Abstract
Research over the last decades has provided strong evidence for the pivotal role of the tumor-associated blood and lymphatic vasculature in supporting immunoevasion and in subverting T cell–mediated immunosurveillance. Conversely, tumor blood and lymphatic vessel growth is in part regulated by the immune system, with infiltrating innate as well as adaptive immune cells providing both immunosuppressive and various angiogenic signals. Thus, tumor angiogenesis and escape of immunosurveillance are two cancer hall-marks that are tightly linked and interregulated by cell constituents from compartments secreting both chemokines and cytokines. In this review, we discuss the implication and regulation of innate and adaptive immune cells in regulating blood and lymphatic angiogenesis in tumor progression and metastases. Moreover, we also highlight novel therapeutic approaches that target the tumor vasculature as well as the immune compartment to sustain and improve therapeutic efficacy in cancer.
Keywords: tumor blood and lymphatic vessels, angiogenesis, innate and adaptive immune cells, metabolism, antiangiogenic therapy, immunotherapy
INTRODUCTION
Similar to developing and growing organs, tumors require blood vessels to access oxygen and nutrients. Tumors at their initial stage (i.e., in situ carcinomas) grow avascular and encapsulated so that a basal lamina separates the tumor mass from the peritumoral tissue. In this situation, blood vessels do not enter and are not present in the lesion (1, 2). These tumors can remain in this dormant state for decades. Indeed, the discovery of dormant tumors during the autopsies of individuals who died from nononcological causes reinforces the idea that actually only a subset of these lesions progress to a vessel-dependent state of exponential growth (3). When this occurs, a vascular network infiltrates the lesion, a process known as angiogenic switch, and the tumor undergoes a malignant transition: Cancer cells can now cross the vessel wall and exploit the hematic route to disseminate and reach distant organs where they form metastasis. Notably, however, the angiogenic switch can occur at different stages during tumorigenesis, depending on the tumor type and the environment (4). The onset of neovascularization is a multistep process that can occur by different mechanisms and is orchestrated by a wealth of activating and inhibiting factors whose balance will dictate whether endothelial cells (ECs) are in a quiescent or activated state (4–6). Sprouting angiogenesis is the most common and best-studied mechanism by which new vascular branches arise from preexisting capillaries or postcapillary venules. In addition, tumors also use additional routes of vessel expansion such as vasculogenesis, vascular mimicry, intussusception, and vascular co-option to cope with oxygen and nutrient demands during propagation (for a review, see 1).
However, although blood vessel formation is tightly regulated during physiological conditions, tumors have lost the appropriate balances between positive and negative angiogenic controls. Once tumor angiogenesis is induced, it remains activated, leading to a continually and abnormally expanding tumor vasculature (7, 8). Tumor blood vessels are far from being normal (9–11). This is due to (a) the loss of the appropriate balances between positive and negative angiogenic factors, which lead to excessive proangiogenic signaling, i.e., the physiological response to oxygen shortage, namely hypoxia; and (b) because angiogenic pathways are often downstream of oncogene activation. They are rather aberrant and leaky and have loose endothelial junctions, a discontinuous endothelial lining, and a defective basement membrane, with blind ends and scarce pericyte coverage. These features are ultimately all signs of poor vessel maturation and functionality, with the consequence that a tumor remains constantly hypoxic, which leads to a negative feedback loop whereby proangiogenic signals never stop. Dysfunctional vessels characterized by a poor blood flow ultimately end up forming bulging and thicker vessels, where clotting events and hemostasis are landmarks. It follows that tumors with high vessel density can be also very hypoxic and vice versa, depending on their vascular functionality and metabolic demand.
From a therapeutic point of view, the initial concept to starve the tumor to death, as it was proposed more than 40 years ago by Judah Folkman, has now been revised (9, 12). Strategies leading to nonproductive angiogenesis and tumor vessel normalization represent the opposite side of the coin. The former strategy was initially described when the inhibition of Delta-like 4 in ECs, releasing Notch-1–mediated lateral inhibition, displayed excessive, dysfunctional vessel sprouting (13, 14). Although some tumors grew slowly due to inefficient blood supply as the result of this non-productive angiogenesis, the approach was soon abandoned because Delta-like 4 blockade could lead to the formation of vascular neoplasms (15), and Notch-1 haplodeficiency was associated with the formation of vascular tumors and lethal hemorrhage in mice (16). The latter, namely tumor vessel normalization, was first hypothesized by Rakesh Jain in 2005 (10) (Figure 1). The idea was that drugs that heal the aberrant vessels of the tumor can alleviate hypoxia and increase the efficacy of conventional therapies if vessel perfusion is reestablished. In addition, a normalized tumor vessel, with a smoothly aligned endothelium, continuous basement membrane, and well-covered pericytes, enables to a lesser extent cancer cells to sneak into the circulation and metastasize to distant organs (17–19). The concept comes with some limitations because it is difficult to predict the precise regimen (dose and time window) that will lead to vessel normalization instead of vessel pruning. Indeed, the same strategy such as blockade of vascular endothelial growth factor (VEGF), given at different doses or at the same dose in different tumors, can elicit vessel disruption and then worsen hypoxia or vessel normalization, and thus, tumor reoxygenation (20). In this respect, accessible biomarkers that predict the outcome of antiangiogenic drugs are needed (11, 21).
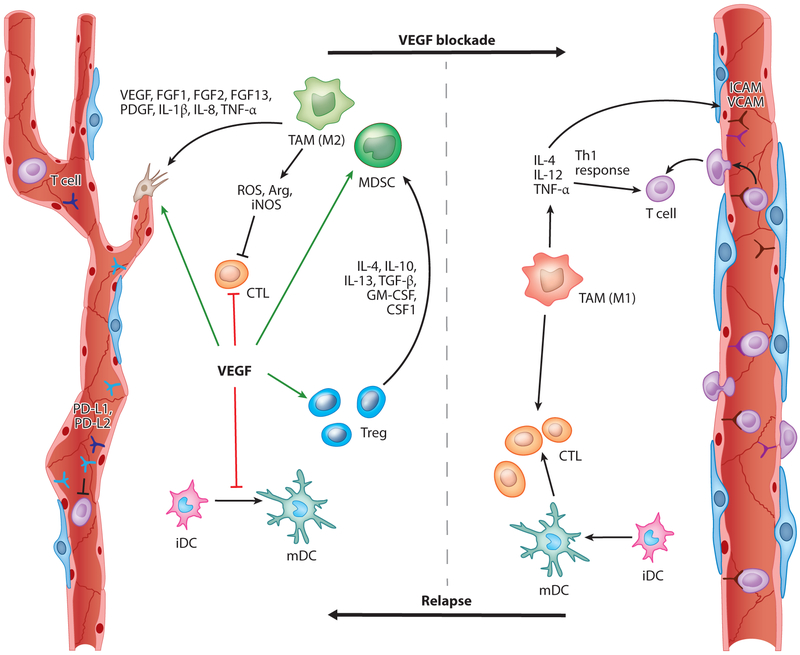
Figure 1.
VEGF/VEGFR signaling controls angiogenesis and tumor immunity. VEGF facilitates several aspects of vessel formation and also promotes immunosuppression by acting on different cell types. In endothelial cells, VEGF inhibits the expression of the T cell adhesion molecules VCAM and ICAM and induces expression of the PD-1 ligands PD-L1 and PD-L2 that interact with PD-1 onT cells, resulting in reduced T cell proliferation and effector function. VEGF also directly impairs DC maturation and induces PD-L1 expression on mature DCs. It inhibits the proliferation and effector function of CTLs but induces the proliferation of Tregs. Tregs in turn recruit MDSCs and TAMs, which produce ROS, iNOS, and Arg to suppress T cell proliferation, viability, and activity. In contrast, inhibition of VEGF signaling enables enhanced T cell infiltration due to vessel normalization accompanied with an increase of ICAM and VCAM, which enhances DC maturation and thus provides more intratumoral effector T cells. VEGF/VEGFR blockade also increases the presence of Th1/M1-polarized myeloid cells (e.g., macrophages, neutrophils). Taken together, anti-VEGF therapy should promote an antitumor response by affecting the vasculature and the immune system. Continuous vessel pruning, however, induces hypoxic areas that drive the recruitment and polarization of immunosuppressive and angiogenic myeloid cells. Abbreviations: Arg, arginase; CSF1, colony-stimulating factor 1; CTL, cytotoxic T cell; DC, dendritic cell; FGF, fibroblast growth factor; G- or M-MDSC, granulocytic or monocytic myeloid-derived suppressor cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; ICAM, intercellular adhesion molecule; iDC, immature DC; IL, interleukin; iNOS, nitric oxide synthase; mDC, mature DC; PDGF, platelet-derived growth factor; PD-L1/2, programmed death-ligand 1/2; ROS, reactive oxygen species; TAM, tumor-associated macrophage; TGF-β, transforming growth factor-beta; Th1, T helper 1; TNF-α, tumor necrosis factor-α; Treg, regulatory T cell; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Another important route for cancer cell dissemination is the lymphatic circulation. Cancer cell dissemination from lymphatic tumor vessels to regional draining lymph nodes is an important indicator of tumor aggressiveness for most human malignancies (22, 23). The primary function of lymphatics is not to carry oxygen or essential nutrients. They instead absorb extravasated proteinrich fluids, lipids, macromolecules, and immunocompetent cells from the interstitial spaces within tissues. Normal and functioning lymphatic vessels thus maintain plasma volume, prevent increases in tissue pressure, and allow leukocyte trafficking, thereby also playing a key role in the proper functioning of the immune system. In a tumor, the persistent activation of lymphangiogenic signaling pathways leads to dysfunctional lymphatic vessels, resulting in an increased tumor interstitial fluid pressure. Uncontrolled tumor interstitial fluid pressure impairs the uptake of therapeutics by the tumor but also promotes mechanical forces that trigger cancer cell proliferation and invasion (17, 24).
Although cancer cells are certainly an important source of angiogenic and lymphangiogenic factors, recruited leukocytes and all tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) play a key role in these processes at both the primary and metastatic sites. Here, we review the latest advances on how various immune cells, including macrophages, affect tumor angiogenesis and lymphangiogenesis.
INNATE IMMUNE CELLS AND TUMOR BLOOD ANGIOGENESIS
Tumors, in part owing to their hypoxic and acidic nature, recruit a substantial amount of different innate immune cells that can comprise up to 30% of the entire tumor population. TAMs, monocytic or granular myeloid-derived suppressor cells (M-MDSCs and G-MDSCs, respectively), and TANs are most commonly found and often associated with increased intratumoral vessel density (25–27). Indeed, besides tumor cells and cancer-associated fibroblasts, myeloid cells become a pivotal source of growth factors and chemokines to promote angiogenesis (2, 28), as shown in multiple mouse tumor models of skin, cervical, breast, and brain cancers (25, 29–34). Because of their high plasticity, these cells can either convey proinflammatory or anti-inflammatory features, but in the tumor setting, they are commonly found to support immunosuppression and angiogenesis (26, 35, 36) (Figure 1).
While TAMs are generally protumoral [M2/T helper (Th)2-like], macrophage polarization toward a proinflammatory, antitumoral (M1/Th1-like) phenotype induced tumor blood vessel normalization in several preclinical tumor models or human tumors. This triggered an adaptive immune response against the tumor inhibiting cancer growth and metastasis; this synergized with the effects of standard treatment such as chemotherapy (37–39). One of the first seminal studies underscoring the functional importance of TAMs in tumor angiogenesis was conducted in the endogenous mouse mammary virus polyoma middle T-antigen (PyMT) tumor model and then confirmed in other tumor model systems (35, 40, 41). VEGF-producing TAMs were sufficient to facilitate the angiogenic switch and the progression to malignancy. This is because inactivation of TAMs by blocking the CSF1/CSF1R pathway, broadly depleting TAMs by clodronate liposomes, or genetically deleting VEGF in macrophages, delayed the angiogenic switch, whereas genetic restoration of the macrophage population rescued the angiogenic phenotype (35, 40, 41).
TAMs and TANs also express various proteases, including the matrix metalloproteinase 9 (MMP9). MMP9 was shown to release and thus increase the bioavailability of extracellular matrix–sequestered VEGF, thus providing an alternative mechanism of VEGF-induced angiogenesis by innate immune cells in pancreatic, cervical, and brain tumor models (33, 34, 42). Blocking MM9 by genetic or pharmacological depletion inhibited the angiogenic switch in all three tumor models. Tie2-expressing macrophages (TEMs) belong to a subgroup of TAMs that is often closely aligned to tumor vessels through EC expression of the Tie2 receptor ligand angiopoietin-2 (Ang2) (43). The number of TEMs correlates with vascular density in several tumor models and certain human tumors (44). Furthermore, selective ablation of TEMs by antibody-mediated neutralization of the Tie2 ligand Ang2 or by virtue of Tie2 promoter–driven thymidine kinase expression in mammary, pancreatic neuroendocrine, and brain tumor mouse model systems underscores their significant contribution in tumor angiogenesis (45, 46). Notably, early studies had revealed that hypoxia-induced Ang2 in concert with VEGF strongly induced the angiogenic switch in co-opted tumor vessels of glioblastomas (47).
Besides TAMs, neutrophils, or granule-containing cells, which are the most abundant white blood cells and the first cells to be recruited to injuries, produce factors that regulate angiogenesis. Neutrophils are normally the first to fight invading pathogens by several means including the generation of neutrophil extracellular traps (NETs), which are webs of fibers composed of chromatin and serine proteases that trap and kill extracellular microbes (48, 49). TANs, like TAMs, can exist as Th1 (N1) or Th2 (N2) polarized cells based on their anti- or protumor activity (29, 50). In tumors and metastases, neutrophils secrete proangiogenic factors and proteases similar to those of macrophages, most predominantly VEGF, fibroblast growth factor (FGF), and MMPs (28, 51, 52). Neutrophils contain granules with different compounds and factors. They can also harbor VEGF-enriched granules that are released upon tumor necrosis factor (TNF) stimulation in vitro, suggesting an alternative and fast route of VEGF availability to promote blood vessel growth (53). As described above, neutrophils could secrete MMP9 to liberate extracellular matrix–sequestered VEGF in dysplastic pancreatic islets of Rip1Tag2 mice that was sufficient to induce the angiogenic switch (42, 54), whereas pharmacological neutrophil depletion impaired the angiogenic switch in these pancreatic islet lesions (54). In addition, granulocyte colony-stimulating factor (G-CSF)–stimulated neutrophils upregulate the expression of Bv8 (prokineticin 2), which stimulates EC survival, migration, and proliferation but also functions as a chemoattractant for neutrophils, providing a positive feedback loop for neutrophil recruitment and activation (36).
Immature Gr1+ immune cells in mice with either a mononuclear or granular morphology also convey immunosuppressive functions in tumors and were therefore named M-MDSCs and G-MDSCs, respectively (26). This is because MDSCs have predominantly been studied for their ability to suppress human CD3+ and mouse CD4+ or CD8+ T cells (55). Most studies relating to their proangiogenic activities during tumor progression have not differentiated between neutrophils and MDSCs but solely depicted them as Gr1+CD11b+ cells. What these studies have revealed so far is that tumor-associated Gr1+CD11b+ cells display angiogenic properties and promote blood vessel growth in various tumor models partly by producing VEGF and MMP9 (56–58). In addition, they produce additional chemokines and cytokines such as CXCL1, CXCL8, interleukin (IL)-1b, and IL-6 that promote tumor neovascularization (51).
Taken together, all these heterogeneous innate immune cell constituents produce various but overlapping angiogenic mediators that control many aspects of vessel formation. The most prominent factors commonly found in these cells are growth factors and cytokines [e.g., basic fibroblast growth factor (bFGF)], tumor necrosis factor-alpha (TNF-α) and -beta (TGF-β), platelet-derived growth factor, placental growth factor (PlGF), neuropilin 1 (Nrp1), CXCL chemokines (CXCL8, 12), semaphorins, and various proteases, including MMP2, MMP7, MMP9, and MMP14, as well as cysteine cathepsin proteases (33, 39, 42, 59–66).
Because distinct myeloid cells redundantly express these angiogenic factors, it is conceivable that innate immune cells may compensate for the loss of other myeloid subpopulations during progression and targeted therapy. In line with this concept, neutrophils can compensate for macrophages to support tumor angiogenesis in tumor-bearing CCR2 knockout mice (67). Targeting GR1+ immune cells in pancreatic neuroendocrine tumors relapsing from anti-VEGF therapy did not further sensitize angiogenic inhibition because the enhanced recruitment of TAMs compensated for the lack of neutrophils and MDSCs (68).
It is important to note that not only innate immune cells but also adaptive immune cells can regulate tumor angiogenesis, although their specific implications still remain obscure. Like myeloid cells, B cells can directly promote angiogenesis by producing proangiogenic factors such as VEGF, FGF2, and MMP9 (69), or indirectly by polarizing macrophages to a Th2 immunosuppressive and proangiogenic phenotype in an immunoglobulin G (IgG)-dependent manner (70). On the other hand, T cells, dependent on the subtype, can negatively or positively control tumor angiogenesis. CD8+ cytotoxic T cells and CD4 Th1 cells produce interferon-gamma (IFN-γ) that restrains EC proliferation and induces the production of angiostatic chemokines CXCL9, 10, and 11 in TAMs (28, 71). In contrast, regulatory T cells (Tregs) suppress IFN-γ–expressing CD4 Th1 cells and secrete VEGF via hypoxia-induced CCL28, which both contribute to a proangiogenic tumor environment (72).
IMMUNE CELLS AND LYMPHANGIOGENESIS
Strong evidence that TAMs are involved in tumor lymphangiogenesis is based on the observation that macrophage depletion in several tumor types abates the formation of lymphatic vessels (73, 74). TAMs can promote lymphangiogenesis by expressing VEGFC and VEGFD that bind to VEGFR3 on lymphatic endothelial cells (LECs) (Figure 2). VEGFR3 activation leads to enhanced proliferation and survival of LECs by activation of protein kinase Akt, extracellular signal–related kinases Erk1 and Erk2, focal adhesion kinase, and NF-κB (75, 76). This process is stimulated by cancer cells that activate macrophage-derived lymphangiogenesis by producing IL-1α in a highly specific manner (77). Studies in patients with stage 1 (thus in situ) squamous cell carcinoma showed that CD163+ (alternatively activated) macrophages are recruited to the peritumoral, nonlesion skin, where they release VEGFC, which is linked to increased lymphatic density and reorganization (78). Complementing these findings, IL-8 was upregulated in squamous cell carcinoma compared to normal and adjacent nontumor skin, suggesting that this cytokine may be involved in TAM recruitment because firm adhesion of monocytes to inflamed ECs greatly depends on IL-8 (79).
Figure 2.
The role of macrophages in tumor lymph angiogenesis. Adhesion of circulating VEGFR3+ monocytes to tumor BECs in response to cancer cell (and stromal cell) secreted IL-8 (blue circles) favors monocyte extravasation. Once inside the tumor parenchyma, monocyte differentiation into TAMs, which in human tumors are mostly CD163+, elicits their production of VEGFA, VEGFC, and VEGFD (purple circles) upon TNF-α/TNFR1 signaling (yellow circles depict TNF-α). Autocrine stimulation of VEGFR3 by its cognate ligands VEGFC and VEGFD further increases VEGFC production. VEGFA, VEGFC, and VEGFD as well as MMPs, uPA, and plasmin favor the migration of tip-LECs and the formation of new lymphatic sprouts. They also contribute to the junction disassembling of LECs and thus to the promotion of cancer cell intravasation through the lymphatics. TEMs are in close in proximity to the tumor lymphatics but not in lymphatics of normal tissue. These perilymphatic macrophages (that share other lymphatic markers such as PROX-1, LYVE-1, PDPN, and VEGFR3) support new sprout growth in a paracrine manner, but it is still debated if they can integrate into the vessel wall. Chemotherapy will also act on TAMs and induce the initiation of a cathepsin B/heparinase cascade that leads to enhanced VEGFC release by TAMs and thus lymph angiogenesis and cancer cell intravasation. Mirroring this, radiotherapy induces the release of CSF1 (orange circles) by cancer cells that boosts the recruitment and differentiation of VEGFR3+ (prolymph angiogenic) TAMs. Abbreviations: BEC, blood endothelial cell; CSF1, colony-stimulating factor 1; IL-8, interleukin 8; LEC, lymphatic endothelial cell; LYVE-1, lymphatic vessel endothelial hyaluronan receptor 1; MMP, matrix metalloproteinase; PDPN, podoplanin; PROX-1, prospero homeobox protein 1; TAM, tumor-associated macrophage; TEM, Tie2-expressing macrophage; Tip-LEC, lymphatic endothelial tip cell; TNF-α, tumor necrosis factor-alpha; TNFR1, tumor necrosis factor receptor 1; uPA, urokinase-type plasminogen activator; VEGFA, vascular endothelial growth factor A; VEGFC, vascular endothelial growth factor C; VEGFD, vascular endothelial growth factor D; VEGFR3, vascular endothelial growth factor receptor 3.
This observation highlights the existence of cross talk between squamous cell carcinoma and macrophages in driving progression toward malignancy. In vitro evidence further supports the communication between cancer cells and macrophages during the lymphangiogenic process (Figure 2). Zhang et al. (80) showed that Lewis lung carcinoma cells induce alternative activation of cocultured macrophages; these in turn induced VEGFC expression in cancer cells. The induction of VEGFC transcription, production, and release by TAMs has been ascribed to TNFR1. TNF-α–overexpressing tumors display augmented density of both lymphatics and blood vessels. VEGFR3-blocking antibodies or the replacement of wild-type TAMs with TNFR1-deficient TAMs inhibited TNF-α–induced lymphangiogenesis and lymphatic metastases to lymph nodes without affecting TNF-α–stimulated angiogenesis. This emphasizes the importance of TNF-α stimulation of TAMs in the induction of VEGFC and the following activation of VEGFR3 on LECs (81). Interestingly, a study in cervical cancer patients shows that the fraction of TAMs that mostly release VEGFC (and VEGFD) also express VEGFR3 on the cell surface (thus sharing a marker with LECs). Their VEGFR3-positive monocyte progeny did not produce VEGFC unless stimulated with TNF-α [as in the study by Ji et al. (81)] or with the VEGFR3 ligand VEGFD (75). This suggests that VEGFR3 on monocytes and TAMs can initiate a positive loop to foster the production of its cognate ligands VEGFC and VEGFD that in turn work in a paracrine manner on LECs.
However, VEGFR3 is not always found in all tumor types in either mouse or human TAMs (82, 83). Besides VEGFC and VEGFD, TAMs also secrete VEGFA, which is more characterized for its role in angiogenesis, although this factor also plays an important function in lymphangiogenesis. First, VEGFA recruits TAMs mostly via the activation of VEGFR1 on macrophages (82, 84), but it also directly induces the proliferation and migration of LECs via VEGFR2 activation (85). VEGFA also promotes tumor and peritumoral lymphangiogenesis (86) as well as sentinel lymph node lymphangiogenesis in a model of chemically induced skin carcinogenesis (87). In addition to their release of lymphangiogenic growth factors, TAMs regulate lymphangiogenesis indirectly by the production of enzymes, such as MMPs, plasmin, and urokinase plasminogen activator, that contribute to matrix remodeling and growth factor activation (88). Similar to what has been previously described for TEMs in the process of tumor blood vessel formation (46, 89), perilymphatic macrophages might support the emerging lymphatics so that only a small fraction of TAMs that reside in close proximity to the vessels is relevant for lymphangiogenesis (M. Mazzone, unpublished data). Once in the perilymphatic space, TAMs sustain lymphangiogenesis but also lymphatic metastasis by fostering cancer cell intravasation (90, 91). A study in breast cancer patients has revealed that TEMs are associated with lymphatic vessels in the tumor but not in the peritumoral tissue. Importantly, while TEMs within the tumor express lymphatic markers such as LYVE-1, podoplanin (PDPN), VEGFR3, and PROX-1, myeloid cells in the non-neoplatic tissue did not, suggesting that a phenotypic switch is impinged by the tumor microenvironment (92). Isolated TEMs were angiogenic and lymphangiogenic in vitro and expressed high levels of VEGFA, VEGFC, and VEGFD. In vitro blockade of VEGF receptors and Tie2 substantially impaired their (lymph)angiogenic potential, thus indicating the role of these pathways. A close look at the association of TEMs with lymphatic vessels revealed that elongated cells may be either very proximal or even integrated into the vessel wall. Macrophages proximal to the lymphatics not only sustain lymphangiogenesis but also promote cancer cell intravasation (92). An in vitro study showed that IL-1β released by perilymphatic macrophages can contribute to this step (91); however, many other factors can be involved in this process in vivo.
Although the paracrine communication of TAMs with LECs is well recognized (93–95), the physical contribution of macrophages (or myeloid cells in general) to the vessel wall during pathological lymphangiogenesis is a matter of debate (96). A previous study shows that macrophages can form lymphatic vessel–like structures that are positive for LYVE-1, PROX-1, and PDPN (97). Yet, in vitro cultured monocytes were shown to acquire endothelial markers such as CD31, VE-cadherin, and Tie2 (98); however, the in vivo relevance of this observation is uncertain (99). In the lymphangiogenesis field, however, there is still an open debate about the possibility of perilymphatic TAM integration. Analysis of the literature suggests that macrophages can also transdifferentiate in vitro into vessel-like structures, an action accompanied by downregulation of hematopoietic markers such as CD45 and CX3CR1 (100, 101). Similarly, in the Rip1Tag2 mouse model of insulinoma and in the TRAMP-C1 prostate cancer transplantation model, F4/80+ LYVE-1+ TAMs directly integrate into lymphatic vessels and presumably lose their macrophage features upon integration (96). Because no cell fusion events between macrophages and LECs were detected by genetic tracing experiments, the underlying idea is that TAMs can transdifferentiate into LECs. In a different context, Maruyama et al. (97) provided evidence that transplanted CD11b+ macrophages infiltrate the corneal stroma and transdifferentiate into LEC clusters that join existing lymphatic vessels.
On the contrary, He et al. (102) demonstrated that genetically marked bone marrow–derived cells do not incorporate into lymphatic vessels during subcutaneous Lewis lung carcinoma, melanoma, or VEGFC-induced lymphangiogenesis. A limitation of all these studies is that either transplantations into the cornea (97) or irradiation of recipient mice (102, 103) were applied before evaluating the physical contribution of macrophages to the vessel wall, thus leading to a nonphysiological perturbation of the host and to hyperinflammatory conditions. Finally, the possibility that this process is context and tumor dependent may hold true. Overall, the proposed transdifferentiation requires further investigation and confirmation in vivo. Finally, adaptive immunity still plays a poorly characterized role in the formation of tumor lymphatics. Although tumor lymphangiogenesis was recently shown to promote T cell infiltration and potentiate immunotherapy in melanoma (104), the reverse cross talk—or how T cells regulate lymphangiogenesis in the context of cancer—is not well understood. It has been reported that inhibition of Th1, Th2, or Th17 cytokines increases VEGFA and VEGFC expression and, thus, lymph node lymphangiogenesis in a mouse model of inflammation (105). Mechanistically, the absence of T cells induced hypoxia-inducible factor 1 alpha (HIF-1α) in macrophages, which in turn enhanced VEGFA and VEGFC levels (105). A more recent study (using a model of tail lymphatic disruption) shows that upon lymphatic injury, CD4+ T cells get activated into a mixed Th1 and Th2 phenotype by dendritic cells in the regional lymph nodes and then migrate to the injury site to initiate lymphedema pharmacological inhibition of T cell release from the lymph nodes or genetic depletion of CD4+ cells. This resulted in reduced lymphedema, suggesting that CD4+ T cells impair lymphatic function after lymphatic injury (106). Another mechanism whereby T cells negatively regulate lymphatic function and lymphangiogenesis is through IFN-γ secretion, leading to the suppression of lymphatic-specific genes in LECs and consequently causing marked reduction in lymph node lymphangiogenesis (107). Thus, inflammation elicits a T cell–dependent, self-limiting response that dampens T cell trafficking. Instead, the role of T cells and adaptive immunity in the regulation of tumor lymphangiogenesis and lymphatic metastatic spread is completely unstudied and will require thorough investigation in light of the latest focus of cancer biology on T cell–mediated immunotherapies.
MACROPHAGE-INDUCED LYMPHANGIOGENESIS IN RESISTANCE TO THERAPY
Another important aspect in cancer biology is how tumors escape the deleterious effects of chemotherapeutic drugs and irradiation, thus leading to therapy resistance. Starting from the observation that healthy mice treated with paclitaxel, FOLFOX, or gemcitabine (but not cisplatin) and breast cancer patients after paclitaxel chemotherapy showed increased levels of VEGFC, Alishekevitz et al. (108) showed that chemotherapy-educated macrophages secrete cathepsin B that cleaves proheparanase into its active form. In turn, heparinase induced VEGFC expression by TAMs and thus endorsed tumor lymphangiogenesis and lymphatic metastasis. The induction of the cathepsin B–mediated cascade is ascribed to a population of VEGFR3+ TAMs in tumors (108). This is in favor of a positive feedback loop, where VEGFC enhanced its own signaling through TAMs. Importantly, based on these results, chemotherapy would presumably introduce the risk of fostering metastasis, whereas the combination of paclitaxel and VEGFC/VEGFR3 blockade would both directly inhibit lymphangiogenesis and block the prometastatic activity of macrophages. This was indeed the case in tumor-bearing mice treated with chemotherapy (108). Similarly, radiotherapy stimulated cancer cells to produce higher levels of CSF1, resulting in the enhanced infiltration of (lymph)angiogenic myeloid cells into the tumor site (109). More data are required to corroborate these findings in patients. Nevertheless, these studies highlight how TAM recruitment following a wound repair situation as it occurs after chemotherapy or radiation therapy can lead to treatment failure and/or resistance. Thus, TAM-targeting agents should be tested in combination with a specific type of chemotherapeutic drugs or irradiation regimens.
METASTASIS-ASSOCIATED MACROPHAGES IN ANGIOGENESIS
Although angiogenesis at the primary tumor site has been studied for more than 50 years, much less attention has been paid to the molecular mechanisms and cellular players controlling the angiogenic switch in metastasis. A considerable amount of work has proven that TAMs take part in each step of cancer growth and tumor angiogenesis (110, 111), but much less is known about the distinct role of metastasis-associated macrophages (MAMs). Several studies demonstrated that myeloid cells, and in particular MAMs, are important for the preparation of the metastatic niche via the release of matrix proteins at the metastatic sites. For this reason, these cells are also entrained by the primary tumor into the premetastatic niche before the lodging of cancer cells (112–115). In breast cancer, the release of CCL2 (also known as monocyte chemoattractant protein 1, MCP1) by cancer cells and stromal cells in the lung is important to recruit macrophages to the parenchymal tissue (116). Upon CCL2/CCR2 interaction, CCL3 is released by the same macrophages. In an autocrine manner, CCL3 binding to CCR1 and its activation enhance interaction of MAMs with metastasizing cancer cells, at least in part, through the engagement of integrin α4 (117). As a result, prolonged MAM retention enhances extravasation of cancer cells and therefore metastasis via macrophage-borne VEGFA that loosens the endothelial junctions and allows cancer cell extravasation (116). These MAMs are enriched for the expression of VEGFR1. Activation of this receptor in MAMs has been shown to be important for metastatic growth but not for cancer cell extravasation (116, 118). Indeed, VEGFR1 blockade was able to decrease the total metastatic burden and the average size of the lesion despite an unchanged number of metastatic nodules (119). One possible explanation for these observations is that MMP9 is downstream of VEGFR1 activation. MMP9 activity released by MAMs (and to a minor extent by cancer cells or other stromal cells) sustained the angiogenic growth in metastatic lesions (118). Together with MMP9, the coexpression of CSF1 (also known as macrophage colony-stimulating factor, M-CSF), another VEGFR1 downstream effector, is required for metastatic growth (119), likely because CSF1 is a key cytokine for macrophage function, survival, proliferation, and differentiation (120). Interestingly, we have shown that under physiological conditions, CSF2 (also known as granulocyte-macrophage colony-stimulating factor, GM-CSF) keeps the levels of caveolin-1 elevated in interstitial macrophages of the lungs; thus, it follows that caveolin-1 downregulates VEGFR1 exposure on the membrane of these macrophages, likely through the formation of caveolae (119). Together, macrophage-associated caveolin-1 is critical for restraining metastasis. It represents an intrinsic antimetastatic surveillance mechanism in the pulmonary microenvironment, whereby its upregulation prevents excessive exposure of VEGFR1 at the cell surface and thereby limits downstream MMP9 and CSF1 expression, angiogenesis, and finally metastatic growth (118). Because the lung has the physiology to encounter dangerous signals from the air, it is not surprising that blocking the metastasis by this axis in macrophages was seen in the lung but not in the liver (118). Further effort will be required to understand how the prometastatic axis represented by the CCL2/CCR2–CCL3/CCR1 axis in MAMs is specific for breast cancer and lung metastasis or whether this pathway is also observed in other tumors and/or metastatic sites.
Elegant work by Mazzieri et al. (46) has shown that Ang2 antibodies not only inhibited primary tumor growth and metastatic dissemination of a breast cancer mouse model but also directly suppressed the progression from a micro- to a macrometastatic stage independently of primary tumor growth. This was, at least in part, ascribed to the fact that at both the primary tumor and metastatic sites, Tie2 expression by TEMs is instrumental to sustain their association with sprouting vessels in response to Ang2 expression by blood endothelial cells (BECs). Mirroring these findings and using a different mouse model, Srivastava et al. (121) have proven that Ang2 reduced the growth of preseeded metastases by decreasing vessel density and increasing pericyte coverage at the metastatic sites in an adjuvant setting (after primary tumor resection).
Interestingly, a combination of Ang2 and low-dose chemotherapy completely regressed metastasis in a model that is refractory to maximal doses of paclitaxel. Mechanistically, Ang2 signaling in ECs is able to elicit an inflammatory response via endothelial production of CCL2 that recruits CCR2+Tie2 MAMs and indirectly via endothelial expression of adhesive molecules such as ICAM1, which is instrumental for inflammatory cell intravasation (121). Given the observation that anti-Ang2 antibodies also sensitized anti-VEGF treatment in metastatic lesions that were generally resistant to anti-VEGF alone, it is relevant to note that in this context, blocking Ang2 shielded the effects of Bv8 on ECs (121) [Bv8 having been released by CD11b+Gr1+ myeloid cells and conferring resistance to VEGF-targeted therapies (122, 123)]. Further investigation is needed to understand which inflammatory pathways are specifically important for the angiogenic switch in micrometastasis, whether these are the same as for the metastatic growth of existing lesions, and whether these targets can be translated into human cancers.
Although we have started to gain a better understanding of the implication of macrophages in angiogenesis at metastatic sites, we still know very little about the contribution of all other immune cells (and especially cells of adaptive immunity) in this process. Studies in mice using sarcoma, melanoma, and pancreatic cancer models have all pointed to CD4+ T cells and/or CD8+ cytotoxic T cells as the main executors of metastatic dormancy (124–126). However, to which extent this depends on blood vessel expansion and angiogenesis remains to be elucidated.
INFLAMMATION, HYPOXIA, AND METABOLISM IN THE CONTEXT OF TUMOR ANGIOGENESIS
When inflammatory cells infiltrate into tumors, they encounter different oxygen tensions fluctuating from 60 mmHg (i.e., 8% oxygen) to almost anoxic conditions (almost no oxygen), depending on the tumor type. Yet, the oxygen tension of most tumors varies from anoxia to 7.5 mmHg (i.e., 1% oxygen), a condition known as hypoxia (127). In addition, immune cells also face an acidic tumor environment due to the increased anaerobic glycolysis of tumor cells. Thus, low oxygen tension and different metabolic fingerprints of specific cancer cell types will greatly affect metabolite availability and cause metabolic restrictions (128–130). Oxygen and metabolite availability can thus define what is coined a metabolic niche. It follows that TAMs (and other immune cells) display specific alterations in metabolic gene expression because they are forced to adapt their metabolism in relation to the metabolic niche they encounter (38, 128–132). The questions of how these different niches are causatively linked to a phenotypic shift of inflammatory cells and how this impinges on tumor progression have gained much attention in the last few years. As the focus of this review is on how cancer inflammation controls angiogenesis, we describe how differing metabolite and oxygen availabilities can affect the angiogenic properties of TAMs and other immune cells within the tumor.
Control of Inflammation by Hypoxia in the Process of Tumor Angiogenesis
Several mechanisms can drive myeloid cell accumulation into the hypoxic niche of the tumor. For example, HIF-1α stabilization under low oxygen tension promotes the transcriptional induction of CXCL12 (also known as SDF1) (133) and its receptor CXCR4 (134) in ECs (133) and myeloid cells (33), respectively, thus allowing recruitment and retention of bone marrow–derived angiogenic cells (33). In a similar way, the ligand for Tie2, Ang2, is produced by angiogenic tumor vessels and is a chemoattractant for TEMs. Hypoxia upregulates Tie2 expression on TEMs and Ang2 binding to Tie2 to downregulate their antitumor functions and promote their proangiogenic functions (46, 135). Depleting TEMs or silencing Tie2 in macrophages greatly abates the angiogenic sprouting of several tumors (46, 99). Hypoxic cancer cells and stromal cells also upregulate semaphorin 3A (Sema3A) that engages neuropilin 1 (Nrp1)+ TAMs into the hypoxic niche via a VEGFR1/PlexinA1/PlexinA4 signaling platform. Once in the hypoxic niche, hypoxia-driven Nrp1 downregulation in TAMs will retain them in loco via a PlexinA1/PlexinA4 pathway, therefore countering external migratory signals (136). Here, TAMs become angiogenic and immunosuppressive (82, 126, 137). Nrp1 genetic knockout or knockin in macrophages of an Nrp1 form that does not signal through Sema3A strongly reduces angiogenesis, promotes cytotoxic T cell responses, and reduces tumor growth and metastasis in mouse pancreatic, lung, and breast cancer models (138). Similar findings were confirmed by Miyauchi et al. (139) in gliomas, where both genetic knockout of Nrp1 in microglia and macrophages and systemic pharmacological inhibition of Nrp1 via a compound named EG00229 had a strong antitumoral effect via the reshaping of the inflammatory response. Human mast cells have also been shown to express Nrp1 (as well as Nrp2, VEGFR1, VEGFR2, Tie1, and Tie2) and to release a large array of angiogenic and lymphangiogenic molecules such as VEGFA, VEGFC, and VEGFD (140). Immunologically activated human basophils selectively produce VEGFA but not VEGFC and VEGFD. However, they also produce Ang1 that activates Tie2 on human mast cells or on BECs and LECs, promoting a mast cell–mediated cascade that leads to indirect or direct tumor angiogenesis and lymphatic angiogenesis (140–142).
Although hypoxia-induced release of growth factors, such as VEGF, and of chemokines, such as CXCL12 and CCL2, is important for the recruitment of specific monocyte subsets (127, 143, 144), the effect of hypoxia on the macrophage phenotype is less understood. This may be because of the complex interaction between cell autonomous signaling pathways induced by hypoxia in macrophages and their response to hypoxia-induced stimuli coming from neighboring cancer and stromal cells (127, 144). It is well established that HIF-1α has an important role in the phenotypic response to hypoxia. HIF-1α positively controls diverse inflammatory responses by promoting glycolysis and energy production in myeloid cells at the inflammatory hypoxic site (145). As a consequence, macrophages display reduced activation and motility when HIF-1α is genetically deleted (145). However, whether metabolic changes in HIF-1α knockout macrophages impinge on cancer progression and tumor angiogenesis is unknown. Instead, it has been shown that TAMs react to hypoxia by increased expression of HIF-1α–mediated proangiogenic genes such as VEGFA (40, 132, 146). VEGFA release by TAMs is responsible for tumor blood vessel dysfunction and abnormalities and the consequent increase in tumor hypoxia. This has been shown by the genetic deletion of VEGF in myeloid cells, which results in tumor vessel normalization and restores tumor perfusion, thus resulting in a better response to chemotherapy (40). Besides its induction of VEGF, HIF-1α in TAMs mediates the suppression of adaptive immunity, as the loss of HIF-1α in myeloid cells directly abrogates hypoxia-induced suppression of T cell activation in an inducible nitric oxide synthase (iNOS)–dependent manner (146).
Although hypoxic TAMs upregulate both HIF-1α and HIF-2α (146, 147), it seems clear that HIF-1α has an impact only on the expression of angiogenic (and metabolic) genes (40, 132, 146), whereas hypoxia-induced HIF-2α has a different function (148). Myeloid cell–specific loss of HIF-2α in murine hepatocellular and colitis-associated colon carcinoma models reduces TAM infiltration due to impaired expression of CXCR4 and CSF1R. This alteration is linked to a drop in cancer cell proliferation and tumor progression, while blood vessel density is unchanged (148). Additional data and the analysis of different tumor models are required to support a general idea that HIF-2α is more relevant for the recruitment of TAMs and their effect on cancer cells, whereas HIF-1α is more related to angiogenic and immune functions of TAMs. Based on a noncancer study, we reported that in conditions of muscle or cardiac ischemia, the downregulation of the HIF-prolyl hydroxylase PHD2 by genetic deletion of one allele or by activation of Tie2 signaling in macrophages increases NF-κB activity that leads to the transcription of CXCL12 and platelet-derived growth factor B (149, 150). These are at least partly responsible for the recruitment of mural cells around blood vessels and thus for the arteriogenic process (149, 150). However, we could not observe a difference in tumor vessel coverage when analyzing myeloid cell–specific Phd2-haplodeficient mice (17), supporting the notion that different macrophage subsets in different tissues can rely on alternative pathways in support of their angiogenic activity.
HIF-1 and HIF-2 are not only stabilized by hypoxia but also by paracrine stimuli and signaling pathways that involve kinase, phosphatases, and other interacting molecules (151–153). It has been shown that mainly Pl3Kγ and partly Pl3Kδ elicit AKT activity in TAMs, which leads to HIF- 1α and HIF-2α accumulation and the production of several angiogenic factors. Loss of Pl3Kγ in TAMs was sufficient to induce, also in hypoxia, HIF-1α and HIF-2α degradation via the 26S proteasome pathway, which resulted in reduced growth and distant dissemination of subcutaneous Lewis lung carcinoma tumors (151). The attention to these results is also due to the fact that researchers have defined Pl3Kγ as a macrophage-specific mediator of resistance to therapies such as immunotherapy and antiangiogenic therapy (68, 154, 155). However, the role of HIF proteins and hypoxic TAMs in this resistance process remains to be further characterized.
In addition, because it is not yet well understood how the HIF-dependent hypoxic response regulates tumor angiogenesis through the phenotypic alterations of T cells, this will likely be an important topic in the near future. A recent publication provides evidence that HIF-1 but not HIF-2 is essential for the cytotoxic activity of CD8+ cells and for their ability to cross the endothelial barrier, a limiting step for their recruitment into the tumor (156). However, although HIF-1 in T cells appears to mediate cytotoxicity (157, 158), hypoxic tumor niches prevent cytotoxic T cell activation via the influence of other hypoxic cells and the paracrine interaction of ligands and receptors that are controlled by hypoxia (159, 160). It is more surprising that tumors in T cell–specific HIF-1α knockout mice display less tortuous and more perfused vessels (with increased tumor vessel normalization); this is based on reduced expression of the HIF-1α target VEGFA (156). It is not clear whether or not the effect on blood vessels in T cell–specific HIF-1α or VEGFA knockout mice is epiphenomenal and thus whether the increased tumor growth in these mice is rather due to angiogenesis-independent mechanisms. Indeed, other reports show that reduction of vessel normalization, not the induction, increases T cell infiltration (161, 162). More intriguingly, activation of CD4+ T cells by immunocheckpoint inhibitors correlates with vessel normalization, and adoptive Th1 transfer in xenopatients resolves primary tumor hypoxia via vessel normalization (161). The intertwined cross talk between hypoxia, T cells, and blood vessels and how it affects disease outcome certainly warrants future in-depth analysis.
Control of Inflammation by Metabolism in the Process of Tumor Angiogenesis
Despite the growing interest in immunometabolism, how metabolic pathways and metabolite availability affect immune cell phenotype in a cancer context remains poorly explored. Even less is known about how metabolic pathways in immune cells affect the formation of a tumor vascular network (128, 130, 163). Interestingly, angiogenic and metabolic genes exhibited the highest-expression differences between hypoxic and normoxic TAMs (164). We found that REDD1 (regulated in development and DNA damage response 1), a physiologic inhibitor of the mechanistic target of rapamycin (mTOR), is synergically induced in TAMs by hypoxia and soluble stimuli from the tumor. REDD1 upregulation in hypoxic TAMs will excessively inhibit the mTOR pathway to a lower extent than it does in healthy tissues (131). As a consequence, mTOR release upon genetic knockout of REDD1 leads to enhanced glucose uptake. In this way, REDD1 knockout TAMs are able to subtract glucose from newly forming blood vessels and compete with tumor-associated ECs for glucose (131). Reduction of glucose availability and decreased glycolysis in ECs foster endothelial quiescence and the stabilization of blood vessels (165). Conversely, in tumors where REDD1 is excessively induced in hypoxic TAMs (which turn off the mTOR pathway), competition between ECs and macrophages is in favor of blood vessels; the glycolytic endothelium is thus hyperactive and less sessile (165). This is the first proof that in a tumor, both the surge of angiogenic factors and metabolic cross talk play in favor of an abnormal and dysfunctional vascular network. REDD1 knockout in TAMs reinstalls mTOR activity and thus reestablishes glucose up-take by macrophages and glucose competition with ECs. This results in more normalized, mature tumor blood vessels that prevent hypoxia and cancer cell intravasation (131).
Not only can macrophages affect angiogenesis, but a mutual control exists whereby ECs in turn sustain the M2-like, proangiogenic phenotype of macrophages. They install a positive feedback loop in cancer that keeps blood vessels hyperbranched, leaky, dysfunctional, tortuous, and poorly covered. This mutual regulation of EC and macrophage behavior at least partly relies on a metabolic pathway. At the vascular niche of the tumor, in particular in glioblastoma, EC-specific production of IL-6 and a more diffuse release of CSF1 promote an M2-like, alternative activation of TAMs (166). This alternative activation depends on the downstream activation by both IL-6 and CSF1 of the peroxisome proliferator-activated receptor gamma (PPAR-γ), a key transcriptional factor involved in the control of lipid uptake and glucose metabolism. In TAMs, PPAR-γ binds the promoter of HIF-2α, inducing its transcriptional (hypoxia-independent) accumulation. HIF-2α is then associated with alternative macrophage activation (153). EC-specific knockout of IL-6 was sufficient to reduce microvascular proliferation in the tumor, to promote extensive necrosis, to decrease arginase-1 expression by TAMs (a marker of M2 activation), and to increase the survival of glioblastoma-bearing mice. This observation may indeed provide a link between obesity, macrophage polarization, tumor angiogenesis, and cancer because polyunsaturated fatty acids bind and activate PPAR-γ, and long-chain polyunsaturated fatty acid status has recently been related to the pathogenesis of obesity (167). If true, could PPAR-γ antagonists be used in immunotherapy to block M2-like macrophage polarization and thus abnormal angiogenesis and cancer malignancy? Phenolic acids such as oleuropein found in olives inhibit adipogenesis and adipocyte lipolysis. This means that mice fed a high-fat diet will display less circulating fatty acid and reduced body weight gain when treated with oleuropein (168). In B16 melanoma-bearing mice subjected to a high-fat diet, cancer and metastasis are more pronounced than in mice on a lean diet, but oleuropein strongly abrogates the effect conferred by the high-fat diet on tumor progression. Interestingly, this was associated with a reduction in M2 macrophage polarization as well as a consequent decrease in VEGFA, VEGFC, and VEGFD expression, altogether leading to the inhibition of tumor angiogenesis and lymphangiogenesis (168). Although more observational than mechanistic, this study underlines the strong effect of systemic metabolism on tumor inflammation, angiogenesis, and lymphangiogenesis.
An important metabolic pathway in macrophages implies the degradation of the amino acid arginine. Although arginase-1 is more abundantly expressed in M2-like, protumoral macrophages and degrades arginine into ornithine and urea, iNOS or Nos2 is enriched in M1-like, antitumoral macrophages and utilizes arginine and NADPH to generate citrullin and nitric oxide. Arginine depletion by macrophage-borne arginase-1 has been linked to the inhibition of antitumor T cell responses (127, 169). Similarly, nitric oxide release by hypoxic TAMs is also immune suppressive (147). Although the effect of TAM-associated arginase-1 on tumor blood vessels is unexplored, a recent report has shown that in several pancreatic cancer patients and in several mouse tumor models, local radiotherapy of the tumor induces iNOS in TAMs (because of a direct effect of γ-irradiation on macrophages). Instead, it was more unexpected that iNOS was responsible for tumor blood vessel normalization, EC activation, and recruitment of host T cells as well as increased delivery of adoptively transferred antitumor and cytotoxic T cells, all of which were accompanied by a reduction of Th2 (protumoral) and an increase of Th1 (antitumoral) cytokines (170). These results demonstrate the positive antitumor effects of macrophage-borne nitric oxide on blood vessels and the indirect effects on T cell activation and recruitment (170) that are in sharp contrast to the immunosuppressive function of nitric oxide release by hypoxic TAMs (likely in avascular areas of the tumor) (147). Altogether, tumor context, association with therapy, and localization of immune cells and their interaction within different compartments can give rise to opposite effects (171).
Another amino acid involved in defining the phenotypic features of TAMs and their impact on tumor blood vessels is glutamine. Its role has always been considered proinflammatory, as the amino acid has been widely recognized as an important metabolic fuel for immune cells as well as a required foundation for lymphocyte and macrophage functions (172). However, in certain circumstances, glutamine supplementation is clearly anti-inflammatory (173, 174). This is likely due to the effect that glutamine availability has on the induction of glutamine synthetase, the enzyme responsible for glutamine production starting from glutamate. We found that an anti-inflammatory stimulus such as IL-4 as well as glutamine deprivation induce glutamine synthetase in mouse and human macrophages (38). Deletion of glutamine synthetase results in glutamate reroute to the GABA shunt with accumulation of succinate at the tricarboxylic acid (TCA) cycle. Succinate directly and indirectly (through HIF-1) sustains a rewiring of TAMs into M1-like macrophages. This leads to increased T cell recruitment and activation but also tumor blood vessel normalization, with increased perfusion, reduced permeability, and prevention of cancer cell dissemination (38). In this case, tumor vessel normalization (despite a reduction in vessel number) likely fosters a feedback loop of enhanced host T cell response, as described above (161, 162, 170).
Overall, it is apparent that the hypoxia/oxygen-sensing and metabolic machinery are contestants in the same game. Finally, given the large armamentarium of metabolic drugs currently tested in clinical settings (175), the repurposing of these molecules as a strategy to reshape inflammation and thus the angiogenic (and immune) landscape within the tumor is a novel frontier of cancer therapy.
MYELOID CELL–INDUCED ANGIOGENESIS IN RESISTANCE TO THERAPY
A variety of standard and targeted therapies, including those that reduce blood vessel density, generate intratumoral hypoxia. Therapeutically induced low oxygen tension instigates the same course of action as naturally arising hypoxia to mobilize innate immune cells from the bone marrow and retain them at the tumor site (33, 46). Thus, intratumoral innate immune cells not only sustain angiogenesis but also possess the capacity to protect tumors from the deleterious effects of anti-VEGFR therapy by stimulating VEGF-independent pathways, as shown in several preclinical tumor studies. In addition to VEGF, TAMs and neutrophils express other proangiogenic factors like FGF1, FGF2, MMP9, and Ang2, and enhance those in response to VEGF inhibition (73, 176, 177) (Figure 1). Specifically, Gr1+ immune cells, including TANs and MDSCs, have been found to be enriched in several tumor types relapsing from antiangiogenic therapy where they convey a proangiogenic relapse from VEGF blockade by secreting increased levels of angiogenic mediators including Bv8 (68, 178). As shown recently, the enhanced attraction of neutrophils seems to be in part orchestrated by CXCL5-producing CX3CR1+ Ly6Clo monocytes that are recruited to the tumor site due to the upregulation of CX3CL1 on tumor ECs in response to antiangiogenic therapy; this triggers transmigration of these monocytes across the endothelium (179).
In addition, accumulating TEMs in pancreatic tumors of the Rip1Tag2 mice undergoing VEGFR2 blockade contributed to a proangiogenic relapse that could be suppressed with a dual ANG2/VEGFR2 inhibitor targeting both TEMs and VEGFR2 (176). CXCR4+ TEMs were also found to participate in the revascularization of MMTV-PyMT mammary tumors that had under-gone treatment with the vascular-disrupting agent combretastatin A4 phosphate (CA4P). CA4P is known to induce substantial intratumoral hypoxia due to tumor vessel obstruction and, thus, enhanced HIF-induced CXCL12 expression and subsequent infiltration of CXCR4+ TEMs (180). Combination of CA4P and a CXCR4 inhibitor blocked TEM accumulation and enhanced CA4P-induced tumor necrosis concomitant with reduced tumor growth, and consequently, sustained response. These results demonstrate that therapeutically induced hypoxia can induce expression and secretion of Ang2 and CXCL12, which in turn mediate Tie2-mediated VEGF-independent angiogenic activity of TEMs in tumors.
It is important to emphasize, however, that the different innate cell populations in tumors appear to express quite a similar profile of multiple angiogenic factors; thus, it is conceivable that they can compensate for each other in regulating angiogenesis. Interestingly, in the pancreatic Rip1Tag2 tumor model, depleting specific myeloid subpopulations resulted in increases in non-targeted myeloid cells, creating an oscillating pattern of resistance (68). Although most tumor model systems will respond to antiangiogenic therapy by slowing down tumor growth, pancreatic neuroendocrine tumors in Rip1Tag2 mice first respond very well to VEGF signaling blockade exhibiting reduced density of vessels, which are overall normalized and show growth stasis. Sub-sequently, they relapse and reinstate growth within 2–8 weeks, depending on the specific drug regimen (177, 181, 182). By comparing innate immune cells in responding and relapsing pancreatic neuroendocrine tumors, it became apparent that macrophages, monocytes, and neutrophils all exerted angiostatic and immunostimulating features in responding tumors when associated with the upregulation of CXCL14 and other angiostatic chemokines (Figure 1). This led to an influx of cytotoxic CD8 cells, whereas in relapsing tumors, myeloid cells converted back into an immune-suppressive and angiogenic phenotype, and CD8 influx ceased. Importantly, myeloid cells activated their PI3Kγ pathway, which disabled their repolarization and consequently promoted the proangiogenic tumor relapse (68). In support of these results, myeloid PI3Kγ signaling was shown to induce a transcriptional program that promoted immunosuppression by inhibiting NF-κB and activating C/EBPβ (155). Thus, pharmacological inhibition of myeloid PI3Kγ/d improved and sustained the tumor response to antiangiogenic therapy by converting all innate immune cells to an angiostatic and immune-stimulatory state associated with enhanced cytotoxic T cell infiltration and activity (68). These results further support the emerging proposition that angiogenesis and inflammation are functionally interregulated and that immune cells play a pivotal role in regulating both processes.
In line with these observations, tumors can also endorse the adaptive immune system to escape from antiangiogenic therapy. Recent studies have shown that relapsing tumors upregulated the negative immune checkpoint regulator, programmed cell death-ligand 1 (PD-L1), in tumor and stromal cell constituents (183, 184). As a result of PD-L1 binding PD-1 on the surface of activated T cells, T cell anergy or exhaustion was produced, which thereby triggered immunosuppression. Combining immunotherapy using anti-PD-L1 with antiangiogenic therapy (either anti-VEGF or anti-VEGF/Ang2) had reciprocally beneficial effects in that immunotherapy targeted evasion from antiangiogenic therapy, whereas vascular normalization elicited by antiangiogenic treatment could increase lymphocyte infiltration and activation (183, 184). Surprisingly, in successfully treated tumors, antiangiogenic immunotherapy induced high endothelial venule (HEV)-like structures that are normally found in secondary lymphoid organs and specialized to facilitate lymphocyte trafficking (185). Indeed, intratumoral HEVs substantially enhanced cytotoxic T cell infiltration and activity, and thereby furthered tumor cell destruction, leading to overall improved outcome (183). These preclinical studies support the notion that immune checkpoint inhibitors can sensitize and prolong efficacy of the VEGF signaling blockade and, conversely, antiangiogenic therapy can improve immunotherapy by supporting vascular changes such as vessel normalization and HEV formation in tumors.
Further support for this concept stems from a recent study by Tian and colleagues (161, 186) who demonstrated that intratumoral T lymphocyte infiltration promoted blood vessel normalization; in addition, a normalized vasculature has the ability to enhance T cell infiltration. Mice deficient in CD4 and CD8 T cells involved tumors with more abnormal tumor vessels and hypoxic areas than those of CD4- and CD8-proficient mice. Checkpoint immunotherapy (anti-PD1 and/or anti-CTLA4) or adoptive Th1 transfer generating activated cytotoxic T cells in tumor model systems was sufficient to induce blood vessel normalization and reduce both hypoxia and metastases. These results were congruent with a normalization plus T cell receptor signaling pathway signature obtained by transcriptional profiling of patient tumors and was associated with good prognosis. These studies provide evidence in patient-derived tumors that blood vessel normalization and T lymphocyte infiltration provide positive feedback loops conferred by each compartment. They also underscore the notion that tumor vessels can be modified by the immune system, which enables enhanced T cell infiltration and improves immunotherapies.
CONCLUSION: FROM MOUSE TO HUMAN—ADVANTAGES AND LIMITATIONS
Although the mouse tumor model systems discussed in this review have greatly helped to elucidate the various mechanistic underpinnings of myeloid-directed tumor angiogenesis and adaptive tumor resistance, it remains to be validated to which extent some of these mechanisms are also functionally implicated and significant in the human tumor setting. Notably, a number of clinical trials ( NCT03024437, NCT02659384, NCT02873962, and NCT02017717) are already evaluating combinatorial approaches of VEGF/VEGFR and PD-1/PD-L1 inhibition for a number of cancer types, such as renal cell carcinoma, colorectal cancer, ovarian cancer, and recurrent glioblastoma. Thus, ongoing clinical trials already combine antiangiogenic agents and immunotherapies such as immune checkpoint blockade or target innate immune cells as well as various approaches to enhance infiltration and activation of T cells. These trials will be instrumental to validate the concept of targeting the functional and regulatory interaction between the immune system and vascular system in cancer (187–189).
ACKNOWLEDGMENTS
This work was supported by grants from the European Research Council CoG ImmunoFIT (#773208) (to M.M.), the Flemish Government FWO (#G066515N) and Methusalem (to M.M.), the Belgian Foundation Against Cancer (#2014–197) (to M.M.), the National Institutes of Health/National Cancer Institute (NIH/NCI R01CA201537) (to G.B.), and the Flemish Government FWO (#G0A0818N) (to G.B.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Leite de Oliveira R, Hamm A, Mazzone M. 2011. Growing tumor vessels: more than one way to skin a cat—implications for angiogenesis targeted cancer therapies. Mol. Aspects Med 32:71–87 [DOI] [PubMed] [Google Scholar]
- 2.De Palma M, Biziato D, Petrova TV. 2017. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17:457–74 [DOI] [PubMed] [Google Scholar]
- 3.Black WC, Welch HG. 1993. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N. Engl. J. Med 328:1237–43 [DOI] [PubMed] [Google Scholar]
- 4.Bergers G, Benjamin LE. 2003. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3:401–10 [DOI] [PubMed] [Google Scholar]
- 5.Baeriswyl V, Christofori G. 2009. The angiogenic switch in carcinogenesis. Semin. Cancer Biol 19:329–37 [DOI] [PubMed] [Google Scholar]
- 6.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. 2011. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med 17:347–62 [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P 2005. Angiogenesis in life, disease and medicine. Nature 438:932–36 [DOI] [PubMed] [Google Scholar]
- 8.Baluk P, Hashizume H, McDonald DM. 2005. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev 15:102–11 [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. 2011. Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain RK. 2005. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62 [DOI] [PubMed] [Google Scholar]
- 11.Jain RK. 2013. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol 31:2205–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkman J 1974. Proceedings: tumor angiogenesis factor. Cancer Res 34:2109–13 [PubMed] [Google Scholar]
- 13.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, et al. 2006. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444:1032–37 [DOI] [PubMed] [Google Scholar]
- 14.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, et al. 2006. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444:1083–87 [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, et al. 2010. Chronic DLL4 blockade induces vascular neoplasms. Nature 463:E6–7 [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Turkoz A, Jackson EN, Corbo JC, Engelbach JA, et al. 2011. Notch1 loss of heterozygosity causes vascular tumors and lethal hemorrhage in mice. J. Clin. Investig 121:800–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leite de Oliveira R, Deschoemaeker S, Henze AT, Debackere K, Finisguerra V, et al. 2012. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 22:263–77 [DOI] [PubMed] [Google Scholar]
- 18.Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, et al. 2009. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136:839–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xian X, Håkansson J, Ståhlberg A, Lindblom P, Betsholtz C, et al. 2006. Pericytes limit tumor cell metastasis. J. Clin. Investig 116:642–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, et al. 2015. Role of vascular density and nor-malization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. PNAS 112:14325–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambrechts D, Claes B, Delmar P, Reumers J, Mazzone M, et al. 2012. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol 13:724–33 [DOI] [PubMed] [Google Scholar]
- 22.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. 2000. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res 60:4324–27 [PubMed] [Google Scholar]
- 23.Mainiero MB. 2010. Regional lymph node staging in breast cancer: the increasing role of imaging and ultrasound-guided axillary lymph node fine needle aspiration. Radiol. Clin. N. Am 48:989–97 [DOI] [PubMed] [Google Scholar]
- 24.Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, et al. 2006. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia 8:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murdoch C, Muthana M, Coffelt SB, Lewis CE. 2008. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 8:618–31 [DOI] [PubMed] [Google Scholar]
- 26.Talmadge JE, Gabrilovich DI. 2013. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 13:739–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. 1996. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56:4625–29 [PubMed] [Google Scholar]
- 28.Lewis CE, Harney AS, Pollard JW. 2016. The multifaceted role of perivascular macrophages in tumors. Cancer Cell 30:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ. 2006. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br. J. Cancer 94:101–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coussens LM, Tinkle CL, Hanahan D, Werb Z. 2000. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103:481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du R, Lu KV, Petritsch C, Liu P, Ganss R, et al. 2008. HIF1αinduces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13:206–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giraudo E, Inoue M, Hanahan D. 2004. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J. Clin. Investig 114:623–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, et al. 2006. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66:11238–46 [DOI] [PubMed] [Google Scholar]
- 34.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, et al. 2007. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450:825–31 [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A 2010. Molecular pathways linking inflammation and cancer. Curr. Mol. Med 10:369–73 [DOI] [PubMed] [Google Scholar]
- 36.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. 2009. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16:183–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, et al. 2011. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331:1612–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmieri EM, Menga A, Martin-Pérez R, Quinto A, Riera-Domingo C, et al. 2017. Pharmacologic or genetic targeting of glutamine synthetase skews macrophages toward an M1-like phenotype and inhibits tumor metastasis. Cell Rep 20:1654–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, et al. 2011. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19:31–44 [DOI] [PubMed] [Google Scholar]
- 40.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, et al. 2008. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456:814–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, et al. 2010. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115:1461–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, et al. 2000. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol 2:737–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. 2007. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol 28:519–24 [DOI] [PubMed] [Google Scholar]
- 44.Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, et al. 2013. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology 57:1416–25 [DOI] [PubMed] [Google Scholar]
- 45.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, et al. 2005. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8:211–26 [DOI] [PubMed] [Google Scholar]
- 46.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, et al. 2011. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19:512–26 [DOI] [PubMed] [Google Scholar]
- 47.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, et al. 1999. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284:1994–8 [DOI] [PubMed] [Google Scholar]
- 48.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. 1999. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab. Investig 79:213–23 [PubMed] [Google Scholar]
- 49.Bird L 2016. Tumour immunology: neutrophils help tumours spread. Nat. Rev. Immunol 16:74–75 [DOI] [PubMed] [Google Scholar]
- 50.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, et al. 2015. MET is required for the recruitment of anti-tumoural neutrophils. Nature 522:349–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang W, Ferrara N. 2016. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol. Res 4:83–91 [DOI] [PubMed] [Google Scholar]
- 52.Coffelt SB, de Visser KE. 2015. Immune-mediated mechanisms influencing the efficacy of anticancer therapies. Trends Immunol 36:198–216 [DOI] [PubMed] [Google Scholar]
- 53.Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. 1997. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 90:4153–61 [PubMed] [Google Scholar]
- 54.Nozawa H, Chiu C, Hanahan D. 2006. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. PNAS 103:12493–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, et al. 2008. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–44 [DOI] [PubMed] [Google Scholar]
- 56.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. 2008. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Investig 118:3367–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Huang J, Ren X, Gorska AE, Chytil A, et al. 2008. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13:23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan PY, Wang GX, Yin B, Ozao J, Ku T, et al. 2008. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 111:219–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams RH, Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol 8:464–78 [DOI] [PubMed] [Google Scholar]
- 60.de Palma M, Coussens LM. 2008. Immune cells and inflammatory mediators as regulators of tumor angiogenesis In Angiogenesis: An Integrative Approach from Science to Medicine, ed. Figg WD, Folkman J, 225–37. New York: Springer Sci. Bus. Media [Google Scholar]
- 61.Betsholtz C, Lindblom P, Bjarnegard M, Enge M, Gerhardt H, Lindahl P. 2004. Role of platelet-derived growth factor in mesangium development and vasculopathies: lessons from platelet-derived growth factor and platelet-derived growth factor receptor mutations in mice. Curr. Opin. Nephrol. Hypertens 13:45–52 [DOI] [PubMed] [Google Scholar]
- 62.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, et al. 2004. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104:3472–82 [DOI] [PubMed] [Google Scholar]
- 63.Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G. 2000. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res 60:7163–69 [PubMed] [Google Scholar]
- 64.Joyce JA, Hanahan D. 2004. Multiple roles for cysteine cathepsins in cancer. Cell Cycle 3:1516–19 [DOI] [PubMed] [Google Scholar]
- 65.Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146:873–87 [DOI] [PubMed] [Google Scholar]
- 66.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. 2003. The expression of matrix metalloproteinase-2 and −9 in human gliomas of different pathological grades. Brain Tumor Pathol 20:65–72 [DOI] [PubMed] [Google Scholar]
- 67.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, et al. 2008. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia 10:329–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. 2015. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep 11:577–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang C, Lee H, Pal S, Jove V, Deng J, et al. 2013. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLOS ONE 8:e64159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, et al. 2010. FcRγactivation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17:121–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, et al. 2009. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, et al. 2011. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475:226–30 [DOI] [PubMed] [Google Scholar]
- 73.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, et al. 2007. Anti-PIGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–75 [DOI] [PubMed] [Google Scholar]
- 74.Zumsteg A, Christofori G. 2012. Myeloid cells and lymphangiogenesis. Cold Spring Harb. Perspect. Med 2:a006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, et al. 2002. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol 161:947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tammela T, Petrova TV, Alitalo K. 2005. Molecular lymphangiogenesis: new players. Trends Cell Biol 15:434–41 [DOI] [PubMed] [Google Scholar]
- 77.Watari K, Shibata T, Kawahara A, Sata K, Nabeshima H, et al. 2014. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLOS ONE 9:e99568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moussai D, Mitsui H, Pettersen JS, Pierson KC, Shah KR, et al. 2011. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J. Investig. Dermatol 131:229–36 [DOI] [PubMed] [Google Scholar]
- 79.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, et al. 1999. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398:718–23 [DOI] [PubMed] [Google Scholar]
- 80.Zhang B, Zhang Y, Yao G, Gao J, Yang B, et al. 2012. M2-polarized macrophages promote metastatic behavior of Lewis lung carcinoma cells by inducing vascular endothelial growth factor-C expression. Clinics 67:901–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, et al. 2014. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat. Commun 5: 4944. [DOI] [PubMed] [Google Scholar]
- 82.Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, et al. 2010. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J. Clin. Investig 120:2684–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du X, Gao Y, Sun P, Chen Y, Chang H, Wei B. 2018. CD163+/CD68+ tumor-associated macrophages in angiosarcoma with lymphedema. Int. J. Clin. Exp. Pathol 11:2106–11 [PMC free article] [PubMed] [Google Scholar]
- 84.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, et al. 2004. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig 113:1040–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, et al. 2004. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the α1β1 and α2β1 integrins. FASEB J 18:1111–13 [DOI] [PubMed] [Google Scholar]
- 86.Björndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, et al. 2005. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res 65:9261–68 [DOI] [PubMed] [Google Scholar]
- 87.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. 2005. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med 201:1089–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mantovani A, Romero P, Palucka AK, Marincola FM. 2008. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 371:771–83 [DOI] [PubMed] [Google Scholar]
- 89.Forget MA, Voorhees JL, Cole SL, Dakhlallah D, Patterson IL, et al. 2014. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PLOS ONE 9:e98623. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Karaman S, Detmar M. 2014. Mechanisms of lymphatic metastasis. J. Clin. Investig 124:922–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Storr SJ, Safuan S, Ahmad N, El-Refaee M, Jackson AM, Martin SG. 2017. Macrophage-derived interleukin-1beta promotes human breast cancer cell migration and lymphatic adhesion in vitro. Cancer Immunol. Immunother 66:1287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bron S, Henry L, Faes-Van’t Hull E, Turrini R, Vanhecke D, et al. 2016. TIE-2-expressing monocytes are lymphangiogenic and associate specifically with lymphatics of human breast cancer. Oncoimmunology 5:e1073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, et al. 2002. Overexpression of hypoxia-inducible factor 1α is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin. Cancer Res 8:1831–7 [PubMed] [Google Scholar]
- 94.Mantovani A 2010. La mala educacíon of tumor-associated macrophages: diverse pathways and new players. Cancer Cell 17:111–12 [DOI] [PubMed] [Google Scholar]
- 95.Mantovani A 2010. Role of inflammatory cells and mediators in tumor invasion and metastasis. Cancer Metastas. Rev 29:241. [DOI] [PubMed] [Google Scholar]
- 96.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. 2009. Myeloid cells contribute to tumor lymphangiogenesis. PLOS ONE 4:e7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, et al. 2005. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig 115:2363–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y. 2006. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells 24:2733–43 [DOI] [PubMed] [Google Scholar]
- 99.De Palma M, Venneri MA, Roca C, Naldini L. 2003. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat. Med 9:789–95 [DOI] [PubMed] [Google Scholar]
- 100.Ran S, Montgomery KE. 2012. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers 4:618–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kerjaschki D 2005. The crucial role of macrophages in lymphangiogenesis. J. Clin. Investig 115:2316–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, et al. 2004. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res 64:3737–40 [DOI] [PubMed] [Google Scholar]
- 103.Zumsteg A, Christofori G. 2009. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr. Opin. Oncol 21:60–70 [DOI] [PubMed] [Google Scholar]
- 104.Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, et al. 2017. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med 9:eeal4712. [DOI] [PubMed] [Google Scholar]
- 105.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, et al. 2013. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 27:1114–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.García Nores GD, Ly CL, Cuzzone DA, Kataru RP, Hespe GE, Torrisi JS, et al. 2018. CD4+ T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun 9(1):1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, et al. 2011. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34:96–107 [DOI] [PubMed] [Google Scholar]
- 108.Alishekevitz D, Gingis-Velitski S, Kaidar-Person O, Gutter-Kapon L, Scherer SD, et al. 2016. Macrophage-induced lymphangiogenesis and metastasis following paclitaxel chemotherapy is regulated by VEGFR3. Cell Rep 17:1344–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu J, Escamilla J, Mok S, David J, Priceman S, et al. 2013. CSF1R signaling blockade stanches tumorinfiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 73:2782–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis CE, Pollard JW. 2006. Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–12 [DOI] [PubMed] [Google Scholar]
- 111.Ruffell B, Coussens LM. 2015. Macrophages and therapeutic resistance in cancer. Cancer Cell 27:462–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang X, Gao L, Wang S, Lee CK, Ordentlich P, Liu B. 2009. HDAC inhibitor SNDX-275 induces apoptosis in erbB2-overexpressing breast cancer cells via down-regulation of erbB3 expression. Cancer Res 69:8403–11 [DOI] [PubMed] [Google Scholar]
- 114.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, et al. 2009. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, et al. 2011. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. PNAS 108:302–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, et al. 2011. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, et al. 2015. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med 212:1043–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Celus W, Di Conza G, Oliveira AI, Ehling M, Costa BM, et al. 2017. Loss of caveolin-1 in metastasis-associated macrophages drives lung metastatic growth through increased angiogenesis. Cell Rep 21:2842–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qian BZ, Zhang H, Li J, He T, Yeo EJ, et al. 2015. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J. Exp. Med 212:1433–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chitu V, Stanley ER. 2006. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol 18:39–48 [DOI] [PubMed] [Google Scholar]
- 121.Srivastava K, Hu J, Korn C, Savant S, Teichert M, et al. 2014. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer Cell 26:880–95 [DOI] [PubMed] [Google Scholar]
- 122.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, et al. 2009. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. PNAS 106:6742–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, et al. 2007. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol 25:911–20 [DOI] [PubMed] [Google Scholar]
- 124.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, et al. 2010. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Investig 120:2030–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, et al. 2007. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450:903–7 [DOI] [PubMed] [Google Scholar]
- 126.Müller-Hermelink N, Braumüller H, Pichler B, Wieder T, Mailhammer R, et al. 2008. TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13:507–18 [DOI] [PubMed] [Google Scholar]
- 127.Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. 2014. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene 33:1743–54 [DOI] [PubMed] [Google Scholar]
- 128.Mazzone M, Menga A, Castegna A. 2018. Metabolism and TAM functions-it takes two to tango. FEBS J 285:700–16 [DOI] [PubMed] [Google Scholar]
- 129.Henze AT, Mazzone M. 2016. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig 126:3672–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. 2014. Metabolism of stromal and immune cells in health and disease. Nature 511:167–76 [DOI] [PubMed] [Google Scholar]
- 131.Wenes M, Shang M, Di Matteo M, Goveia J, Martin-Pérez R, et al. 2016. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab 24:701–15 [DOI] [PubMed] [Google Scholar]
- 132.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, et al. 2014. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res 74:24–30 [DOI] [PubMed] [Google Scholar]
- 133.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, et al. 2004. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med 10:858–64 [DOI] [PubMed] [Google Scholar]
- 134.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. 2003. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425:307–11 [DOI] [PubMed] [Google Scholar]
- 135.Lewis CE, De Palma M, Naldini L. 2007. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res 67:8429–32 [DOI] [PubMed] [Google Scholar]
- 136.Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, et al. 2012. miR-511–3p modulates genetic programs of tumor-associated macrophages. Cell Rep 1:141–54 [DOI] [PubMed] [Google Scholar]
- 137.Casazza A, Mazzone M. 2014. Altering the intratumoral localization of macrophages to inhibit cancer progression. Oncoimmunology 3:e27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, et al. 2012. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO Mol. Med 4:234–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miyauchi JT, Caponegro MD, Chen D, Choi MK, Li M, Tsirka SE. 2018. Deletion of neuropilin 1 from microglia or bone marrow-derived macrophages slows glioma progression. Cancer Res 78:685–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marone G, Varricchi G, Loffredo S, Granata F. 2016. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol 778:146–51 [DOI] [PubMed] [Google Scholar]
- 141.Morisada T, Oike Y, Yamada Y, Urano T, Akao M, et al. 2005. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 105:4649–56 [DOI] [PubMed] [Google Scholar]
- 142.Fiedler U, Augustin HG. 2006. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 27:552–58 [DOI] [PubMed] [Google Scholar]
- 143.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, et al. 2013. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24:695–709 [DOI] [PubMed] [Google Scholar]
- 144.Lewis C, Murdoch C. 2005. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am. J. Pathol 167:627–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, et al. 2003. HIF-1αis essential for myeloid cell-mediated inflammation. Cell 112:645–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, et al. 2009. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood 114:844–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, et al. 2010. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res 70:7465–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, et al. 2010. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Investig 120:2699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Takeda Y, Costa S, Delamarre E, Roncal C, de Oliveira RL, et al. 2011. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479:122–U53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hamm A, Veschini L, Takeda Y, Costa S, Delamarre E, et al. 2013. PHD2 regulates arteriogenic macrophages through TIE2 signalling. EMBO Mol. Med 5:843–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Joshi S, Singh AR, Zulcic M, Durden DL. 2014. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α and HIF2αstability and tumor growth, angiogenesis, and metastasis. Mol. Cancer Res 12:1520–31 [DOI] [PubMed] [Google Scholar]
- 152.Di Conza G, Trusso Cafarello S, Loroch S, Mennerich D, Deschoemaeker S, et al. 2017. The mTOR and PP2A pathways regulate PHD2 phosphorylation to fine-tune HIF1α levels and colorectal cancer cell survival under hypoxia. Cell Rep 18:1699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, et al. 2010. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev 24:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, et al. 2016. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 539:443–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, et al. 2016. PI3Kγ is a molecular switch that controls immune suppression. Nature 539:437–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, et al. 2017. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell 32:669–83.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, et al. 2016. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell 166:1117–31.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gropper Y, Feferman T, Shalit T, Salame TM, Porat Z, Shakhar G. 2017. Culturing CTLs under hypoxic conditions enhances their cytolysis and improves their anti-tumor function. Cell Rep 20:2547–55 [DOI] [PubMed] [Google Scholar]
- 159.Hasan A, Mazzone M. 2015. Sixty shades of oxygen—an attractive opportunity for cancer immunotherapy. Ann. Transl. Med 3:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hatfield SM, Sitkovsky M. 2015. Oxygenation to improve cancer vaccines, adoptive cell transfer and blockade of immunological negative regulators. Oncoimmunology 4:e1052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, et al. 2017. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544:250–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, et al. 2008. Vascular normalization in Rgs5- deficient tumours promotes immune destruction. Nature 453:410–14 [DOI] [PubMed] [Google Scholar]
- 163.Pearce EJ, Pearce EL. 2018. Immunometabolism in 2017: Driving immunity: all roads lead to metabolism. Nat. Rev. Immunol 18:81–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. 2014. Functional relationship between tumor-associated macrophages and macrophage colony-stimulating factor as contributors to cancer progression. Front. Immunol 5:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, et al. 2016. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30:968–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Q, He Z, Huang M, Liu T, Wang Y, et al. 2018. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun 9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fekete K, Gyorei E, Lohner S, Verduci E, Agostoni C, Decsi T. 2015. Long-chain polyunsaturated fatty acid status in obesity: a systematic review and meta-analysis. Obes. Rev 16:488–97 [DOI] [PubMed] [Google Scholar]
- 168.Song H, Lim DY, Jung JI, Cho HJ, Park SY, et al. 2017. Dietary oleuropein inhibits tumor angiogenesis and lymphangiogenesis in the B16F10 melanoma allograft model: a mechanism for the suppression of high-fat diet-induced solid tumor growth and lymph node metastasis. Oncotarget 8:32027–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chang CI, Liao JC, Kuo L. 2001. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res 61:1100–6 [PubMed] [Google Scholar]
- 170.Klug F, Prakash H, Huber PE, Seibel T, Bender N, et al. 2013. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24:589–602 [DOI] [PubMed] [Google Scholar]
- 171.Rivera LB, Bergers G. 2013. Location, location, location: macrophage positioning within tumors determines pro- or antitumor activity. Cancer Cell 24:687–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Newsholme P 2001. Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr 131:2515S–22S [DOI] [PubMed] [Google Scholar]
- 173.Hubert-Buron A, Leblond J, Jacquot A, Ducrotte P, Dechelotte P, Coeffier M. 2006. Glutamine pretreatment reduces IL-8 production in human intestinal epithelial cells by limiting IκBα ubiquitination. J. Nutr 136:1461–65 [DOI] [PubMed] [Google Scholar]
- 174.da Silva R, Levillain O, Brosnan JT, Araneda S, Brosnan ME. 2013. The effect of portacaval anastomosis on the expression of glutamine synthetase and ornithine aminotransferase in perivenous hepatocytes. Can. J. Physiol. Pharmacol 91:362–68 [DOI] [PubMed] [Google Scholar]
- 175.Clem BF, O’Neal J, Klarer AC, Telang S, Chesney J. 2016. Clinical development of cancer therapeutics that target metabolism. QJM 109:367–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rigamonti N, Kadioglu E, Keklikoglou I, Wyser Rmili C, Leow CC, De Palma M. 2014. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep 8:696–706 [DOI] [PubMed] [Google Scholar]
- 177.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. 2005. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8:299–309 [DOI] [PubMed] [Google Scholar]
- 178.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. 2008. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol 18:372–78 [DOI] [PubMed] [Google Scholar]
- 179.Jung K, Heishi T, Khan OF, Kowalski PS, Incio J, et al. 2017. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J. Clin. Investig 127:3039–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, et al. 2011. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J. Clin. Investig 121:1969–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pietras K, Hanahan D. 2005. A multitargeted, metronomic, and maximum-tolerated dose “chemoswitch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol 23:939–52 [DOI] [PubMed] [Google Scholar]
- 182.Allen E, Walters IB, Hanahan D. 2011. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin. Cancer Res 17:5299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, et al. 2017. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med 9:eaak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassara A, Wyser Rmili C, et al. 2017. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med 9:eaak9670. [DOI] [PubMed] [Google Scholar]
- 185.Ager A, May MJ. 2015. Understanding high endothelial venules: lessons for cancer immunology. Oncoimmunology 4:e1008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thienpont B, Lambrechts D. 2017. It’s T time for normal blood vessels. Dev. Cell 41:125–26 [DOI] [PubMed] [Google Scholar]
- 187.Khan KA, Kerbel RS. 2018. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol 15:310–24 [DOI] [PubMed] [Google Scholar]
- 188.Kroemer G, Galluzzi L. 2015. Combinatorial immunotherapy with checkpoint blockers solves the problem of metastatic melanoma—an exclamation sign with a question mark. Oncoimmunology 4:e1058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Campesato LF, Merghoub T. 2017. Antiangiogenic therapy and immune checkpoint blockade go hand in hand. Ann. Transl. Med 5:497. [DOI] [PMC free article] [PubMed] [Google Scholar]