716. doi: 10.1177/1545968317718267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray VL, Yang C-L, McCombe Waller S, Rogers MW. Lateral Perturbation-Induced Stepping: Strategies and Predictors in Persons Poststroke. J Neurol Phys Ther. 2017;41(4). doi: 10.1097/NPT.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roerdink M, Geurts AC, de Haart M, Beek PJ. On the relative contribution of the paretic leg to the control of posture after stroke. Neurorehabil Neural Repair. 2009;23(3):267–274. doi: 10.1177/1545968308323928 [DOI] [PubMed] [Google Scholar]
- 17.Hilliard MJ, Martinez KM, Janssen I, et al. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89(9):1708–1713. doi: 10.1016/j.apmr.2008.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yungher DA, Morgia J, Bair WN, et al. Short-term changes in protective stepping for lateral balance recovery in older adults. Clin Biomech (Bristol, Avon). 2012;27(2):151–157. doi: 10.1016/j.clinbiomech.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knorr S, Brouwer B, Garland SJ. Validity of the Community Balance and Mobility Scale in community-dwelling persons after stroke. Arch Phys Med Rehabil. 2010;91(6):890–896. doi: 10.1016/j.apmr.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 20.Hafsteinsdóttir T, Rensink M, Schuurmans M. Clinimetric properties of the Timed Up and Go Test for patients with stroke: a systematic review. Top Stroke Rehabil. 2014;21(13):197–210. [DOI] [PubMed] [Google Scholar]
- 21.Yiu J, Miller WC, Eng JJ, Liu Y. Longitudinal analysis of balance confidence in individuals with stroke using a multilevel model for change. Neurorehabil Neural Repair. 2012;26(8):999–1006. doi: 10.1177/1545968312437941 [DOI] [PubMed] [Google Scholar]
- 22.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63 http://www.ncbi.nlm.nih.gov/pubmed/8418551. [DOI] [PubMed] [Google Scholar]
- 23.Roll R, Kavounoudias A, Roll JP. Cutaneous afferents from human plantar sole contribute to body posture awareness. Neuroreport. 2002;13(15):1957–1961. http://www.ncbi.nlm.nih.gov/pubmed/12395099. [DOI] [PubMed] [Google Scholar]
- 24.Mille ML, Johnson ME, Martinez KM, Rogers MW. Age-dependent differences in lateral balance recovery through protective stepping. Clin Biomech (Bristol, Avon). 2005;20(6):607–616. doi: 10.1016/j.clinbiomech.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 25.Patton JL, Hilliard MJ, Martinez K, Mille ML, Rogers MW. A simple model of stability limits applied to sidestepping in young, elderly and elderly fallers. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3305–3308. doi: 10.1109/IEMBS.2006.260199 [DOI] [PubMed] [Google Scholar]
- 26.Bair WN, Prettyman MG, Beamer BA, Rogers MW. Kinematic and behavioral analyses of protective stepping strategies and risk for falls among community living older adults. Clin Biomech (Bristol, Avon). 2016;36:74–82. doi: 10.1016/j.clinbiomech.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Addison O, Inacio M, Bair WN, Beamer BA, Ryan AS, Rogers MW. Role of Hip Abductor Muscle Composition and Torque in Protective Stepping for Lateral Balance Recovery in Older Adults. Arch Phys Med Rehabil. 2017;98(6):1223–1228. doi: 10.1016/j.apmr.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inacio M, Ryan AS, Bair WN, Prettyman M, Beamer BA, Rogers MW. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr. 2014;14:37. doi: 10.1186/1471-2318-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirker SG, Jenner JR, Simpson DS, Wing AM. Changing patterns of postural hip muscle activity during recovery from stroke. Clin Rehabil. 2000;14(6):618–626. http://www.ncbi.nlm.nih.gov/pubmed/11128737. [DOI] [PubMed] [Google Scholar]
- 30.Kirker SG, Simpson DS, Jenner JR, Wing AM. Stepping before standing: hip muscle function in stepping and standing balance after stroke. J Neurol Neurosurg Psychiatry. 2000;68(4):458–464. http://www.ncbi.nlm.nih.gov/pubmed/10727481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allet L, Kim H, Ashton-Miller J, De Mott T, Richardson JK. Step length after discrete perturbation predicts accidental falls and fall-related injury in elderly people with a range of peripheral neuropathy. J Diabetes Complicat. 2014;28(1):79–84. doi: 10.1016/j.jdiacomp.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mille ML, Rogers MW, Martinez K, et al. Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol. 2003;90(2):666–674. doi: 10.1152/jn.00974.2002 [DOI] [PubMed] [Google Scholar]
- 33.Rogers MW, Hedman LD, Johnson ME, Cain TD, Hanke TA. Lateral stability during forward-induced stepping for dynamic balance recovery in young and older adults. J Gerontol A Biol Sci Med Sci. 2001;56(9):M589–94. https://www.ncbi.nlm.nih.gov/pubmed/11524454. [DOI] [PubMed] [Google Scholar]
- 34.Schinkel-Ivy A, Inness EL, Mansfield A. Relationships between fear of falling, balance confidence, and control of balance, gait, and reactive stepping in individuals with sub-acute stroke. Gait Posture. 2016;43:154–159. doi: 10.1016/j.gaitpost.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beninato M, Portney LG, Sullivan PE. Using the International Classification of Functioning, Disability and Health as a framework to examine the association between falls and clinical assessment tools in people with stroke. Phys Ther. 2009;89(8):816–825. doi: 10.2522/ptj.20080160 [DOI] [PubMed] [Google Scholar]
- 36.Rogers MW, Johnson ME, Martinez KM, Mille ML, Hedman LD. Step training improves the speed of voluntary step initiation in aging. J Gerontol A Biol Sci Med Sci. 2003;58(1):46–51. https://www.ncbi.nlm.nih.gov/pubmed/12560410. [DOI] [PubMed] [Google Scholar]