Cardiopulmonary exercise testing (CPET) is often helpful to shed light on the mechanisms of exercise intolerance in different clinical populations. Although specific response patterns are rarely pathognomonic, an integrative approach considering metabolic and mechanical–ventilatory responses in addition to limiting symptoms has been valuable to guide further investigations [1].
Short abstract
A flattened or decreasing O2 pulse trajectory during incremental CPET is commonly found in patents with low exercise stroke volume but not in those with severely impaired muscle O2 utilisation. This finding should prompt additional cardiovascular work-up. http://bit.ly/2HRE739
To the Editor:
Cardiopulmonary exercise testing (CPET) is often helpful to shed light on the mechanisms of exercise intolerance in different clinical populations. Although specific response patterns are rarely pathognomonic, an integrative approach considering metabolic and mechanical–ventilatory responses in addition to limiting symptoms has been valuable to guide further investigations [1].
Noninvasive CPET, however, remains poorly informative regarding the central haemodynamic responses to exertion [2]. This represents a major test drawback, particularly for patients with multiple potential causes of exercise intolerance. Thus, identification of a noninvasive CPET variable able to provide insights into exertional stroke volume remains an important unmet clinical need.
Oxygen pulse (oxygen uptake (V′O2)/heart rate (HR) ratio) has the potential to help fill this gap. According to the Fick principle, O2 pulse depends on both stroke volume and arteriovenous O2 difference (Ca−vO2). Notably, stroke volume usually plateaus (or asymptotes) at mid-range incremental exercise [3]. In contrast, Ca−vO2 is well described by a linear function of V′O2 up to peak exercise [4]. In other words, O2 pulse from mid-exercise onward is mainly determined by Ca−vO2. Consequently, an early plateau of a low stroke volume that cannot be adequately compensated by a wider Ca−vO2 tends to level-off (of even diminish) O2 pulse [5, 6]. Therefore, a flattened or decreasing O2 pulse trajectory could suggest impaired stroke volume, i.e., cardiocirculatory dysfunction.
Provided these assertions prove true, we reasoned that O2 pulse trajectory abnormalities would be significantly more frequent in a disease primarily associated with low stroke volume (pulmonary arterial hypertension (PAH)) [7] than another characterised by poor muscle O2 extraction and high mixed venous oxygen content (mitochondrial myopathy (MM)) [8]. Confirmation of this hypothesis would hold important clinical implications, as detraining, a main confounder in the interpretation of CPET, is associated with much milder impairment in muscle O2 utilisation compared with MM.
In this context, we performed a retrospective, single-centre study in which 21 treatment-naïve PAH patients and 15 patients with biopsy-proven MM (ragged-red muscle fibres, abnormally low mitochondrial electron transport chain activity and chronic progressive external ophthalmoplegia with normal resting echocardiogram) were included. Subjects had undergone CPET either for clinical reasons (assessment of exercise capacity) or in the context of a previous clinical trial [9] and had given their written informed consent for use of data in research studies.
Incremental CPET was performed on a cycle ergometer following a “ramp” protocol (10 W·min−1). Pulmonary gas exchange and ventilatory variables were obtained from calibrated signals derived from rapidly responding gas analysers and a pneumotachograph (CardiO2 Cycle; Medical Graphics Corp., St Paul, MN, USA). Increases <0.5 mL per beat or progressive decrements in O2 pulse that persisted up to exercise cessation defined a “flattened” or a “decreasing” trajectory, respectively.
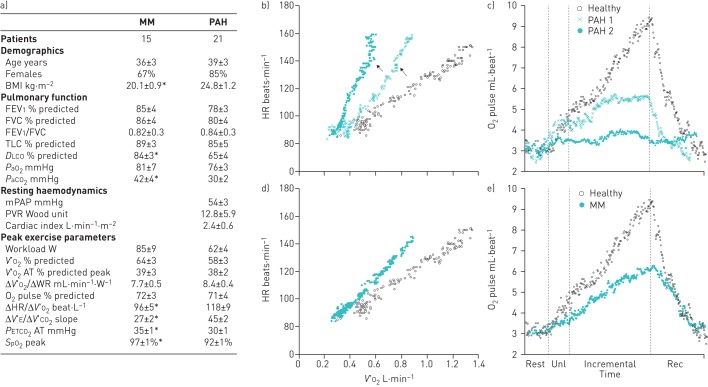
Demographics, pulmonary function, haemodynamics and exercise variables are reported in the figure 1a. The groups were well matched by sex, age and peak V′O2 (% predicted). ΔV′O2/work rate and V′O2 at the estimated lactate threshold (% predicted peak V′O2) were similarly reduced in PAH compared to MM patients. As expected, however, PAH patients showed lower peak O2 saturation by pulse oximetry, lower end-tidal CO2 at the anaerobic threshold and higher ventilation (V′E)–carbon dioxide output (V′CO2) slope.
FIGURE 1.
a) Demographics, pulmonary function, resting haemodynamics and cardiopulmonary exercise testing parameters for patients with mitochondrial myopathy (MM) and pulmonary arterial hypertension (PAH). Data are presented as mean±sem unless otherwise stated. Independent samples Student's t-test was used to compare parametric data between groups. b–e) Key cardiovascular responses during noninvasive cardiopulmonary exercise testing in two representative patients with PAH and a patient with MM. An age- and sex-matched healthy control was also added for comparison. This representation should be considered simply as a reference of expected values and kinetics for the variables of interest: how subjects would have performed had they not been ill. Note that while O2 pulse was downwardly displaced in the MM patient without a discernible change in curve appearance, PAH patients showed a flattened (PAH 1) or decreasing (PAH 2) trajectories at the late stages of exercise (c and e). Those abnormalities coincided with upward inflections of heart rate (HR) as a function of O2 uptake (V′O2) (arrows) in patients with PAH (b), which is not seen in the representative MM patient (d). BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity of the lung for carbon monoxide; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; V′O2: oxygen uptake; AT: anaerobic threshold; WR: work rate; V′E: minute ventilation; V′CO2: carbon dioxide output; PETCO2: end-tidal carbon dioxide tension; SpO2: oxygen saturation measured by pulse oximetry; unl: unloaded exercise; rec: recovery. *: p<0.05.
There were no between-group differences in peak O2 pulse (figure 1a). In line with our hypothesis, however, an abnormal O2 pulse trajectory was found in 14 (67%) out of 21 PAH (13 flattened and one decreasing) but only three (20%) out of 15 MM patients (p<0.001) (see figure 1c and e for representative subjects). In other words, 14 (82.3%) out of 17 patients showing an abnormal O2 pulse trajectory had PAH.
As shown in figure 1b and d for representative subjects, flattening O2 pulse was systematically related to an upward inflection in HR as a function of V′O2. Two MM patients presenting with abnormal O2 pulse trajectory showed a simultaneous plateau in both V′E and V′O2 tracings towards the end of incremental exercise, suggesting that O2 pulse plateau was actually a result of severe ventilatory constraint. By excluding those two patients from the analysis, the presence of abnormal O2 pulse trajectory as a surrogate of abnormal cardiovascular response had a sensitivity of 67%, specificity of 93% and positive predictive value of 93%.
Although using O2 pulse trajectory as a surrogate of stroke volume abnormality during exercise has been previously discussed [6], there is little evidence to support the underlying assumption that this does not reflect impaired muscle O2 utilisation, i.e. low Ca−vO2. Thus, the present study innovates by testing this concept in patients suffering from a disease characterised by profound muscle dysfunction (MM) and who arguably constitute the ideal population to test whether low Ca−vO2 throughout exercise could also level off O2 pulse. Our results showing that a flattened or decreasing O2 pulse is rarely found in these patients but is frequently seen in PAH give support to the notion that abnormal O2 pulse trajectory predominantly reflects impaired stroke volume. Of course, care should be taken to assure that O2 pulse level-off was not caused by a plateau in V′E (and consequently, V′O2) as this would lead to a false positive for primary central cardiocirculatory limitation [10]. In our study, all tests were evaluated by two blinded, independent researchers, but we recognise that observer variability may be a limitation in nonexperienced centres.
We fully recognise the inferential nature of these data owing to the lack of concomitant measurements of stroke volume and Ca−vO2. For instance, whether a flat O2 pulse curve in a minority of MM patients represents critically low O2 utilisation at higher rates of ATP regeneration and/or exercise-induced abnormalities in stroke volume needs further investigation. Conversely, it is unclear why a third of our PAH patients had low peak O2 pulse but normal curve morphology, but this surely demonstrates that a seemingly normal O2 pulse curve should not be unrestrictedly seen as evidence of preserved exertional stroke volume.
In addition, even though subjects were selected based on their primary diagnosis rather than their exercise profile, it is possible that MM patients referred for CPET differ from those in whom exercise tests were not performed. Therefore, the possibility of selection bias cannot be completely overruled. Future studies using invasive CPET and encompassing a larger spectrum of disease severity, both for pulmonary hypertension and MM patients, could certainly provide additional pathophysiological information about our findings.
Notwithstanding these limitations, there is no question that impaired muscle O2 extraction is the dominant pathophysiological feature of MM. However, PAH epitomises a disease in which inability to increase stroke volume on exertion dominates the clinical picture despite some evidence of mild muscle dysfunction [11]. In fact, a steep decrease in mixed venous O2 saturation as a function of exercise intensity is found in these patients [12, 13]. Thus, our patients would tend to present with increased submaximal Ca−vO2. Notably, hypoxaemia, another cause of poor O2 delivery, was not related to O2 pulse abnormalities in our sample, which corroborates the notion that impaired stroke volume was the primary mechanism behind those abnormalities.
Although we were specifically interested in the submaximal O2 pulse behaviour, there are other CPET variables that might help in suggesting cardiocirculatory impairment (e.g. a very early lactate threshold or a sudden downward inflection in V′O2/work rate slope) [1]. Poor ventilatory efficiency (high V′E/V′CO2 or higher V′E–V′CO2 slope/peak V′O2 ratio) has also been used to discriminate central cardiovascular dysfunction from pure muscle deconditioning [14]. However, we do not expect this to hold true when applied to a variety of respiratory disorders, as a high “wasted” ventilation because of increased dead space and/or a low arterial carbon dioxide tension set point would similarly increase V′E–V′CO2 [15]. Therefore, analysis of O2 pulse trajectory and simultaneous changes in ΔHR/Δ V′CO2 linearity might prove particularly helpful when evaluating patients with respiratory diseases who are able to increase their ventilation in response to incremental exercise.
To conclude, in this hypothesis-generating study, a flattened or decreasing O2 pulse trajectory during incremental CPET was commonly found in PAH, a disease characterised by impaired central haemodynamics and low exercise stroke volume. Conversely, these features were rarely seen in MM, a disease typified by severely impaired muscle O2 utilisation. As peripheral muscle detraining is associated with much milder impairment in muscle O2 utilisation compared to MM, “deconditioning” per se would be unlikely to explain a flattened or decreasing O2 pulse trajectory in individual subjects. Thus, such findings should suggest the need for additional cardiovascular work-up in patients who show no evidence of severe concomitant ventilatory constraint. Further studies involving direct measurement of cardiac output and Ca−vO2 during exercise are needed in order to confirm our findings as well as to validate them across different levels of disease severity and even in populations distinct from the ones we studied.
Acknowledgements
The authors would like to thank the whole team of the Clinical Pulmonary Function and Clinical Exercise Physiology Laboratory at Federal University of Sao Paulo (Sao Paulo, Brazil) for their direct or indirect assistance in collecting the data analysed in this manuscript.
Footnotes
Conflict of interest: L.H. Degani-Costa has nothing to disclose.
Conflict of interest: L.E. Nery has nothing to disclose.
Conflict of interest: M.T. Rodrigues has nothing to disclose.
Conflict of interest: A.C. Gimenes has nothing to disclose.
Conflict of interest: E.V. Ferreira has nothing to disclose.
Conflict of interest: J.S. Ota-Arakaki has nothing to disclose.
Conflict of interest: R.P. Ramos has nothing to disclose.
Conflict of interest: J.A. Neder has nothing to disclose.
References
- 1.ERS Task Force, Palange P, Ward SA, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007; 29: 185–209. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Arena R, Halle M, et al. Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016; 133: e694–e711. [DOI] [PubMed] [Google Scholar]
- 3.Stringer WW, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol 1997; 82: 908–912. [DOI] [PubMed] [Google Scholar]
- 4.Stringer WW, Whipp BJ, Wasserman K, et al. Non-linear cardiac output dynamics during ramp-incremental cycle ergometry. Eur J Appl Physiol 2005; 93: 634–639. [DOI] [PubMed] [Google Scholar]
- 5.Whipp BJ, Higgenbotham MB, Cobb FC. Estimating exercise stroke volume from asymptotic oxygen pulse in humans. J Appl Physiol 1996; 81: 2674–2679. [DOI] [PubMed] [Google Scholar]
- 6.Nery LE, Wasserman K, French W, et al. Contrasting cardiovascular and respiratory responses to exercise in mitral valve and chronic obstructive pulmonary diseases. Chest 1983; 83: 446–453. [DOI] [PubMed] [Google Scholar]
- 7.Neder JA, Ramos RP, Ota-Arakaki JS, et al. Exercise intolerance in pulmonary arterial hypertension. The role of cardiopulmonary exercise testing. Ann Am Thorac Soc 2015; 12: 604–612. [DOI] [PubMed] [Google Scholar]
- 8.Tarnopolsky M. Exercise testing in metabolic myopathies. Phys Med Rehabil Clin N Am 2012; 23: 173–186. [DOI] [PubMed] [Google Scholar]
- 9.Gimenes AC, Neder JA, Dal Corso S, et al. Relationship between work rate and oxygen uptake in mitochondrial myopathy during ramp-incremental exercise. Braz J Med Biol Res 2010; 44: 354–360. [DOI] [PubMed] [Google Scholar]
- 10.Chuang ML, Lin IF, Huang SF, et al. Patterns of oxygen pulse curve in response to incremental exercise in patients with chronic obstructive pulmonary disease–an observational study. Sci Rep 2017; 7: 10929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farina S, Correale M, Bruno N, et al. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev 2018; 27: 170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson S, Johnson M. Is systemic oxygen extraction on exercise impaired in pulmonary arterial hypertension? Eur Respir J 2013; 42: Suppl. 57, 4833. [Google Scholar]
- 13.Deboeck G, Niset G, Lamotte M, et al. Exercise testing in pulmonary arterial hypertension and in chronic heart failure. Eur Respir J 2004; 23: 747–751. [DOI] [PubMed] [Google Scholar]
- 14.Rozenbaum Z, Khoury S, Aviram G, et al. Discriminating circulatory problems from deconditioning – echocardiogram and cardio-pulmonary exercise test analysis. Chest 2017; 151: 431–440. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell DE, Laveneziana P, Webb K, et al. Chronic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med 2014; 35: 51–69. [DOI] [PubMed] [Google Scholar]