Abstract
Glioblastoma are highly immunosuppressive brain tumors that are known for their T cell paucity. In a recent issue of Nature Medicine, Chongsathidkiet et al. (2018) discovered a brain-specific mechanism of tumors to escape immunosurveillance by trapping T cells in the bone marrow through the loss of sphingosine-1-phosphate (S1P) receptor on the T cell surface.
Cancer cells are genetically distinct from normal cells and thus prompt the induction of tumor-reactive T cell responses. Cancer cells, however, have various tricks up their sleeves to become “invisible” to the immune system. While the presence of tumor-infiltrating lymphocytes (TILs), and in particular CD8+ T cells, are positive prognostic markers in multiple solid tumors, these cells fail to effectively eliminate cancer cells. One reason for the impaired immune control is the curtailing of effector functions of infiltrating T cells by a broad spectrum of immunosuppressive mechanisms that are present in the tumor microenvironment (Thommen and Schumacher, 2018). Immunosuppression is particularly severe among glioblastomas (GBMs), aggressive brain tumors with a meager prognosis, which harbor a relatively low number of somatic mutations and contain very few T cells when compared with other tumor types (Lim et al., 2018).
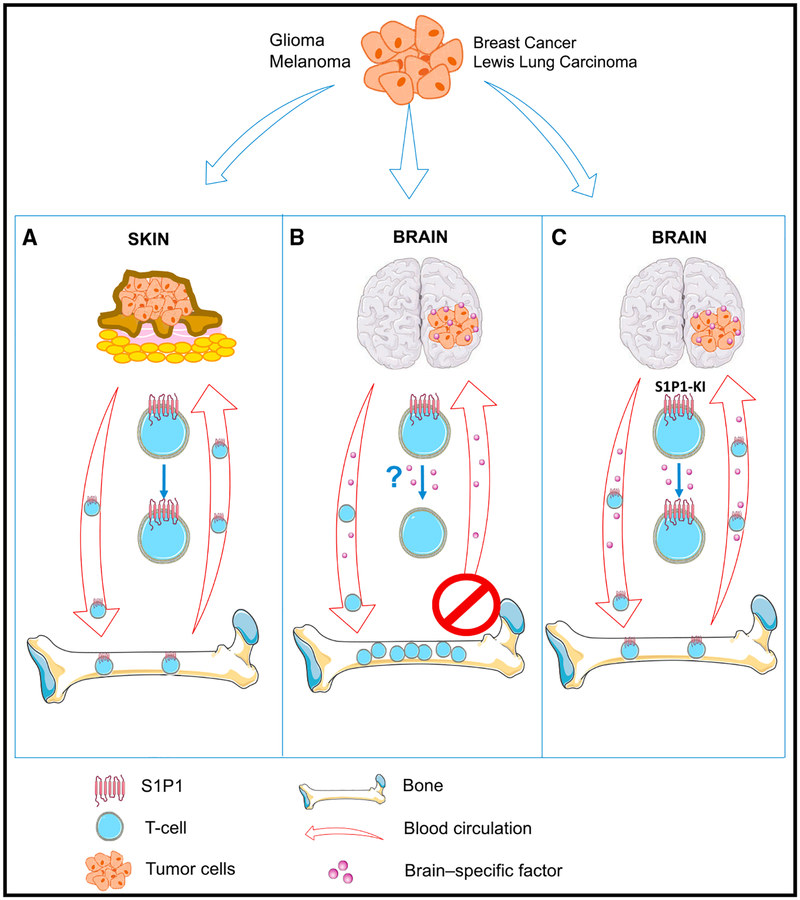
In the recent issue of Nature Medicine, Chongsathidkiet et al. (2018) have discovered a new mechanism for the paucity of infiltrating T cells in intracranial tumors. GBMs lock away T cells in the bone marrow to escape immunosurveillance (Figure 1). The researchers observed that newly diagnosed GBM patients already show signs of severe lymphopenia, virtually equivalent to patients with AIDS. Whereas most people harbor more than 1,000 CD4+ T cells/microliter in the blood, the majority of GBM patients were found to have CD4+ T cell counts of 200 or less. T cells were also sparse in the spleen, leading to splenic contraction which suggested that T cell production in the bone marrow may be diminished. Instead, the investigators found the opposite. Bone marrow aspirates from treatment-naive GBM patients and GBM-bearing mice revealed more T cells in the bone marrow than in the blood, while controls had matching T cell counts between blood and bone marrow. Interestingly, this phenomenon was not dependent on the tumor type but rather on the brain environment in which the tumors grew. Intracranial injection not only of GBMs but also of melanoma, breast carcinomas, and Lewis lung carcinomas in mice induced T cell trapping in the bone marrow, while subcutaneous growth of these tumors did not evoke this phenomenon (Figure 1).
Figure 1. Tumor-Induced Brain-Specific T Cell Sequestration in the Bone Marrow.
(A) S1P1+ T cells are produced in the bone marrow and normally cycle through the body where they also infiltrate tumors like those grown subcutaneously. (B) Intracranially injected tumors likely secrete a factor that promotes S1P1 loss on the T cell surface leading to T cell sequestration in the bone marrow and subsequent leukopenia. (C) Genetic blockade of S1P1 internalization on T cells in intracranial tumor-bearing SD1 P1-knockin mice (S1P1-KI) hinders S1P1 loss on the T cell surface, unlocks T cell entrapment, and thus enables T cell release into the peripheral blood.
Next, the team investigated whether T cell accumulation is caused by homing to, or sequestration in, the bone marrow. Adoptive transfer of naive T cells in mice bearing intracranial, but not subcutaneous, tumors revealed preferential T cell accumulation in the bone marrow, but only within 24 hr, suggesting T cell trapping rather than specific homing to the bone marrow. Why are then T cells stashed away in the bone marrow? The investigators speculated a potential role of the sphingosine-1-phosphate (S1P)-S1P receptor (S1P1) axis because (1) S1P1 is highly expressed on T cells and regulates T cell chemotaxis (Garris et al., 2014); (2) a gradient of the ligand S1P, being higher in blood and lymph than in interstitial fluids of tissues, guides the trafficking pattern of lymphocytes and helps maintain an appropriate frequency of circulating lymphocytes (Tiper et al., 2016); and (3) S1P-S1P1 signaling mediates T cell egress from the bone marrow in alymphoplastic mice lacking thymus and secondary lymphoid organs mirroring the lymphoid organ contraction in GBM-bearing mice and patients (Maeda et al., 2010).
Initial observations revealed that S1P levels were unaltered in plasma and tumors, and so was the expression of its receptor S1P1 in bone marrow-residing T cells. However, the team found severely reduced S1P1 protein levels on the cell surface of T cells in the bone marrow suggesting a post-translational mechanism for the S1P1 drop. These results were congruent with those from GBM patients compared to healthy age-matched controls. In order to test the functional significance of these observations, S1P1 levels were genetically manipulated in T cells. S1P1-deficient T cells accumulated within 2 hr in the bone marrow of glioma-bearing mice. In contrast, adoptive transfer of T cells from S1P1-knockin mice (S1P1-KI) harboring a stabilized form of S1P1 on the cell surface (which cannot be internalized) did not lead to preferential T cell sequestration in the bone marrow of glioma-bearing mice, demonstrating that the loss of S1P1 on the T cell surface is responsible for the T cell trapping in the bone marrow (Figure 1).
Given that the loss of S1P1 on the T cell surface only occurs in the presence of tumors growing in the intracranial compartment and is dependent on the environment rather than on the tumor type, the question arises as to the brain-specific mechanistic underpinnings that down-regulate S1P1 surface levels of T cells to trap them in the bone marrow. Although the i nvestigators do not provide an answer, they speculate that either enhanced internalization and/or impaired recycling of S1P1 may cause this phenomenon. This is likely caused by a factor released from the tumor-bearing microenvironment into the blood where it diminishes S1P1 within 24 hrfrom the T cell surface. It is important to note that a tumor has to grow in the brain in order to provoke these effects, suggesting the involvement of both tumor-intrinsic factors and a host response from the brain. Such a brain-specific and tumor-related immunosuppressive mechanism was recently described in a neoantigen-expressing melanoma model. Neoantigenspecific T cells were actively deleted when melanoma cells were implanted in the brain, and the T cells that had escaped deletion failed to execute cytotoxic functions, even after encountering the cognate neoantigen in peripheral lymphoid organs (Jackson et al., 2016). By contrast, immune function was mostly preserved in mice with subcutaneous tumors. The brain-dependent immune suppression was apparently mediated by microglial cells, the resident brain macrophages in the brain, which secreted and released the cytokine TGFß into the circulation. The factthat pharmacological inhibition of TGFß signaling partially reverses the immune suppression but does not prolong the survival of mice with brain tumors may have been in part due to the lack of sufficient T cells in brain tumors (Jackson et al.,2016).
Is it then enough to convey a strong immune response to brain tumors when T cells get back into the circulation? Although brain tumor-bearing S1P1-KI mice displayed a higher number of activated TILs, it was not sufficient to improve survival. This may not be too surprising given that S1P1 loss is predominantly observed on naive T cells, but these results also suggest that additional immunosuppressive modes creating T cell dysfunction like T cell anergy are put in place by these tumors. Indeed, treatment of tumor-bearing S1P1-KI mice with T cell activating agents such as an agonist for 4–1BB (CD137, TNFSR9), a costimulatory molecule that activates CTL and induces the cytokine INFy (Vinay and Kwon, 2014), or blockade of PD1, a negative immune checkpoint regulator (Lim et al., 2018), had additive effects and was able to prolong median survival and produce a 50% long-term survival rate. Importantly, monotherapies of these drugs have no prominent effect in GBM patients, arguing that T cell trapping in the bone marrow may be a limiting factor for successful immunotherapy in glioma patients and likely patients with brain métastasés (Lim et al., 2018). Although there are no current pharmacological means to stabilize S1P1 on T cells, the investigators pointed to a translatable indirect route of T cell release from the bone marrow by treating glioma-bearing mice with granulocyte colony stimulating factor (G-CSF). G-CSF decreased bone marrow T celi counts, reversed T cell lymphopenia, and in combination with an 4–1BB agonist, yielded survival improvement. Taken together, this study revealed a brain-specific mechanism of T cell dysfunction by locking T cells away in the bone marrow. This mechanism appears to be not only applicable to primary brain tumors but also likely to brain métastasés, which make up to 50% of all central nervous system lesions. In the future, it will be important to unravel the tumor-induced brain-specific factor that facilitates the loss of S1P1 on the T cell surface in the hope that by finding this key, it will open new pharmacological opportunities to prevent S1P1 internalization and thus enhance the effects of immunotherapies in brain cancer patients.
REFERENCES
- Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Färber SH, Woroniecka K, Elsamadicy AA, Dechant CA, Kemeny HR, et al. (2018). Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med 24, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris CS, Blaho VA, Hla T, and Han MH (2014). Sphingosine-1-phosphate receptor 1 signalling in T cells: trafficking and beyond. Immunology 142, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CM, Kochel CM, Nirschl CJ, Durham NM, Ruzevick J, Alme A, Francica BJ, Elias J, Daniels A, Dubensky TW Jr., et al. (2016). Systemic tolerance mediated by melanoma brain tumors is reversible by radiotherapy and vaccination. Clin. Cancer Res. 22,1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M, Xia Y, Bettegowda C, and Weller M (2018). Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol 15, 422–442. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Seki N, Sato N, Sugahara K, and Chiba K (2010). Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int. Immunol 22, 515–525. [DOI] [PubMed] [Google Scholar]
- Thommen DS, and Schumacher TN (2018). T cell dysfunction in cancer. Cancer Cell 33, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiper IV, East JE, Subrahmanyam PB, and Webb TJ (2016). Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection. Pathog. Dis 74, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay DS, and Kwon BS (2014). 4–1 BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 47, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]