Abstract
Background and Aims:
Pancreatic intraepithelial neoplasia is associated with chronic pancreatitis (CP) changes on EUS. The objective of this study was to determine whether CP changes were more common in high-risk individuals (HRIs) than in control subjects and whether these changes differed among higher-risk subsets of HRIs.
Methods:
HRIs and control subjects were identified from an endoscopy database. HRIs were defined as having predisposing mutations or a family history (FH) of pancreatic ductal adenocarcinoma. HRIs were classified as vHRIs who met Cancer of the Pancreas Screening (CAPS) criteria for high risk and mHRIs who did not. Multivariable logistic regression was used to adjust for confounders and CP risk factors.
Results:
Sixty-five HRIs (44 vHRIs, 21 mHRIs) and 118 control subjects were included. HRIs were included for FH (25), Lynch syndrome (5), Peutz-Jeghers syndrome (2), and mutations in BRCA1/2 (26), PALB2 (3), ATM (3), and CDKN2A (1). After adjustment for relevant variables, HRIs were 16 times more likely to exhibit 3 or more CP changes than control subjects (95% confidence interval, 2.6–97.0; P = .003). HRIs were also more likely to have hypoechoic foci (odds ratio, 8.0; 95% confidence interval, 1.9–32.9; P = .004). vHRIs and mHRIs did not differ in frequency of 3 or more CP changes on EUS.
Conclusions:
HRIs were more likely to exhibit CP changes and hypoechoic foci on EUS compared with control subjects. HRIs with these findings may require closer surveillance. HRIs who did or did not meet CAPS criteria did not differ with regard to CP findings, supporting a more inclusive approach to screening. (Gastrointest Endosc 2019;89:842–51.)
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer-related deaths in the United States.1 Prognosis has remained poor because PDAC often presents in later stages.1,2 Because survival depends on the initial stage,2 early detection is key. Although most PDAC cases are sporadic, screening the general population is not feasible given significant cost. However, some are considered high-risk individuals (HRIs) because of either significant family history (FH) of PDAC or a pre-disposing genetic mutation or syndrome. In these patients, screening is pursued to prevent PDAC or detect it at early stages where prognosis is improved.
In 2013 the International Cancer of the Pancreas Screening (CAPS) Consortium published consensus recommendations on screening HRIs.3 The CAPS consortium recommended rigorous criteria meant to identify HRIs.3 However, other high-risk groups that are not included in these criteria are recognized in published screening protocols.4–13 For screening modality, magnetic resonance imaging/MRCP and EUS were recommended by the CAPS consortium.3 Successful screening was defined as detection of T1N0M0 PDAC or high-grade pre-cursor lesions, including pancreatic intraepithelial neoplasia (PanIN).3 PDAC is detected at very low rates, ranging from 0% to 12.5%,4–7,9,11–17 and can present during screening as metastatic disease.6,9 Given the known association between PanIN and PDAC18 and the increased frequency of PanIN in familial PDAC,19 there is benefit in detecting this precursor lesion during screening.
Chronic pancreatitis (CP) changes may be associated with underlying PanIN. EUS is the most appropriate modality to detect these changes, which are quantified through 9 widely used criteria.20 PanIN lesions are believed to cause localized duct obstruction leading to lobulocentric atrophy, which, when multifocal, presents on EUS as CP changes.21 Histopathologic studies on pancreatectomy specimens from HRIs with CP changes shows PanIN associated with loss of acinar parenchyma and lobulocentric atrophy.21–23 Through its relation to PanIN, the presence of CP changes may indicate a higher risk of PDAC. Takenaka et al24 found that among patients with sporadic intraductal papillary mucinous neoplasms, prior CP changes on EUS were associated with invasive carcinoma.
These findings suggest that CP changes on EUS, particularly in patients without clinical CP, may help predict PDAC risk. Among HRIs, studies have shown anywhere from 0% to 60% of patients had CP changes on EUS,6,10–15,17,22,25 although only 2 studies10,22 compared HRIs with normal-risk populations. It is unclear whether CP changes on EUS differ between HRIs who do or do not meet the CAPS criteria for high PDAC risk. Presence of CP changes among more “moderate-risk” HRIs who do not meet these criteria could support a more inclusive screening approach.
In this study our primary aim was to determine whether CP changes were more prevalent in HRIs when compared with individuals not at high risk for PDAC. Among HRIs, we further sought to investigate whether CP changes differed between higher and moderate-risk subsets of HRIs.
METHODS
Study design
We performed a single-center retrospective study from December 2012 to December 2017 examining CP changes on EUS in HRIs and control subjects. Approval for this study was obtained from the Stanford University Institutional Review Board (IRB no. 19286).
Consecutive HRIs who underwent screening EUS at our center were included. Patients who met the CAPS criteria were classified as very high-risk individuals (vHRIs) and included patients with the following:
Two or more relatives with PDAC, including 1 first-degree relative (FDR)
Peutz-Jeghers syndrome (PJS)
BRCA2, PALB2, or CDKN2A mutation or Lynch syndrome (LS) with at least 1 FDR with PDAC
At our center, we regularly screen high-risk groups who did not meet the CAPS criteria. These individuals were classified as moderately high-risk individuals (mHRIs) and included patients with the following:
Three or more non-FDRs with PDAC
BRCA1, BRCA2, PALB2, CDKN2A, or ATM mutation or LS regardless of FH of PDAC
HRIs were excluded if they had prior PDAC, pancreatic surgery, or clinical evidence of other pancreatic disease.
Consecutive patients undergoing EUS for nonpancreatobiliary indications were included as control subjects. Reasons for exclusion were FH of PDAC, prior pancreatic disease or surgery, lipase/amylase elevation, CA 19–9 elevation, or prior pancreatobiliary imaging abnormalities. For HRIs and control subjects, incomplete description of the pancreas on EUS led to exclusion.
Data collection
We retrospectively analyzed the Stanford University Medical Center endoscopy database to identify HRIs and control subjects who underwent EUS during our study period. EUS was performed by 1 of 4 operators who each had at least 3 years of experience. Information on CP changes was abstracted from EUS reports. We used the standard 9 criteria for CP: hyperechoic strands, hyperechoic foci, lobularity, cysts, ductal dilation, ductal irregularity, hyperechoic duct walls, visible side branches, and intraductal stones. At our center, in agreement with consensus criteria, we defined lobularity as well-circumscribed structures with an enhancing rim and a relatively echo-poor center.26 We further defined hypoechoic foci to represent echo-poor foci without this enhancing rim. Beyond CP changes and hypoechoic foci, we also documented solid pancreatic lesions. The decision to sample pancreatic abnormalities was based on operator discretion depending on technical feasibility and safety. For patients with more than 1 EUS, information was gathered from each procedure. Our primary outcome of interest was the presence of 3 or more CP changes, which is a commonly used threshold for the diagnosis of CP.20,26–28
From the electronic medical record, we gathered information on age, gender, race, body mass index (BMI), diabetes, and history of smoking. For patients with multiple EUS procedures, the most recent EUS with the greatest number of CP changes was used to determine age. We determined presence of significant alcohol use (>2 drinks per day) and any alcohol use. We further quantified alcohol use using fluid ounces per week, where each standard drink contains .6 fluid ounces.
Comparisons and statistical methods
Data are presented as mean ± standard deviation or frequency (%). For univariate analyses the Pearson χ2 test or the Fisher exact test was used for categorical variables, whereas the 2-sample t test with unequal variances was used for continuous variables.
We first compared HRIs with control subjects. An analysis of variables was conducted by performing univariate logistic regression analyses with 3 or more CP changes as the outcome variable and the following independent variables: age, male gender, white race, BMI, diabetes, smoking history, any prior alcohol use, and number of EUS procedures. Any variable associated with being an HRI and with 3 or more CP changes at a P < .15 was a potential confounder. Number of EUS procedures, male gender, and any alcohol use were found to be potential confounders in our cohort. These variables were included along with other classic CP risk factors in the final multivariable logistic regression model. Classic CP risk factors were age, male gender, any alcohol use, history of smoking, and history of diabetes. When comparing HRIs and control subjects, we similarly performed 5 separate logistic regression models with the 4 most common CP changes (hyperechoic strands, lobularity, cysts, hyperechoic duct walls) and hypoechoic foci as outcome variables. Potential confounders differed based on outcome variable and included male gender, age, number of EUS, BMI, and any alcohol use. Potential confounders were included with classic CP risk factors in final multivariable logistic regression models.
In a similar fashion, we performed logistic regression analyses investigating 3 pairwise comparisons: control subjects versus mHRIs, control subjects versus vHRIs, and mHRIs versus vHRIs. Outcome variables of interest were 3 or more CP changes, hyperechoic strands, lobularity, cysts, hyperechoic duct walls, and hypoechoic foci. Potential confounders varied based on comparison and outcome variable and included male gender, number of EUS, any alcohol use, age, and BMI. Classic CP risk factors and potential confounders were included in final multivariable logistic regression models.
All statistical analyses were performed with the Stata/IC 15.1 statistical package (StataCorp LP, College Station, Tex). A P < .05 was considered to be statistically significant.
RESULTS
We identified 65 HRIs and 118 control subjects meeting our inclusion criteria. Table 1 describes FH and mutation information for HRIs. Among HRIs, reason for high-risk status included FH (25), BRCA1 (6), BRCA2 (19), PALB2 (3), ATM (3), BRCA2 and ATM (1), CDKN2A (1), LS (5), and PJS (2). Forty-four vHRIs met CAPS criteria and 21 mHRIs did not. Of 118 control subjects, EUS indications included pathology of the esophagus (16), stomach (32), liver (1), gall-bladder (2), spleen (1), duodenum (27), regional lymph nodes (22), mesenteric/retroperitoneal mass (7), and pre-transplant evaluation (1). No HRIs and 17 control subjects were excluded for incomplete description of the pancreas on EUS.
TABLE 1.
Characteristics of HRIs (n = 65)
| No. of cases | Percent of total | |
|---|---|---|
| HRI with mutation | 40 | 61.54 |
| BRCA1 | 6 | |
| BRCA2 | 19 | |
| PALB2 | 3 | |
| ATM | 3 | |
| CDKN2A | 1 | |
| BRCA2+ATM | 1* | |
| Lynch syndrome | 5 | |
| PJS | 2 | |
| vHRIs | 44 | 67.69 |
| vHRIs with FH | 24 | |
| 2+ FDRs | 12 | |
| 1 FDR and 1+ non-FDR | 12 | |
| vHRIs with mutation | 20 | |
| BRCA2 and 1+ FDR | 12* | |
| PALB2 and 1+ FDR | 1 | |
| CDKN2A and 1+ FDR | 1 | |
| Lynch syndrome and 1+ FDR | 4 | |
| PJS | 2 | |
| mHRIs | 21 | 32.31 |
| mHRIs with FH | 1 | |
| 0 FDR and 3+ non-FDRs | 1 | |
| mHRIs with mutation | 20 | |
| BRCA1 and 1+ non-FDR | 3 | |
| BRCA1 with no FH | 3 | |
| BRCA2 and 1+ non-FDR | 5 | |
| BRCA2 with no FH | 3 | |
| PALB2 with any/no FH | 2 | |
| CDKN2A with any/no FH | 0 | |
| Lynch syndrome with any/no FH | 1 | |
| ATM with any/no FH | 3 |
HRI, High-risk individual; FH, family history; vHRI, HRIs who meet the Cancer of the Pancreas Screening consortium criteria; mHRI, HRIs who do not meet the Cancer of the Pancreas Screening consortium criteria; FDR, first-degree relative.
One vHRI had ATM and BRCA2 mutations
CP changes in HRIs and control subjects
Table 2 shows baseline demographics of control subjects and HRIs. HRIs were more likely to be women (72% vs 49%; P = .002) and have any alcohol use (58% vs 36%; P = .003) than control subjects. HRIs also underwent more EUS procedures than control subjects (1.6 vs 1.1; P < .001).
TABLE 2.
Baseline demographics of HRIs and control subjects
| Demographic | Control subjects (n = 118) | HRIs (n = 65) | P value |
|---|---|---|---|
| Female | 58 (49) | 47 (72) | .002† |
| White | 72 (61) | 45 (69) | .239† |
| BMI | 26.2 ± 5.9 | 27.3 ± 5.8 | .224* |
| History of diabetes | 15 (13) | 6 (9) | .480† |
| History of smoking | 46 (39) | 21 (32) | .370† |
| Alcohol use | |||
| >2 drinks/day | 5 (4) | 3 (5) | 1‡ |
| Any alcohol use | 42 (36) | 38 (58) | .003† |
| Fluid oz/wk | 1.4 ± 3.9 | 2.0 ± 2.8 | .242* |
| No. of EUS procedures | 1.1 ± .4 | 1.6 ± 1.1 | <.001* |
Values are mean ± standard deviation or n (%). Each standard drink (1 can beer, 1 glass wine, 1 shot liquor) has .6 fluid oz alcohol.
HRIs, High-risk individuals; BMI, body mass index.
Two-sample t-test with unequal variance.
Pearson χ2 test.
Fisher exact test.
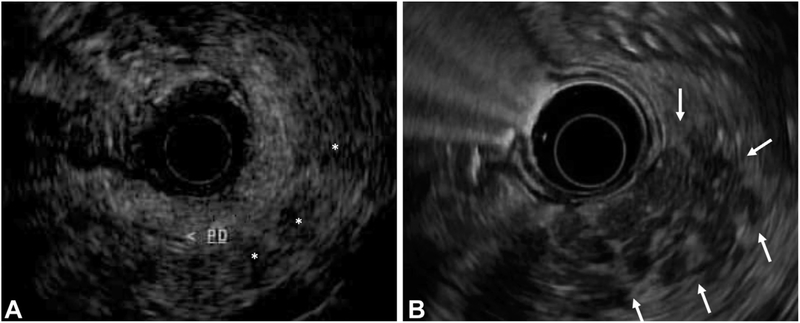
Table 3 shows the results of univariate and multivariable analyses comparing HRIs and control subjects. Twelve of 65 HRIs (18%) had 3 or more CP changes, compared with 2 of 118 control subjects (2%). After controlling for potential confounders and classic CP factors, HRIs had 16 times the odds of having 3 or more CP changes compared with control subjects (odds ratio [OR], 15.8; 95% confidence interval [CI], 2.6–97.0; P = .003). HRIs were more likely to exhibit individual CP changes, including hyperechoic strands (OR, 15.4; 95% CI, 4.3–55.1; P < .001), lobularity (OR, 7.6; 95% CI, 2.0–28.8; P = .003), cysts (OR, 36.2; 95% CI, 4.1–318; P = .001), and hyperechoic duct walls (OR,37.2; 95% CI, 6.6–209; P < .001). HRIs were also more likely to have hypoechoic foci on EUS (OR, 8.0; 95% CI,1.9–32.9; P = .004). These differences between HRIs and control subjects persisted in subsets of HRIs who did or did not have any alcohol use (Supplementary Table 1, available online at www.giejournal.org). Representative EUS images of hypoechoic foci and lobularity are shown in Figure 1.
TABLE 3.
EUS findings in HRIs and control subjects
| Outcome variable | Control subjects* (n = 118) | HRIs* (n = 65) | Unadjusted P value | Adjusted OR (95% CI) | Adjusted P value |
|---|---|---|---|---|---|
| Hyperechoic strands | 5 (4) | 22 (34) | <.001† | 15.4 (4.3–55.1) | <.001§ |
| Lobularity | 5 (4) | 14 (22) | <.001† | 7.6 (2.0–28.8) | .003‡,§ |
| Cysts | 1 (1) | 16 (25) | <.001† | 36.2 (4.1–318) | .001§,ǁ |
| Hyperechoic duct walls | 3 (3) | 15 (23) | <.001† | 37.2 (6.6–209) | <.001** |
| Hypoechoic foci | 3 (3) | 13 (20) | <.001† | 8.0 (1.9–32.9) | .004§ |
Univariate analyses with unadjusted P values and multivariable logistic regression analysis with adjusted OR and P values are shown.
HRIs, High-risk individuals; OR, odds ratio; CI, confidence interval; CP, chronic pancreatitis.
Values are n (%).
Pearson χ2 test.
Potential confounders are ‡male gender
number of EUS
any ethanol use
age and
body mass index.
Figure 1.
Representative images of hypoechoic foci and lobularity taken from the pancreas body using a GF-UE160-AL5 radial array echoendoscope (Olympus America, Center Valley, Pa). A, Hypoechoic foci (white asterisks), defined as echo-poor foci without enhancing rim; the main pancreas duct (PD) is labeled. B, Area of lobularity (white arrows), defined as well-circumscribed >5-mm structures with enhancing rim and relatively echo-poor center.
CP changes in vHRIs and mHRIs
Table 4 shows baseline demographics of control subjects, mHRIs, and vHRIs. Female gender was more common in mHRIs and vHRIs compared with control subjects but did not differ between mHRIs and vHRIs. vHRIs were more likely to have any alcohol use than control subjects. vHRIs underwent more EUS studies than control subjects (1.8 vs 1.1; P < .001), but there was no difference in this finding between vHRIs and mHRIs or mHRIs and control subjects.
TABLE 4.
Baseline demographics of vHRIs, mHRIs, and control subjects
| Demographic | Control subjects (n = 118) | mHRIs (n = 21) | vHRIs (n = 44) | P values | ||
|---|---|---|---|---|---|---|
| Control subjects vs mHRIs | Control subjects vs vHRIs | mHRIs vs vHRIs | ||||
| Female | 58 (49) | 17 (81) | 30 (68) | .007† | .031 † | .282† |
| White | 72 (61) | 11 (52) | 34 (77) | .457† | .053† | .051† |
| BMI | 26.2 ± 5.9 | 28.0 ± 5.8 | 26.9 ± 5.9 | .184* | .484* | .461* |
| History of diabetes | 15 (13) | 1 (5) | 5 (11) | .466‡ | 1‡ | .655‡ |
| History of smoking | 46 (39) | 6 (29) | 15 (34) | .364† | .568† | .656† |
| Alcohol use | ||||||
| >2 drinks/day | 5 (4) | 0(0) | 3 (7) | 1‡ | .449‡ | .545‡ |
| Any alcohol use | 42 (36) | 12 (57) | 26 (59) | .062† | .007† | .937† |
| Fluid oz/wk | 1.4 ± 3.9 | 1.1 ± 1.4 | 2.4 ± 3.2 | .477* | .092* | .020* |
| No. of EUS procedures | 1.1 ± .4 | 1.4 ± .7 | 1.8 ± 1.2 | .071* | <.001* | .127* |
Values are mean ± standard deviation or n (%). Each standard drink (1 can beer, 1 glass wine, 1 shot liquor) has .6 fluid oz alcohol.
vHRI, Very high-risk individual; mHRI, moderately high-risk individual; BMI, body mass index.
Two-sample t test with unequal variance.
Pearson χ2 test.
Fisher exact test
Table 5 shows results of univariate and multivariable analyses comparing EUS changes in vHRIs, mHRIs, and control subjects. Nine of 44 vHRIs (20%) and 3 of 21 mHRIs (14%) had 3 or more CP changes. When adjusted for potential confounders and classic risk factors, mHRIs had 61 times the odds (OR, 60.9; 95% CI, 3.3–1129; P = .006) and vHRIs had 17 times the odds (OR, 16.6; 95% CI, 1.8–151; P = .013) of having 3 or more CP changes compared with control subjects. When comparing vHRIs with mHRIs, there was no difference in this finding even after multivariable adjustment (OR, .35; 95% CI, .4–3.5; P = .374).
TABLE 5.
EUS findings in vHRIs, mHRIs, and control subjects
| Outcome variable | Control subjects* (n = 118) | mHRIs* (n = 21) | vHRIs* (n = 44) | Control subjects vs mHRIs | ||
|---|---|---|---|---|---|---|
| Unadjusted P value | Adjusted OR (95% CI) | Adjusted P value | ||||
| Hyperechoic strands | 5 (4) | 3 (14) | 19 (43) | .069‡ | 10.8 (1.4–83.4) | .022§ |
| Lobularity | 5 (4) | 4 (19) | 10 (23) | .030‡ | 29.5 (2.6–332) | .006§ |
| Cysts | 1 (1) | 2 (10) | 14 (32) | .060‡ | 7.3 (.3–186) | .227** |
| Hyperechoic duct walls | 3 (3) | 3 (14) | 12 (27) | .044‡ | 13.2 (1.5–119) | .022¶ |
| Hypoechoic foci | 3 (3) | 1 (5) | 12 (27) | .485‡ | 2.5 (.2–36.6) | .508** |
| Control subjects vs vHRIs | vHRIs vs mHRIs | ||||
|---|---|---|---|---|---|
| Unadjusted P value | Adjusted OR (95% CI) | Adjusted P value | Unadjusted P value | Adjusted OR (95% CI) | Adjusted P value |
| <.001† | 21.7 (5.3–89.2) | <.001ǁ | .021† | 4.4 (1.02–19.0) | .047** |
| .001‡ | 8.2 (1.6–42.0) | .012§,¶,ǁ | 1‡ | .8 (.1–4.7) | .802§,ǁ,†† |
| <.001‡ | 52.3 (5.3–516) | .001ǁ,‡‡ | .051† | 3.5 (.7–18.3) | .137** |
| <.001‡ | 37.5 (6.3–223) | <.001††,‡‡ | .350‡ | 2.2 (.5–10.0) | .324** |
| <.001‡ | 10.7 (2.5–46.1) | .001ǁ | .046‡ | 6.3 (.67–58.5) | .107** |
Univariate analyses with unadjusted P values and multivariable logistic regression analysis with adjusted OR and P values are shown.
vHRI, Very high-risk individual; mHRI, moderately high-risk individual; OR, odds ratio; CI, confidence interval; CP, chronic pancreatitis.
Values are n (%).
Pearson χ2 test.
Fisher exact test.
Potential confounders are §male gender
any ethanol use
number of EUS
none
body mass index
age.
Of the most common CP changes, vHRIs were more likely than control subjects to exhibit all changes. mHRIs were more likely than control subjects to have hyperechoic strands (OR, 10.8; 95% CI, 1.4–83.4; P = .022), lobularity (OR, 29.5; 95% CI, 2.6–332; P = .006), and hyperechoic duct walls (OR, 13.2; 95% CI, 1.5–119; P = .022) but did not differ from control subjects with regard to cysts. When comparing vHRIs with mHRIs, vHRIs were more likely to exhibit hyperechoic strands (OR, 4.4; 95% CI, 1.02–19.0; P = .047) but did not differ from mHRIs with regard to lobularity, cysts, or hyperechoic duct walls.
vHRIs were more likely to exhibit hypoechoic foci than control subjects (OR, 10.7; 95% CI, 2.5–46.1; P = .001). There was no difference between mHRIs and control subjects or mHRIs and vHRIs with regard to this finding.
Solid lesions
FNA was performed on 4 HRIs and 2 control subjects for incidental pancreatic cysts, from which no cancers were diagnosed. Two additional HRIs exhibited solid pancreatic lesions on EUS and had FNA performed. In 1 case, PDAC was found in a 59-year-old white woman classified as an mHRI with a pathogenic BRCA2 mutation and no FH of PDAC. Before PDAC screening, the patient had no symptoms. Baseline EUS showed a diffusely hyperechoic pancreas suggestive of fatty infiltration with 1 CP change of lobularity. The patient was lost to follow-up and presented 2 years later with liver function test elevation and magnetic resonance imaging showing a new pancreatic tail lesion and liver lesions concerning for metastases. FNA of liver lesions confirmed stage IV metastatic PDAC. The patient subsequently underwent radiation and chemotherapy but had progressive disease and died 2 years after cancer diagnosis. We re-reviewed the available images from the baseline EUS and found no evidence of a missed solid lesion at that time.
The other case was a 71-year-old white man classified as a vHRI with 2 FDRs with PDAC and no known genetic mutation. Baseline EUS showed a 14 × 10 mm cyst communicating with the main pancreatic duct. There was a small solid nodule at the side of the cyst, which was too small to perform biopsy sampling. FNA showed elevated carcinoembryonic antigen. Given imaging and carcinoembryonic antigen results, there was concern for high-risk intraductal papillary mucinous neoplasm. Distal pancreatectomy was recommended, but the patient moved to another state and was lost to follow-up.
DISCUSSION
In this study, we found that HRIs had 16 times the odds of having CP changes on EUS compared with control subjects without pancreatobiliary disease. This association persisted even after controlling for potential confounders and classic CP risk factors. When examining specific EUS findings, HRIs were more likely to exhibit hyperechoic stranding, lobularity, cysts, hyperechoic duct walls, and hypoechoic foci. When comparing vHRIs who met the CAPS high-risk criteria with mHRIs who did not, there was no difference in frequency of 3 or more CP changes, and both high-risk groups exhibited this more frequently than control subjects.
CP changes in asymptomatic HRIs may reflect lobulocentric atrophy associated with PanIN. Brune et al21 examined 8 pancreatectomy specimens obtained from HRIs with CP changes on EUS and found a high density of PanIN lesions associated with lobular units affected by atrophy and loss of acinar cells. Meckler et al29 examined 11 HRIs who underwent pancreatectomy and similarly found multifocal PanIN associated with lobules containing fibrocystic atrophy. This progressive acinar dropout and atrophy is similar to changes seen in animals after pancreatic duct ligation.30 This implies that in HRIs, diffuse PanIN leads to multifocal small duct obstruction resulting in lobulocentric atrophy that is reflected on EUS as CP changes. Although the mechanism of obstruction may be physical in advanced PanIN, obstructive atrophy is seen in flat, low-grade lesions. This suggests alternative mechanisms for obstruction, such as altered expression of mucins31 causing more viscous secretions.
Given its relationship with PanIN, CP changes may be a risk factor for PDAC. LeBlanc et al32 found that increasing CP changes on EUS is associated with advancing PanIN grade. Takenaka et al24 described 69 patients with sporadic intraductal papillary mucinous neoplasms who underwent resection and found that having at least 1 CP finding on EUS was associated with a higher prevalence of invasive carcinoma. These findings suggest that HRIs with CP changes are at higher risk for PDAC and may require closer monitoring.
CP changes on EUS in HRIs have been previously reported, as summarized in Table 6.6,10–15,17,22,25 In agreement with our findings, Canto et al22 and Mizrahi et al10 reported these changes more frequently in HRIs when compared with control subjects. Canto et al22 defined HRIs as having an FH of PDAC or PJS, whereas Mizrahi et al10 examined BRCA2 carriers. Our cohort included individuals with an FH of PDAC, PJS, and LS and BRCA1/2, PALB2, CDKN2A, and ATM carriers. In this diverse group, we found that 18% of HRIs had 3 or more CP changes on EUS. A similar frequency was reported by Verna et al6 (6/31; 19%) and Langer et al14 (17/76; 22%). By contrast, CP changes were more frequently seen in HRIs screened by Canto et al22 (47/78; 60%). Control subjects in this study also had a higher rate of CP changes (23/138; 17%)22 than our control subjects (2/118; 2%) despite meeting similar inclusion criteria. This discrepancy could be related to differences in CP risk factors. HRIs screened by Canto et al22 were more likely to be men (44% vs 27%) or ever smokers (45% vs 33%) than our cohort. In terms of individual CP changes, we found that 22% of HRIs had lobularity and 34% had hyperechoic strands on EUS, which is similar to the 18% and 37% of BRCA2 carriers, respectively, described by Mizrahi et al10 with these findings.
TABLE 6.
Prior studies investigating chronic pancreatitis changes on EUS in HRIs
| Study | HRI characteristics | Screening | No. of HRIs | CP criteria examined | CP results in HRIs | CP results in HRIs |
|---|---|---|---|---|---|---|
| Canto et al 200622 | FH; PJS; BRCA2 | EUS, ERCP, CT | 78 | 3+ CP changes | 47/78 (60%) | 23/138 (17%) |
| Langer et al 200914 | FH; BRCA2 | EUS, MRI | 76 | 3+ CP changes | 17/76 (22%) | |
| 5+ CP changes | 8/76 (11%) | |||||
| Poley et al 200915 | FH; BRCA1/2; LS; CDKN2A | EUS | 44 | 1+ CP changes | 3/10 (30%)* | |
| Verna et al 20106 | FH; PALB2 | EUS, MRI | 31 | 3+ CP changes | 6/31 (19%) | |
| 5+ CP changes | 2/31 (6%) | |||||
| Sud et al 201417 | FH; BRCA1/2; CDKN2A; LS | EUS | 16 | 1+ CP changes | 0/16 (0%) | |
| Harinck et al 201613 | FH; PJS; BRCA1/2; CDKN2A | EUS, MRI | 139 | 1+ CP changes | 20/139 (14%) | |
| Mocci et al 201512 | FH; PJS; LS; CDKN2A; BRCA1/2 | EUS → MRI | 38 | 1+ parenchymal CP changes | 16/38 (42%) | |
| Mizrahi et al 201710 | BRCA2 | EUS | 37 | Rosemont consistent with CP | 5/37 (14%) | 1/92 (1%) |
| Rosemont suggestive of CP | 6/37 (16%) | 2/92 (2%) | ||||
| Lobularity | 7/37 (18%) | 3/92 (3%) | ||||
| Hyperechoic strands | 14/37 (37%) | 6/92 (7%) | ||||
| DaVee et al 201811 | BRCA1/2; p53; PJS; LS; ATM; APC | EUS, MRI, CT | 64 | Hyperechoic strands and foci | 9/64 (14%) | |
| Mild MPD dilation | 2/64 (3%) |
HRI, High-risk individual; CP, chronic pancreatitis; FH, family history; PJS, Peutz-Jeghers syndrome; MRI, magnetic resonance imaging; LS, Lynch syndrome; MPD, main pancreatic duct.
In Poley et al, only looked at CP changes in 10 patients with pathologic findings on EUS.
In the original description of CP features on EUS by Wiersema et al,27 “focal regions of reduced echogenicity” was considered a CP change. In subsequent validation studies,33 whereas lobularity, defined as echo-poor structures with an enhancing rim,26 is a CP criteria, foci without this surrounding rim are not included. In our study these findings, which we termed hypoechoic foci, were found in 20% of HRIs and were more commonly observed in HRIs than control subjects. Brentnall et al23 reported hypoechoic nodules accompanying CP changes in 7 of 14 HRIs who underwent EUS and noted widespread dysplasia in the 6 who then underwent pancreatectomy. Harinck et al13 found hypoechoic lesions in 8 of 139 HRIs; in the 2 of 8 who underwent resection, pathology revealed multifocal grade 2 pancreatic intraepithelial neoplasia. Studies in dogs further indicate that hypoechoic foci, like CP changes, are seen with ductal obstruction.34 Considering these findings, hypoechoic foci may reflect PanIN-induced duct obstruction and should therefore be documented along with standard CP changes during screening.
In 2013 the CAPS consortium published guidelines for PDAC screening and described rigorous criteria for high-risk groups.3 Within these criteria, individuals with BRCA2, PALB2, and CDKN2A mutations or LS had to have an FDR with PDAC to be considered high risk.3 Consensus on screening BRCA1 carriers was not achieved, and screening ATM carriers was not commented on.3 A Markov model simulating PDAC screening found that life expectancy gains are achieved if relative risk of PDAC exceeded 2.4 (in men) or 2.7 (in women).35 PDAC risk in BRCA1 and BRCA2 carriers is 2 to 436,37 and 4 to 6,37,38 respectively, compared with the general population. Data are limited for ATM carriers, with 1 study showing a nonsignificant PDAC risk of 4.39 Similarly, risk in PALB2 carriers is unclear, perhaps because of low mutation prevalence.40 For LS patients, studies have shown up to an 11-fold elevated PDAC risk.41 Given the risk profile of these groups, many centers, including our own, screen HRIs who do not meet the CAPS criteria (Table 7).4–13,15,17
TABLE 7.
Prior studies that have screened mHRIs not meeting CAPS criteria
| mHRI group | Study |
|---|---|
| BRCA2 with 1+ non-FDR | Verna et al 2010,6 Ludwig et al 2011,4 Canto et al 2012,7 Potjer et al 2013,8 Al-Sukhni et al 2013,9 Sud et al 2014,17 Bartsch et al 2016,5 Mizrahi et al 2017,10 DaVee et al 201811 |
| BRCA2 with no FH | Al-Sukhni et al 2013,9 Mizrahi et al 2017,10 DaVee et al 201811 |
| BRCA1 with any FH | Verna et al 2010,6 Ludwig et al 2011,4 Canto et al 2012,7 Al-Sukhni et al 2013,9 Sud et al 2014,17 Bartsch et al 2016,5 DaVee et al 201811 |
| BRCA1 with no FH | DaVee et al 201811 |
| PALB2 with 1+ non-FDR | Potjer et al 2013,8 Bartsh et al 20165 |
| CDKN2A with 1+ non-FDR | Poley et al 2009,15 Verna et al 2010,6 Potjer et al 2013,8 Al-Sukhni et al 2013,9 Sud et al 2014,17 Harinck et al 2016,13 Mocci et al 201512 |
| CDKN2A with no FH | Poley et al 2009,15 Harinck et al 2016,13 |
| LS with 1+ non-FDR | Verna et al 2010,6 Sud et al 2014,17 Mocci et al 2015,12 DaVee et al 201811 |
| LS with no FH | DaVee et al 201811 |
| ATM with any FH | DaVee et al 201811 |
| ATM with no FH | DaVee et al 201811 |
mHRIs, Moderately high risk individuals; CAPS, Cancer of the Pancreas Screening; FDR, first-degree relative; FH, family history; LS, Lynch syndrome
In this study we classified HRIs as vHRIs who met the CAPS criteria and mHRIs who did not. Twenty of 21 mHRIs were BRCA1/2, PALB2, and ATM or LS patients with no FDRs with PDAC. After adjusting for potential confounders and classic CP risk factors, there was no difference in frequency of 3 or more CP changes on EUS between vHRIs and mHRIs and both groups were more likely to exhibit this finding than control subjects. When examining specific CP changes, vHRIs were more likely to have hyperechoic strands than mHRIs but did not significantly differ in other CP changes. Given the relationship between CP changes and PanIN, these findings indicate potential benefit in broadening screening guidelines to include mHRIs. Of note, the only patient in our cohort who developed PDAC was a BRCA2 carrier who did not meet CAPS criteria.
Certain limitations merit further discussion. Our outcome of interest was CP changes on EUS, and although this might indicate PanIN and PDAC risk, these changes are also more common with age, male gender, smoking, and alcohol use.42 To address these issues, we compared EUS changes in HRIs with control subjects. When selecting control subjects, we did not match based on CP risk factors. However, we did adjust for classic CP risk factors and potential confounders. In our study, we abstracted data from EUS reports and did not re-review EUS images. Therefore, there was a potential for interobserver variability, which is a recognized limitation of EUS.43 Furthermore, because our endoscopists were aware of high-risk status, there was a potential for observer detection bias in reporting CP changes. Detection bias may have also arisen from the relative lack of attention devoted to the pancreas by our endoscopists in control subjects who were undergoing EUS for nonpancreatobiliary indications. These limitations highlight the need for future verification of our findings with carefully designed prospective trials that account for observer bias and interobserver variability. In this study, our sample size did not allow for comparisons of EUS findings between patients with specific mutations. Nonetheless, we provided EUS data on perhaps the most diverse groups of HRIs that has been described to date and further investigated the CAPS recommendations by comparing EUS findings among HRIs who did or did not meet these criteria. Finally, follow-up for HRIs and control subjects after our study period was not available, and future studies examining the long-term outcomes of HRIs with and without CP changes are eagerly awaited.
In conclusion, we found that HRIs were more likely to exhibit CP changes and hypoechoic foci on EUS compared with control subjects. Given the potential relation of these findings to PanIN, we recommend documentation of CP changes and hypoechoic foci during PDAC screening. Individuals with these findings may represent a higher risk subset requiring closer monitoring. In this study we also found no significant difference in nearly all CP changes between HRIs who did or did not meet CAPS criteria. This supports a more inclusive approach in selecting HRIs for screening. Future studies should aim to identify additional biomarkers for risk stratification. Pancreatic juice DNA mutation concentration in humans44 and EUS imaging with targeted contrast microbubbles45 has promise in detecting PDAC. The role for these modalities in the screening of HRIs must still be determined.
Supplementary Material
DISCLOSURE:
All authors disclosed no financial relationships relevant to this publication. Research support for this study was provided to W.G. Park by the National Institutes of Health (grant no. 1 U01 CA210020–01A1).
Abbreviations:
- BMI
body mass index
- CAPS
Cancer of the Pancreas Screening
- CP
chronic pancreatitis
- CI
confidence interval
- FDR
first-degree relative
- FH
family history
- HRI
high-risk individual
- LS
Lynch syndrome
- mHRI
moderately high-risk individual
- OR
odds ratio
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- PJS
Peutz-Jeghers syndrome
- vHRI
very high-risk individual
REFERENCES
- 1.Anon. Pancreatic cancer—cancer stat facts. Available at: https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed February 15, 2018.
- 2.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC pancreatic cancer staging system. Cancer 2007;110:738–44. [DOI] [PubMed] [Google Scholar]
- 3.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol 2011;106:946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartsch DK, Slater EP, Carrato A, et al. Refinement of screening for familial pancreatic cancer. Gut 2016;65:1314–21. [DOI] [PubMed] [Google Scholar]
- 6.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010;16:5028–37. [DOI] [PubMed] [Google Scholar]
- 7.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potjer TP, Schot I, Langer P, et al. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res 2013;19:442–9. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012;16:771–83. [DOI] [PubMed] [Google Scholar]
- 10.Mizrahi M, Tseng JF, Wong D, et al. Chronic pancreatitis-like change in BRCA2 mutation carriers. Pancreas 2017;46:679–83. [DOI] [PubMed] [Google Scholar]
- 11.DaVee T, Coronel E, Papafragkakis C, et al. Pancreatic cancer screening in high-risk individuals with germline genetic mutations. Gastrointest Endosc 2018;87:1443–50. [DOI] [PubMed] [Google Scholar]
- 12.Mocci E, Guillen-Ponce C, Earl J, et al. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer 2015;51:1911–7. [DOI] [PubMed] [Google Scholar]
- 13.Harinck F, Konings ICAW, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016;65:1505–13. [DOI] [PubMed] [Google Scholar]
- 14.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009;58:1410–8. [DOI] [PubMed] [Google Scholar]
- 15.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009;104:2175–81. [DOI] [PubMed] [Google Scholar]
- 16.Schneider R, Slater EP, Sina M, et al. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer 2011;10:323–30. [DOI] [PubMed] [Google Scholar]
- 17.Sud A, Wham D, Catalano M, et al. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas 2014;43:458–61. [DOI] [PubMed] [Google Scholar]
- 18.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol 2003;16:996–1006. [DOI] [PubMed] [Google Scholar]
- 19.Shi C, Klein AP, Goggins M, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res 2009;15: 7737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catalano MF, Lahoti S, Geenen JE, et al. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998;48:11–7. [DOI] [PubMed] [Google Scholar]
- 21.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 2006;30: 1067–76. [PMC free article] [PubMed] [Google Scholar]
- 22.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006;4:766–81. [DOI] [PubMed] [Google Scholar]
- 23.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 1999;131:247–55. [DOI] [PubMed] [Google Scholar]
- 24.Takenaka M, Masuda A, Shiomi H, et al. Chronic pancreatitis finding by endoscopic ultrasonography in the pancreatic parenchyma of intraductal papillary mucinous neoplasms is associated with invasive intraductal papillary mucinous carcinoma. Oncology 2017;93:61–8. [DOI] [PubMed] [Google Scholar]
- 25.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004;2:606–21. [DOI] [PubMed] [Google Scholar]
- 26.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009;69:1251–61. [DOI] [PubMed] [Google Scholar]
- 27.Wiersema MJ, Hawes RH, Lehman GA, et al. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993;25:555–64. [DOI] [PubMed] [Google Scholar]
- 28.Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultra-sound chronic pancreatitis criteria to the endoscopic secretin-stimulated pancreatic function test. Dig Dis Sci 2007;52:1206–10. [DOI] [PubMed] [Google Scholar]
- 29.Meckler KA, Brentnall TA, Haggitt RC, et al. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol 2001;25:1047–53. [DOI] [PubMed] [Google Scholar]
- 30.Bhutani MS, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009;38:820–4. [DOI] [PubMed] [Google Scholar]
- 31.Levi E, Klimstra DS, Andea A, et al. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol 2004;57:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeBlanc JK, Chen J-H, Al-Haddad M, et al. Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis? A retrospective study of pathologic correlation. Pancreas 2014;43: 849–54. [DOI] [PubMed] [Google Scholar]
- 33.LeBlanc JK, Chen J-H, Al-Haddad M, et al. Endoscopic ultrasound and histology in chronic pancreatitis. Pancreas 2014;43:440–4. [DOI] [PubMed] [Google Scholar]
- 34.Bhutani M, Ahmed I, Verma D, et al. An animal model for studying endoscopic ultrasound changes of early chronic pancreatitis with histologic correlation: a pilot study. Endoscopy 2009;41:352–6. [DOI] [PubMed] [Google Scholar]
- 35.Pandharipande PV, Heberle C, Dowling EC, et al. Targeted screening of individuals at high risk for pancreatic cancer: results of a simulation model. Radiology 2015;275:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002;94:1358–65. [DOI] [PubMed] [Google Scholar]
- 37.Mocci E, Milne RL, Méndez-Villamil EY, et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol Biomarkers Prev 2013;22:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 1999;91:1310–6. [DOI] [PubMed] [Google Scholar]
- 39.Geoffroy-Perez B, Janin N, Ossian K, et al. Cancer risk in heterozygotes for ataxia-telangiectasia. Int J Cancer 2001;93:288–93. [DOI] [PubMed] [Google Scholar]
- 40.Chaffee KG, Oberg AL, McWilliams RR, et al. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet Med 2018;20:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol 2012;30: 958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrone MC, Arcidiacono PG, Perri F, et al. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease: Do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis? Pancreatology 2010;10:597–602. [DOI] [PubMed] [Google Scholar]
- 43.Del Pozo D, Poves E, Tabernero S, et al. Conventional versus Rosemont endoscopic ultrasound criteria for chronic pancreatitis: interobserver agreement in same day back-to-back procedures. Pancreatology 2012;12:284–7. [DOI] [PubMed] [Google Scholar]
- 44.Suenaga M, Yu J, Shindo K, et al. Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res 2018;24:2963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foygel K, Wang H, Machtaler S, et al. Detection of pancreatic ductal adenocarcinoma in mice by ultrasound imaging of thymocyte differentiation antigen 1. Gastroenterology 2013;145:885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.