Abstract
Preventive healthcare is the focus of a large proportion of UK small animal veterinary consultations. The evidence base for how to optimise these consultations is limited. Therefore, evidence-based practical recommendations are needed for veterinary surgeons conducting these consultations. The aim of this study was to use an evidence-based methodology to develop the first consensus recommendations to improve dog and cat preventative healthcare consultations (PHCs).
Evidence from multiple sources was systematically examined to generate a list of 18 recommendations. Veterinary surgeons and pet owners with extensive experience of PHCs were recruited to an anonymous panel to obtain consensus on whether these recommendations would improve PHCs. A Delphi technique was followed during three rounds of online questionnaire, with consensus set at 80 per cent agreement or disagreement with each recommendation. Thirteen of the original 18 recommendations reached consensus (>80per cent agreement), while the five remaining recommendations did not reach consensus.
Globally, these are the first evidence-based recommendations developed specifically in relation to small animal general practice PHCs, generated via a Delphi panel including both veterinary surgeons and pet owners. Future work is needed to understand how these recommendations can be implemented in a range of veterinary practice settings.
Keywords: consultations, preventative healthcare, preventative medicines, Delphi technique, small animal, companion animal, vaccination, general practice
Introduction
Preventative healthcare is the focus of approximately one-third of small animal consultations in the UK.1 Recent research has identified preventative healthcare consultations (PHCs) to be particularly complex,1 including both different content and discussion of a greater number of problems in comparison with other consultation types.2 As a result, PHCs can be under significant time pressures.3 Perspectives on the preferred content and structure of these consultations may differ within and between owners and veterinary surgeons.4 Suggestions have been proposed to improve the consistency and value of these consultations for clients, including the use of checklists5 and promotion of pet health plans.6 A recent survey confirmed that a wide range of strategies are being tried by veterinary surgeons in UK practices,7 but work has not yet been done to determine their effectiveness.
In the absence of good quality evidence, expert opinion can be used to develop recommendations or guidelines for veterinary practitioners. Published guidance typically combines a narrative literature review with opinion from the expert authors.8–10 Ideally, the sources of evidence included should be critically appraised, for example, references 11 12, and the method by which expert opinion has been reached described in detail with recognised methods to achieve consensus.13 Expert opinion has been used to generate recommendations about how preventative medicines should be used.9 14 15 However, recommendations have not been developed for what should happen during the PHCs, and the evidence on which to base any recommendations appears weak.16
Since its development by Dalkey and Helmer,17 the Delphi technique has been widely used as a method for exploring and achieving agreement, or consensus, on a real-world topic.18–20 This qualitative methodology involves combining the knowledge and opinions of a carefully selected panel of ‘experts’ within a specific field and the completion of iterative rounds of an online survey to obtain group consensus on answers to questions that are highly uncertain.18–21 The online nature of the research allows panellists to be anonymous to each other, but not to the researchers, minimising the risk of response bias,20 facilitating the involvement of groups from a range of backgrounds and avoiding domination of the panel by an individual or subgroup.22 Delphi panels in human healthcare frequently include patients with experience of the condition for which guidance is being developed.23–25 Their involvement ensures that the content is relevant and incorporates their experiences as experts in managing their own conditions.26 27
Previous use of the Delphi technique to generate consensus on diverse topics in veterinary medicine includes: the preferred methodology for replacing a bovine uterine prolapse13; behavioural signs of pain in cats28; and development of learning objectives for an undergraduate neurology course.29 To the authors’ knowledge, owners have not previously been directly involved in the development of veterinary guidelines or recommendations, including those using a Delphi technique.
The aim of this study was to develop consensus recommendations based to improve dog and cat PHCs. The objective was to use a Delphi technique to determine whether consensus could be reached on a range of evidence-based statements that could improve PHCs for dogs and cats.
Materials and methods
The study was conducted in accordance with the Declaration of Helsinki and ethical approval was granted by the ethics committee at the School of Veterinary Medicine and Science, University of Nottingham (Reference number: 1521 150813). Data were handled in accordance with EU Data Protection Directive 95/46/EC.
Explanation and justification of the Delphi technique
The Delphi technique was chosen to create theses recommendations due to the limited evidence base on which to draw when developing practical recommendations on the complex topic of improving PHCs. The online format permitted involvement of geographically dispersed individuals, and the anonymous nature of the panel permitted both owners and veterinary surgeons to work together without risk of domination by individuals perceived to have greater expertise or power,22 or the undue influence of strong personality traits.18 The relatively small panel size required ensured the Delphi technique could be conducted in a limited time period, and expert panellists could be recruited from previous research conducted by the authors.
Development of recommendations
Recommendations to be used in the Delphi technique were derived from three data sources: a systematic review to identify measures of success for canine and feline PHCs16; transcripts of interviews with pet owners and veterinary surgeons about PHCs3 4 30 31; and a survey of veterinary surgeons about what currently happens during booster consultations.7 Each of the data sources were comprehensively reviewed, and a list of statements was developed. Any data that suggested an improvement that could be made to any aspect of the owner or veterinary surgeon experience of attending or delivering a PHC was eligible for inclusion in a statement. Statements were independently compiled, then collated and categorised according to inductively derived, agreed subheadings relating to different aspects of the PHC experience. A total of 337 statements were then reviewed and organised into 20 different subheadings with a composite unique recommendation for each. The composite recommendations were then reviewed and iterative changes made to ensure the wording best reflected the data from which they had been derived. A final list of 18 recommendations was agreed on and included in the online questionnaires sent to the Delphi panel.
Identifying and recruiting eligible panellists
Both veterinary surgeons and pet owners were included in the Delphi panel. As they would be the most likely end-user of the recommendations, it was decided that veterinary surgeons should make up the majority of the panel, but sufficient pet owners should be included to prevent consensus being reached based on veterinary surgeon opinion alone. A split of 75 per cent veterinary surgeons/25 per cent pet owners was aimed for.
Veterinary surgeon inclusion criteria were: (A) veterinary surgeon respondents to an earlier survey about dog and cat booster consultations7 who had consented to be contacted about future research and had provided a valid email address; (B) who were currently working in the UK in a practice where they were performing PHCs; and (C) who were on an adjusted pro rata basis performing at least 35 dog and/or cat booster consultations per 40-hour working week. To calculate this latter figure, data on average hours worked per week and average number of PHCs conducted per week were extracted from data provided during the survey and used to calculate an adjusted number of consultations per 40-hour week (by calculating: (40/average hours worked per week) × the number of consultations per week). This figure ranged from 5 to 157 consultations per 40-hour week (median 25, mean 27) with respondents doing 35 or more consults per 40 hour week considered for inclusion in the panel.
Eligible participants were contacted by email to ask if they would be interested in being involved in an anonymous online panel to develop recommendations to improve dog and cat PHCs. The email included a link to a short online questionnaire (SurveyMonkey) to collect the respondents’ name, role in the practice, practice type and preferred email address.
Pet owner inclusion criteria were: (A) residents of the UK who are currently the owner of at least two dogs and/or cats; (B) experience of attending at least five dog or cat PHCs; and (C) attendance at a dog or cat PHC in the three months prior to being contacted. Owners were recruited via: posts on social media describing eligibility criteria and email contact with owners who had previous been involved in research about preventative healthcare with the Centre for Evidence-based Veterinary Medicine. Eligibility criteria were checked with each potentially eligible pet owner by email.
Consensus
It was agreed the consensus level used would be ≥80 per cent agreement or disagreement. This would mean if 80 per cent or more of the panellists agreed or disagreed on a specific recommendation, then this would be classed as ‘consensus reached’. Where consensus was reached, that recommendation was not included in any further rounds of the questionnaire. Where <80 per cent of respondents concurred to agree or disagree, any free-text comments relating to that question were read. Where a recommendation had not reached consensus but participants had not provided suggestions for rewording, the recommendation was included in round 2 unchanged. If less than 80 per cent of the panellists agreed or disagreed on a recommendation once all rounds had been completed, then it would be classed as ‘consensus not reached’. The results for all 18 recommendations will be reported.
Design of the questionnaire
The questionnaire was developed in SurveyMonkey, an online survey provider, by NJR and ZB and pretested with researchers within the Centre for Evidence-based Veterinary Medicine. In the main study, each panel participant received a unique link to each round of the questionnaire by email with a clear explanation of what they were expected to do and the approximate length of time it would take to complete the task. Participants were able to save the results of the questionnaire part way through and return to finish it at a later date, provided they logged in from the same device and had cookies enabled on that device. Questionnaire links were active until closed by the researchers at a date specified to participants. Responses were monitored daily by NJR and ZB. If participants had not responded by a standardised time point, they were sent a reminder email. A second reminder was then sent to those who had still not completed the questionnaire prior to the link being closed. Participants were given two weeks to respond to each round of the survey. All participants were invited to participate in all rounds, independent of whether they had completed the previous round.
Questionnaire rounds
Questionnaire round 1
For each of the 18 recommendations presented, participants were asked to choose one of four options: ‘Agree’; ‘Disagree’; ‘Reword’; or ‘I need more information’. A free-text box after each recommendation gave participants the opportunity to suggest rewording or request additional information, and an open-text box at the end of the questionnaire allowed participants to suggest any additional recommendations they thought should be included. The full questionnaire is available in supplementary material.
Responses were collected in SurveyMonkey, and then transferred to an Excel spreadsheet (Microsoft Office 2013) that was not linked to participant details. Comments relating to both rewording and requests for information were discussed and were used to reword the recommendations where appropriate. Any suggestions for additional recommendations were discussed and included in round 2 if: (A) they were suggested by more than one participant, or (B) were supported by the existing evidence base.
Questionnaire rounds 2 and 3
Recommendations were again displayed as in round 1. Reworded recommendations were included in revised form. Participants were invited to comment or suggest additional recommendations for inclusion. The cut-off for consensus, and data handling, were performed as above.
Recommendations that had reached consensus after round 2 were categorised as ‘Consensus reached’ and were removed from round 3. In addition, recommendations that had still not reached consensus after a second round of consideration by the panel and for which no relevant suggestions for rewording had been suggested were classified as ‘Consensus not reached’ and were removed from round 3. Remaining recommendations were reworded as before if appropriate using free text comments.
The same process as above was then performed for the remaining recommendation(s) in round 3. After the third consensus round, recommendations that has not reached at least 80 per cent agreement or disagreement remained as ‘Consensus not reached’.
Results
Development of the recommendations
The original list of 18 recommendations included in round 1 of the questionnaire and the sources of evidence from which these were drawn are listed in table 1.
Table 1.
List of the 18 recommendations alongside the evidence sources from which each recommendation came
| Original recommendation entered into round 1 of the Delphi panel | Evidence source(s)* | ||||
| 1 | 2 | 3 | 4 | 5 | |
| To improve consistency across their practice, the practice team should agree on the purpose of their preventative healthcare consultations and what they should include. | ✓ | ✓ | ✓ | ✓ | ✓ |
| The practice team should agree on the role of each member of the team (vet, vet nurse, receptionist and so on) in the practice preventative healthcare strategy. | ✓ | ✓ | ✓ | ✓ | ✓ |
| The practice team should agree how details of the costs of preventative healthcare will be communicated to owners. | ✗ | ✓ | ✓ | ✓ | ✓ |
| Prior to each preventative healthcare consultation, practices should make clear to owners the risks associated with preventative medicines and discuss alternatives. | ✗ | ✗ | ✓ | ✓ | ✗ |
| Practices should make clear to owners the benefits of preventative healthcare and medicines to the individual animal, to the pet population and to public health. | ✗ | ✗ | ✓ | ✓ | ✓ |
| The time allocated for each preventative healthcare consultation should be tailored to the individual patient and adjusted for patient age, species and known pre-existing conditions. | ✓ | ✓ | ✓ | ✓ | ✓ |
| Each patient should be allocated at least 15 min for a preventative healthcare consultation. | ✓ | ✓ | ✓ | ✓ | ✓ |
| Prior to each preventative healthcare consultation, the practice should explain to owners what may happen and what topics may be discussed. | ✗ | ✓ | ✓ | ✓ | ✓ |
| Prior to each preventative healthcare consultation, the practice should encourage owners to consider any questions they have about their pet’s health or preventative healthcare. | ✗ | ✓ | ✓ | ✓ | ✓ |
| Prior to each preventative healthcare consultation, the practice should make it clear to owners that the content of the consultation may vary dependent on species, breed, age and health of the patient and well as the needs and experience of the owner(s). | ✓ | ✓ | ✓ | ✓ | ✓ |
| Prior to each preventative healthcare consultation, the practice should make it clear to owners that they can choose which veterinary surgeon they see. | ✗ | ✗ | ✗ | ✓ | ✗ |
| Prior to each preventative healthcare consultation, the practice should encourage owners to consider any questions they have about their pet’s health or preventative healthcare. | ✗ | ✓ | ✓ | ✓ | ✓ |
| At the start of each preventative healthcare consultation, owners should be directly asked how much they understand about preventative healthcare and medicines. | ✗ | ✓ | ✓ | ✓ | ✓ |
| During each preventative healthcare consultation, owners should be encouraged to ask any questions they have about their pet’s health or preventative healthcare. | ✗ | ✓ | ✓ | ✓ | ✗ |
| During each preventative healthcare consultation, a full clinical examination should be undertaken by a veterinary surgeon. | ✓ | ✓ | ✓ | ✓ | ✓ |
| As part of each preventative healthcare consultation, patients should be weighed and have their body condition score assessed using a scale agreed by the practice team. | ✓ | ✓ | ✓ | ✓ | ✓ |
| During each preventative healthcare consultation, owners should be made aware of both normal and abnormal findings from a clinical examination. | ✗ | ✗ | ✓ | ✓ | ✓ |
| During each preventative healthcare consultation, it must be ensured that owners understand the rationale behind any recommendations made and alternatives discussed where appropriate. | ✗ | ✓ | ✓ | ✓ | ✓ |
| At the end of a preventative healthcare consultation, a written summary of the findings and a plan for managing the patient’s healthcare needs should be given to owners. | ✗ | ✗ | ✓ | ✓ | ✓ |
*Key: 1=previous CEVM research; 2=systematic review; 3=interviews with veterinary surgeons; 4=interviews with owners; 5=survey.
Panellists recruited
Thirty-seven eligible veterinary surgeons were identified and contacted. Of these, 28 replied to the invitation to participate, with 26 of these agreeing to take part. Four hundred and fifty-five people engaged with the owner-targeted social media post; of these, nine eligible owners responded and five agreed to participate. A further seven eligible owners who had previously been involved in CEVM research were contacted by email and three agreed to take part.
Questionnaire rounds
Twenty-two of the 26 veterinary surgeons and seven of the eight owners completed at least one questionnaire, an overall response rate of 85 per cent. Demographic characteristics of these participants are given in online supplementary tables S1 and S2. Twenty respondents (16 veterinary surgeons and 4 owners) took part in all three rounds; two veterinary surgeons and two owners participated in two rounds (with one of each participating in rounds one and two, or rounds two and three); and three veterinary surgeons participated in only the first round of the process. Every respondent who took part completed all the questions in the round(s) in which they were involved.
vetrec-2018-104970supp001.pdf (317KB, pdf)
An overview of the results from the questionnaire rounds is presented in figure 1; changes to the recommendations made throughout the process are shown in online supplementary table S3, and the final results are presented in table 2. Contrary to instructions, further emphasised during rounds 2 and 3, many of the suggestions provided by participants for rewording statements related to the feasibility of implementing the recommendations in their own setting. Seven of the 18 recommendations were reworded. No suggestions for additional recommendations were incorporated as they generally related to refinements of the existing recommendations or were not supported by the existing evidence.
Figure 1.
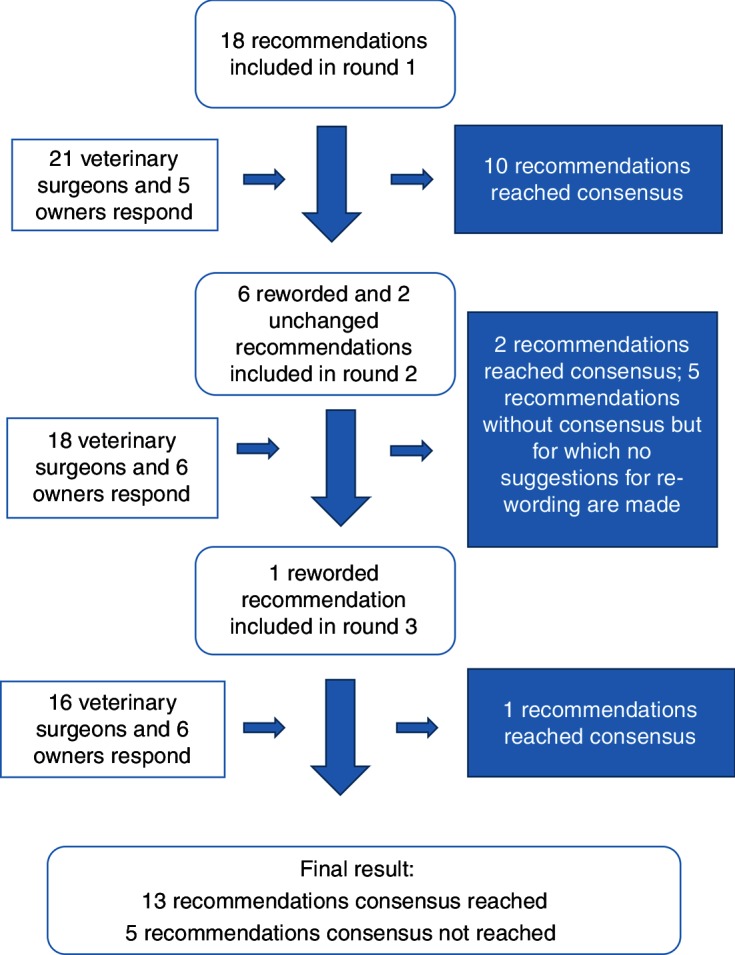
Flow chart detailing the three rounds of questionnaire conducted as part of the Delphi process.
Table 2.
Final list of 18 recommendations alongside details of level of consensus reached for each recommendation
| Final recommendation | Number (percentage) of respondents who agreed with this recommendation during the last round in which it was presented for consideration | Consensus reached? |
| To improve consistency across their practice, the practice team should agree on the purpose of their preventative healthcare consultations and what they should include. | 24/26 (92) | Yes |
| The practice team should agree on the role of each member of the team (vet, vet nurse, receptionist and so on) in the practice preventative healthcare strategy. | 21/26 (81) | Yes |
| The practice team should agree how details of the costs of preventative healthcare will be communicated to owners. | 23/26 (88) | Yes |
| The practice team should agree how potential risks associated with preventative medicines will be communicated to owners. | 23/24 (96) | Yes |
| Practices should make clear to owners the benefits of preventative healthcare and medicines to the individual animal, to the pet population and to public health. | 24/26 (92) | Yes |
| Each patient should be at allocated at least 15 min for a preventative healthcare consultation. | 17/24 (71) | No |
| The time allocated for each preventative healthcare consultation should be tailored to the individual patient and adjusted for patient age, species and known pre-existing conditions. | 22/26 (85) | Yes |
| Prior to, or at the start of, each preventative healthcare consultation, the practice should explain to owners what may happen and what topics may be discussed. | 19/22 (86) | Yes |
| Prior to each preventative healthcare consultation, the practice should encourage owners to consider any questions they have about their pet’s health or preventative healthcare. | 23/26 (88) | Yes |
| Prior to each preventative healthcare consultation, the practice should make it clear to owners that the content of the consultation may vary dependent on species, breed, age and health of the patient. | 17/24 (71) | No |
| Prior to each preventative healthcare consultation, the practice should make it clear to owners that they can choose which veterinary surgeon they would like to see. | 19/24 (79) | No |
| At the start of each preventative healthcare consultation, owners should be asked how much they understand about preventative healthcare and medicines. | 16/24 (67) | No |
| During each preventative healthcare consultation, owners should be encouraged to ask any questions they have about their pet’s health or preventative healthcare. | 25/26 (96) | Yes |
| During each preventative healthcare consultation, a full clinical examination should be undertaken by a veterinary surgeon or veterinary nurse. | 21/24 (88) | Yes |
| As part of each preventative healthcare consultation, patients should be weighed and have their body condition score assessed using a scale agreed by the practice team. | 25/26 (96) | Yes |
| During each preventative healthcare consultation, owners should be made aware of both normal and abnormal findings from a clinical examination. | 25/26 (96) | Yes |
| During each preventative healthcare consultation, it must be ensured that owners understand the rationale behind any recommendations made and alternatives discussed where appropriate. | 25/26 (96) | Yes |
| At the end of a preventative healthcare consultation, a written summary of the findings and a plan for managing the patient’s healthcare needs should be offered to owners. | 18/24 (75) | No |
The final result of the process was that 13 recommendations had reached consensus (all >80 per cent agreement that they would help to improve PHCs) and five had not reached consensus (table 2). Where recommendations were put to the panel more than once, percentage agreement increased with each time. Therefore, the percentages reported are both the highest percentage agreement achieved, and the percentage agreement in the final round in which the recommendation was included. Percentage agreement with the 18 recommendations ranged from 67 per cent to 96 per cent.
Discussion
To the authors’ knowledge, these are the first evidence-based recommendations developed specifically in relation to small animal general practice consultations. This novel study uses the Delphi technique, which is still relatively new to veterinary research and, to the authors’ knowledge, is the first to involve pet owners in the development of veterinary guidance. These recommendations fill a gap in the existing evidence on canine and feline preventative healthcare and provide an important contribution to the literature on companion animal consultation satisfaction.
Thirteen of the 18 recommendations reached consensus of >80 per cent agreement. The other five recommendations each reached a consensus of at least 67 per cent. Our research did not explore reasons why those recommendations did not reach consensus, but a factor may have been concern about how practical they would be to implement. Despite being asked not to consider their feasibility, the majority of comments provided by both veterinary surgeon and owner panellists related to the feasibility or practicality of implementing these recommendations. This suggests these aspects are an important factor in the decisions made about PHCs. Therefore, determining the feasibility of implementing any of these recommendations in a range of practice settings and documenting both potential positive and negative impacts of their implementation will form an important next step in PHC research. Until these data are available, practitioners should consider whether, and how, to implement these recommendations prior to using them and should monitor the effect of any changes made in their specific practice setting.
Given the frequency with which preventative healthcare is discussed in UK companion animal practice,1 it is perhaps surprising that recommendations did not previously exist for how those consultations might be optimised. Indeed, the research base from which these recommendations were drawn is relatively scant. However, consistent themes were identified across the different evidence sources that have been incorporated into these recommendations. We determined that multiple practice staff are involved in preventative healthcare delivery.31 Reflecting this, many of the recommendations were worded to include the practice team, rather than just veterinary surgeons. PHCs in the UK are highly variable in length and content.2–4 7 32 Therefore, several recommendations highlight aspects of the consultation such as a complete clinical examination and weight checks that multiple evidence sources suggested should be core.4 7 16 Interviews with owners3 4 and evidence relating to veterinarian satisfaction with PHCs emphasised the importance of good communication.33 This led to recommendations that focus on the importance of both listening to and communicating with owners and the wider veterinary team. The 18 initial recommendations put to the Delphi panel for review therefore contained a carefully considered distillation of the best available evidence.
Agreement may have been more likely for recommendations that the veterinary panellists were already following or that owners had experienced. For example, in a recent survey, Robinson et al 7 established that 88.7 per cent of respondents always or almost always weigh dogs during PHCs and 76 per cent perform always or almost always perform a body condition score on those patients. It is perhaps unsurprising that the recommendation to weigh and body condition score all patients reached 96 per cent consensus in round 1. The same survey identified that only 44.3 per cent of respondents currently have 15 min allocated for a PHC, which may explain why the recommendation to allocate at least 15 min failed to reach consensus with only 71 per cent agreement. This suggests that published case studies from practices where these recommendations are already being followed may be useful to help overcome feasibility concerns.
It has been argued that the Delphi technique exemplifies the definition of evidence-based medicine by combining the best available evidence with expert opinion22; however, the technique is not completely standardised. The initial list of recommendations for presentation to the panel can be developed by the research team,23 28 34 or the panel can generate recommendations themselves during the first round of the process.19 20 We opted for the former approach, both to ensure that the recommendations were as evidence-based as possible and to limit the time that the process would take. Okoli and Pawlowski20 advocate inclusion of 10–18 participants in a Delphi panel but suggest numbers should be increased if diverse groups are included. Due to the involvement of both owners and veterinary surgeons in our panel, we aimed to include at least 20 panellists but invited more in anticipation of some dropout occurring over the length of the process. Sumsion35 advocates a minimum response rate of 70 per cent to maintain rigour. In the current study, we achieved an overall response rate of 85 per cent from the participants who expressed interest in being involved, with response per round not dropping below 76 per cent. A universally agreed level of consensus does not exist,19 but consensus of greater than 70 per cent is thought to equate to strong agreement.20 We opted for a slightly higher cut-off for consensus at 80 per cent to ensure that recommendations with which all owners disagreed could not reach consensus. This 80 per cent level has been used in other veterinary Delphi research.34 The number of rounds in a Delphi also varies, but three to four rounds are most commonly described21; three were needed in this instance to reach a decision on all recommendations.
Metrics such as test–retest reliability are not relevant in Delphi panels19 since it is both expected and necessary that participants will change their mind during the process.20 Instead, the validity of the results of a Delphi are dependent both on the experts included20 and the response rate.19 Evidence reviewed22 suggests that inclusion of diverse and heterogeneous members in a Delphi panel, particularly the involvement of lay members, improves the quality of the decisions made. Our Delphi included both veterinary surgeons and owners who were particularly experienced in participating in general practice PHCs. This is different to the composition of many of the groups who produce veterinary guidelines, where participation typically necessitates holding a relevant specialist level veterinary qualification.8 Problems associated with excluding patients from evidence-based healthcare research are increasingly recognised.27 Recruitment of owners to participate in our research was relatively simple, and their input was very helpful. We hope this will lead to other researchers including this important group on future veterinary guideline or recommendation development panels.
This research has clear strengths and some weaknesses. These recommendations were developed through comprehensive evidence review and consultation using a diverse expert Delphi panel. The involvement of general practitioners and owners in the development in the process should have ensured that the recommendations agreed are highly relevant and should aid their generalisability. The Delphi process itself has weaknesses, including a lack of clearly standardised methodology. By its nature, the process can involve only a small number of people and may be highly sensitive to factors such as panel composition and question design.18 21 22 The composition of our panel could be criticised in that it included only one male owner. However, previous research involving owners4 36 and owner demographic data37 suggests female owners may be more likely than men to be involved in veterinary research and to visit veterinary practices. In addition, most of our owner and veterinary surgeon participants were from England, not other parts of the UK. It is not currently known how attitudes to pet health or standards of veterinary practice vary by UK region so the impact of this on our ability to extrapolate these results to the whole UK is unclear. Several of the recommendations did not reach consensus. It is possible that adding further information or context to accompany the recommendations may have helped consensus to be reached. Bishop et al 38 successfully employed this strategy in the development of consensus about which children should be referred for specialist assistance with language impairment.
These are the first evidence-based recommendations aimed at improving small animal PHCs. Future work is needed to understand when and how these recommendations can be implemented in a range of veterinary practice settings. Collection of data from practices where recommendations are implemented will be useful in determining their impact on a range of veterinary surgeon, owner, patient and practice-level outcomes. This ‘audit’ step is vital to ensure that the recommendations do have a positive influence on the patients involved in these consults. These recommendations were developed by veterinary surgeons and practitioners in the UK.
Acknowledgments
The authors are grateful to all the owners and veterinary surgeons involved in the Delphi panel.
Footnotes
Funding: This study was supported by a grant from MSD Animal Health. NJR and ZB’s time was funded by MSD Animal Health, and RD and MLB’s time was covered by The University of Nottingham. The topic of study, study design, statistical analysis, interpretation of the results, decision to publish and writing of the manuscript were undertaken independently of the funders.
Competing interests: None declared.
Ethics approval: Ethical approval was granted by the ethics committee at the School of Veterinary Medicine and Science, University of Nottingham (reference number: 1521 150813).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Robinson NJ, Brennan ML, Cobb M, et al. Capturing the complexity of first opinion small animal consultations using direct observation. Vet Rec 2015;176:48 10.1136/vr.102548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson NJ, Brennan ML, Cobb M, et al. Investigating preventive-medicine consultations in first-opinion small-animal practice in the United Kingdom using direct observation. Prev Vet Med 2016;124:69–77. 10.1016/j.prevetmed.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 3. Belshaw Z, Robinson NJ, Dean RS, et al. "I Always Feel Like I Have to Rush…" Pet Owner and Small Animal Veterinary Surgeons' Reflections on Time during Preventative Healthcare Consultations in the United Kingdom. Vet Sci 2018;5 10.3390/vetsci5010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belshaw Z, Robinson NJ, Dean RS, et al. Owners and veterinary surgeons in the united kingdom disagree about what should happen during a small animal vaccination consultation. Vet Sci 2018;5:7 10.3390/vetsci5010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lefebvre S. Critically appraised topic: improving preventive pet care. Banfield Journal 2012;8:3–8. [Google Scholar]
- 6. Ravetz G. Prevention is better than cure: promoting pet health plans. Veterinary Business Journal 2017;170:16–19. [Google Scholar]
- 7. Robinson NJ, Belshaw Z, Brennan ML, et al. Topics discussed, examinations performed and strategies implemented during canine and feline booster vaccination consultations. Veterinary Record. [DOI] [PubMed] [Google Scholar]
- 8. Bhatti SF, De Risio L, Muñana K, et al. International Veterinary Epilepsy Task Force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet Res 2015;11:176 10.1186/s12917-015-0464-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Day MJ, Horzinek MC, Schultz RD, et al. WSAVA Guidelines for the vaccination of dogs and cats. J Small Anim Pract 2016;57:E1–45. 10.1111/jsap.2_12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moriello KA, Coyner K, Paterson S, et al. Diagnosis and treatment of dermatophytosis in dogs and cats.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol 2017;28:266–e68. 10.1111/vde.12440 [DOI] [PubMed] [Google Scholar]
- 11. Boller M, Fletcher DJ. Prelude to RECOVER: time is up for veterinary CPR guidelines. J Vet Emerg Crit Care 2012;22:143–4. 10.1111/j.1476-4431.2012.00732.x [DOI] [PubMed] [Google Scholar]
- 12. Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol 2010;21:233–48. 10.1111/j.1365-3164.2010.00889.x [DOI] [PubMed] [Google Scholar]
- 13. Wapenaar W, Griffiths H, Lowes J, et al. Developing evidence-based guidelines using expert opinion for the managment of uterine prolapse in cattle. Cattle Practice 2011;19:17–21. [Google Scholar]
- 14. Pennisi MG, Hartmann K, Addie DD, et al. Lungworm disease in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2015;17:626–36. 10.1177/1098612X15588455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sainz Á, Roura X, Miró G, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors 2015;8:75 10.1186/s13071-015-0649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robinson NJ, Belshaw Z, Brennan ML, et al. Measuring the success of canine and feline preventative healthcare consultations: a systematic review. Prev Vet Med 2018;158:18–24. 10.1016/j.prevetmed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 17. Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manage Sci 1963;9:458–67. 10.1287/mnsc.9.3.458 [DOI] [Google Scholar]
- 18. C-C HSU, Sandford BA. The Delphi technique: making sense of consensus Practical Assessment. Research and Evaluation 2007;12:1–8. [Google Scholar]
- 19. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008–15. [PubMed] [Google Scholar]
- 20. Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manage 2004;42:15–29. 10.1016/j.im.2003.11.002 [DOI] [Google Scholar]
- 21. Donohoe HM, Needham RD. Moving best practice forward: Delphi characteristics, advantages, potential problems, and solutions. International Journal of Tourism Research 2009;11:415–37. 10.1002/jtr.709 [DOI] [Google Scholar]
- 22. Powell C. The Delphi technique: myths and realities. J Adv Nurs 2003;41:376–82. 10.1046/j.1365-2648.2003.02537.x [DOI] [PubMed] [Google Scholar]
- 23. Mukerji G, Halperin I, Hunter K, et al. Developing a set of indicators to monitor quality in ambulatory diabetes care using a modified Delphi panel process. Int J Qual Health Care 2018;30:65–74. 10.1093/intqhc/mzx167 [DOI] [PubMed] [Google Scholar]
- 24. Schmitt J, Lange T, Günther KP, et al. Indication criteria for total knee arthroplasty in patients with osteoarthritis - a multi-perspective consensus study. Z Orthop Unfall 2017;155:539–48. 10.1055/s-0043-115120 [DOI] [PubMed] [Google Scholar]
- 25. Lin YK, Chen CW, Lee WC, et al. Development and pilot testing of an informed consent video for patients with limb trauma prior to debridement surgery using a modified Delphi technique. BMC Med Ethics 2017;18:67 10.1186/s12910-017-0228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Breejen EM, Hermens RP, Galama WH, et al. Added value of involving patients in the first step of multidisciplinary guideline development: a qualitative interview study among infertile patients. Int J Qual Health Care 2016;28:299–305. 10.1093/intqhc/mzw020 [DOI] [PubMed] [Google Scholar]
- 27. Greenhalgh T, Snow R, Ryan S, et al. Six ’biases' against patients and carers in evidence-based medicine. BMC Med 2015;13:200 10.1186/s12916-015-0437-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merola I, Mills DS. Behavioural signs of pain in cats: an expert consensus. PLoS One 2016;11:e0150040 10.1371/journal.pone.0150040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin YW, Volk HA, Penderis J, et al. Development of learning objectives for neurology in a veterinary curriculum: part I: undergraduates. BMC Vet Res 2015;11:2 10.1186/s12917-014-0315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belshaw Z, Robinson NJ, Dean RS, et al. Motivators and barriers for dog and cat owners and veterinary surgeons in the United Kingdom to using preventative medicines. Prev Vet Med 2018;154:95–101. 10.1016/j.prevetmed.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 31. Belshaw Z, Robinson NJ, Dean RS, et al. Owner and veterinary surgeon perspectives on the roles of veterinary nurses and receptionists in relation to small animal preventive healthcare consultations in the United Kingdom. Vet Rec 2018;183:296 10.1136/vr.104773 [DOI] [PubMed] [Google Scholar]
- 32. Robinson NJ, Dean RS, Cobb M, et al. Consultation length in first opinion small animal practice. Vet Rec 2014;175:486 10.1136/vr.102713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw JR, Adams CL, Bonnett BN, et al. Veterinarian satisfaction with companion animal visits. J Am Vet Med Assoc 2012;240:832–41. 10.2460/javma.240.7.832 [DOI] [PubMed] [Google Scholar]
- 34. Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458 10.1136/bmjopen-2016-011458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sumsion T. The Delphi technique: an adaptive research tool. British Journal of Occupational Therapy 1998;61:153–6. 10.1177/030802269806100403 [DOI] [Google Scholar]
- 36. Belshaw Z, Asher L, Dean RS. The attitudes of owners and veterinary professionals in the United Kingdom to the risk of adverse events associated with using non-steroidal anti-inflammatory drugs (NSAIDs) to treat dogs with osteoarthritis. Prev Vet Med 2016;131:121–6. 10.1016/j.prevetmed.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. THE PEOPLE’S DISPENSARY FOR SICK ANIMALS. (2018) PDSA Animal Wellbeing (PAW) Report. 2017. Available online https://www.pdsa.org.uk/media/3291/pdsa-paw-report-2017_printable-1.pdf (Accessed 9th Apr 2018).
- 38. Bishop DV, Snowling MJ, Thompson PA, et al. CATALISE: a multinational and multidisciplinary delphi consensus study. Identifying language impairments in children. PLoS One 2016;11:e0158753 10.1371/journal.pone.0158753 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
vetrec-2018-104970supp001.pdf (317KB, pdf)


