Abstract
Background
Non-invasive prenatal testing (NIPT) using cell-free fetal DNA from maternal plasma for fetal aneuploidy identification is expanding worldwide. The objective of this study was to evaluate the clinical utility of NIPT for the detection of trisomies 21, 18, and 13 of high-risk fetus in a large Korean population.
Methods
This study was performed retrospectively, using stored maternal plasma from 1,055 pregnant women with singleton pregnancies who underwent invasive prenatal diagnosis because of a high-risk indication for chromosomal abnormalities. The NIPT results were confirmed by karyotype analysis.
Results
Among 1,055 cases, 108 cases of fetal aneuploidy, including trisomy 21 (n = 57), trisomy 18 (n = 42), and trisomy 13 (n = 9), were identified by NIPT. In this study, NIPT showed 100% sensitivity and 99.9% specificity for trisomy 21, and 92.9% sensitivity and 100% specificity for trisomy 18, and 100% sensitivity and 99.9% specificity for trisomy 13. The overall positive predictive value (PPV) was 98.1%. PPVs for trisomies 21, 18, and 13 ranged from 90.0% to 100%.
Conclusion
This study demonstrates that our NIPT technology is reliable and accurate when applied to maternal DNA samples collected from pregnant women. Further large prospective studies are needed to adequately assess the performance of NIPT.
Keywords: Prenatal Screening, Cell-free DNA, Massively Parallel Sequencing
Graphical Abstract
INTRODUCTION
Conventionally, prenatal screening for trisomy 21 is determined using ultrasound measurement of fetal nuchal translucency thickness and biochemical measurement of pregnancy-associated plasma protein-A, α-fetoprotein, unconjugated estriol, human chorionic gonadotropin, and inhibin A in the first and second trimesters. This screening method yields a detection rate of 96%, but it has a high false-positive rate (FPR) of 5%, causing unnecessary invasive diagnostic procedures and associated fetal loss.1
In 2011, non-invasive prenatal testing (NIPT) based on analysis of cell-free DNA (cfDNA) obtained from maternal plasma was introduced into clinical practice as a new approach for screening for common fetal aneuploidies. Its clinical introduction has been successfully reported in many clinical validation studies using shotgun massively parallel sequencing, targeted massively parallel sequencing, and single-nucleotide polymorphism-based sequencing.2,3,4,5,6,7 In a recent meta-analysis, NIPT showed a high detection rate of more than 99%, with a low FPR of less than 1% for trisomy 21.8
Although NIPT clinical services were introduced in Korea in 2015, a large-scale clinical study is still lacking. A previous report described reliable results for fetal aneuploidy detection using 447 Korean samples enrolled in a multicenter study. However, there was only one sample for trisomy 18 and one sample for trisomy 13 among the 15 common fetal aneuploidies that were tested by NIPT.9 Therefore, the sample size was insufficient for validating the clinical value of NIPT.
The objective of this study was to validate the clinical utility of NIPT performance in detecting trisomies 21, 18, and 13 of high-risk fetus using 1,055 samples for the purpose of a large-scale clinical service at a single Korean center.
METHODS
Study population
This study was a nested case-control study of women with singleton pregnancies who received prenatal care in the Department of Obstetrics and Gynecology at Cheil General Hospital. For the current study, 1,055 pregnant women with euploidy, trisomy 21, trisomy 18, or trisomy 13 were enrolled. Exclusion criteria included women who had fetuses with an additional chromosomal abnormality, were without karyotype results, had known maternal aneuploidy or cancer, had a multiples pregnancy, or had a pregnancy by oocyte donation. All subjects had an invasive diagnostic test, such as chorionic villus sampling (CVS) or amniocentesis. The evaluation of fetal chromosomes was performed by karyotyping and quantitative fluorescence polymerase chain reaction (QF-PCR). All maternal peripheral blood samples were drawn prior to performing the invasive procedure.
cfDNA preparation and massively parallel shotgun sequencing
Approximately ten milliliters of maternal peripheral blood was collected in EDTA tubes and centrifuged at 1,600 × g for 10 minutes. The plasma supernatant was re-centrifuged at 16,000 × g for 10 minutes and one-milliliter aliquots of the supernatant were made for DNA extractions. The plasma aliquots were stored at −80°C until analysis. The cfDNA was extracted from one to four milliliters of maternal plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany). The DNA concentration was determined using the Qubit2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA). The DNA library was prepared using the Ion Xpress PlusFragment Library Kit (Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions. The size distribution and concentration of the libraries were analyzed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The clonal amplification and enrichment steps were performed using the Ion Chef or Ion OneTouch instruments (Life Technologies). The resulting libraries were sequenced using the Ion S5 XL system (Life Technologies).
Data analysis
The data analyses of massively parallel shotgun sequencing were performed using a novel algorithm and bioinformatics pipeline based on z-score calculations. The control group was a set of reference samples of known euploidy. Z-scores at or above 3 were classified as high-risk for trisomy 21, trisomy 18, or trisomy 13. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated with standard formulas for binomial distributions.
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. Appropriate Institutional Review Board approval for this study was obtained from the Ethics Committee at Cheil General Hospital (CGH-IRB-2013-3-1). All subjects provided written informed consent for the collection of samples and subsequent analysis.
RESULTS
A total of 1,055 pregnant women were enrolled in this study. Based on the karyotype results from invasive prenatal testing, 947 of the fetuses were euploid and 108 were aneuploid. The cases of aneuploidy included 57 cases of trisomy 21, 42 cases of trisomy 18, and 9 cases of trisomy 13. The clinical characteristics of the study subjects are summarized in Table 1. The median maternal age was 36 years (range, 22–48 years) and the median fetus gestational age was 16.1 weeks, with a range from 10 to 21.4 weeks. Forty four percent of the tests were performed in the first trimester and 56% of the tests were performed in the second trimester. The most common indications for testing were advanced maternal age (41.8%), multiple indications (25.2%), or abnormal ultrasound findings (17.1%). Regarding the indications for testing, the prevalences of trisomy 21 and trisomy 18 were 0.5% (2/442) and 1.6% (7/442) in the group with advanced age, 13.9% (37/266) and 8.3% (22/266) in the group with multiple indications, and 7.7% (14/181) for both in the group with ultrasound anomalies.
Table 1. Clinical characteristics of study subjects.
| Clinical characteristics | Data | |
|---|---|---|
| Maternal age, yr | 36 (22–48) | |
| < 35 | 342 (32.4) | |
| ≥ 35 | 713 (67.6) | |
| Gestational age, wk | 16.1 (10–21.4) | |
| 1st trimester | 462 (43.8) | |
| 2nd trimester | 593 (56.2) | |
| Body mass index, kg/m2 | 21.4 (14.9–53.5) | |
| Gender-ratio of fetus (men:women) | 550:505 (52:48) | |
| Indication for karyotyping | ||
| Advanced maternal age | 442 (41.8) | |
| Ultrasound abnormality | 181 (17.1) | |
| Positive serum screening | 128 (12.1) | |
| Multiple indication | 266 (25.2) | |
| Others | 38 (3.6) | |
| Chromosomal karyotype | 1,055 (100) | |
| Normal | 947 (89.8) | |
| Trisomy 13 | 9 (0.8) | |
| Trisomy 18 | 42 (4.0) | |
| Trisomy 21 | 57 (5.4) | |
Values are median (range) or number (%).
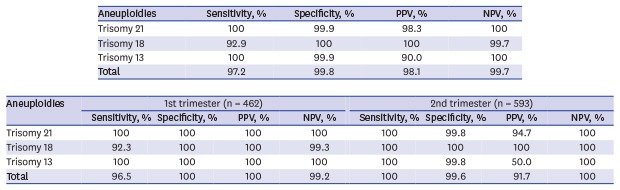
The NIPT performance showed 100% sensitivity and 99.9% specificity for the detection of trisomy 21, 92.9% sensitivity and 100% specificity for trisomy 18, and 100% sensitivity and 99.9% specificity for trisomy 13. The PPVs for trisomy 21, trisomy 18 and trisomy 13 were 98.3%, 100%, and 90.0%, respectively. The pooled sensitivity, specificity and PPV for trisomies 21, 18, and 13 were 97.2%, 99.8%, and 98.1%, respectively (Table 2).
Table 2. Performance of non-invasive prenatal testing in detection of trisomies 21, 18, and 13.
| Aneuploidies | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|
| Trisomy 21 | 100 | 99.9 | 98.3 | 100 |
| (57/57) | (997/998) | (57/58) | (997/997) | |
| Trisomy 18 | 92.9 | 100 | 100 | 99.7 |
| (39/42) | (1,013/1,013) | (39/39) | (1,013/1,016) | |
| Trisomy 13 | 100 | 99.9 | 90.0 | 100 |
| (9/9) | (1,045/1,046) | (9/10) | (1,045/1,045) | |
| Total | 97.2 | 99.8 | 98.1 | 99.7 |
| (105/108) | (945/947) | (105/107) | (945/948) |
PPV = positive predictive value, NPV = negative predictive value.
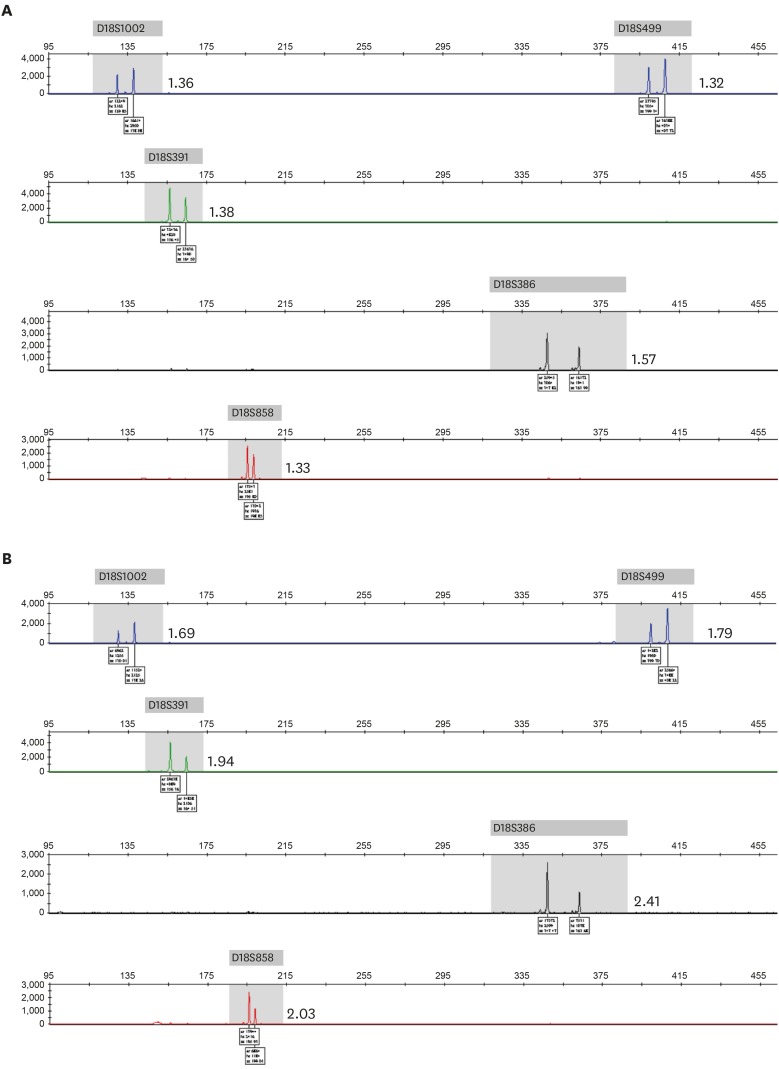
When analyzing NIPT results according to the trimester of the pregnancy, sensitivity in both trimesters was at 100%, but specificity was higher in the first trimester than in the second trimester for the detection of trisomy 21 and trisomy 13. For trisomy 18, the specificity was the same in both trimesters, at 100%, but sensitivity was higher in the second trimester than in the first trimester of pregnancy. For trisomies 21, 18, and 13, the pooled sensitivity was higher in the second trimester than in the first, but the pooled specificity and PPV were lower in the second trimester (Table 3). There were two false-positive results for trisomy 21 and trisomy 13, which were confirmed with normal karyotypes in amniocentesis results. For trisomy 18, there were three false-negative cases with placental mosaicism in CVS results. These cases showed low-level mosaicism for trisomy 18 in the QF-PCR analysis of uncultured chorionic villi samples, but showed complete trisomy 18 in QF-PCR and karyotype analysis (data not shown) of long-term cultured chorionic villi samples (Fig. 1).
Table 3. Performance of non-invasive prenatal testing according to the pregnancy trimester.
| Aneuploidies | 1st trimester (n = 462) | 2nd trimester (n = 593) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
| Trisomy 21 | 100 | 100 | 100 | 100 | 100 | 99.8 | 94.7 | 100 |
| (39/39) | (423/423) | (39/39) | (423/423) | (18/18) | (574/575) | (18/19) | (574/574) | |
| Trisomy 18 | 92.3 | 100 | 100 | 99.3 | 100 | 100 | 100 | 100 |
| (36/39) | (423/423) | (36/36) | (423/426) | (3/3) | (590/590) | (3/3) | (590/590) | |
| Trisomy 13 | 100 | 100 | 100 | 100 | 100 | 99.8 | 50.0 | 100 |
| (8/8) | (454/454) | (8/8) | (454/454) | (1/1) | (591/592) | (1/2) | (591/591) | |
| Total | 96.5 | 100 | 100 | 99.2 | 100 | 99.6 | 91.7 | 100 |
| (83/86) | (376/376) | (83/83) | (376/379) | (22/22) | (569/571) | (22/24) | (569/569) | |
PPV = positive predictive value, NPV = negative predictive value.
Fig. 1. QF-PCR results of a chorionic villus sample with placental mosaicism for trisomy 18. (A) Skewed allele ratios in an uncultured chorionic villus sample, demonstrating mosaicism for trisomy 18. (B) Skewed allele ratios in a long-term cultured chorionic villus sample, demonstrating trisomy 18. Allele ratios are shown.
QF-PCR = quantitative fluorescence polymerase chain reaction.
The NIPT result was obtained in 100% of the cases for euploidy and trisomy. However, 51 cases failed the initial NIPT testing due to technical reasons, 48 of which were successfully rerun without using a second aliquot of plasma. Among these cases were one trisomy 21 and two trisomy 18. The remaining three cases required a third run in order to obtain results.
DISCUSSION
We demonstrated the clinical utility of NIPT technology for the detection of fetal trisomies 21, 18, and 13 during the first or second trimester of pregnancy in a Korean population. The present study evaluated the sensitivity and specificity of NIPT with fetal karyotype results rather than the pregnancy outcome, which has been used in previous studies. As a result, NIPT showed high sensitivities of 100%, 92.9%, and 100%, with a low FPR of 0.09%, 0%, and 0.09% for trisomies 21, 18, and 13, respectively. These results were similar to those of other population-based studies with various NIPT methods.8 Therefore, our results suggest that NIPT provides a high-quality and accurate results for non-invasive detection of trisomies 21, 18, and 13 using fetal cfDNA from maternal plasma.
Recently, a meta-analysis reported test performance between high-risk, routine and mixed groups.8 In the high-risk group, previous studies have demonstrated that NIPT can detect 99% of trisomy 21 pregnancies, with a FPR of less than 1%, but the test performance for trisomies 18 and 13 is less accurate. Among individual studies for trisomy 21, the sensitivity varied from 96.7% to 100% and the FPR varied from 0% to 0.94%. For trisomy 18, the sensitivity varied between 92.3% and 100% and the FPR was between 0% and 0.22%. For trisomy 13, the sensitivity was between 87.5% and 100%, with a FPR from 0% to 0.25%. Our presented data obtained with the high-risk pregnancy group were also within these ranges.
The discordant results between NIPT and invasive diagnostic tests are most likely due to placental mosaicism.10 In this study, there were two false positive results for trisomy 21 and trisomy 13 and three false negative results for trisomy 18. However, the false-positive results that were identified at the time of amniocentesis could not be confirmed for placental mosaicism. The false-negative results that were identified by CVS showed peak patterns of mosaic trisomy 18 in QF-PCR of uncultured villi samples, but showed peak patterns of complete trisomy 18 in QF-PCR of long-term cultured villi samples. In the QF-PCR test, uncultured villi samples include cells from both the cytotrophoblast, which is the origin of fetal cfDNA, and the mesoderm, whereas long-term cultured villi samples include cells from only the mesoderm.11 Therefore, the discordant results for trisomy 18 are likely to arise from 1) normal cells derived from cytotrophoblast and trisomic cells derived from the mesoderm or 2) low-level mosaic cells for trisomy that are derived from the cytotrophoblast and trisomic cells that are derived from the mesoderm.
In addition to sensitivity and specificity, the test failure rate is an important criterion for evaluating test performance quality. The failed results arise as a consequence of low fetal fractions or other technical reasons.12 The test failure rate varies depending on the sequencing method and the reported rates ranged from 0.7% to 8.1% in previous reports of similar scale.7,13,14 In this study, although 4.8% of initial tests failed to provide a result because of a technical reason that caused low read counts, 94% of these were resolved after a repeat run. Only three cases required additional run beyond the repeat run and were successfully analyzed. Therefore, these cases were not rated as a test failure. However, in clinical practice, 99.7% of cases can yield results within a turn-around time of seven to ten calendar days. If a result cannot be reported within this time period, the test is considered a failure, implying that this could occur with 0.3% of samples.
In clinical practice, the limitations of NIPT such as false test results and test failure should be explained to the pregnant woman. NIPT is a screening test, and both false positive and false negative results occur. Therefore, pregnant women with abnormal NIPT results are strongly recommended the diagnostic confirmation with CVS or amniocentesis. And also, pregnant women with failed test results need an invasive diagnostic testing rather than re-test of NIPT because of the increased risk for fetal aneuploidy.
To our knowledge, this is the largest clinical validation study to estimate the accuracy of non-invasive detection of common fetal trisomies performed in Korea. The strength of this study involves the pairing of NIPT and karyotype results from a large number of pregnant women in a single center, with the presence of a relatively large number of abnormal cases (10.2%) when comparing to previous studies.7,14,15,16,17 Also, we confirmed placental mosaicism in false-negative cases using QF-PCR and CVS karyotyping results. However, this study was designed using stored samples and is still considered to be a small sample size for drawing valid conclusions when comparing to recently published studies.18,19,20 Therefore, further studies with a large prospective cohort are required to validate the use of NIPT for the detection of a broader spectrum of fetal chromosomal abnormalities.
ACKNOWLEDGMENTS
We thank the following scientists of Cheil General Hospital and Women's Healthcare Center who took the time and effort to contribute to this study: Jin Woo Kim, Do Jin Kim, Bom Yi Lee, JuYeon Park, Shin Young Kim, Ji Hyae Lim, Yeon Woo Lee, Ah Rum Oh, and Shin Yeong Lee.
Footnotes
Funding: This study was supported by a grant from the Korea Health Technology R&D Project, through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1336). Also, this study was supported by SK Telecom.
Disclosure: Jungsun Park, Taegyun Yun, and Dong Yoon Park are employees of the SK Telecom and other authors have nothing to disclose. Involvement of SK employees did not compromise the scientific integrity of this work.
- Conceptualization: Lee DE, Ryu HM.
- Data curation: Lee DE.
- Formal analysis: Lee DE.
- Methodology: Lee DE, Kim H.
- Software: Park DY, Park J, Yun T.
- Writing - original draft: Lee DE.
- Writing - review & editing: Ryu HM.
References
- 1.Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005;353(19):2001–2011. doi: 10.1056/NEJMoa043693. [DOI] [PubMed] [Google Scholar]
- 2.Sehnert AJ, Rhees B, Comstock D, de Feo E, Heilek G, Burke J, et al. Optimal detection of fetal chromosomal abnormalities by massively parallel DNA sequencing of cell-free fetal DNA from maternal blood. Clin Chem. 2011;57(7):1042–1049. doi: 10.1373/clinchem.2011.165910. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808. doi: 10.1056/NEJMoa1311037. [DOI] [PubMed] [Google Scholar]
- 4.Norton ME, Brar H, Weiss J, Karimi A, Laurent LC, Caughey AB, et al. Non-Invasive Chromosomal Evaluation (NICE) Study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–137.e8. doi: 10.1016/j.ajog.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Verweij EJ, Jacobsson B, van Scheltema PA, de Boer MA, Hoffer MJ, Hollemon D, et al. European non-invasive trisomy evaluation (EU-NITE) study: a multicenter prospective cohort study for non-invasive fetal trisomy 21 testing. Prenat Diagn. 2013;33(10):996–1001. doi: 10.1002/pd.4182. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaides KH, Syngelaki A, Gil M, Atanasova V, Markova D. Validation of targeted sequencing of single-nucleotide polymorphisms for non-invasive prenatal detection of aneuploidy of chromosomes 13, 18, 21, X, and Y. Prenat Diagn. 2013;33(6):575–579. doi: 10.1002/pd.4103. [DOI] [PubMed] [Google Scholar]
- 7.Pergament E, Cuckle H, Zimmermann B, Banjevic M, Sigurjonsson S, Ryan A, et al. Single-nucleotide polymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstet Gynecol. 2014;124(2 Pt 1):210–218. doi: 10.1097/AOG.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):302–314. doi: 10.1002/uog.17484. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Jung H, Han SH, Lee S, Kwon J, Kim MG, et al. An adaptive detection method for fetal chromosomal aneuploidy using cell-free DNA from 447 Korean women. BMC Med Genomics. 2016;9(1):61. doi: 10.1186/s12920-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao J, Wang T, Wang BJ, Liu YH, Li H, Zhang J, et al. Confined placental origin of the circulating cell free fetal DNA revealed by a discordant non-invasive prenatal test result in a trisomy 18 pregnancy. Clin Chim Acta. 2014;433:190–193. doi: 10.1016/j.cca.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Donaghue C, Mann K, Docherty Z, Ogilvie CM. Detection of mosaicism for primary trisomies in prenatal samples by QF-PCR and karyotype analysis. Prenat Diagn. 2005;25(1):65–72. doi: 10.1002/pd.1086. [DOI] [PubMed] [Google Scholar]
- 12.Comas C, Echevarria M, Rodríguez MA, Prats P, Rodríguez I, Serra B. Initial experience with non-invasive prenatal testing of cell-free DNA for major chromosomal anomalies in a clinical setting. J Matern Fetal Neonatal Med. 2015;28(10):1196–1201. doi: 10.3109/14767058.2014.947579. [DOI] [PubMed] [Google Scholar]
- 13.Gil MM, Quezada MS, Bregant B, Ferraro M, Nicolaides KH. Implementation of maternal blood cell-free DNA testing in early screening for aneuploidies. Ultrasound Obstet Gynecol. 2013;42(1):34–40. doi: 10.1002/uog.12504. [DOI] [PubMed] [Google Scholar]
- 14.Oepkes D, Page-Christiaens GC, Bax CJ, Bekker MN, Bilardo CM, Boon EM, et al. Trial by Dutch laboratories for evaluation of non-invasive prenatal testing. Part I-clinical impact . Prenat Diagn. 2016;36(12):1083–1090. doi: 10.1002/pd.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang F, Ren J, Chen F, Zhou Y, Xie J, Dan S, et al. Noninvasive Fetal Trisomy (NIFTY) test: an advanced noninvasive prenatal diagnosis methodology for fetal autosomal and sex chromosomal aneuploidies. BMC Med Genomics. 2012;5:57. doi: 10.1186/1755-8794-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke WL, Zhao WH, Wang XY. Detection of fetal cell-free DNA in maternal plasma for Down syndrome, Edward syndrome and Patau syndrome of high risk fetus. Int J Clin Exp Med. 2015;8(6):9525–9530. [PMC free article] [PubMed] [Google Scholar]
- 17.Qi G, Yi J, Han B, Liu H, Guo W, Shi C, et al. Noninvasive prenatal testing in routine clinical practice for a high-risk population: experience from a center. Medicine (Baltimore) 2016;95(41):e5126. doi: 10.1097/MD.0000000000005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597. doi: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Liu H, Peng C, Deng T, Fu X, Chung C, et al. Clinical experience of non-invasive prenatal chromosomal aneuploidy testing in 190,277 patient samples. Curr Mol Med. 2016;16(8):759–766. doi: 10.2174/1566524016666161013142335. [DOI] [PubMed] [Google Scholar]
- 20.Taneja PA, Snyder HL, de Feo E, Kruglyak KM, Halks-Miller M, Curnow KJ, et al. Noninvasive prenatal testing in the general obstetric population: clinical performance and counseling considerations in over 85 000 cases. Prenat Diagn. 2016;36(3):237–243. doi: 10.1002/pd.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]